Introduction
Lung cancer is a common type of clinical malignant
tumor with high incidence and mortality in the respiratory system.
Lung cancer seriously affects the health and life of patients
(1,2).
With the highest incidence among all the subtypes of lung cancer,
non-small cell lung cancer is a main cause of death in patients
(3,4).
Metastasis is common in malignant tumors, and bone metastasis is
the most common type, and bone metastases is observed in
approximately 20–40% of patients with lung cancer (5). Therefore, how to improve the treatment
of bone metastases in lung cancer patients is the key to improving
their quality of life and survival.
At present, treatment with bisphosphonate drugs is
an important method in the treatment of bone metastasis from lung
cancer (6). Studies have shown that
(7,8)
bisphosphonates can significantly relieve bone pain and collapse
caused by bone metastasis. With direct antitumor effects,
bisphosphonates can inhibit the proliferation and diffusion of
cancer cells, induce the apoptosis of cancer cells, and inhibit
angiogenesis. With direct antitumor activities, zoledronic acid can
effectively inhibit the proliferation of small cell lung cancer
cells (9,10). Ibandronate has been proved to be
effective in the treatment of bone cancer pain and bone collapse
caused by bone metastases.
In this study, lung cancer bone metastases rat model
was established to explore the therapeutic effect of zoledronic
acid and ibandronate. The results are as follows.
Materials and methods
Subjects
A total of 124 female SD rats were purchased from
Experimental Animal Center of Jilin University [certificate number:
SCXK (Kyrgyzstan) 2008-0004]. LAD0011 feed was provided by Trophic
Animal Feed High-Tech Co., Ltd. (Nantong, China). All the rats were
aged 8.5 weeks on average, weighed 200–225 g and raised at indoor
temperature of 21.5±0.5°C and humidity of 45–65% with fluorescent
lighting. All rats were allowed to access food and water freely.
The study was approved by the Ethics Committee of Wuwei People's
Hospital (Wuwei, China).
Rat model establishment
Rat model was constructed according to the methods
described by Miller et al (11). Briefly, H1299 cells were cultured with
Roswell Park Memorial Institute (RPMI) medium (Invitrogen; Thermo
Fisher Scientific, Grand Island, NY, USA) containing 10% fetal
bovine serum in an incubator (37°C, 5% carbon dioxide). Adjusting
concentration, 100 µl (1×105) was injected into each rat
by left ventricular. Rat model established 5 days. In the first 10
min before model establishment, rats were injected with 10 mg/kg
low molecular weight heparin enoxaparin (1 mg/ml) through tail vein
to prevent thrombosis. After model construction, rats were kept in
a laminar flow cage and the activities were monitored.
Treatment
The 124 rats with lung cancer bone metastasis were
randomly divided into A, B and C groups (n=30). Group A was treated
with ibandronate combined with zoledronic acid, group B was treated
with zoledronic acid monotherapy, and group C was treated with
ibandronate monotherapy. Rats in group A were injected
subcutaneously with zoledronic acid at a dose of 0.1 mg/kg and
ibandronate at a dose of 10 µg/kg, once per week for 12 weeks; rats
in group B were injected subcutaneously with zoledronic acid, and
rats in group C were injected subcutaneously with ibandronate, the
same method as the treatment group. No treatment was performed for
the remaining 34 rats, so those rats were used as the control
group.
Observation indicators
The 150 SD rats were subjected to radioisotope
scanning, and radioisotope accumulation in bone indicated positive
bone metastasis. Based on this, 124 rats showed bone metastasis.
Changes of body weight were observed. Therapeutic effect was
evaluated by comparing the pain behavior of treated rats and
control group. Improved pain behavior was defined as effective
treatment. Evaluation of physical changes in rats was performed
using a modified tail suspension test (TST).
Observation of pain behavior: Paw withdrawal latency
(PWL): Rats were transferred to observation cage. When rats became
quiet, light was focused on the middle of the toes. The upper limit
of PWL was set as 20 sec to avoid burns. The period from the
beginning of the irradiation to rats showing lifted legs or escaped
was taken as the thermal pain threshold. This experiment was
performed 3 times with an interval of 10 min to calculate the
average time. PWL determined at 1 day before model construction was
used as the baseline value.
