Introduction
Liver cancer is the fifth and seventh most common
malignancy in men and women, respectively. Approximately 85% of all
cases occur in developing countries (1). Cholangiocarcinoma (CCA) is the second
most common type of liver cancer. It accounts for 15% of all
primary liver cancers, and 3% of all gastrointestinal tumors
worldwide (2,3). There are two types of CCA. Intrahepatic
CCA is highly malignant and its incidence is increasing worldwide.
Extrahepatic CCA is less malignant and more stable in incidence
(4). While CCA is uncommon in the
United States and Europe, it is very common in Thailand and other
Southeast Asian countries. The overall incidence of CCA in the USA
is 2.9 cases/100,000 patients (4),
while in the North and Northeast regions of Thailand it is 30.9
cases/100,000 patients (5). Liver
fluke (Opisthorchis viverrini) is a well-established
contributory factor to CCA in Thailand. Chronic inflammation
induced by liver fluke infection causes malignant transformation of
the bile duct (6). The primary
treatment for CCA is surgical resection. However, patients often
present at a late stage, which makes surgical resection much more
difficult. Despite this difficulty, 25% of these cases undergo the
procedure successfully (5,7). The 5-year patient survival rates are
20–32% for intrahepatic CCA and 30–42% for extrahepatic CCA
(8). This difference is most likely
due to microscopic metastatic lesions and the frequency of positive
surgical margins (9). Despite
extensive research and various new treatment modalities, such as
immunotherapy or signal transduction inhibitors that have been
developed to treat tumors, these have not been effective in CCA
treatment. Thus, other novel modalities of treatment are needed. In
this study, we have explored a new direction for treating CCA by
targeting the metabolic vulnerability of the disease.
In intrahepatic CCA, tumor cells proliferate rapidly
(10) and thus require significant
amounts of amino acids, such as arginine, to support their growth.
Advanced malignancies, including: hepatocellular carcinoma (HCC),
melanoma, prostate cancer, pancreatic cancer, mesothelioma, renal
cell carcinoma, sarcoma, and small cell lung cancer, are deficient
in argininosuccinate synthetase (ASS) expression, the rate-limiting
enzyme in arginine synthesis (11).
Generally, arginine is synthesized from citrulline through two-step
reactions catalyzed by ASS and argininosuccinate lyase (12). Tumor cells that have low ASS
expression are unable to synthesize arginine and must depend on
extracellular arginine (13). Thus,
arginine becomes an essential amino acid. Depletion of arginine in
the extracellular environment can be achieved by the arginine
deiminase (ADI) enzyme (14), a
bacterial enzyme which degrades arginine to citrulline. Pegylated
ADI has been created by conjugating ADI with polyethylene glycol
(ADI-PEG20) to improve its half-life and to suppress its
immunogenicity. Treatment of arginine auxotrophic cancers with
ADI-PEG20 can result in tumor cell death due to lack of ASS
(11,15). Several phase I/II clinical trials have
demonstrated promising clinical benefits with low toxicity when
using ADI-PEG20 to treat patients with ASS-deficient cancers, such
as HCC, metastatic melanoma, and mesothelioma (16). A Phase III clinical trial was
undertaken to treat HCC patients with ADI-PEG20 (11). As reported at ASCO 2016, the
randomized trial showed no overall survival benefit linked to
ADI-PEG20 treatment compared to placebo; however, some patients
appeared to benefit from the treatment.
This study aimed to investigate the expression of
ASS in intrahepatic CCA, and to explore whether ADI-PEG20 could be
useful for CCA treatment. We determined the levels of ASS
expression at both the mRNA and protein level and assessed cell
proliferation via Ki-67 expression in CCA specimens from Thai
patients. We also used two CCA cell lines derived from Thai
patients as in vitro models to test the inhibitory effect of
ADI-PEG20 and correlate with ASS expression. Silencing of ASS
expression was also carried to further confirm that ASS expression
is a key determinant for the antitumor effect of ADI-PEG20.
Materials and methods
Patients and tissue samples
A total of 40 CCA patients, comprising of 24 males
and 16 females with a median age of 60 years (range 48–73 years)
was recruited for this study. All cases underwent surgical
resection. The clinicopathological features of the patients were
collected including gender, age, type of CCA, histopathological
differentiation, TNM staging, lymphovascular invasion, perineural
invasion and viral hepatitis status. Information regarding liver
fluke infection was obtained from questionnaires. Only 2 cases were
reported. No specific test for liver fluke infection was performed.
Paraffin-embedded tissues representing 40 CCA patients, 38 of which
were intrahepatic CCA and 2 of which were perihilar CCA cases, were
obtained from Chulabhorn hospital, and from Srinagarind hospital,
which is affiliated to Khon Kaen Medical University. The
histological types of the CCA tissues were classified according to
the World Health Organization classification (17). This study was conducted according to
the Helsinki declaration for international health research, and was
approved by the Human Research Ethics Committee of Chulabhorn
Research Institute, Bangkok, Thailand (project no. 013/2559 on 17
August 2016). All the subjects gave written informed consent for
participation prior to enrollment in the study.
Cell culture and treatment
Two human CCA cell lines (RmCCA-1 and HuCCA) and a
human fibroblast cell line (BJ-1) were used in this study. The
RmCCA-1 and HuCCA cell lines were established from intrahepatic CCA
specimens derived from Thai patients. The characterization of these
two cell lines has previously been published (18,19). These
CCA cell lines were maintained in DMEM media supplemented with 10%
FBS and Penicillin/Streptomycin. The cells were obtained from the
Chulabhorn Research Institute, Thailand. BJ-1 cells were obtained
from the ATCC. The BJ-1 cells were maintained on EMEM supplemented
with 10% FBS and Penicillin/Streptomycin.
For ADI-PEG20 treatment, cells were seeded and
allowed to attach overnight at 37°C, then treated for 3 days with
0.1 µg/ml of ADI-PEG20 (kindly provided by Polaris Pharmaceuticals
Inc., San Diego, CA, USA). Controls did not receive ADI-PEG20.
For treatment with arginine-free medium, the medium
was prepared as described in Savaraj et al (20) with minor modifications. Briefly, the
medium was pretreated with 0.1 µg/ml of ADI-PEG20 for 3 days prior
to use.
Where ASS siRNA treatment is indicated, cells were
pretreated with 50 nM of either pooled non-target scramble control
siRNA (siNT) or 3 unique 27mer siRNA obtained from OriGene
Technologies, Inc. (Rockville, MD, USA; cat. no. SR300322). The
transfection was accomplished using INTERFERin
(Polyplus-transfection, New York, USA) according to the
manufacturer's protocol. After 3 days of siRNA transfection
with/without ADI treatment, cells were harvested and assayed for
ASS expression by western blot, and for study of growth inhibition
or apoptotic effect of ADI-PEG20 treatment.
Immunohistochemistry
The ASS expression level and Ki-67 proliferation
index were determined for paraffin-embedded CCA tissues (3-µm
sections). Sections were de-paraffinized in xylene and washed
sequentially with 100% and 95% ethanol. Endogenous peroxidase
activity was blocked by incubating slides in 3% hydrogen peroxide
for 20 min at room temperature. Antigen retrieval was carried out
with target retrieval solution (Dako; Agilent Technologies, Inc.,
Santa Clara, CA, USA) for 20 min at 90°C, followed by the blocking
of non-specific binding with the biotin blocking system (Dako;
Agilent Technologies, Inc.) for 10 min. The primary antibodies used
were anti-ASS (mouse monoclonal, 1:100 dilution; BD Biosciences,
Franklin Lakes, NJ, USA) and anti-Ki-67 (MIB-1, mouse monoclonal,
1:100 dilution; Dako; Agilent Technologies, Inc.). Sections were
incubated with primary antibodies overnight at room temperature in
a moist chamber. Subsequently, the sections were washed 3 times in
PBS and incubated with biotinylated anti-mouse immunoglobulins
(Dako; Agilent Technologies, Inc.) for 15 min, followed by
incubation with streptavidin peroxidase conjugate (Dako; Agilent
Technologies, Inc.) for 15 min. The color reaction was developed
with Liquid DAB Substrate-Chromogen (1:50 v/v; Dako; Agilent
Technologies, Inc.). The sections were counterstained with
hematoxylin, dehydrated with graded ethanol and mounted with
Cytoseal-60. For the negative controls, the primary antibody was
replaced with antibody diluent at the appropriate dilution.
