Introduction
Gastric cancer (GC) is a type of gastrointestinal
cancer that is commonly diagnosed at an advanced stage. Gastric
cancer remains the second leading cause of cancer mortality in
China (1). Invasion and metastasis
are hallmarks of cancer, and affect the mortality rates of GC
(2).
12-LOX is an isozyme of the LOX superfamily, and
previous studies have suggested that LOX isozymes, including
12-LOX, are implicated in tumor progression (3). 12-LOX, or its metabolic product,
12-HETE, promotes progression and metastasis in several types of
solid tumors, including prostate cancer (4), breast cancer (5), colon cancer (6) and melanoma (7). The role of 12-LOX in the invasion and
metastasis of human GC, and its underlying mechanism, remain to be
elucidated.
Tumor cells acquire invasive/metastatic properties
due to epithelial-mesenchymal transition (EMT), whereby the
epithelial cell layers lose polarity and cell-cell adhesion due to
remodeling of the cytoskeleton (8).
EMT is involved in a variety of processes that characterize tumor
progression, including cell invasion and metastasis (9,10). The
hallmarks of EMT are the upregulation of N-cadherin and the
downregulation of E-cadherin expression (11). N-cadherin is a member of the cadherin
superfamily, which regulates cell-cell adhesion (12), and has been demonstrated to increase
the motility and migration abilities of a number of types of cancer
cells (13–15) and be a marker of EMT (16,17).
E-cadherin is a Ca2+-dependent cell-cell adhesion
molecule that is important for maintaining the integrity and
polarity of epithelial cells (18).
Numerous studies have indicated that downregulation of E-cadherin
results in tumor progression, metastasis and a poor prognosis for
patients with GC (19–22), cervical carcinoma (23), colorectal cancer (24) and cholangiocarcinoma (25).
The aim of the present study was to investigate the
role of 12-LOX in the invasion and metastasis of human GC, and to
determine whether the effects of 12-LOX are mediated through
EMT.
Patients and methods
Patient selection and tissue
preparation
The present study was approved by the Ethics
Committee of the Fujian Medical University Union Hospital (Fuzhou,
China; reference no. 2014KY031) and written informed consent was
obtained from each patient. A total of 105 paraffin-embedded GC
tissue samples and 43 adjacent normal gastric mucosa tissue samples
were randomly selected from the Department of Pathology, Fujian
Medical University Union Hospital (Fuzhou, China), for
retrospective study. The GC cases consisted of 80 men and 25 women,
aged 34–81 years (mean age, 57 years), between October 2011 and
December 2014. Tumors were staged according to the 7th edition of
the AJCC Cancer Staging Manual: Stomach (26). The clinicopathological characteristics
of the patients are summarized as follows (some data missing):
Tumor size (<5 cm, 53 cases; ≥5 cm, 52 cases); tumor invasion
(T1-T2, 28 cases; T3-T4, 76 cases); differentiation (poor, 52
cases; middle-well, 42 cases); clinical stage (I+II, 44 cases;
III+IV, 61 cases); lymph nodes metastasis (negative, 26 cases;
positive, 78 cases). All patients were treated by radical surgical
resection and had not received chemotherapy or radiotherapy prior
to surgery.
Immunohistochemistry
Formalin-fixed and paraffin-embedded specimens from
the Department of Pathology were sectioned at a thickness of 4-µm,
then deparaffinized twice in 100% dimethylbenzene for 30 min, then
rehydrated in a graded ethanol series (100, 95, 70 and 50%). The
slides were placed in antigen retrieval buffer (sodium citrate 10
mM, pH 6.0) and microwaved at high power for 15 min, followed by
blocking endogenous peroxidase activity in 0.3% hydrogen peroxide
for 20 min at room temperature. Protein expression was detected
using the following primary antibodies: 12-LOX (dilution 1:100;
cat. no. GTX80966), E-cadherin (dilution, 1:500; cat. no.
GTX100443) and N-cadherin (dilution 1:150; cat. no. GTX12221; all
GeneTex, Inc., Irvine, CA, USA), which were incubated with the
sections overnight at 4°C. The slides were then incubated with
secondary antibodies for 20 min in a humidified chamber at 37°C.
All slides were stained with 10% 3′-diaminobenzidine (OriGene
Technologies, Inc., Beijing, China) for 2 min at room temperature,
washed with PBS and then stained with 0.1% hematoxylin for 3 min at
room temperature. The sections were washed again with PBS and then
washed with running tap water for 10 min. All the slides were
observed under a light microscope (magnification, ×400) in 10
randomly selected fields of view, and qualitatively scored by 2
pathologists. The final score was based on the percentage of
positively stained cells as follows: 0, 1%; 1, 1–25%; 2, 25–50%; 3,
50–75%; and 4, >75%. This was multiplied by the intensity of the
staining, which was scored as follows: 0, none; 1, weak; 2,
moderate, and 3, strong. Scores of ≤3 were considered to indicate
negative expression, whereas scores ≥4 were considered to indicate
positive expression.
Cell culture
The human GC cell line, SGC-7901 (The Department of
Gastroenterology, Fujian Medical University Union Hospital), was
cultured in DMEM (HyClone; GE Healthcare, Chicago, IL, USA)
supplemented with 10% fetal bovine serum (HyClone; GE Healthcare)
at 37°C in 5% CO2.
Lentivirus vector construction and
transfection
The lentivirus vector for 12-LOX gene overexpression
was constructed by Shanghai GeneChem Co., Ltd. (Shanghai, China).
An empty green fluorescent protein (GFP) vector was used as a
negative control (Shanghai GeneChem Co., Ltd.). A total of
6×104 SGC-7901 cells were seeded in each well of a
6-well plate. When confluency reached 30–40%, the 12-LOX
overexpression vector (2×107 TU/ml) or the empty GFP
vector (2×107 TU/ml) was transfected into the SGC-7901
cells. The status of cells was observed by fluorescence microscope
(magnification, ×200) for 8–12 h, according to the lentivirus
vector manufacturer's instructions, and the medium was replaced.