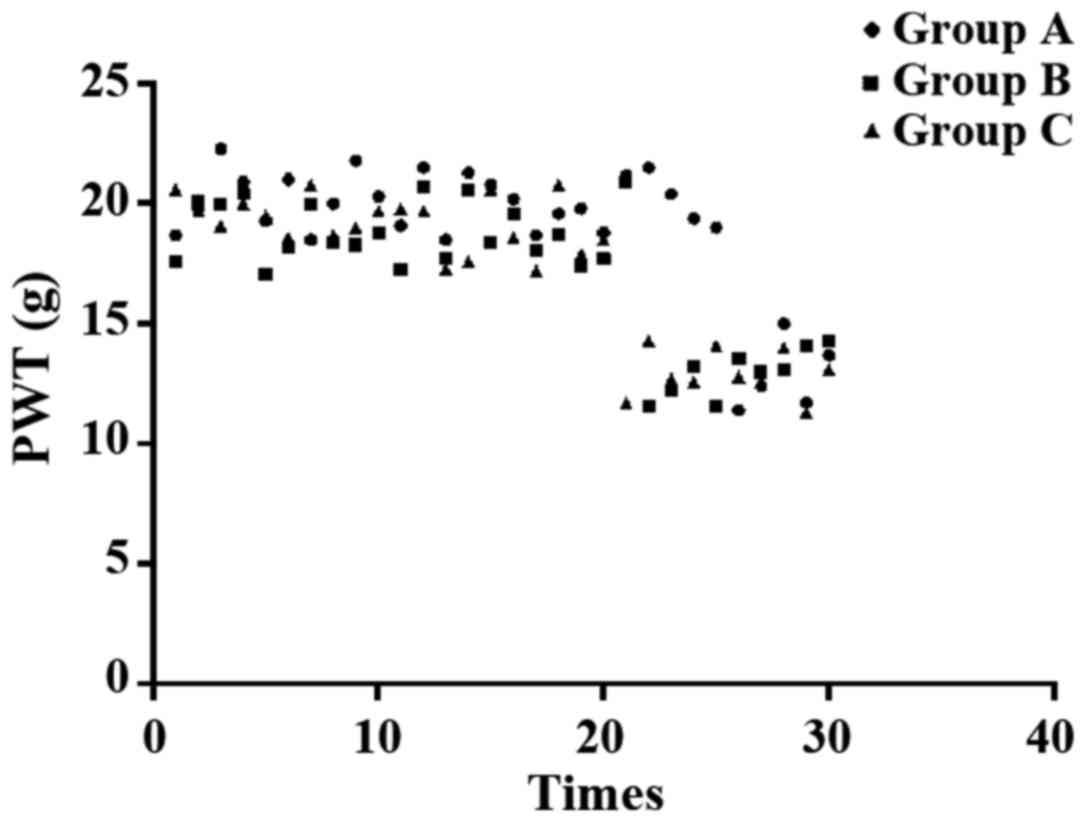
Paw withdrawal mechanical threshold (PWT): The rat
foot was stimulated by von Frey filaments with different
thresholds, and the upper limited was set as 26 g. Rapid paw
withdrawals during or right after stimulation were treated as
positive reaction. The von Frey filament provided 3 positive
reactions in 5 tests with an interval of 10 sec was used as PWT
threshold.