Slides were viewed by a pathologist using an Axio
Imager Z2 Microscope (Zeiss GmbH, Jena, Germany) equipped with a
HXG40c Baumer digital camera and the HistoFAXS® Version
4.1 (TissueGnostics GmbH, Vienna, Austria) image analysis system.
In the same tissue sections, the tumor areas were selected and
identified from the invasive areas, whereas the surrounding
non-tumor areas were selected from those in the vicinity of the
tumor areas but with apparent normal cells. At least 4 different
regions of tumor and non-tumor areas were selected for each
specimen.
ASS expression in CCA specimens was classified as
low or high according to a previous report (21). Briefly, IHC analysis of ASS and Ki-67
expression in CCA specimens was determined by HistoFAXS software
Version 4.1 and viewed at 100× magnification. The measurement
parameters included mean intensity and percentage of stained cells.
For ASS expression, the mean intensity of ASS in tumor areas was
normalized to the mean intensity of ASS expression in non-tumor
areas from the same specimen. The mean ASS expression ratio
(tumor/non-tumor areas) from 40 patients was 0.77, where levels
below or above 0.77 were classified as low- or high-ASS expression,
respectively. For Ki-67 expression in each specimen, the mean
intensity and percentage of stained cells was determined from at
least 4 different regions of tumor areas.
In situ immunocytochemistry
All cell lines were seeded and allowed to attach
overnight at 37°C on chamber slides. After treatment for 3
consecutive days, cells were fixed on a permeabilized membrane by
incubation with 95% ethanol for 10 min, followed by incubation in
antibody diluent (Dako; Agilent Technologies, Inc.) for 10 min. The
immunocytochemical detection of ASS and Ki-67 was performed as
described for immunohistochemical detection.
Western blot analysis
Cells for total cell lysate production were
harvested using a cell-scraper in cold PBS. Cell were lysed with
RIPA buffer (Cell Signaling Technology, Inc., Danvers, MA, USA)
plus protease inhibitor cocktail (EMD Millipore, Billerica, MA,
USA), passed several times through a 23G needle, and centrifuged as
previously described (14). For each
cell lysate, the protein concentration was determined using the
Bio-Rad Protein Assay (Bio-Rad Laboratories, Inc., Hercules, CA,
USA), according to the manufacturer's protocol. Twenty micrograms
of protein was electrophoresed on 12% polyacrylamide gels
containing sodium dodecyl sulfate and transferred to a
nitrocellulose membranes by electro-blotting (GE Healthcare Life
Sciences, Little Chalfont, UK). The membrane was then washed and
incubated with monoclonal antibodies against ASS (1:1,000; BD
Biosciences). Detection of ASS expression was done using an ECL
Western Blotting Detection kit (GE Healthcare Life Sciences),
according to the manufacturer's instructions. Expression was
normalized to internal controls, using a mouse antibody against
β-actin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany).
Gene expression analysis by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cells using a
PerfectPure RNA Cultured Cell kit (5 PRIME, Hamburg, Germany)
according to the manufacturer's instructions. One-step RT-qPCR was
performed with a LightCycler 480 (Roche Diagnostics GmbH, Mannheim,
Germany). Briefly, RNA (20 ng) was mixed with 1X Quantitect SYBR
Green RT-PCR Master Mix (Qiagen GmbH, Hilden, Germany), 0.2 µl
QuantiTect RT mix (Qiagen GmbH) and ASS primers (forward:
5′-GAGGATGCCTGAATTCTACA-3′ and reverse: 5′-GTTGGTCACCTTCACAGG-3′)
in a total volume of 20 µl. The RT-qPCR conditions were as follows:
50°C for 20 min, 95°C for 15 min followed by 45 cycles of: 95°C for
15 sec, 58°C for 20 sec and 72°C for 30 sec. Expression of
ASS was quantified using the ΔΔCq method and normalized to
GAPDH (forward primer: 5′-TCTTCCAGGAGCGAGATCC-3′ and reverse
primer: 5′-TTGTCATGGATGACCTTGGC-3′).
Growth inhibitory assay
Cell viability was determined by
3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl tetrazolium bromide (MTT)
assay. Cells treated with ADI-PEG20 (0.05–1 µg/ml) were incubated
with 20 µl MTT (5 mg/ml in PBS) at 37°C for 4 h. The medium was
then removed and 200 µl dimethylsulfoxide (DMSO) was added to each
well followed by incubation for 5 min at room temperature.
Viability was assessed using a microplate reader (SoftMax Pro;
Molecular Devices, Sunnyvale, CA, USA) at 570 nm. Relative cell
viability was determined and expressed as percentage of
control.
Apoptosis assay
The percentage of cells undergoing apoptosis after
ADI-PEG20 treatment was determined using the Muse™ Annexin V and
Dead Cell kit (EMD Millipore). Treated and control cells were
collected in medium containing 1% FBS, mixed with the Muse Annexin
V and Dead Cell Reagent and incubated in the dark at room
temperature for 30 min according to the kit instructions. The
percentage of apoptotic cells was determined using a Muse Cell
Analyzer (EMD Millipore).
Cell cycle analysis
Cell cycle arrest was determined using the Muse™
Cell Cycle kit (EMD Millipore), according to the manufacturer's
instructions. Briefly, control and treated cells were fixed with
ice-cold 70% ethanol at −20 °C for 3 h, washed with PBS and stained
with PI/RNAse reagent for 30 min. The percentage of cells in each
phase of the cell cycle was analyzed using a Muse Cell Analyzer
(EMD Millipore).
Statistical analysis
Data were expressed as the mean ± SE. All
statistical analyses were performed using the Stata software
package (version 10.0; StataCorp LP, College Station, TX, USA). A
paired t-test was used to compare the immunohistochemistry data for
ASS expression between non-tumor and tumor cells. The Chi-square
test was used to determine differences between categorical
variables. For the in vitro study, the Student's t-test was
used to compare the two sets of data and analysis of variance was
used to compare >3 sets of data using the Bonferroni test for
post hoc analysis. Pearson's correlation was used to assess
correlation between proliferation and expression level of ASS or
CCA stage.