When confluency reached 90%, the cells was transferred into a
culture flask. Following transfection, cells were used in
subsequent experiments after 2 passages. Three groups of cells were
used in the subsequent experiments: Untransfected cells (control
group), cells transfected with the empty GFP vector (LV-vector
group) and cells transfected with the 12-LOX expression vector
(LV-12-LOX group).
Wound-healing assay
A total of 4×105 cells were seeded into
each well of a 6-well plate and allowed to grow in DMEM
supplemented with 10% fetal bovine serum (both HyClone; GE
Healthcare, Chicago, IL, USA) until ~100% confluent. A 200-µl
pipette tip was used to scratch across the cell monolayer. Cellular
debris was removed by washing with PBS three times, and the plate
was cultured for another 24 h. Images were captured at 0 and 24 h
after wounding using an inverted light microscope. The extent of
wound-healing was quantified using the following formula: (W0
h-W24 h)/W0 h, where W0 h
and W24 h represent the width of the wound at 0 and 24
h, respectively.
Cell invasion and migration assay
A 24-well plate containing Transwell inserts with a
pore size of 8 µm (Merck KGaA, Darmstadt, Germany) was coated with
Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) for the invasion
assay and left uncoated for the migration assay. The following
steps were then performed for the two assays. A total of
1.5×104 cells suspended in serum-free DMEM were placed
in the upper chamber. DMEM containing 10% fetal bovine serum was
placed in the lower chamber as a chemoattractant. The cells were
incubated for 24 h at 37°C prior to removal of cells remaining on
the upper side of the chamber with a cotton swab. Cells on the
lower side of the membrane were fixed in 4% paraformaldehyde for 10
min at room temperature and dyed with 0.1% crystal violet for 15
min at room temperature. The number of cells was then counted under
an inverted light microscope (magnification, ×400) by a technician
blinded to the experimental settings in 5 randomly selected fields
of view in each plate. The experiments were repeated 3 times.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
RT-qPCR was used to detect the mRNA expression of
12-LOX and N-cadherin in the transfected and untransfected cells.
Total RNA was extracted using TRIzol (Thermo Fisher Scientific,
Inc., Waltham, MA, USA). cDNA was synthesized using 2 µg total RNA
using the PrimeScript™ RT reagent kit (Takara
Biotechnology Co., Ltd. Dalian, China). RT-qPCR analysis was
performed using SYBR® Premix Ex Taq™ II (Tli
RNaseH Plus; Takara) with 7500 Fast Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The PCR amplification
was performed under the following conditions: 95°C for 2 min, then
45 cycles of denaturation at 95°C for 15 sec, annealing at 63°C for
15 sec and elongation at 72°C for 20 sec. The primer sequences for
12-LOX, N-cadherin and GAPDH are listed in Table I (Shanghai Yaolin Bio-Technology Co.,
Ltd., Shanghai, China). The gene expression levels were normalized
to GAPDH and are presented as relative fold change compared to the
control (27). The data were analyzed
using the 2−ΔΔCq method. A total of 3 independent repeat
experiments were performed.
 | Table I.Primer sequences for 12-LOX,
N-cadherin and GAPDH. |
Table I.
Primer sequences for 12-LOX,
N-cadherin and GAPDH.
| Gene | Primer (5′-3′) |
|---|
| 12-LOX | Forward:
ATGGCCCTCAAACGTGTTTAC |
|
| Reverse:
GCACTGGCGAACCTTCTCA |
| N-cadherin | Forward:
GCGTCTGTAGAGGCTTCTGG |
|
| Reverse:
AAATCTGCAGGCTCACTGCT |
| GAPDH | Forward:
CATCAGCAATGCCTCCTGCAC |
|
| Reverse:
TGAGTCCTTCCACGATACCAA |
|
| AGTT |
Western blotting analysis
Total protein was extracted using cell lysis buffer
(Beyotime Institute of Biotechnology, Haimen, China) containing 1
mmol/l phenylmethylsulfonyl fluoride. Protein concentration was
determined using a bicinchoninic acid assay kit (Beyotime Institute
of Biotechnology). A total of 40 µg protein per lane was subjected
to 10% SDS-PAGE and transferred to a nitrocellulose membrane (GE
Healthcare, IL, Chicago). The membrane was blocked in 5% skimmed
milk dissolved by PBS for 2 h at room temperature and subsequently
incubated with the following rabbit polyclonal primary antibodies
overnight at 4°C: 12-LOX (dilution 1:80; cat. no. GTX80966),
N-cadherin (dilution 1:80; cat. no. GTX12221; both GeneTex, Inc.)
or GAPDH (dilution 1:1,500; cat. no. TA309157; OriGene
Technologies, Inc.). The membrane was then incubated with the
appropriate HRP-conjugated Goat anti-Rabbit IgG (H+L) (dilution
1:2,000; cat. no. ZB2301; OriGene Technologies, Inc.) for 1 h at
room temperature. The proteins were visualized using an ECL Advance
Detection system (Origene Technologies, Inc.).
Statistical analysis
Data are expressed as the mean ± standard deviation.