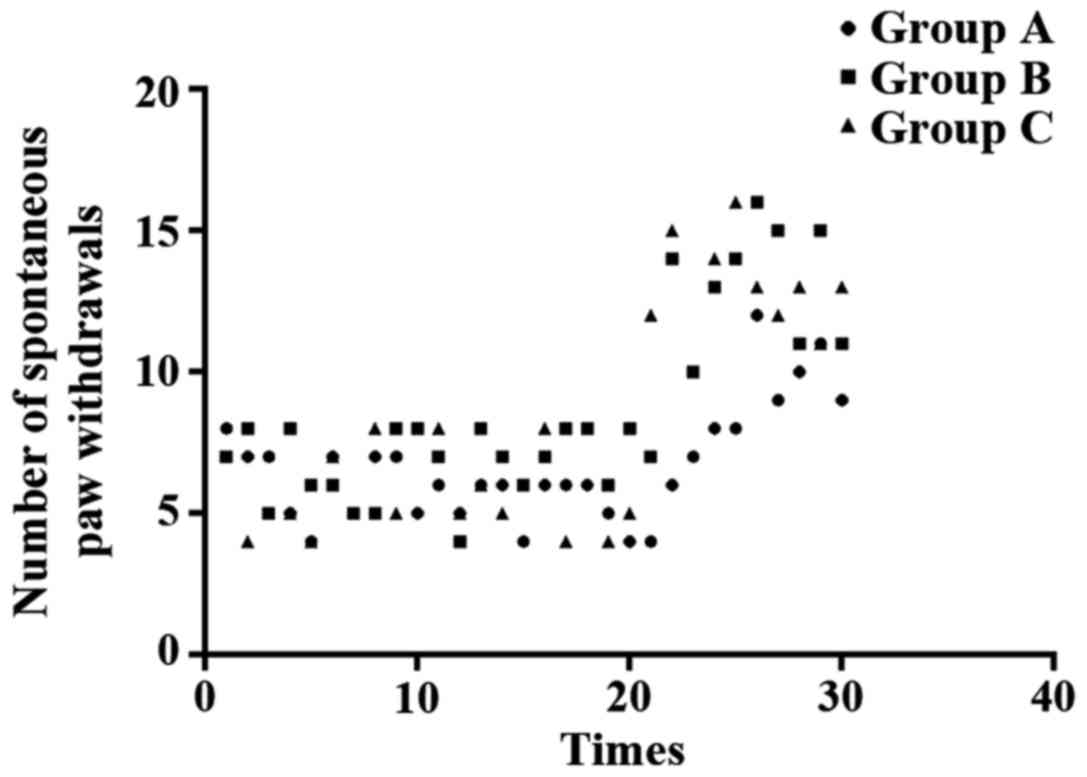
Observation of spontaneous paw withdrawals: Rats
were kept in cage and were allowed to move freely. Walking gait and
the number of paw withdrawals within 90 sec were observed.
Statistical analysis
SPSS 19.0 (SPSS Inc., Chicago, IL, USA) was used for
all statistical analysis. Measurement data were expressed as mean ±
SD, and comparison among multiple groups were performed using ANOVA
and the post hoc was Dunnett test. P<0.05 was considered to
indicate a statistically significant difference.
Results
General information
All the selected rats were healthy female rats with
mild temper. All rats were fed with LAD0011 feed. Model
construction on all 150 rats was performed at the same time, and
model was successfully constructed in 124 rats. All 124 rats showed
no significant changes in weight and length after model
construction (P<0.05). The average age of rats was 8.5±0.2
weeks. The nutritional level was good and the average glucose
concentration was 86.2±11.6 (Table
I).
 | Table I.Comparison of basic information of
rats. |
Table I.
Comparison of basic information of
rats.