Results
Immunohistochemical analysis of ASS
expression in CCA specimens and its relationship to
clinicopathological parameters
In order to investigate whether CCA may be an
arginine auxotrophic cancer, the levels of ASS expression in 40
human CCA specimens were determined using immunohistochemistry. Our
results showed that the intensity of cytoplasmic ASS staining in
cancer cells was much lower than in the surrounding non-tumor liver
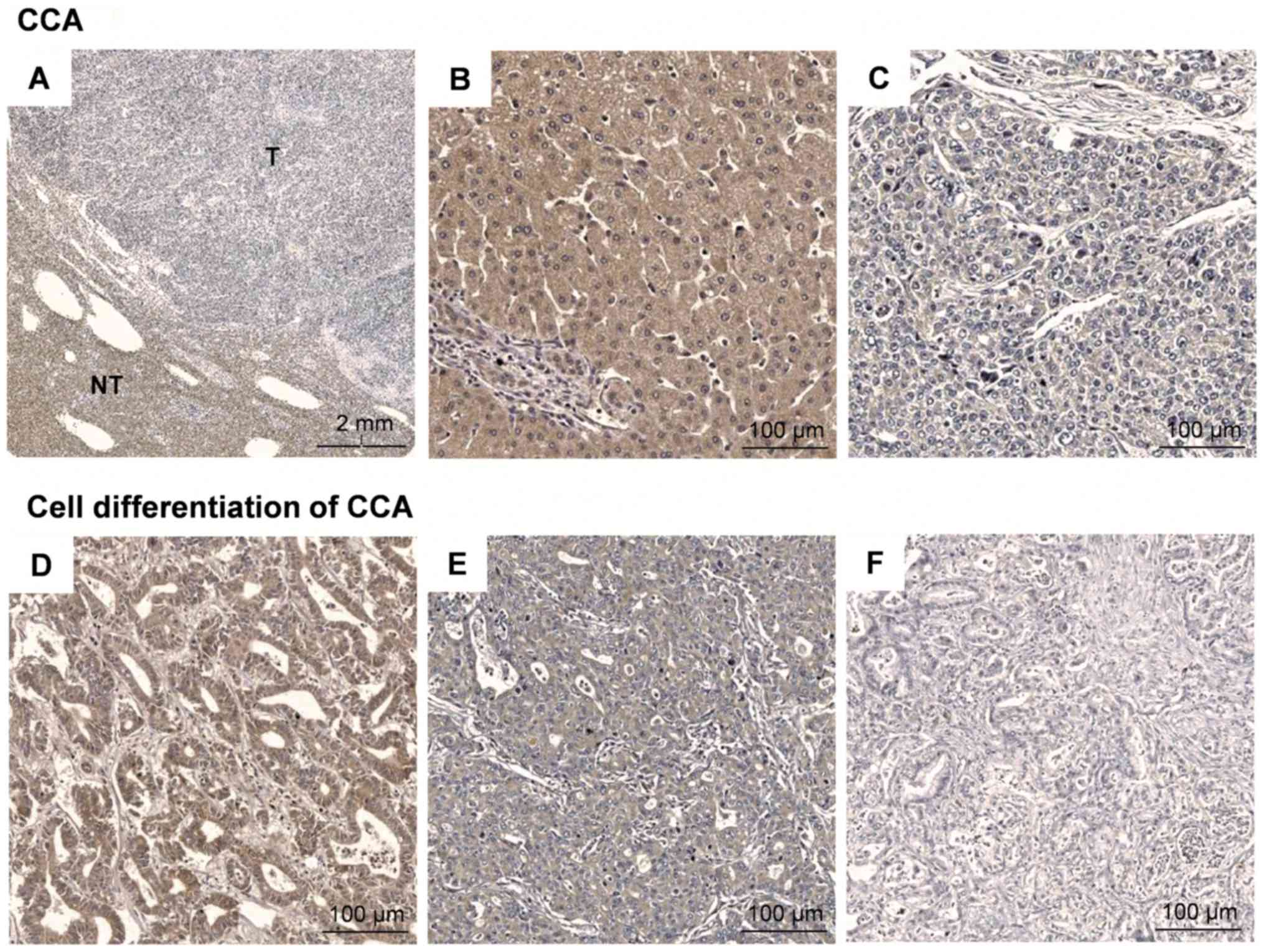
cells in specimens from CCA patients (Fig. 1A-C). The level of ASS expression,
quantified as the relative intensity of ASS staining, is summarized
in Table I. In CCA, the mean
intensity of ASS staining in tumor cells was 1.3-fold lower than
for non-tumor cells (33.56 vs. 44.23, P<0.05). The mean of the
ASS expression ratio (tumor/non-tumor) in CCA was 0.77. In an
attempt to assess the association of ASS expression and
clinicopathological features of CCA, the level of ASS expression
stratified by the level below or above the mean intensity ratio was
arbitrarily categorized as low- or high-ASS expressed groups,
respectively (a ratio above 0.77 was categorized as high; a ratio
lower than 0.77 was categorized as low). As shown in Table II, approximately 45% of the CCA
samples were classified as the low ASS-expressed group. It is
interesting to note that the level of ASS expression showed a
significant association with cell differentiation in CCA (P=0.030)
(Table II), and was also associated
with poor pathological features of CCA (Fig. 1D-F). However, no association between
the level of ASS expression and gender, age, viral hepatitis
status, lymphovascular invasion, lymph node metastasis, or TNM
stage was observed. These results suggest that a reduction of ASS
expression could potentially be a molecular marker for potential
CCA treatment by arginine deprivation.
 | Table I.Immunohistochemical detection of ASS
expression in CCA specimens derived from Thai patients. |
Table I.
Immunohistochemical detection of ASS
expression in CCA specimens derived from Thai patients.
| CCA specimens | Level of ASS
expression (Arbitrary unit) |
|---|
| Tumor tissue |
33.56±2.44a |
|
| 32.57
(3.99–87.87) |
| Surrounding
non-tumor tissue | 44.23±2.27 |
|
| 42.89
(22.69–86.58) |
| Ratio
(tumor/non-tumor) | 0.77±0.04 |
|
| 0.84
(0.11–1.23) |
 | Table II.Association between the level of ASS
and clinicopathological features of CCA. |
Table II.
Association between the level of ASS
and clinicopathological features of CCA.
|
|
| ASS expression
ratioa |
|
|---|
|
|
|
|
|
|---|
| Variable | Patients (n) | Low (n) | High (n) | P-value |
|---|
| All cases | 40 | 18 | 22 | – |
| Sex |
|
|
| 0.154 |
|
Male | 24 | 13 | 11 |
|
|
Female | 16 | 5 | 11 |
|
| Age (years) |
|
|
| 0.057 |
|
<60 | 20 | 12 | 8 |
|
|
≥60 | 20 | 6 | 14 |
|
| Viral status
(Hepatitis B or C) |
|
|
| 0.949 |
|
Yes | 18 | 8 | 10 |
|
| No | 22 | 10 | 12 |
|
| Cell
differentiation |
|
|
| 0.030 |
|
Well | 22 | 8 | 14 |
|
|
Moderately | 13 | 5 | 8 |
|
|
Poorly | 5 | 5 | 0 |
|
| Lymphovascular
invasion |
|
|
| 0.900 |
|
Yes | 33 | 15 | 18 |
|
| No | 7 | 3 | 4 |
|
| Lymph node
metastasis |
|
|
| 0.775 |
|
Yes | 19 | 9 | 10 |
|
| No | 21 | 9 | 12 |
|
| TNM stage |
|
|
| 0.673 |
|
I–II | 3 | 1 | 2 |
|
|
III–IV | 37 | 17 | 20 |
|
Histologic correlation of Ki-67
expression in CCA
The Ki-67 proliferative index is widely used as a
prognostic marker in many types of cancers. To assess the
associations between Ki-67 and the histopathological parameters of
CCA, immunohistochemical staining for Ki-67 was performed in 40
human CCA specimens. As shown in Fig.
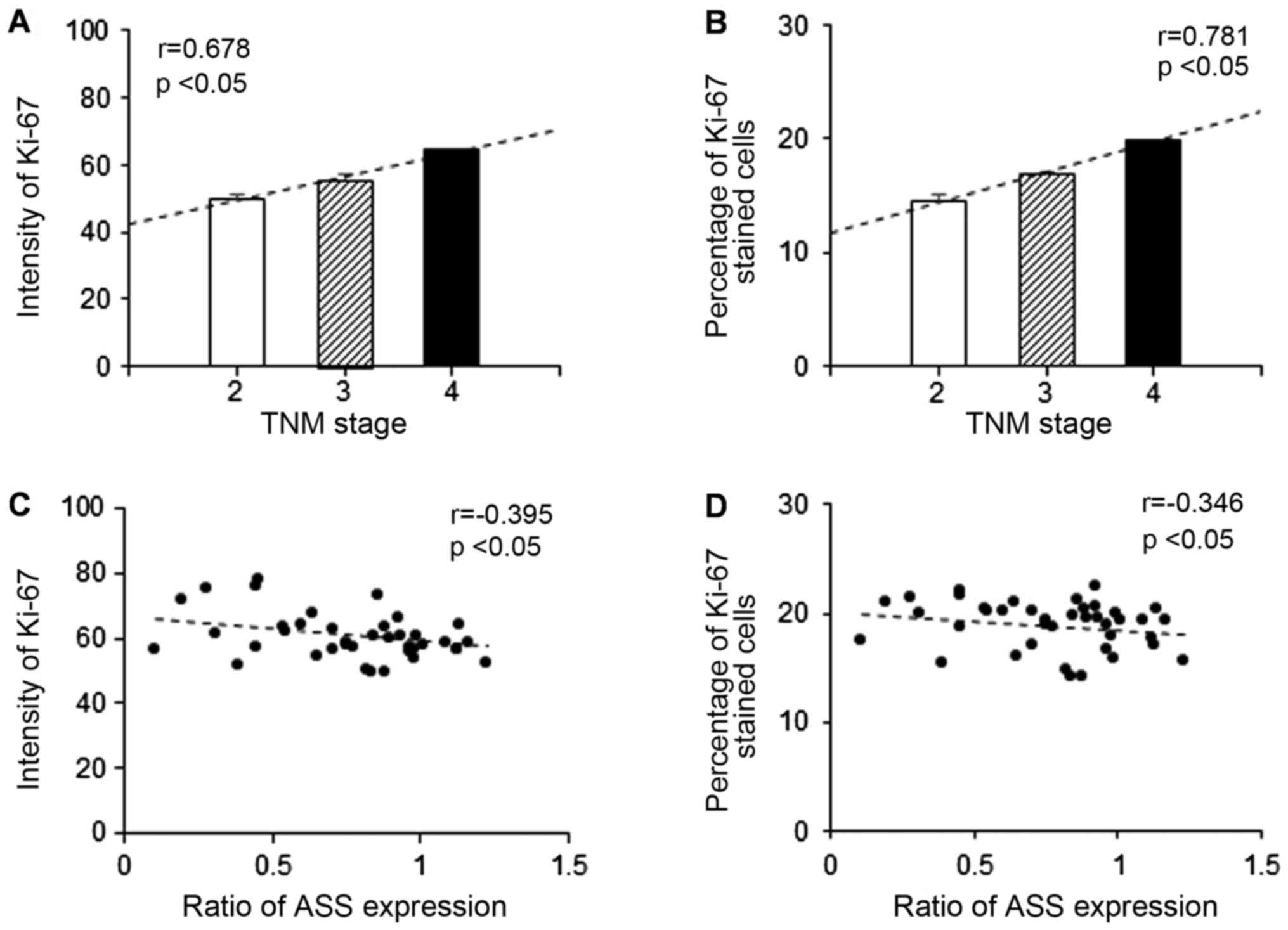
2A, the mean intensities of Ki-67 staining for stages 2, 3, and
4 were 49.97, 55.52 and 62.69, respectively. An increased
expression of Ki-67 was positively correlated with advanced stage
CCA specimens (r=0.678, P<0.05; Fig.