The differentiation between expression levels and
clinicopathological parameters were analyzed using the
χ2 test. The associations between groups were compared
using analysis of variance followed by the
Least-Significant-Difference test. Statistical analyses were
performed using SPSS 19.0 software (IBM Corp., Armonk, NY, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of 12-LOX, N-cadherin and
E-cadherin in GC tissue
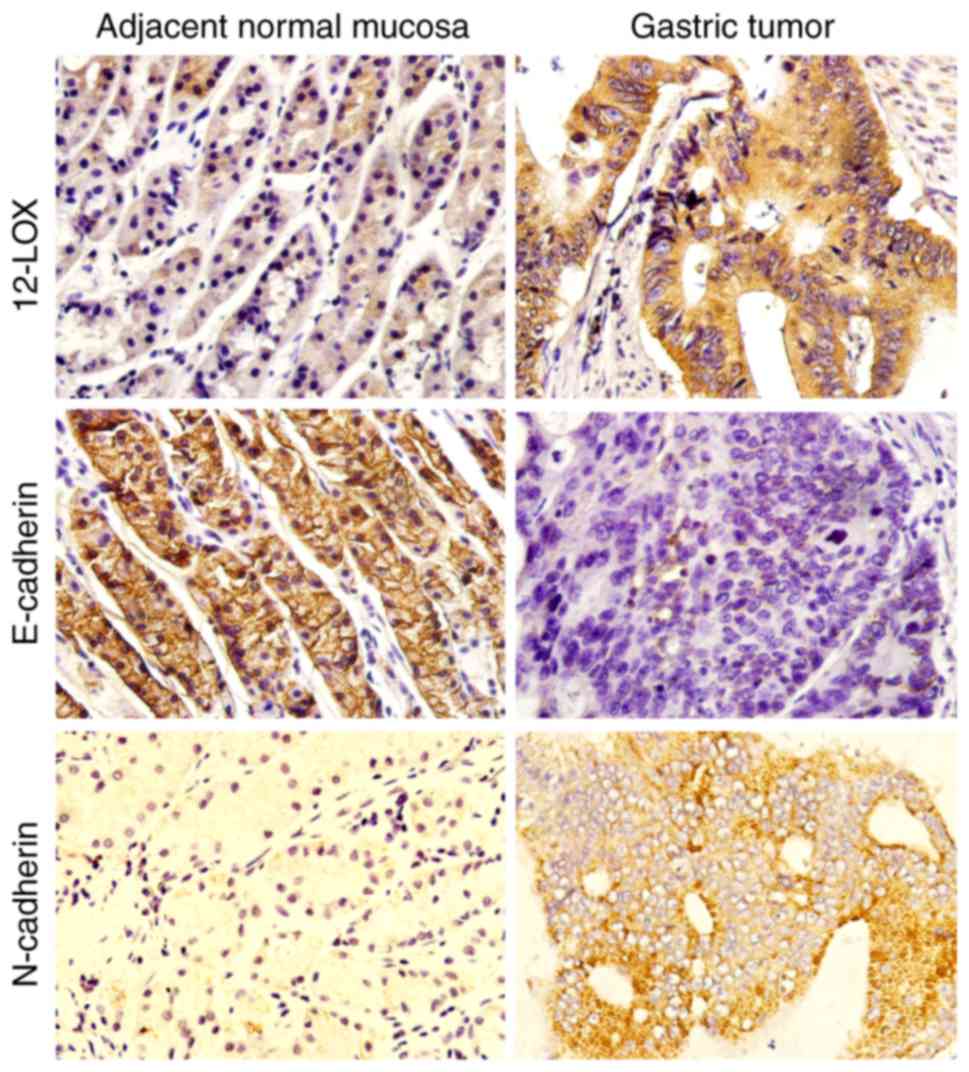
Immunohistochemical analysis was used to evaluate
the protein expression levels of 12-LOX, N-cadherin and E-cadherin
in GC tissue and adjacent normal mucosa tissue. It was demonstrated
that 12-LOX and N-cadherin were mainly expressed in the cytoplasm
of tumor cells, whereas E-cadherin was localized in the cell
membrane (Fig. 1). The protein
expression level of 12-LOX and N-cadherin was significantly
increased in GC tissue compared with that in adjacent normal
gastric mucosa tissue (P<0.05; Table
II). By contrast, the expression of E-cadherin was
significantly decreased in GC tissues compared with that in
adjacent normal gastric mucosa tissue (P<0.05). The associations
between patient clinicopathological characteristics and the protein
expression levels of 12-LOX, N-cadherin and E-cadherin were
examined. As demonstrated in Table
III, there was a close association between the levels of
E-cadherin protein expression and two factors, patient age and
tumor differentiation (P<0.05), whereas the level of N-cadherin
protein expression was associated with tumor size (P<0.05).
12-LOX expression was higher in tumors ≥5 cm, with a depth of
invasion of T3-T4 and clinical stage of III+IV, however, these
differences were not statistically significant (P>0.05). The
expression of 12-LOX was positively associated with that of
N-cadherin in GC tissues (r=0.263; P=0.007; Table IV).
 | Table II.Expression of 12-LOX, N-cadherin and
E-cadherin in 105 cases of gastric cancer and 43 gastric normal
mucosa tissues. |
Table II.
Expression of 12-LOX, N-cadherin and
E-cadherin in 105 cases of gastric cancer and 43 gastric normal
mucosa tissues.
| Protein
expression | Gastric cancer
tissue | Gastric normal
mucosa tissue | P-value |
|---|
| 12-LOX |
|
|
|
|
Positive | 65 | 14 | 0.001 |
|
Negative | 40 | 29 |
|
| N-cadherin |
|
|
|
|
Positive | 63 | 11 | <0.001 |
|
Negative | 42 | 32 |
|
| E-cadherin |
|
|
|
|
Positive | 41 | 24 | 0.046 |
|
Negative | 64 | 19 |
|
 | Table III.Association between 12-LOX,
E-cadherin and N-cadherin protein expression level and
clinicopathological features in 105 cases of gastric cancer (some
patient data is missing). |
Table III.
