Introduction
Osteosarcoma, as a kind of malignant tumor derived
from malignant interstitial cells, has certain osteoid
characteristics, including a strong migration capacity and frequent
systemic metastasis (1). The
pathogenesis of osteosarcoma remains unclear and the osteosarcoma
of osteoblasts is a clinically common form at present, which
generally occurs in the metaphysis of long-tubular bone, but seldom
in the axial skeleton (2,3). The malignant grade of osteosarcoma is
high, micro-lesion metastasis is possible during diagnosis, while
lung tissue is a common metastatic site (4). Prior to the application of
chemotherapeutic drugs, amputation was commonly used in the
treatment of osteosarcoma. The rise and development of chemotherapy
drugs has been useful in the treatment of osteosarcoma. However,
drug resistance of chemotherapy drugs delays the treatment of
osteosarcoma (5). Icariin (ICA), also
known as Epimedium brevicornu, is sweet and warm. It has the
effect of nourishing kidney and strengthening yang, strengthening
tendons and bones, dispelling wind and eliminating dampness,
expelling furuncle and resolving carbuncle. ICA is often clinically
used in the treatment of flaccid bones and muscles and rheumatic
arthralgia (6–8). Recent findings have shown that ICA has
the effect of promoting osteoblast proliferation and increasing ALP
activity in osteoblasts, thus promoting the calcification of bone
matrix and enhancing bone strength (9). Zhang et al (10) found that ICA can significantly
increase osteocytes and promote osteocyte differentiation. Zhang
et al (11) found that ICA can
increase the apoptosis of osteoclastoma. However, to the best of
our knowledge, there is no report currently available on the effect
of ICA on osteosarcoma cells and its mechanism.
In this study, the effect of ICA on osteosarcoma
cell proliferation and its mechanism were studied to clarify the
mechanism of ICA for osteosarcoma cells, in order to provide new
ideas for the efficacious development of ICA and clinical treatment
of osteosarcoma.
Materials and methods
Instruments and materials
Osteosarcoma cancer cell line 143B (Cell Bank of the
Chinese Academy of Sciences, Shanghai, China), methyl thiazolyl
tetrazolium (MTT) and DMSO (both from Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany), ICA (Aladdin Shanghai Biochemical Technology
Co., Ltd., Shanghai, China), TRIzol kit and reverse transcription
kit (both from Invitrogen, Carlsbad, CA, USA), VEGA ELISA kit and
MMP-9 ELISA kit (both from R&D Systems, Inc., Minneapolis, MN,
USA), rabbit anti-DKK1, rabbit anti-caspase-3, rabbit
anti-β-catenin, rabbit anti-GSK3β, rabbit anti-pGSK3β (Ser9) and
GAPDH (all from Cell Signaling Technology, Inc., Danvers, MA, USA),
ECI luminescent solution (Invitrogen), inversed fluorescent
microscope (Thermo Fisher Scientific GmbH, Dreieich, Germany), cell
culture flask (Corning Inc., Corning, NY, USA), pipettor
(Eppendorf, Hamburg, Germany), PCR instrument (Applied Biosystems,
Foster City, CA, USA), UV imaging system (Biometra, Goettingen,
Germany), and electronic balance (BP121S; Sartorius AG, Goettingen,
Germany) were used in the study. Any other equipment and reagents
were previously described in the relevant section.
Detection of inhibitory effect of ICA
on osteosarcoma cell growth via MTT assay
After osteosarcoma cancer cell line 143B was revived
(12), it was placed in the
incubator, and cells in the logarithmic growth phase were
inoculated onto the 96-well plate after subculture
(8×103/well). [Of note, the 143B cell line used in the
present study is possibly identified as osteosarcoma cell line
HTK-osteosarcoma cell line (13);
however, it did not affect the conclusions drawn in the study.]
After inoculation for 24 h, the serum was deprived for 24 h and ICA
was added at different concentrations (20, 10, 5, 2, 1 and 0.1 µM).
The blank control group and the positive drug group (cisplatin)
were then set up. After incubation with medication for 24 h, 1% MTT
was added to incubate the cells for 4 h in the dark. The medium was
discarded and 150 µl dimethyl sulfoxide was added to each well.