| Parameters | Control | A | B | C | P-value |
|---|
| Weight after model
construction (g) | 219.8±10.2 | 222.4±11.3 | 207.6±12.5 | 226.1±10.6 | 0.785 |
| Weight before model
construction (g) | 169.4±7.3 | 176.5±6.8 | 164.2±7.5 | 182.1±7.2 | 0.645 |
| Length (cm) | 18.3±1.2 | 19.5±1.1 | 17.9±1.3 | 20.4±0.9 | 0.693 |
| Age (week) | 8.1±0.4 | 8.5±0.2 | 8.4±0.3 | 8.6±0.1 | 0.899 |
| Glucose (mmol/l) | 82.4±11.3 | 79.8±12.1 | 86.2±11.6 | 84.1±11.5 | 0.685 |
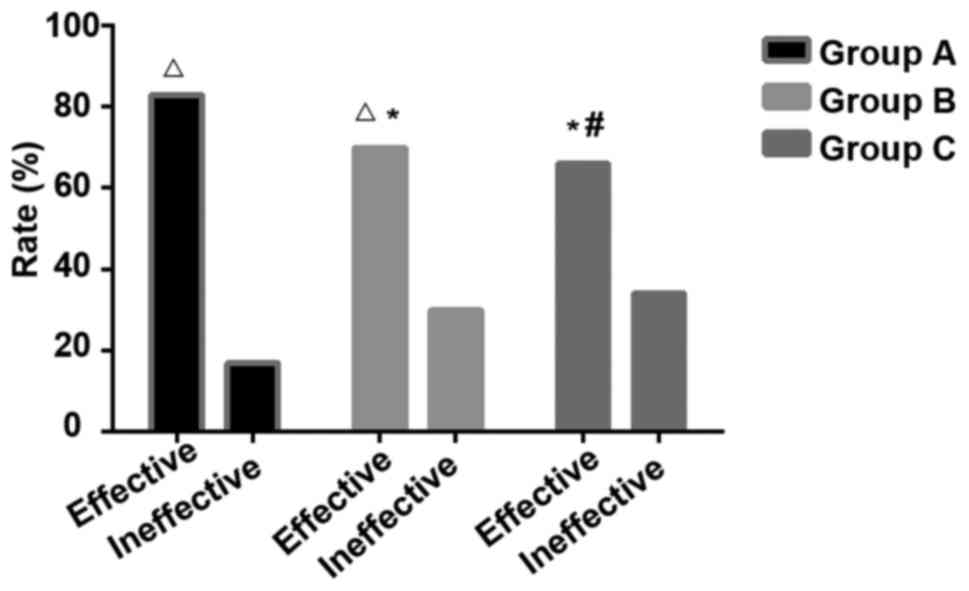
Treatment effect
The mean value of the rats in the control group was
set as standard (spontaneous paw withdrawals 8.3 times, PWL 4.6
sec, PWT 15.7 g), The improvement of pain behavior in rats was used
to evaluate the effect of drug treatment. Twenty-five rats in group
A showed different degrees of improvements, and 5 rats showed no
improvement, and the effective rate was 83.3%; 21 rats in group B
showed different degrees of improvements, and 9 rats showed no
improvement, and the effective rate was 70%; 20 rats in group C
showed different degrees of improvements, and 10 rats showed no
improvement, and the effective rate was 66.7%. Significant
differences in effective rate were found among the 3 groups, A
showed highest effective rate (P<0.05), but no significant
differences were found between group B and C (P>0.05) (Fig. 1). Pain behavioral observation is shown
in Figs. 2–4.
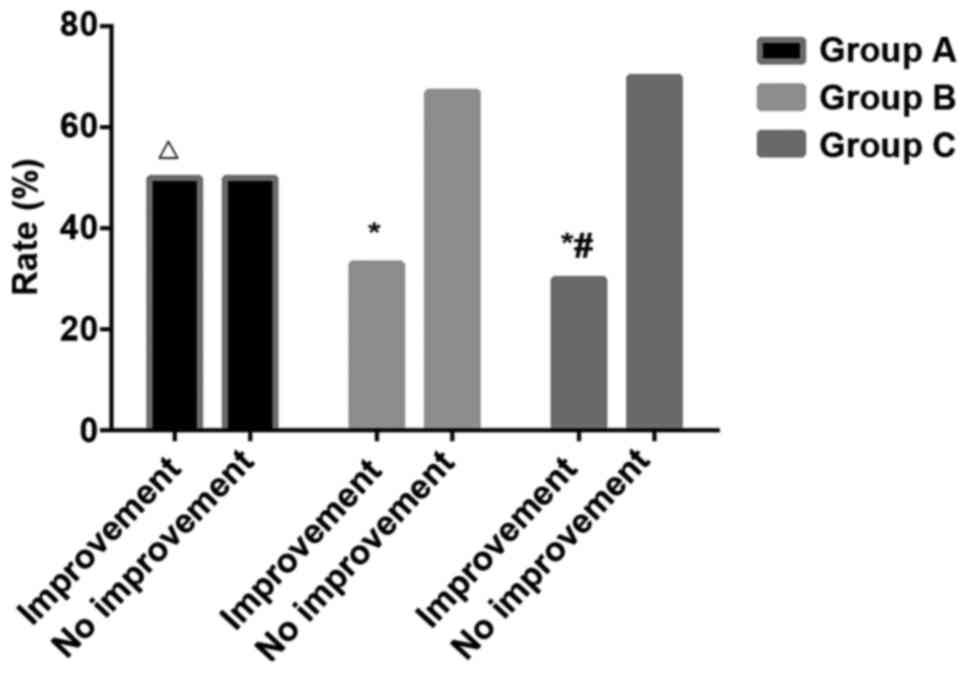
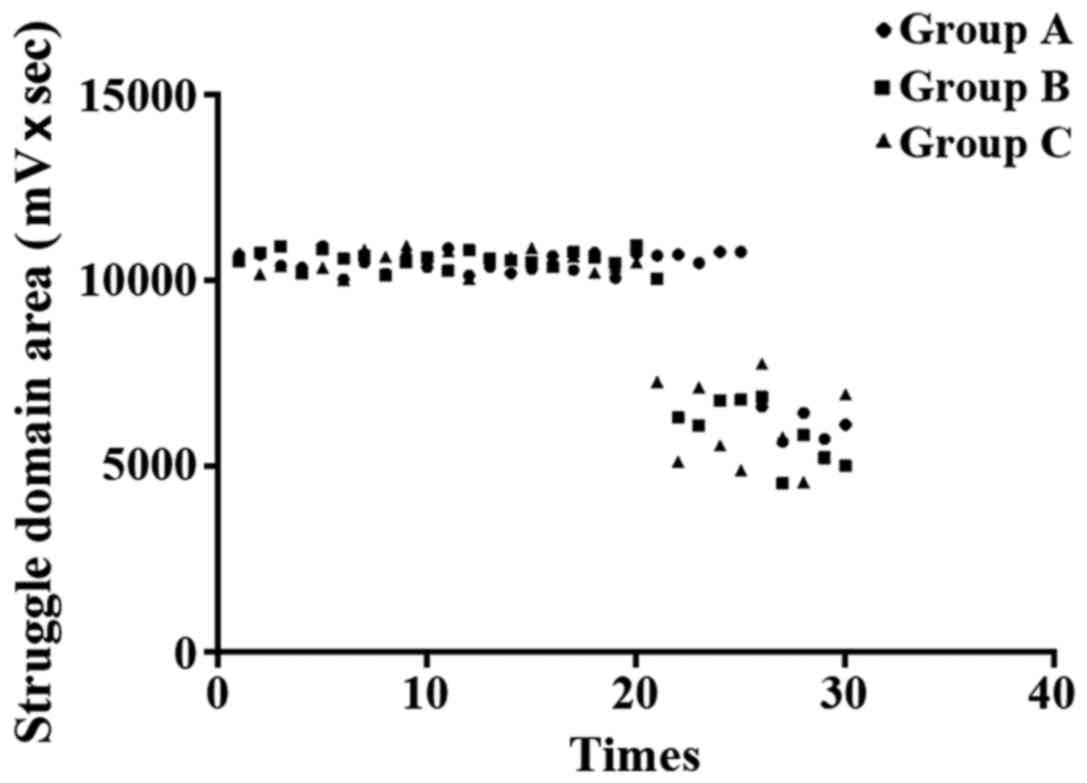
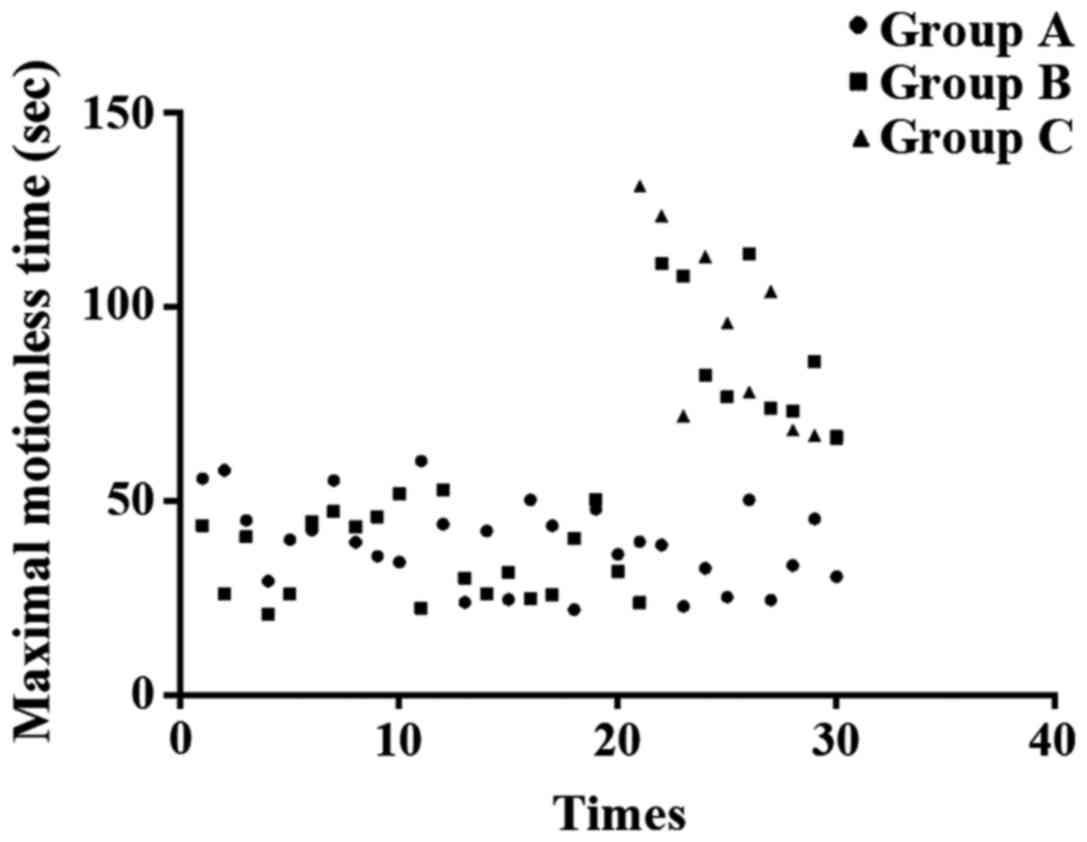
Comparison of physical improvement. With mean value