Association between 12-LOX,
E-cadherin and N-cadherin protein expression level and
clinicopathological features in 105 cases of gastric cancer (some
patient data is missing).
|
| 12-LOX
expression | E-cadherin
expression | N-cadherin
expression |
|---|
|
|
|
|
|
|---|
| Characteristic | Low, n | High, n | P-value | Low, n | High, n | P-value | Low, n | High, n | P-value |
|---|
| Age, years |
|
|
|
|
|
|
|
|
|
|
≤60 | 18 | 29 | 0.751 | 34 | 13 | 0.031a | 21 | 26 | 0.291 |
|
>60 | 24 | 34 |
| 30 | 28 |
| 20 | 38 |
|
| Sex |
|
|
|
|
|
|
|
|
|
|
Male | 35 | 45 | 0.164 | 46 | 34 | 0.198 | 31 | 49 | 0.912 |
|
Female | 7 | 18 |
| 18 | 7 |
| 10 | 15 |
|
| Tumor size, cm |
|
|
|
|
|
|
|
|
|
|
<5 | 24 | 29 | 0.296 | 32 | 21 | 0.902 | 27 | 26 | 0.011a |
| ≥5 | 18 | 34 |
| 32 | 20 |
| 14 | 38 |
|
| Tumor invasion |
|
|
|
|
|
|
|
|
|
|
T1-T2 | 11 | 17 | 0.986 | 18 | 10 | 0.645 | 14 | 14 | 0.145 |
|
T3-T4 | 30 | 46 |
| 45 | 31 |
| 26 | 50 |
|
|
Differentiation |
|
|
|
|
|
|
|
|
|
|
Poor | 32 | 20 | 0.670 | 14 | 38 | 0.021a | 29 | 23 | 0.121 |
|
Middle-well | 24 | 18 |
| 21 | 21 |
| 30 | 12 |
|
| Clinical stage |
|
|
|
|
|
|
|
|
|
|
I+II | 17 | 27 | 0.811 | 29 | 15 | 0.381 | 19 | 25 | 0.466 |
|
III+IV | 25 | 36 |
| 35 | 26 |
| 22 | 39 |
|
| Lymph node
metastasis |
|
|
|
|
|
|
|
|
|
|
Negative | 9 | 17 | 0.562 | 16 | 10 | 0.908 | 11 | 15 | 0.645 |
|
Positive | 32 | 46 |
| 47 | 31 |
| 29 | 49 |
|
 | Table IV.Association between the expression
levels of 12-LOX and N-cadherin protein expression in 105 gastric
cancer tissues. |
Table IV.
Association between the expression
levels of 12-LOX and N-cadherin protein expression in 105 gastric
cancer tissues.
|
| 12-LOX
expression |
|---|
|
|
|
|---|
| N-cadherin
expression | Low | High | R | P-value |
|---|
| Low | 23 | 18 | 0.263 | 0.007 |
| High | 19 | 45 |
|
|
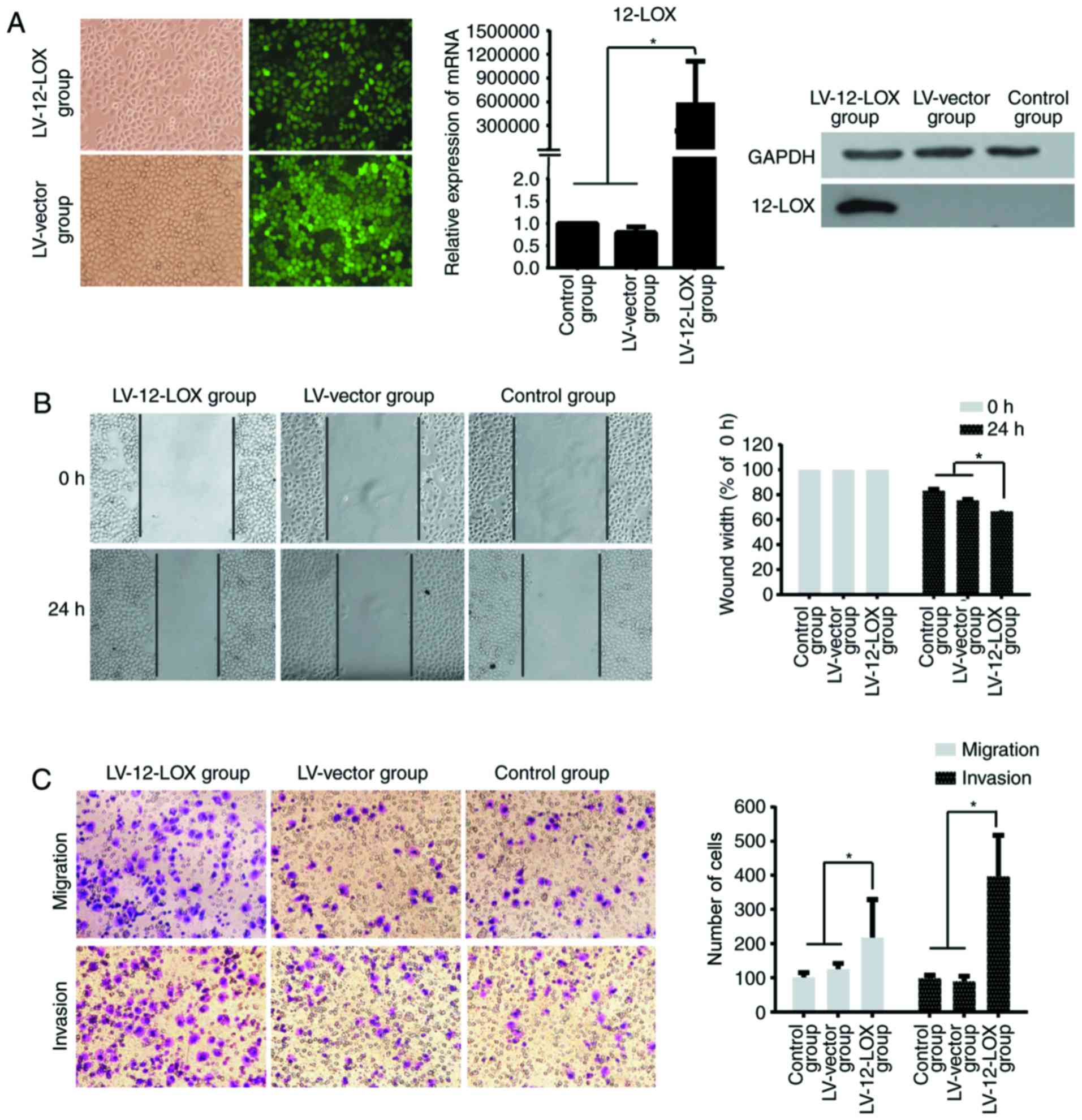
12-LOX promotes the migration and
invasion of GC cells
A stably transfected 12-LOX-overexpressing GC
SGC-7901 cell line was established, and the green fluorescent cells
were >95% confluent prior to fluorescence microscopy
(magnification, ×200). The results of RT-qPCR and western blotting
revealed that the expression of 12-LOX in the LV-12-LOX group was
significantly increased compared with that in the LV-vector and
control groups (Fig. 2A).