2A). In line with the mean intensity of Ki-67, the mean
percentage of Ki-67 stained cells was also positively correlated
with advanced stage CCA specimens (r=0.781, P<0.05) as shown in
Fig. 2B. In addition, the ratio of
ASS expression was negatively correlated (P<0.05) with the mean
level of Ki-67 intensity (r=−0.395; Fig.
2C) and percentage of stained cells (r=−0.346; Fig. 2D).
In vitro study to characterize the level of ASS
expression in human CCA cell lines and their responses to ADI-PEG20
treatment
Level of ASS expression in human CCA
cell lines
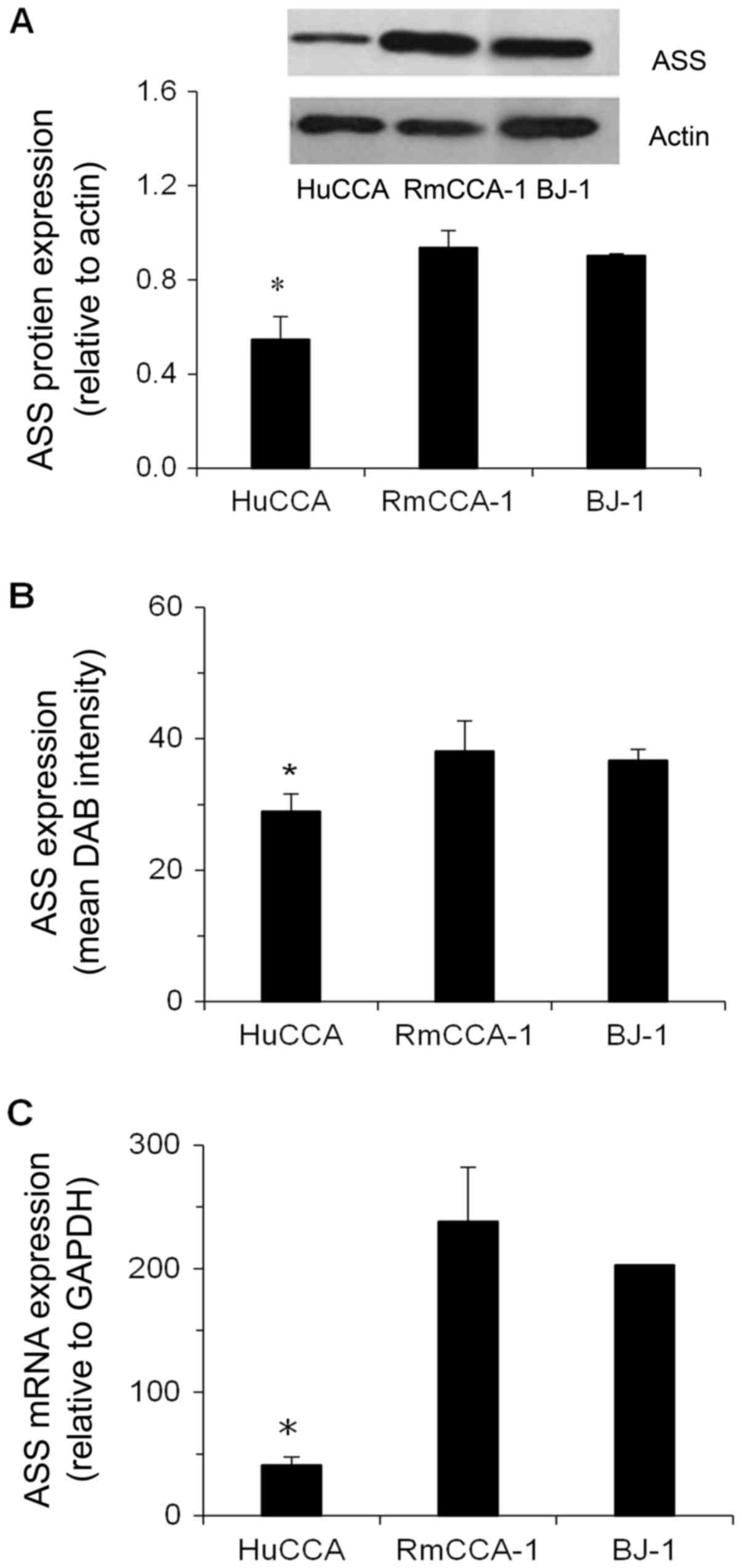
Basal expression of ASS in RmCCA-1 and HuCCA cells
was determined at both the protein and mRNA levels in relation to
that in non-cancer cells (BJ-1). As shown in Fig. 3A, immunoblot detection of the ASS
protein in various cell lines showed that relative ASS protein
expression was significantly lower in HuCCA cells (0.61-fold,
P<0.05), but slightly higher in RmCCA-1 cells (1.04-fold,
P>0.05), compared to that in BJ-1 cells. The cytoplasmic
staining of the ASS protein by in situ immunocytochemistry
in all cell lines showed the same trend as that determined by
immunoblotting (RmCCA-1> BJ-1> HuCCA) (Fig. 3B). In addition, the expression levels
of the ASS protein in all cell lines correlated with the level of
ASS mRNA (Fig. 3C).