of rats in control group as a standard (struggle domain area 7348.6
mv × sec, cumulative immobility time 103.6 sec, single cumulative
immobility time 60.5 sec), physical condition of rats in other
groups were evaluated. Out of 30 rats in group A, 15 showed
physical improvement, and the improvement rate was 50%; 10 out of
30 rats in group B showed physical improvement, and the improvement
rate was 33.3%; 9 out of 30 rats in group C showed physical
improvement, and the improvement rate was 30%. Significant
differences in improvement rate were found among the 3 groups, and
group A showed greatest improvement rate (P<0.05) (Fig. 5). Results of the modified TST are
shown in Figs. 6–8.
Discussion
Lung cancer is a major malignancy that affects human
health. The incidence of lung cancer increases with age, and
occurrence in individuals aged 55 or above is most common (12). Lung cancer is the third malignancy in
the United States and is also a leading cause of death in patients
(13). Smoking is a very important
risk factor, and 70–90% of individuals with lung cancer in the
United States smoke. Although smoking rates have been reduced,
30–40% of individuals have a history of smoking (14). Therefore, it is imperative to improve
the treatment of lung cancer, so as to improve the quality of life
and survival of those patients.
Bone is a major site of lung cancer metastasis, and
~40% of patients with non-small cell lung cancer (NSCLC) showed
bone metastases during the course of disease, and bone tissue