The results of the scratch wound-healing (Fig. 2B) and Transwell (Fig. 2C) assays demonstrated that 12-LOX
promotes the migration and invasion of GC cells in vitro.
Significantly more cells migrated in 24 h in the LV-12-LOX group
compared with those in the LV-vector and control groups
(P<0.05). The number of cancer cells that invaded through the
Matrigel was significantly higher in the LV-12-LOX group compared
with that in the LV-vector and control groups (P<0.05).
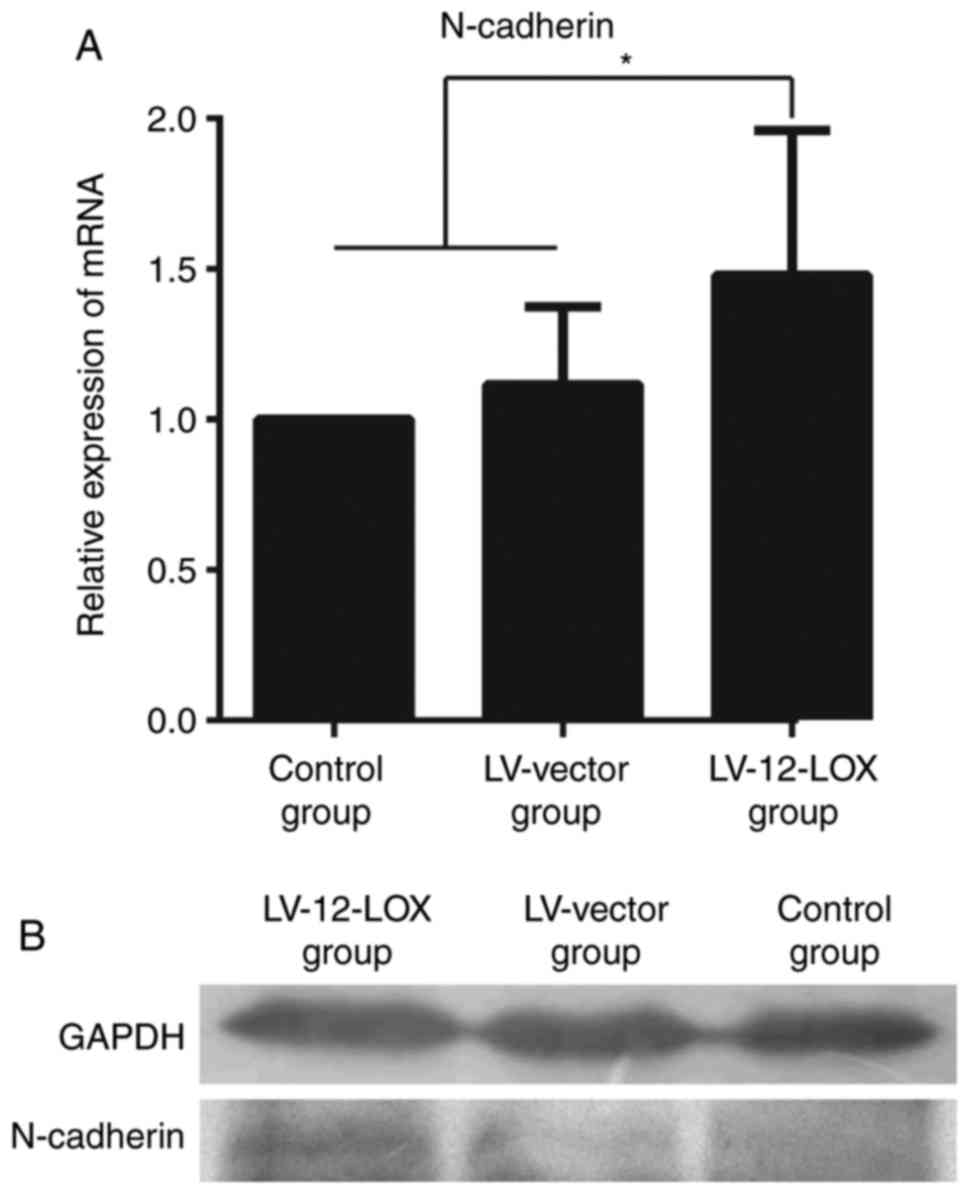
The results of RT-qPCR revealed that the mRNA level
of N-cadherin was significantly increased in the LV-12-LOX group
compared with that in the LV-vector and control groups (Fig. 3A; P<0.05). Western blotting
revealed that the protein expression level of N-cadherin was
markedly increased in the LV-12-LOX group compared with that in the
LV-vector and control groups (Fig.
3B).
Discussion
12-LOX serves an important role in various
inflammatory diseases, including diabetes, atherosclerosis
(28) and nervous system diseases
(29). Previous research has reported
significant functional roles of 12-LOX and its product, 12-HETE, in
the formation, development, invasion and metastasis of several
types of cancer (30–32). Klampfl et al (6) reported that upregulation of 12-LOX
induced a migratory phenotype in colorectal cancer cells. The
available literature regarding the expression and involvement of
12-LOX in GC is limited; however, it has been reported that 12-LOX
may be associated with GC cell apoptosis (33,34).