After vibration for 10 min, the absorbance value at 570 nm was
measured using the microplate reader (Thermo Fisher Scientific;
Waltham, MA, USA).
Effects of ICA on osteosarcoma cell
apoptosis
The 143B colon cancer cell line was cultured as
follows. The cell density was adjusted to 5×106 cells/ml
and added into the 6-well plate, and the cells were then divided
into the blank control, ICA (5 µM) and positive drug groups. After
treatment for 24 h, the cells were washed with pre-cooled PBS
twice, re-suspended and centrifuged at 12,000 × g for 5 min.
Fluorescent solution was added to incubate the cells at room
temperature for 15 min in the dark. The cells were then detected
using a flow cytometer (Thermo Fisher Scientific, Inc., Waltham,
MA, USA).
Detection of the expression of related
genes via semi-quantitative PCR
The cell proteins treated with 5 µM ICA for 24 h
were extracted using a TRIzol kit, and the supernatant was
discarded via centrifugation for 10 min at 6,000 × g. The RNA
integrity was confirmed by agarose gel electrophoresis. The results
of electrophoresis showed that 28S, 18S and 5S bands were clear,
and the brightness of 28S band was approximately twice of that of
18S band, indicating that RNA was intact and can be used for
subsequent experiments. cDNA was obtained via reverse transcription
using the reverse transcription kit. An appropriate internal
reference was selected. The expression levels of c-Myc, cyclin D1
and β-catenin in the Wnt/β-catenin signaling pathway,
apoptosis-related gene caspase-3 and migration-related gene
MMP-9 and VEGF were detected via semi-quantitative PCR.
Reaction conditions were: denaturation, 95°C for 30 sec; annealing,
64°C for 25 sec; and extension 72°C for 30 sec, for a total of 35
cycles. Primers were produced by Tiangen Biotech Co., Ltd.
(Beijing, China). Primer sequences are shown in Table I. After the reaction, agarose gel
electrophoresis was performed, followed by observation using a UV
imaging system.
 | Table I.PCR primer. |
Table I.
PCR primer.
| Gene name | Primer sequences |
|---|
| c-Myc | F:
5′-ATCCAGAGACAAGACATGTAC-3′ |
|
| R:
5′-TTCAGATGTTCTAAGCCTACGG-3′ |
| β-catenin | F:
5′-TGGCGGTTTGCGGTGGAC-3′ |
|
| R:
5′-CCAGTGCAGGGTCCGAGGT-3′ |
| Cyclin D1 | F:
5′-GATGATTGGCATGGCTTT-3′ |
|
| R:
5′-CACCTTCCGTTCCAGTTT-3′ |
| Caspase-3 | F:
5′-GATGGGACTGTGGTTACCGT-3′ |
|
| R:
5′-GGTGAAACTCTTGCCTCGTC-3′ |
| MMP-9 | F:
5′-GATGATTGGCATGGCTTT-3′ |
|
| R:
5′-GCCATACGCTGACCTTTCA-3′ |
| VEGF | F:
5′-GGAGTCCATTGGTGCTTGA-3′ |
|
| R:
5′-ACACCCTTTCCAATGTGCC-3′ |
| GAPDH | F:
5′-GATGATTGGCATGGCTTT-3′ |
|
| R:
5′-CACCTTCCGTTCCAGTTT-3′ |
Detection of the expression of related
proteins via western blot analysis
The cells in the logarithmic growth phase were
inoculated onto the 96-well plate, and the blank control and ICA
groups (5 µM) were established. After medication for 24 h, the
protein was extracted and quantified, followed by separation via
SDS-PAGE and the protein was transferred to PVDF membrane. The
target band was cut off after sealing for 2 h. The target protein
antibody [anti-caspase-3, rabbit monoclonal antibody, catalogue no.
9665; anti-β-catenin, rabbit monoclonal antibody, catalogue no.