lesions were observed in more than 55% of newly diagnosed lung
cancer patients. In this study, a lung cancer bone metastasis model
was established to explore the therapeutic effect of zoledronic
acid and ibandronate. The results of this study show that, compared
with the pain behavior of control group, treatment with zoledronic
acid improved spontaneous paw withdrawal, PWL and PWT in 21 out of
30 rats, and the treatment efficiency is 70%; of the 30 rats
treated with ibandronate, 20 showed improvement, and the effective
rate was 66.7%; of the 30 rats treated with both, the effective
rate reached 83.3%. No significant differences in the effective
rate were found between the treatments with the two drugs alone
(P>0.05). The above results showed that zoledronic acid combined
with ibandronate can effectively improve the symptoms of lung
cancer, and relieve cancer pain in rats. Zoledronic acid is the
most commonly used bisphosphonic acid in the treatment of bone
metastases (5). However, Barrett-Lee
et al (15) found that
zoledronic acid was superior to ibandronic acid in preventing
bone-related events caused by bone metastases. This may be
explained by the different dose, manner of administration, as well
as duration of treatment used in our study. It has been suggested
(16) that zoledronic acid can be
used in the treatment of NSCLC to prevent or delay the occurrence
of bone-related events. Previous findings have shown that, the
therapeutic effect of zoledronic acid on lung cancer is achieved by
inhibiting protein prenylation (17).