The present study demonstrated that 12-LOX was
highly expressed in GC tissue compared with that in adjacent normal
gastric mucosa tissue, indicating that 12-LOX overexpression may
contribute to GC progression. LV-12-LOX GC cells exhibited
significantly enhanced migratory and invasive abilities compared
with the LV-vector and control groups. These results indicate that
12-LOX facilitates GC cell migration and invasion and are
consistent with previous studies that reported that 12-LOX promotes
invasion and metastasis in numerous types of tumor cells (35–37).
However, the specific underlying mechanism remains to be
elucidated.
Immunohistochemical analysis revealed that GC tissue
exhibited increased E-cadherin and decreased N-cadherin protein
expression levels compared with those of adjacent normal gastric
mucosa tissues. Abnormal expression of E-cadherin was demonstrated
to be significantly associated with tumor grade and patient age,
which was also reported by Torabizadeh et al (38). Anbiaee et al (39) and Lazăr et al (40) reported a significant correlation
between the abnormal expression of E-cadherin and tumor grade, but
no correlation with patient age. The present study also revealed a
significant association between the abnormal expression of
N-cadherin and tumor size, suggesting that N-cadherin may promote
the growth of GC.
The present study also demonstrated that the
expression level of 12-LOX was positively associated with that of
N-cadherin in GC tissue. To the best of our knowledge, the
regulation of N-cadherin expression by 12-LOX has not been
previously reported. N-cadherin is a member of the
calcium-dependent cell adhesion molecule family, and a marker of
interstitial cells (41). N-cadherin
is also a marker of EMT (42,43), and serves a key role in tumor cell
migration, invasion and metastasis (44,45). It
has been reported that EMT is involved in the invasion and
metastasis of GC (46), which
indicates that 12-LOX may affect GC progression via EMT.
The present study suggests that EMT may be involved
in the progression of GC, which is in accordance with previous
study results (9,10,46).
Therefore, we hypothesize that 12-LOX promotes the invasion and
metastasis of GC cells via EMT. Han and Xu (47) reported that epithelial membrane
protein 3 is induced by twist family BHLH transcription factor 1/2
and regulates the EMT of GC cells. Song et al (48) demonstrated that the Wnt/β-catenin and
phosphoinositide 3-kinase/protein kinase B signaling pathways
regulate EMT in GC. Chen et al (49) reported that the tumor necrosis
factor-α-inducing protein of Helicobacter pylori induces EMT
in GC cells through activation of the interleukin-6/signal
transducer and activator of transcription 3 signaling pathway.
Numerous other pathways are reportedly involved in EMT, including
transforming growth factor β (50),
notch (51), nuclear factor-κB
(52) and mitogen-activated protein
kinases/extracellular signal-regulated kinase signaling (53). The underlying mechanisms of the
promotion of EMT by 12-LOX require further investigation.
In conclusion, it was demonstrated that EMT may be
involved in the promotion of the invasion and metastasis of human
gastric cancer cells via 12-LOX; therefore, it may be a novel
target for the treatment of gastric cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Key Clinical
Specialty Discipline Construction Program of Fujian, China [grant
no. (2012)149] and the Natural Science Foundation of Fujian
Province, China (grant no. 2015J01476).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CZ performed the experiment, analyzed the experiment
data and wrote the manuscript. MZ was responsible for the
collection of the patient selection and tissue preparation. XW and
JL were responsible for the design of experiment. ZC and YH were
responsible for guiding the experimental technique, analysis of
data and revising the manuscript. FC was accountable for designing
the experiment, performing data interpretation and revising the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Fujian Medical University Union Hospital (Fuzhou,
China; reference no. 2014KY031).
Consent for publication
Written informed consent was obtained from each
patient for the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wadhwa R, Song S, Lee JS, Yao Y, Wei Q and
Ajani JA: Gastric cancer-molecular and clinical dimensions. Nat Rev
Clin Oncol. 10:643–655. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pidgeon GP, Lysaght J, Krishnamoorthy S,
Reynolds JV, O'Byrne K, Nie D and Honn KV: Lipoxygenase metabolism:
Roles in tumor progression and survival. Cancer Metastasis Rev.
26:503–524. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gondek T, Szajewski M, Szefel J,
Aleksandrowicz-Wrona E, Skrzypczak-Jankun E, Jankun J and
Lysiak-Szydlowska W: Evaluation of 12-lipoxygenase (12-LOX) and
plasminogen activator inhibitor 1 (PAI-1) as prognostic markers in
prostate cancer. Biomed Res Int. 2014:1024782014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Singh AK, Kant S, Parshad R, Banerjee N
and Dey S: Evaluation of human LOX-12 as a serum marker for breast
cancer. Biochem Biophys Res Commun. 414:304–308. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Klampfl T, Bogner E, Bednar W, Mager L,
Massudom D, Kalny I, Heinzle C, Berger W, Stättner S, Karner J, et
al: Up-regulation of 12(S)-lipoxygenase induces a migratory
phenotype in colorectal cancer cells. Exp Cell Res. 318:768–778.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kang KH, Ling TY, Liou HH, Huang YK, Hour
MJ, Liou HC and Fu WM: Enhancement role of host 12/15-lipoxygenase
in melanoma progression. Eur J Cancer. 49:2747–2759. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Buehler D, Hardin H, Shan W,
Montemayor-Garcia C, Rush PS, Asioli S, Chen H and Lloyd RV:
Expression of epithelial-mesenchymal transition regulators SNAI2
and TWIST1 in thyroid carcinomas. Mod Pathol. 26:54–61. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang H, Meng F, Liu G, Zhang B, Zhu J, Wu
F, Ethier SP, Miller F and Wu G: Forkhead transcription factor
foxq1 promotes epithelial-mesenchymal transition and breast cancer
metastasis. Cancer Res. 71:1292–1301. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu X, Zhai Y, Kong P, Cui H, Yan T, Yang
J, Qian Y, Ma Y, Wang F, Li H, et al: FAT1 prevents epithelial
mesenchymal transition (EMT) via MAPK/ERK signaling pathway in
esophageal squamous cell cancer. Cancer Lett. 397:83–93. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kamikihara T, Ishigami S, Arigami T,
Matsumoto M, Okumura H, Uchikado Y, Kita Y, Kurahara H, Kijima Y,
Ueno S and Natsugoe S: Clinical implications of N-cadherin
expression in gastric cancer. Pathol Int. 62:161–166. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Natalwala A, Spychal R and Tselepis C:
Epithelial-mesenchymal transition mediated tumourigenesis in the
gastrointestinal tract. World J Gastroenterol. 14:3792–3797. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kokkinos MI, Wafai R, Wong MK, Newgreen
DF, Thompson EW and Waltham M: Vimentin and epithelial-mesenchymal
transition in human breast cancer-observations in vitro and in
vivo. Cells Tissues Organs. 185:191–203. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tomita K, van Bokhoven A, van Leenders GJ,
Ruijter ET, Jansen CF, Bussemakers MJ and Schalken JA: Cadherin
switching in human prostate cancer progression. Cancer Res.