Effect of ADI-PEG20 treatment on cell
viability and cell proliferation of human CCA cell lines
In order to investigate whether there is an
association between the expression of ASS and sensitivity to
arginine deprivation caused by ADI-PEG20 treatment, the
dose-response to ADI-PEG20-induced growth inhibition in CCA cell
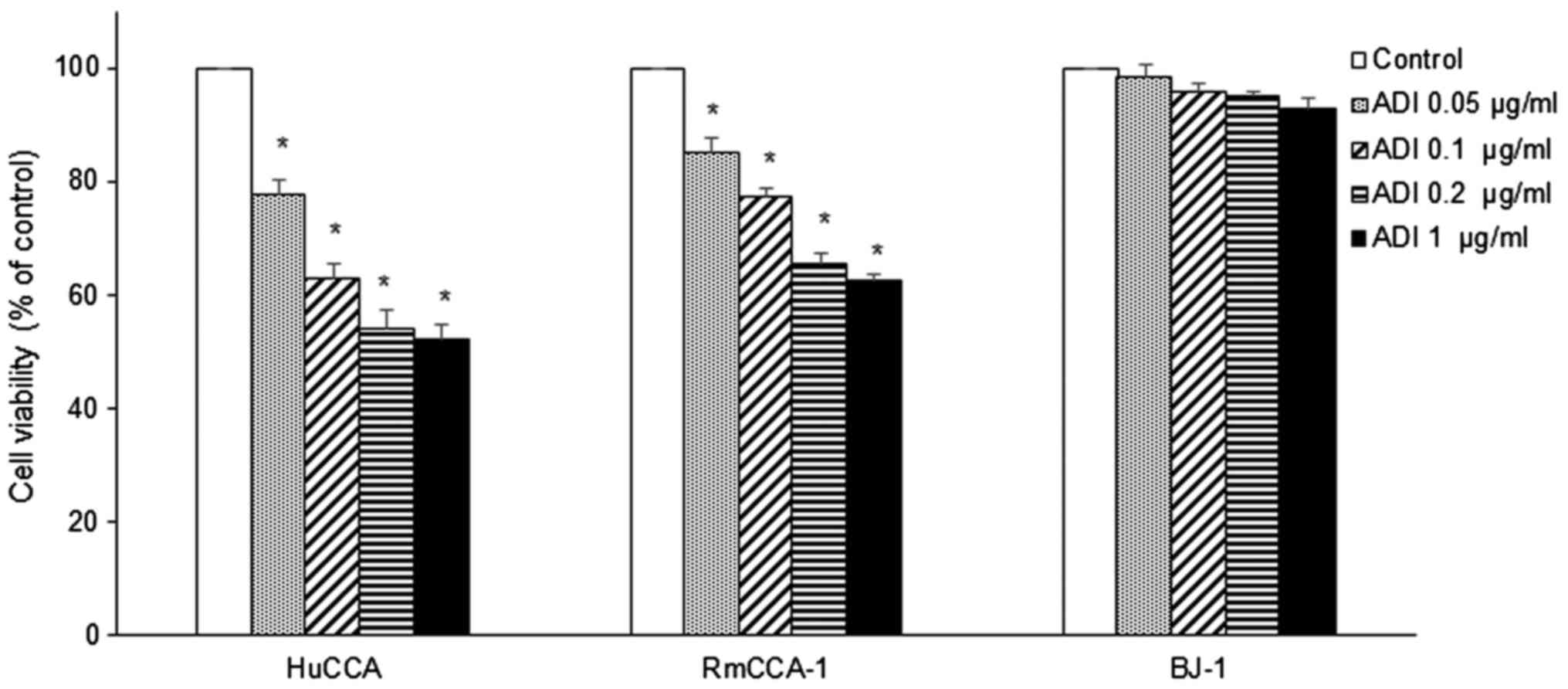
lines was determined. ADI-PEG20 treatment significantly inhibited
growth of RmCCA-1 and HuCCA cells by decreasing both the percentage
of viable cells (Fig. 4) and the
proliferative activity as determined by Ki-67 expression (Fig. 5). As shown in Fig. 4, a statistically significant decrease
in cell viability in both RmCCA-1 and HuCCA cells after ADI-PEG20
treatment was observed in a dose-dependent manner. However, only a
slight decrease in cell viability was found in normal immortalized
cells (BJ-1). Subsequent to ADI-PEG20 treatment, a significant
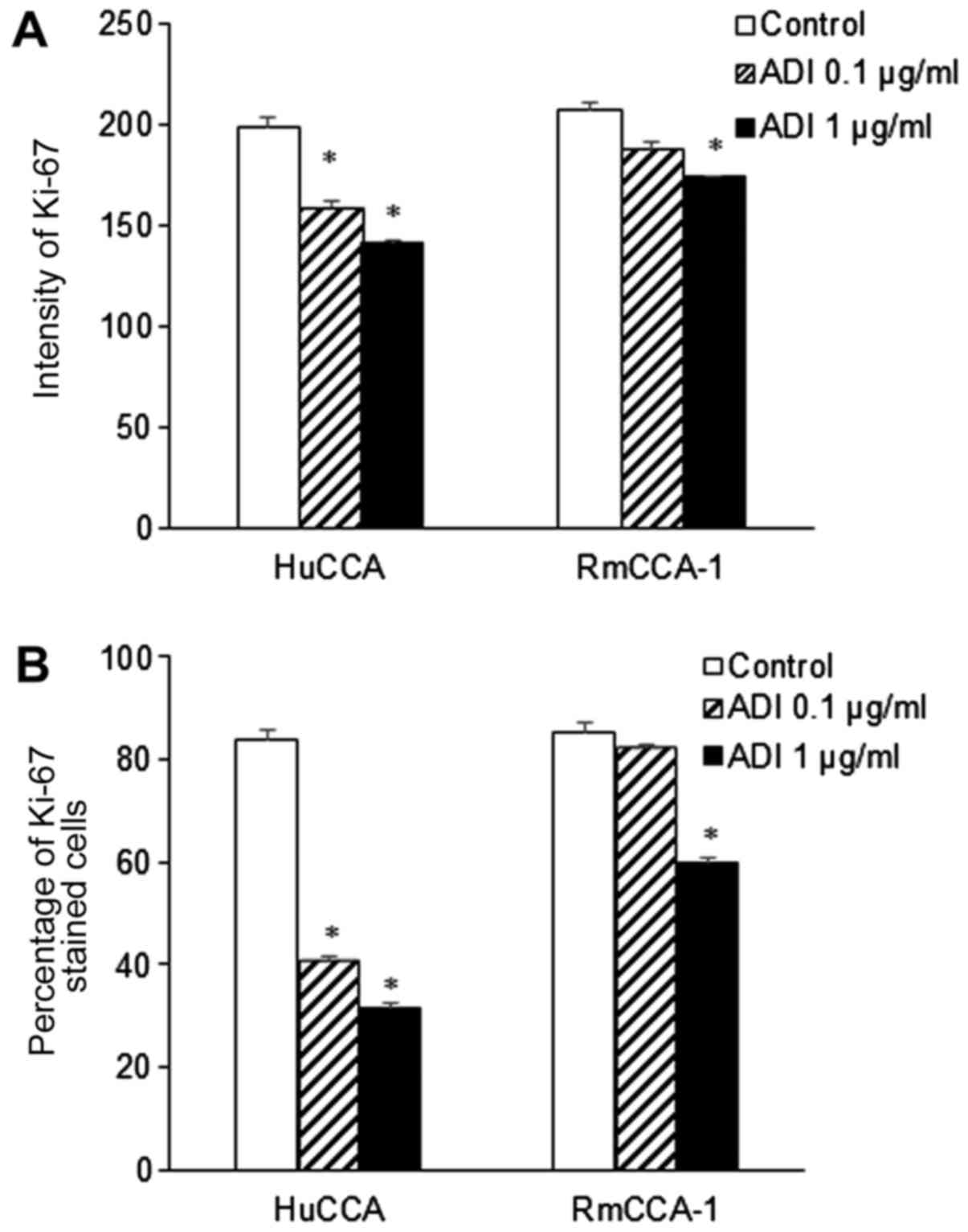
reduction in Ki-67 expression was observed in HuCCA (1.3- and
1.4-fold at 0.1 and 1.0 µg/ml, respectively) and RmCCA-1 cells
(1.2-fold at 1.0 µg/ml) (P<0.05; Fig.
5A). A significant reduction in the percentage of Ki-67 stained
cells (P<0.05) was found in ADI-PEG20-treated HuCCA (2- and
2.2-fold at 0.1 and 1.0 µg/ml, respectively) and RMCCA-1 (1.4-fold
at 1.0 µg/ml) cells as shown in Fig.
5B. As expected, these results suggested that the HuCCA cell
line, which has low ASS expression, is more sensitive to ADI-PEG20
treatment. On the other hand, ADI-PEG20 treatment had no effect on
BJ-1 cells, even though the BJ-1 cell line expressed ASS at a lower
level than RmCCA-1 cells. This is not surprising, since tumor cells
grow rapidly and require more arginine than normal immortalized
cells. Intracellular arginine supply through the urea cycle is
inadequate to support rapid proliferation; therefore, RmCCA-1 cells
grow more slowly and have lower Ki-67 levels.