Silva et al (18) indicated in
their study that zoledronic acid could alter bone microenvironment
and affect the growth of bone and bone tumors, so as to effectively
reduce the risk of bone-related events. Ibandronate can also
effectively reduce the risk of bone-related events in patients with
lung cancer, and it has been reported that ibandronate can inhibit
tumor cell adhesion, invasion, proliferation, and the interactions
with bone matrix metalloproteinases (19).
Although zoledronic acid and ibandronate have good
therapeutic effects on lung cancer, the combined use of zoledronic
acid and ibandronate in the treatment of lung cancer bone
metastases still has not been reported. Our study provided a
reference for follow-up studies. Luedders et al found the
combined use of zoledronic acid and ibandronate showed good
tolerance and safety on renal toxicity in the 6-month treatment of
breast cancer patients with bone metastases (20). Zoledronic acid and ibandronate acid
are both bisphosphonates, and they can also be used in the
treatment of osteoporosis (21,22) and
other bone diseases. More studies are needed to explore the roles
of bisphosphonates in the treatment of bone diseases.
In summary, ibandronate and zoledronic acid have
promising effects on improving the symptoms of bone metastases in
lung cancer, and the combined use of the drugs can significantly
improve the efficacy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the presend
study are available from the corresponding author on reasonable
request.
Authors' contributions
GW and JC established rat model. RM helped with
radioisotope scanning. WX and CY recorded and analyzed pain
behavior. CN observed spontaneous paw withdrawals. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Wuwei People's Hospital (Wuwei, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lortet-Tieulent J, Soerjomataram I, Ferlay
J, Rutherford M, Weiderpass E and Bray F: International trends in
lung cancer incidence by histological subtype: Adenocarcinoma
stabilizing in men but still increasing in women. Lung Cancer.
84:13–22. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tissot C, Couraud S, Tanguy R, Bringuier
PP, Girard N and Souquet PJ: Clinical characteristics and outcome
of patients with lung cancer harboring BRAF mutations. Lung Cancer.
91:23–28. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: KEYNOTE-024 Investigators: Pembrolizumab versus
chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl
J Med. 375:1823–1833. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
D'Antonio C, Passaro A, Gori B, Del
Signore E, Migliorino MR, Ricciardi S, Fulvi A and de Marinis F:
Bone and brain metastasis in lung cancer: Recent advances in
therapeutic strategies. Ther Adv Med Oncol. 6:101–114. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jung RS, Mittal BR, Santhosh S, Sood A,
Bhattacharya A and Kapoor R: Is femoral uptake of Tc99m-methylene
diphosphonate on bone scintigraphy in bronchogenic carcinoma an
alarming sign: A case report and brief review of literature? Lung
India. 31:280–281. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Henry D, Vadhan-Raj S, Hirsh V, von Moos
R, Hungria V, Costa L, Woll PJ, Scagliotti G, Smith G, Feng A, et
al: Delaying skeletal-related events in a randomized phase 3 study
of denosumab versus zoledronic acid in patients with advanced
cancer: An analysis of data from patients with solid tumors.
Support Care Cancer. 22:679–687. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Murakami H, Yamanaka T, Seto T, Sugio K,
Okamoto I, Sawa T, Hirashima T, Takeda K, Atagi S, Fukuoka M, et
al: Phase II study of zoledronic acid combined with docetaxel for
non-small-cell lung cancer: West Japan Oncology Group. Cancer Sci.