60:3650–3654. 2000.PubMed/NCBI
|
|
16
|
Mahmood MQ, Ward C, Muller HK, Sohal SS
and Walters EH: Epithelial mesenchymal transition (EMT) and
non-small cell lung cancer (NSCLC): A mutual association with
airway disease. Med Oncol. 34:452017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Geng XL, Long WH, Hai J, Zhang Y, Zheng W,
Zhang ZY and Du LX: The role of hexokinase 2 in the metastasis of
hepatocellular carcinoma cells. Zhonghua Zhong Liu Za Zhi.
38:739–742. 2016.(In Chinese). PubMed/NCBI
|
|
18
|
van Roy F and Berx G: The cell-cell
adhesion molecule E-cadherin. Cell Mol Life Sci. 65:3756–3788.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao M, Li W, Wang H and Wang G: The
distinct expression patterns of claudin-10, −14, −17 and E-cadherin
between adjacent non-neoplastic tissues and gastric cancer tissues.
Diagn Pathol. 8:2052013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Czyzewska J, Guzińska-Ustymowicz K,
Ustymowicz M, Pryczynicz A and Kemona A: The expression of
E-cadherin-catenin complex in patients with advanced gastric
cancer: Role in formation of metastasis. Folia Histochem Cytobiol.
48:37–45. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yan HB, Wang XF, Zhang Q, Tang ZQ, Jiang
YH, Fan HZ, Sun YH, Yang PY and Liu F: Reduced expression of the
chromatin remodeling gene ARID1A enhances gastric cancer cell
migration and invasion via downregulation of E-cadherin
transcription. Carcinogenesis. 35:867–876. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Y, Chen CQ, He YL, Cai SR, Yang DJ, He
WL, Xu JB and Zan WH: Abnormal expression of E-cadherin in tumor
cells is associated with poor prognosis of gastric carcinoma. J
Surg Oncol. 106:304–310. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang X, Qian Y, Wu H, Xie X, Zhou Q, Wang
Y, Kuang W, Shen L, Li K, Su J, et al: Aberrant expression of
osteopontin and E-cadherin indicates radiation resistance and poor
prognosis for patients with cervical carcinoma. J Histochem
Cytochem. 63:88–98. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yun JA, Kim SH, Hong HK, Yun SH, Kim HC,
Chun HK, Cho YB and Lee WY: Loss of E-Cadherin expression is
associated with a poor prognosis in stage III colorectal cancer.
Oncology. 86:318–328. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Techasen A, Loilome W, Namwat N, Khuntikeo
N, Puapairoj A, Jearanaikoon P, Saya H and Yongvanit P: Loss of
E-cadherin promotes migration and invasion of cholangiocarcinoma
cells and serves as a potential marker of metastasis. Tumour Biol.
35:8645–8652. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Washington K: 7th edition of the AJCC
cancer staging manual: Stomach. Ann Surg Oncol. 17:3077–3079. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen F, Zhuang M, Zhong C, Peng J, Wang X,
Li J, Chen Z and Huang Y: Baicalein reverses hypoxia-induced 5-FU
resistance in gastric cancer AGS cells through suppression of
glycolysis and the PTEN/Akt/HIF-1α signaling patway. Oncol Rep.
33:457–463. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rong S, Cao Q, Liu M, Seo J, Jia L,
Boudyguina E, Gebre AK, Colvin PL, Smith TL, Murphy RC, et al:
Macrophage 12/15 lipoxygenase expression increases plasma and
hepatic lipid levels and exacerbates atherosclerosis. J Lipid Res.
53:686–695. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nagasawa K, Kakuda T, Higashi Y and
Fujimoto S: Possible involvement of 12-lipoxygenase activation in
glucose-deprivation/reload-treated neurons. Neurosci Lett.
429:120–125. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dilly AK, Ekambaram P, Guo Y, Cai Y,
Tucker SC, Fridman R, Kandouz M and Honn KV: Platelet-type
12-lipoxygenase induces MMP9 expression and cellular invasion via
activation of PI3K/Akt/NF-κB. Int J Cancer. 133:1784–1791. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yoshimura R, Inoue K, Kawahito Y,
Mitsuhashi M, Tsuchida K, Matsuyama M, Sano H and Nakatani T:
Expression of 12-lipoxygenase in human renal cell carcinoma and
growth prevention by its inhibitor. Int J Mol Med. 13:41–46.