Effect of ADI-PEG20 treatment on cell
cycle arrest and apoptosis in human CCA cell lines
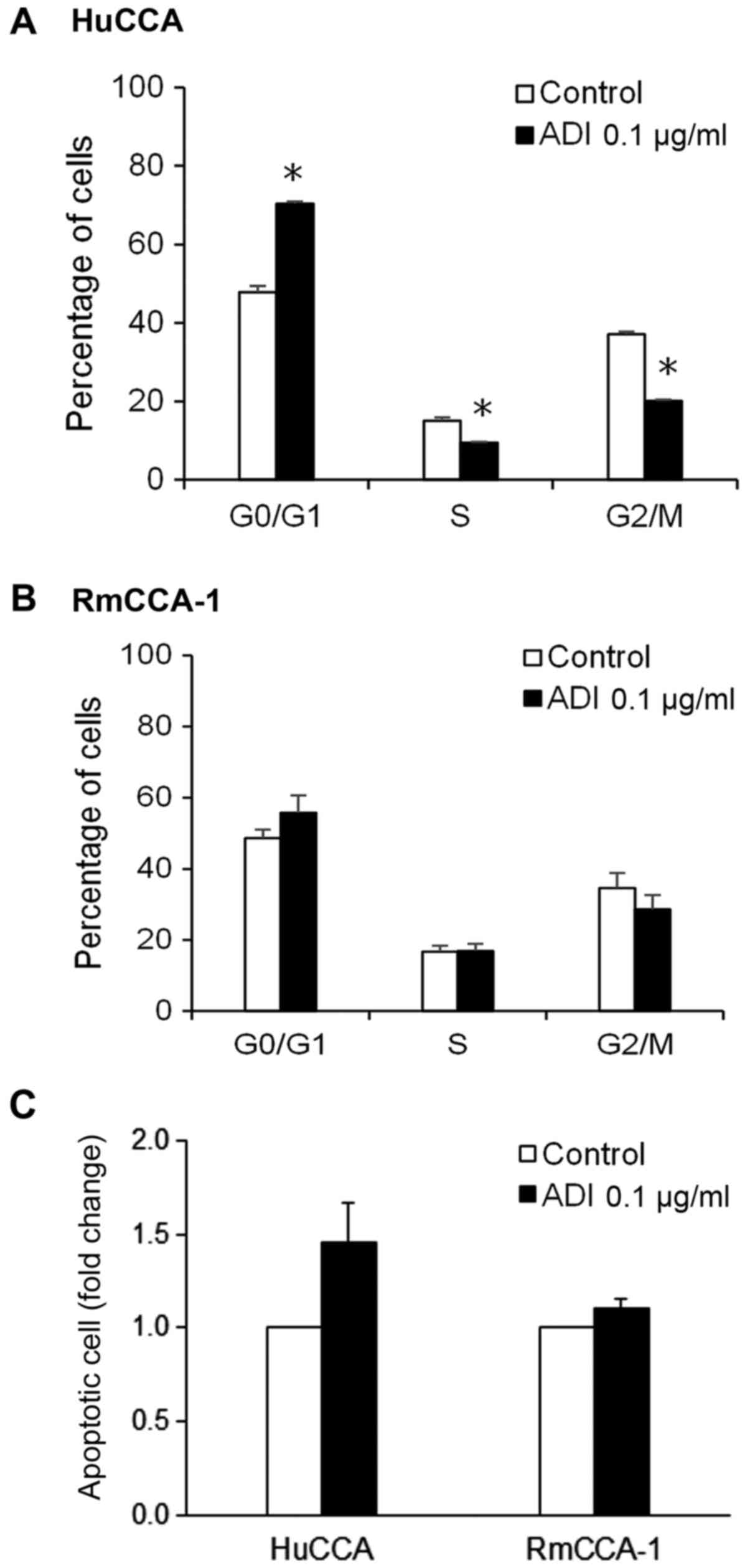
We further determined whether growth inhibition
resulting from ADI-PEG20 was the consequence of cell cycle arrest
and/or apoptotic cell death. Cell cycle analysis was performed
using propidium iodide staining, which corresponds to the DNA
content of the cells. As shown in Fig.
6A, treatment with ADI-PEG20 in HuCCA cells significantly
decreased population of cells in the S and G2/M phases, while it
increased accumulation of cells in the G0/G1 phase, suggesting
G0/G1 arrest in HuCCA cells treated with ADI-PEG20. However, such
effect was not observed in RmCCA-1 cells treated with ADI-PEG20
(Fig. 6B).
The induction of apoptotic cell death by ADI-PEG20
was assessed using Annexin V staining, which is an apoptosis
marker. As shown in Fig. 6C, an
increase in apoptotic cell population was observed in HuCCA cells
treated with ADI-PEG20 treatment, whereas RmCCA-1 cells displayed
only a slight increase in the number of apoptotic cells upon
ADI-PEG20 treatment. The results showed that HuCCA cells, which
have low ASS expression, are more sensitive to ADI-PEG20 treatment
when compared to RmCCA-1 cells, which have a higher level of ASS
expression. These results suggest that the efficacy of ADI-PEG20
treatment in CCA cells depends on the level of ASS expression.
Effect of ADI-PEG20 treatment on ASS
knockdown RmCCA-1 cells
To further confirm that the levels of ASS expression
is an important contributory factor to ADI-PEG20 sensitivity, we
knocked down ASS expression in RmCCA-1 cells using siRNA and
determined the sensitivity to ADI-PEG20 in these transfectants. As
shown in Fig. 7B, ADI-PEG20 treatment
decreased the viability of RmCCA-1siASS cells (20%
reduction) with no effect on the viability of RmCCA-1 cells treated
with pooled non-target scramble control siRNA
(RmCCA-1siNT cells). This data suggests that silencing
of ASS increases sensitivity of these cells to ADI-PEG20
treatment.
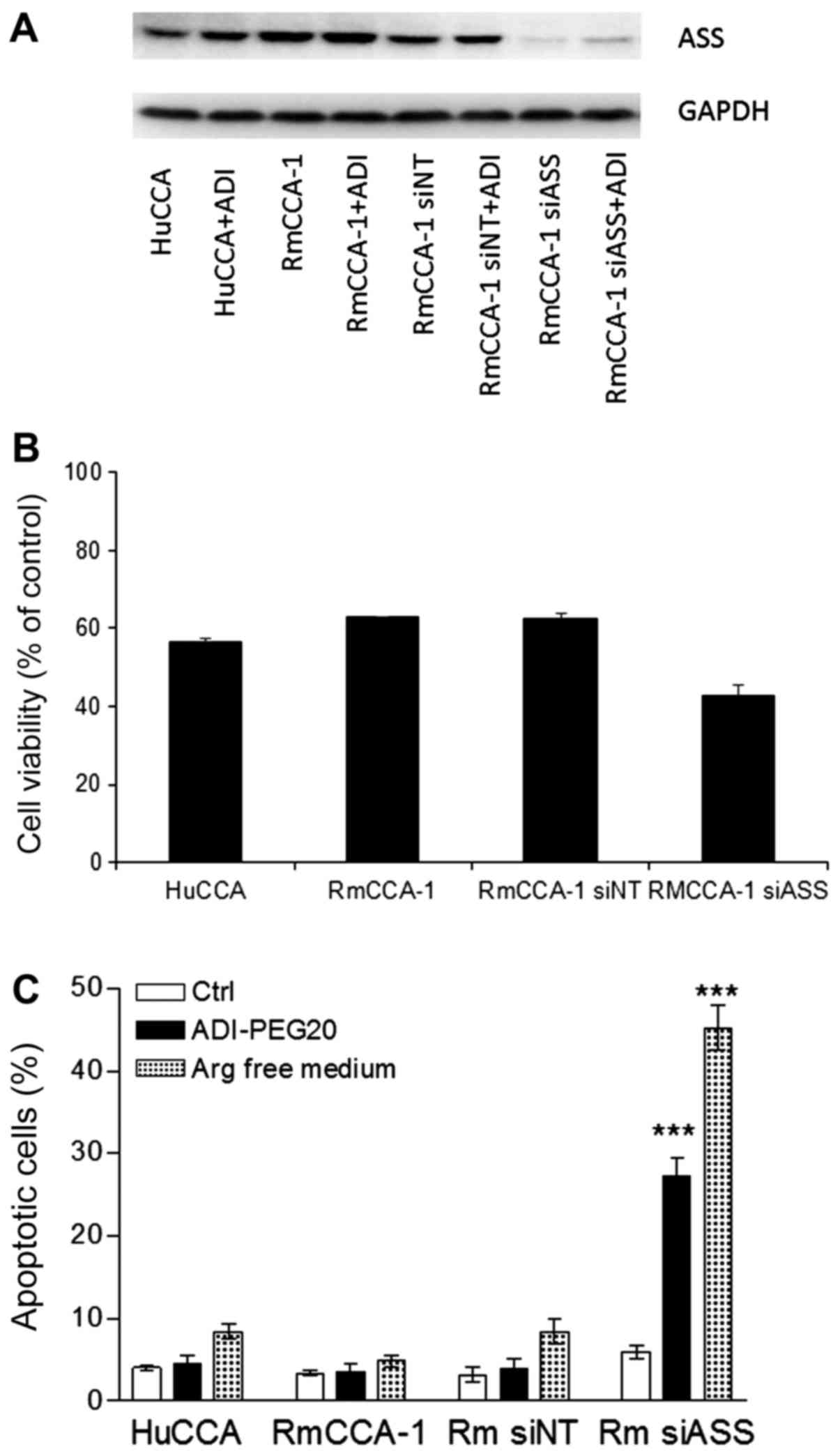 | Figure 7.Effect of arginine deprivation using
ADI-PEG20 treatment or arginine-free medium in ASS knockdown human
CCA cell lines. (A) Immunoblot analysis of ASS protein levels in
HuCCA, RmCCA-1, RmCCA-1siNT and RmCCA-1siASS
with or without treatment with 0.1 µg/ml ADI-PEG20 for 3 days. (B)
The growth inhibitory effect of ADI-PEG20 using MTT assay in HuCCA,
RmCCA-1, RmCCA-1siNT and RmCCA-1siASS cells.