105:989–995. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dimitroulis IA, Dervas A, Vasileiou S and
Toumbis M: Comparative efficacy of ibandronic acid vs. Zoledronic
acid in lung cancer patients with skeletal metastases. Do they
alleviate the patient? J Thorac Oncol. 9:S203–S204. 2014.
|
|
10
|
LeVasseur N, Clemons M, Hutton B, Shorr R
and Jacobs C: Bone-targeted therapy use in patients with bone
metastases from lung cancer: A systematic review of randomized
controlled trials. Cancer Treat Rev. 50:183–193. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miller RE, Jones JC, Tometsko M, Blake ML
and Dougall WC: RANKL inhibition blocks osteolytic lesions and
reduces skeletal tumor burden in models of non-small-cell lung
cancer bone metastases. J Thorac Oncol. 9:345–354. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moyer VA: U.S. Preventive Services Task
Force: Screening for lung cancer: U.S. Preventive Services Task
Force recommendation statement. Ann Intern Med. 160:330–338. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moolgavkar SH, Holford TR, Levy DT, Kong
CY, Foy M, Clarke L, Jeon J, Hazelton WD, Meza R, Schultz F, et al:
Impact of reduced tobacco smoking on lung cancer mortality in the
United States during 1975–2000. J Natl Cancer Inst. 104:541–548.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Humphrey L, Deffebach M, Pappas M, Baumann
C, Artis K, Mitchell JP, Zakher B, Fu R and Slatore C: Screening
for Lung Cancer: Systematic Review to Update the U.S. Preventive
Services Task Force Recommendation [Internet]. U.S. Preventive
Services Task Force Evidence Syntheses, formerly Systematic
Evidence Reviews. 157:7062013.
|
|
15
|
Barrett-Lee P, Casbard A, Abraham J, Hood
K, Coleman R, Simmonds P, Timmins H, Wheatley D, Grieve R,
Griffiths G, et al: Oral ibandronic acid versus intravenous
zoledronic acid in treatment of bone metastases from breast cancer:
A randomised, open label, non-inferiority phase 3 trial. Lancet
Oncol. 15:114–122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hendriks LE, Hermans BC, van den
Beuken-van Everdingen MH, Hochstenbag MM and Dingemans AM: Effect
of bisphosphonates, denosumab, and radioisotopes on bone pain and
quality of life in patients with non-small cell lung cancer and
bone metastases: A systematic review. J Thorac Oncol. 11:155–173.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xie F, Li P, Gong J, Zhang J and Ma J: The
bisphosphonate zoledronic acid effectively targets lung cancer
cells by inhibition of protein prenylation. Biochem Biophys Res
Commun. 467:664–669. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Silva SC, Wilson C and Woll PJ:
Bone-targeted agents in the treatment of lung cancer. Ther Adv Med
Oncol. 7:219–228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu J, Huang W, Zhou R, Jia S, Tang W, Luo
Y and Zhang J: Bisphosphonates in the treatment of patients with
metastatic breast, lung, and prostate cancer: A Meta-analysis.
Medicine (Baltimore). 94:e20142015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Luedders DW, Steinhoff J, Thill M, Rody A
and Bohlmann MK: Lack of difference in acute nephrotoxicity of
intravenous bisphosphonates zoledronic acid and ibandronate in
women with breast cancer and bone metastases. Anticancer Res.
35:1797–1802. 2015.PubMed/NCBI
|
|
21
|
Qaseem A, Forciea MA, McLean RM and
Denberg TD; Clinical Guidelines Committee of the American College
of Physicians: Treatment of low bone density or osteoporosis to
prevent fractures in men and women: A clinical practice guideline
update from the American College of Physicians. Ann Intern Med.
166:818–839. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jamil K, Zacharin M, Foster B and Munns
CF: Protocol for a randomised control trial of bisphosphonate
(zoledronic acid) treatment in childhood femoral head avascular
necrosis due to Perthes disease. BMJ Paediatrics Open.
1:e0000842017.http://bmjpaedsopen.bmj.com/content/1/1/e000084
View Article : Google Scholar : PubMed/NCBI
|