2004.PubMed/NCBI
|
|
32
|
Yoshimura R, Matsuyama M, Mitsuhashi M,
Takemoto Y, Tsuchida K, Kawahito Y, Sano H and Nakatani T:
Relationship between lipoxygenase and human testicular cancer. Int
J Mol Med. 13:389–393. 2004.PubMed/NCBI
|
|
33
|
Wong BC, Wang WP, Cho CH, Fan XM, Lin MC,
Kung HF and Lam SK: 12-Lipoxygenase inhibition induced apoptosis in
human gastric cancer cells. Carcinogenesis. 22:1349–1354. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen FL, Wang XZ, Li JY, Yu JP, Huang CY
and Chen ZX: 12-lipoxygenase induces apoptosis of human gastric
cancer AGS cells via the ERK1/2 signal pathway. Dig Dis Sci.
53:181–187. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nie D, Nemeth J, Qiao Y, Zacharek A, Li L,
Hanna K, Tang K, Hillman GG, Cher ML, Grignon DJ and Honn KV:
Increased metastatic potential in human prostate carcinoma cells by
overexpression of arachidonate 12-lipoxygenase. Clin Exp
Metastasis. 20:657–663. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gao X and Honn KV: Biological properties
of 12(S)-HETE in cancer metastasis. Adv Prostaglandin Thromboxane
Leukot Res. 23:439–444. 1995.PubMed/NCBI
|
|
37
|
Honn KV, Tang DG, Gao X, Butovich IA, Liu
B, Timar J and Hagmann W: 12-lipoxygenases and 12(S)-HETE: Role in
cancer metastasis. Cancer Metastasis Rev. 13:365–396. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Torabizadeh Z, Nosrati A, Sajadi Saravi
SN, Yazdani Charati J and Janbabai G: Evaluation of E-cadherin
expression in gastric cancer and its correlation with
clinicopathologic parameters. Int J Hematol Oncol Stem Cell Res.
11:158–164. 2017.PubMed/NCBI
|
|
39
|
Anbiaee R, Mojir Sheibani K, Torbati P and
Jaam H: Abnormal expression of e-cadherin in gastric
adenocarcinoma, and its correlation with tumor histopathology and
helicobacter pylori infection. Iran Red Crescent Med J. 15:218–222.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lazăr D, Tăban S, Ardeleanu C, Dema A,
Sporea I, Cornianu M, Lazăr E and Vernic C: The immunohistochemical
expression of E-cadherin in gastric cancer; correlations with
clinicopathological factors and patients' survival. Rom J Morphol
Embryol. 49:459–467. 2008.PubMed/NCBI
|
|
41
|
Stemmler MP: Cadherins in development and
cancer. Mol Biosyst. 4:835–850. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ma Y, Zheng X, Zhou J, Zhang Y and Chen K:
ZEB1 promotes the progression and metastasis of cervical squamous
cell carcinoma via the promotion of epithelial-mesenchymal
transition. Int J Clin Exp Pathol. 8:11258–11267. 2015.PubMed/NCBI
|
|
43
|
Zhao J, Yang C, Guo S and Wu Y: GM130
regulates epithelial-to-mesenchymal transition and invasion of
gastric cancer cells via snail. Int J Clin Exp Pathol.
8:10784–10791. 2015.PubMed/NCBI
|
|
44
|
Wang F, Li XK, Xu HY, Shan ZZ, Wang T,
Yang ZC, He W, Wang LX and Fan QX: N-cadherin participated in
invasion and metastasis of human esophageal squamous cell carcinoma
via taking part in the formation of vasculogenic mimicry. Med
Oncol. 32:4802015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yi S, Yang ZL, Miao X, Zou Q, Li J, Liang
L, Zeng G and Chen S: N-cadherin and P-cadherin are biomarkers for
invasion, metastasis, and poor prognosis of gallbladder carcinomas.
Pathol Res Pract. 210:363–368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ye J, Huang J, Xu J, Huang Q, Wang J,
Zhong W and Lin X, Li Y and Lin X: ERp29 controls invasion and
metastasis of gastric carcinoma by inhibition of
epithelial-mesenchymal transition via PI3K/Aktsignaling pathway.
BMC Cancer. 17:6262017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Han M and Xu W: EMP3 is induced by
TWIST1/2 and regulates epithelial-to-mesenchymal transition of
gastric cancer cells. Tumour Biol. 39:10104283177184042017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Song Y, Li ZX, Liu X, Wang R, Li LW and
Zhang Q: The Wnt/β-catenin and PI3K/Akt signaling pathways promote
EMT in gastric cancer by epigenetic regulation via H3 lysine 27
acetylation. Tumour Biol. 39:10104283177126172017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen G, Tang N, Wang C, Xiao L, Yu M, Zhao
L, Cai H, Han L, Xie C and Zhang Y: TNF-α-inducing protein of
Helicobacter pylori induces epithelial-mesenchymal transition (EMT)
in gastric cancer cells through activation of IL-6/STAT3 signaling
pathway. Biochem Biophys Res Commun. 484:311–317. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zavadil J and Böttinger EP: TGF-beta and
epithelial-to-mesenchymal transitions. Oncogene. 24:5764–5774.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Timmerman LA, Grego-Bessa J, Raya A,
Bertrán E, Pérez-Pomares JM, Díez J, Aranda S, Palomo S, McCormick
F, Izpisúa-Belmonte JC and de la Pompa JL: Notch promotes
epithelial-mesenchymal transition during cardiac development and
oncogenic transformation. Genes Dev. 18:99–115. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Huber MA, Azoitei N, Baumann B, Grünert S,
Sommer A, Pehamberger H, Kraut N, Beug H and Wirth T: NF-kappaB is
essential for epithelial-mesenchymal transition and metastasis in a
model of breast cancer progression. J Clin Invest. 114:569–581.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Tashiro E, Henmi S, Odake H, Ino S and
Imoto M: Involvement of the MEK/ERK pathway in EGF-induced
E-cadherin down-regulation. Biochem Biophys Res Commun.
477:801–806. 2016. View Article : Google Scholar : PubMed/NCBI
|