(C) The percentage of apoptotic cells as determined by
fluorescence-activated cell sorting analysis following treatment
with ADI-PEG20 for 3 days or culturing in arginine-free medium for
3 days in HuCCA, RmCCA-1, RmCCA-1siNT and
RmCCA-1siASS cells. The data are presented as the mean ±
standard error of three independent experiments. ***P<0.001 vs.
control. CCA, cholangiocarcinoma; ASS, argininosuccinate
synthetase; ADI-PEG20/ADI, pegylated arginine deiminase; si-, small
interfering; NT, non-target scramble control. |
Results of a fluorescence-activated cell sorting
analysis of cellular apoptosis on ADI-PEG20-treated HuCCA, RmCCA-1,
RmCCA-1siNT and RmCCA-1siASS cells are shown
in Fig. 7C. Treatment with ADI-PEG20
did not significantly induce apoptosis in either the HuCCA or
RmCCA-1 and RmCCAsiNT cell lines, while using
arginine-free medium slightly increased the percentage of apoptotic
cells in both HuCCA and RmCCA-1 cells, as well as its non-target
transfectants. However, treatment with ADI-PEG20 significantly
induced apoptosis in RmCCA-1siASS cells (25%) while
treatment with arginine-free medium increased the apoptotic cells
to 45%. The increase in cell death using arginine-free medium is
most likely due to the fact that the arginine-free duration was
longer, while treatment with ADI-PEG20 results in a gradual loss of
arginine. Taken together, our results strongly suggest that
ADI-PEG20 treatment could be a potential therapy for CCA patients
with low ASS expression.
Discussion
Depleting arginine to halt tumor growth was first
exploited to treat cancer a decade ago, but the approach has never
made it to clinical trials due to the lack of a suitable enzyme to
degrade arginine (16). In the past
few years, ADI-PEG20, a new generation of pegylated ADI, has
entered into clinical trials. Antitumor response following
administration of ADI-PEG20 has been observed primarily in tumors
with low or no ASS expression (11,22).
However, re-expression of the enzyme has been shown, both in
vitro and in vivo, to contribute to ADI-PEG20
resistance, especially in melanoma (23–25). ASS
expression is known to be positively regulated by c-Myc and
negatively regulated by HIF-1α (25).
Epigenetic regulation is also important depending on tumor type
(23).
At present, there is no data on ASS expression in
CCA. In this study, we demonstrated that 45% of CCA samples showed
relatively low ASS protein expression in tumor cells. Low levels of
ASS expression were significantly associated with unfavorable
histopathologic differentiation, but not with gender, age, viral
hepatitis, lymphovascular invasion, lymph node metastasis or TNM
stage. Similar to this finding, it has been shown that in human
pancreatic cancer (26), HCC,
myxofibrosarcoma and bladder cancer, decreased ASS expression
correlated with histopathological grading (27). For instance, a 1.29-fold reduction of
ASS expression in tumor cells of HCC specimens was associated with
poor tumor differentiation (21).
However, there was no association found between ASS expression and
histopathological characteristics of tumors in a cohort study of
lung cancer patients (27). The
differences among the reports could be due to the different methods
used for quantitation. In our study, we quantitated the ASS
expression in tumor cells by comparing it with that in the adjacent
normal cells in the same slides, thus, avoiding the variations
created by staining in different batches.
Ki-67 has been used as a proliferative indicator in
many types of cancers, such as breast cancer, lymphoma and renal
carcinoma as well as CCA (28,29).
Upregulation of Ki-67 has been shown to be associated with poorly
differentiated tumors, advanced stage disease, poor prognosis, and
decreased survival rates in CCA patients (28,30,31). In
line with previous findings, our study showed a significant
association between increased expression of Ki-67, either as
intensity or percentage of Ki-67 stained cells, in addition to
advanced stage disease in CCA samples. Additionally, in CCA samples
the level of ASS expression was found to be inversely correlated
with the proliferative activity, as determined by Ki-67 expression.
Overall, low ASS expression could be associated with poorer
clinicohistopathological findings and a higher Ki-67 proliferative
index in CCA samples (32,33).
In summary, this is the first report to show a
variation in ASS expression in CCA. In 45% of CCA cases, low ASS
expression was associated with poorly differentiated cells and more
aggressive tumors (33,34). In addition, the in vitro study
confirmed that low ASS expression in CCA cells was important for
the efficacy of ADI-PEG20 treatment of CCA. This group of CCA
patients with low ASS expression may be amenable to treatment with
arginine deprivation. Even though this treatment may not eliminate
the tumor cells, it will halt their proliferation. In other tumor
types, a combination of ADI-PEG20 with chemotherapeutic agents has
been shown to improve its therapeutic efficacy with no overt
toxicity (35). Thus, combination
treatments employing ADI-PEG20 with chemotherapeutic agents that
have shown some activity against CCA, such as gemcitabine or
cisplatin/oxaliplatin, may produce a higher response rate and
improve overall survival in patients with this disease (35–37).
Acknowledgements
The authors would like to thank Polaris
Pharmaceuticals Inc. (San Diego, CA, USA) for providing ADI-PEG20
for the present study.
Funding
The present study was supported by The Chulabhorn
Research Institute and The Center of Excellence on Environmental
Health and Toxicology (Bangkok, Thailand) as well as a partial
support from VA Merit Review Award (1BX003328) to Dr. Niramol
Savaraj.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SR performed analysis of western immunoblotting of
ASS, cell cycle arrest and apoptosis in CCA cell lines. PN
conducted the data analysis and manuscript preparation. SW and VP
were responsible for the analysis of mRNA expression and IHC
detection of ASS, respectively. TS and VB contributed to
preparation of tissue slides from CCA specimens and
histopathological evaluation. NS contributed to the study design,
specifically silencing of ASS expression using siRNA. MR, as the
principal investigator, conceived and designed the study and
experiments, sought funding support and served a key role in
developing the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by The Human Research
Ethics Committee of Chulabhorn Research Institute (Bangkok,
Thailand; project no. 013/2559 on 17th August 2016). All of the
subjects gave written informed consent for participation prior to
enrollment in the study.
Consent for publication
Written informed consent was obtained from all
participants.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aljiffry M, Abdulelah A, Walsh M,
Peltekian K, Alwayn I and Molinari M: Evidence-based approach to
cholangiocarcinoma: A systematic review of the current literature.
J Am Coll Surg. 208:134–147. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parkin DM, Ohshima H, Srivatanakul P and
Vatanasapt V: Cholangiocarcinoma: Epidemiology, mechanisms of
carcinogenesis and prevention. Cancer Epidemiol Biomarkers Prev.
2:537–544. 1993.PubMed/NCBI
|
|
4
|
Mosadeghi S, Liu B, Bhuket T and Wong RJ:
Sex-specific and race/ethnicity-specific disparities in
cholangiocarcinoma incidence and prevalence in the USA: An updated
analysis of the 2000–2011 surveillance, epidemiology and end
results registry. Hepatol Res. 46:669–677. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Butthongkomvong K, Sirachainan E,
Jhankumpha S, Kumdang S and Sukhontharot OU: Treatment outcome of
palliative chemotherapy in inoperable cholangiocarcinoma in
Thailand. Asian Pac J Cancer Prev. 14:3565–3568. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Smout MJ, Sotillo J, Laha T, Papatpremsiri
A, Rinaldi G, Pimenta RN, Chan LY, Johnson MS, Turnbull L,
Whitchurch CB, et al: Carcinogenic parasite secretes growth factor
that accelerates wound healing and potentially promotes neoplasia.
PLoS Pathog. 11:e10052092015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Friman S: Cholangiocarcinoma-current
treatment options. Scand J Surg. 100:30–34. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Murakami Y, Uemura K, Sudo T, Hashimoto Y,
Nakashima A, Kondo N, Sakabe R, Ohge H and Sueda T: Prognostic
factors after surgical resection for intrahepatic, hilar, and
distal cholangiocarcinoma. Ann Surg Oncol. 18:651–658. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Patel T: Cholangiocarcinoma-controversies
and challenges. Nat Rev Gastroenterol Hepatol. 8:189–200. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Andersen JB and Thorgeirsson SS: Genetic
profiling of intrahepatic cholangiocarcinoma. Curr Opin
Gastroenterol. 28:266–272. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McAlpine JA, Lu HT, Wu KC, Knowles SK and
Thomson JA: Down-regulation of argininosuccinate synthetase is
associated with cisplatin resistance in hepatocellular carcinoma
cell lines: Implications for PEGylated arginine deiminase
combination therapy. BMC Cancer. 14:6212014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bowles TL, Kim R, Galante J, Parsons CM,
Virudachalam S, Kung HJ and Bold RJ: Pancreatic cancer cell lines
deficient in argininosuccinate synthetase are sensitive to arginine
deprivation by arginine deiminase. Int J Cancer. 123:1950–1955.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Delage B, Fennell DA, Nicholson L, McNeish
I, Lemoine NR, Crook T and Szlosarek PW: Arginine deprivation and
argininosuccinate synthetase expression in the treatment of cancer.
Int J Cancer. 126:2762–2772. 2010.PubMed/NCBI
|
|
14
|
Savaraj N, Wu C, Kuo MT, You M,
Wangpaichitr M, Robles C, Spector S and Feun L: The relationship of
arginine deprivation, argininosuccinate synthetase and cell death
in melanoma. Drug Target Insights. 2:119–128. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Savaraj N, You M, Wu C, Wangpaichitr M,
Kuo MT and Feun LG: Arginine deprivation, autophagy, apoptosis
(AAA) for the treatment of melanoma. Curr Mol Med. 10:405–412.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Phillips MM, Sheaff MT and Szlosarek PW:
Targeting arginine-dependent cancers with arginine-degrading
enzymes: Opportunities and challenges. Cancer Res Treat.
45:251–262. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hahnvajanawong C, Chaiyagool J, Seubwai W,
Bhudhisawasdi V, Namwat N, Khuntikeo N, Sripa B, Pugkhem A and
Tassaneeyakul W: Orotate phosphoribosyl transferase mRNA expression
and the response of cholangiocarcinoma to 5-fluorouracil. World J
Gastroenterol. 18:3955–3961. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rattanasinganchan P, Leelawat K,
Treepongkaruna SA, Tocharoentanaphol C, Subwongcharoen S,
Suthiphongchai T and Tohtong R: Establishment and characterization
of a cholangiocarcinoma cell line (RMCCA-1) from a Thai patient.
World J Gastroenterol. 12:6500–6506. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sirisinha S, Tengchaisri T, Boonpucknavig
S, Prempracha N, Ratanarapee S and Pausawasdi A: Establishment and
characterization of a cholangiocarcinoma cell line from a Thai
patient with intrahepatic bile duct cancer. Asian Pac J Allergy
Immunol. 9:153–157. 1991.PubMed/NCBI
|
|
20
|
Savaraj N, Wu C, Li YY, Wangpaichitr M,
You M, Bomalaski J, He W, Kuo MT and Feun LG: Targeting
argininosuccinate synthetase negative melanomas using combination
of arginine degrading enzyme and cisplatin. Oncotarget.
6:6295–6309. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thongkum A, Wu C, Li YY, Wangpaichitr M,
Navasumrit P, Parnlob V, Sricharunrat T, Bhudhisawasdi V,
Ruchirawat M and Savaraj N: The combination of arginine deprivation
and 5-fluorouracil improves therapeutic efficacy in
argininosuccinate synthetase negative hepatocellular carcinoma. Int
J Mol Sci. 18(pii): E11752017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Beddowes E, Spicer J, Chan PY, Khadeir R,
Corbacho JG, Repana D, Steele JP, Schmid P, Szyszko T, Cook G, et
al: Phase 1 dose-escalation study of pegylated arginine deiminase,
cisplatin, and pemetrexed in patients with argininosuccinate
synthetase 1-deficient thoracic cancers. J Clin Oncol.
35:1778–1785. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Locke M, Ghazaly E, Freitas MO, Mitsinga
M, Lattanzio L, Lo Nigro C, Nagano A, Wang J, Chelala C, Szlosarek
P and Martin SA: Inhibition of the polyamine synthesis pathway is
synthetically lethal with loss of argininosuccinate synthase 1.
Cell Rep. 16:1604–1613. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Long Y, Tsai WB, Wangpaichitr M, Tsukamoto
T, Savaraj N, Feun LG and Kuo MT: Arginine deiminase resistance in
melanoma cells is associated with metabolic reprogramming, glucose
dependence, and glutamine addiction. Mol Cancer Ther. 12:2581–2590.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tsai WB, Aiba I, Long Y, Lin HK, Feun L,
Savaraj N and Kuo MT: Activation of Ras/PI3K/ERK pathway induces
c-Myc stabilization to upregulate argininosuccinate synthetase,
leading to arginine deiminase resistance in melanoma cells. Cancer
Res. 72:2622–2633. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu J, Ma J, Wu Z, Li W, Zhang D, Han L,
Wang F, Reindl KM, Wu E and Ma Q: Arginine deiminase augments the
chemosensitivity of argininosuccinate synthetase-deficient
pancreatic cancer cells to gemcitabine via inhibition of NF-κB
signaling. BMC Cancer. 14:6862014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Walts AE, Bomalaski JS, Ines D and Orsulic
S: Argininosuccinate synthetase (ASS) deficiency in high-grade
pulmonary neuroendocrine carcinoma: An opportunity for personalized
targeted therapy. J Cancer Res Clin Oncol. 141:1363–1369. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Horie S, Endo K, Kawasaki H and Terada T:
Overexpression of MDM2 protein in intrahepatic cholangiocarcinoma:
Relationship with p53 overexpression, Ki-67 labeling, and
clinicopathological features. Virchows Arch. 437:25–30. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao W, Zhang B, Guo X, Zhang X, Hu J, Hu
X and Lu Y: Expression of Ki-67, Bax and p73 in patients with hilar
cholangiocarcinoma. Cancer Biomark. 14:197–202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Iguchi T, Yamashita N, Aishima S, Kuroda
Y, Terashi T, Sugimachi K, Taguchi K, Taketomi A, Maehara Y and
Tsuneyoshi M: A comprehensive analysis of immunohistochemical
studies in intrahepatic cholangiocarcinoma using the survival tree
model. Oncology. 76:293–300. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schiffman SC, Nowacki MR, Spencer L,
McMasters KM, Scoggins CR and Martin RC: Molecular factors
associated with recurrence and survival following hepatectomy in
patients with intrahepatic cholangiocarcinoma: A guide to adjuvant
clinical trials. J Surg Oncol. 109:98–103. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tan GS, Lim KH, Tan HT, Khoo ML, Tan SH,
Toh HC and Ching Ming Chung M: Novel proteomic biomarker panel for
prediction of aggressive metastatic hepatocellular carcinoma
relapse in surgically resectable patients. J Proteome Res.
13:4833–4846. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang H, Lin M, Xiong FX, Yang Y, Nie X and
Zhou RL: Reduced expression of ASS is closely related to
clinicopathological features and post-resectional survival of
hepatocellular carcinoma. Oncol Lett. 1:31–36. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yoon CY, Shim YJ, Kim EH, Lee JH, Won NH,
Kim JH, Park IS, Yoon DK and Min BH: Renal cell carcinoma does not
express argininosuccinate synthetase and is highly sensitive to
arginine deprivation via arginine deiminase. Int J Cancer.
120:897–905. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
You M, Savaraj N, Wangpaichitr M, Wu C,
Kuo MT, Varona-Santos J, Nguyen DM and Feun L: The combination of
ADI-PEG20 and TRAIL effectively increases cell death in melanoma
cell lines. Biochem Biophys Res Commun. 394:760–766. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Feun L, You M, Wu CJ, Kuo MT, Wangpaichitr
M, Spector S and Savaraj N: Arginine deprivation as a targeted
therapy for cancer. Curr Pharm Des. 14:1049–1057. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wangpaichitr M, Wu C, Bigford G,
Theodoropoulos G, You M, Li YY, Verona-Santos J, Feun LG, Nguyen DM
and Savaraj N: Combination of arginine deprivation with TRAIL
treatment as a targeted-therapy for mesothelioma. Anticancer Res.
34:6991–6999. 2014.PubMed/NCBI
|