9582; monoclonal antibody anti-GSK3β, monoclonal antibody, rabbit
12456; anti-pGSK3β (Ser9), monoclonal antibody, rabbit 5558; cyclin
D1, rabbit monoclonal antibody, catalogue no. 2978; and CMYc,
rabbit monoclonal antibody, catalogue no.13987 were procured from
Cell Signaling Technology, Inc., Danvers, MA, USA] was used to
incubate the protein at 4°C overnight. After washing with
Tris-buffered saline Tween-20 (TBST) three times (5 min/time), the
secondary antibody was incubated at room temperature for 2 h. After
washing with TBST again three times, the appropriate amount of ECL
solution (solution A and B were mixed at a ratio of 1:1) was added
in the dark, followed by performing of the experiment, and the
performing time was determined according to the fluorescence
intensity of the protein band. Following development and fixation,
the band was scanned and received gray value analysis via ImageJ
software. The expression levels of related proteins in the
Wnt/β-catenin signaling pathway, p-GSK3β, c-Myc, cyclin D1 and
β-catenin, and apoptosis protein caspase-3 were detected.
Detection of the expression levels of
VEGF and MMP-9 via ELISA
The cells in the logarithmic growth phase were
inoculated onto the 24-well plate, and the blank control and ICA
groups (5 µM) were set up. After medication for 24 h, the
supernatant was collected and the expression levels of VEGF and
MMP-9 in cells were detected using an ELISA kit.
Statistical analysis
Data were presented as mean ± standard deviation and
analyzed by SPSS 19.0 (SPSS Inc., Chicago, IL, USA) software. The
t-test was used for measurement data, while the Chi-square test was
used for enumeration data. One-way analysis of variance (ANOVA) was
used for other data. Bonferronic method was used for pairwise
comparison under homogeneity of variance, while the Welch method
was used under heterogeneity of variance. Dunnett's T3 method was
used for multiple comparisons.
Results
Inhibitory effect of ICA on
osteosarcoma cell growth
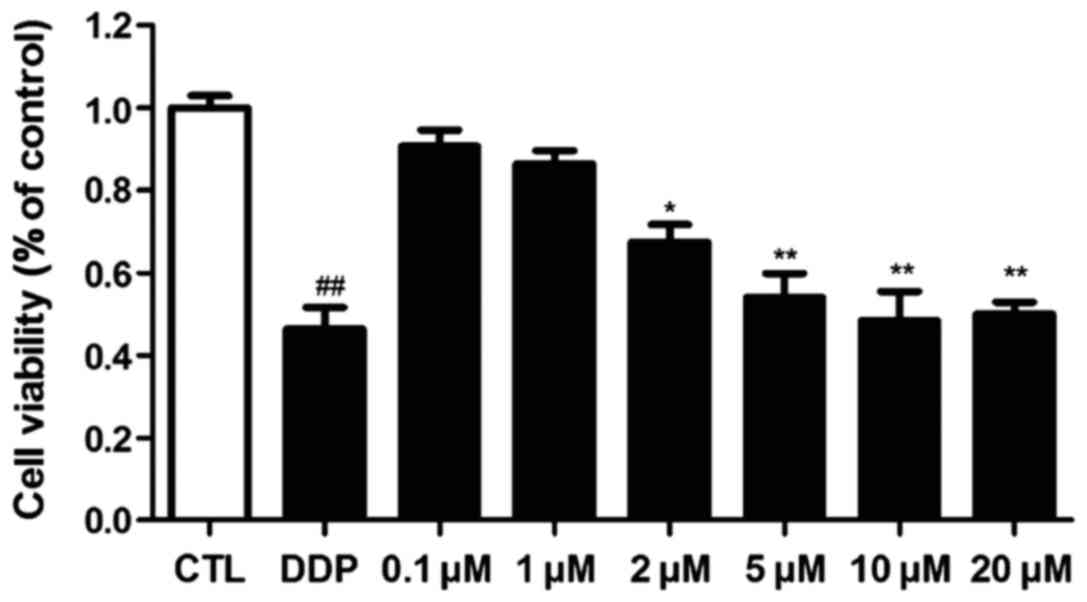
The inhibitory effect of ICA on the osteosarcoma
cell 143B was detected via MTT assay. The results are shown in
Fig. I. ICA concentrations were set
as 20, 10, 5, 2, 1 and 0.1 µM, and the blank control and positive
control groups (cisplatin) were established. The results showed
that ICA significantly inhibited the 143B cell growth when its
concentration reached 5 µM (p<0.01).
Effects of ICA on osteosarcoma cell
apoptosis
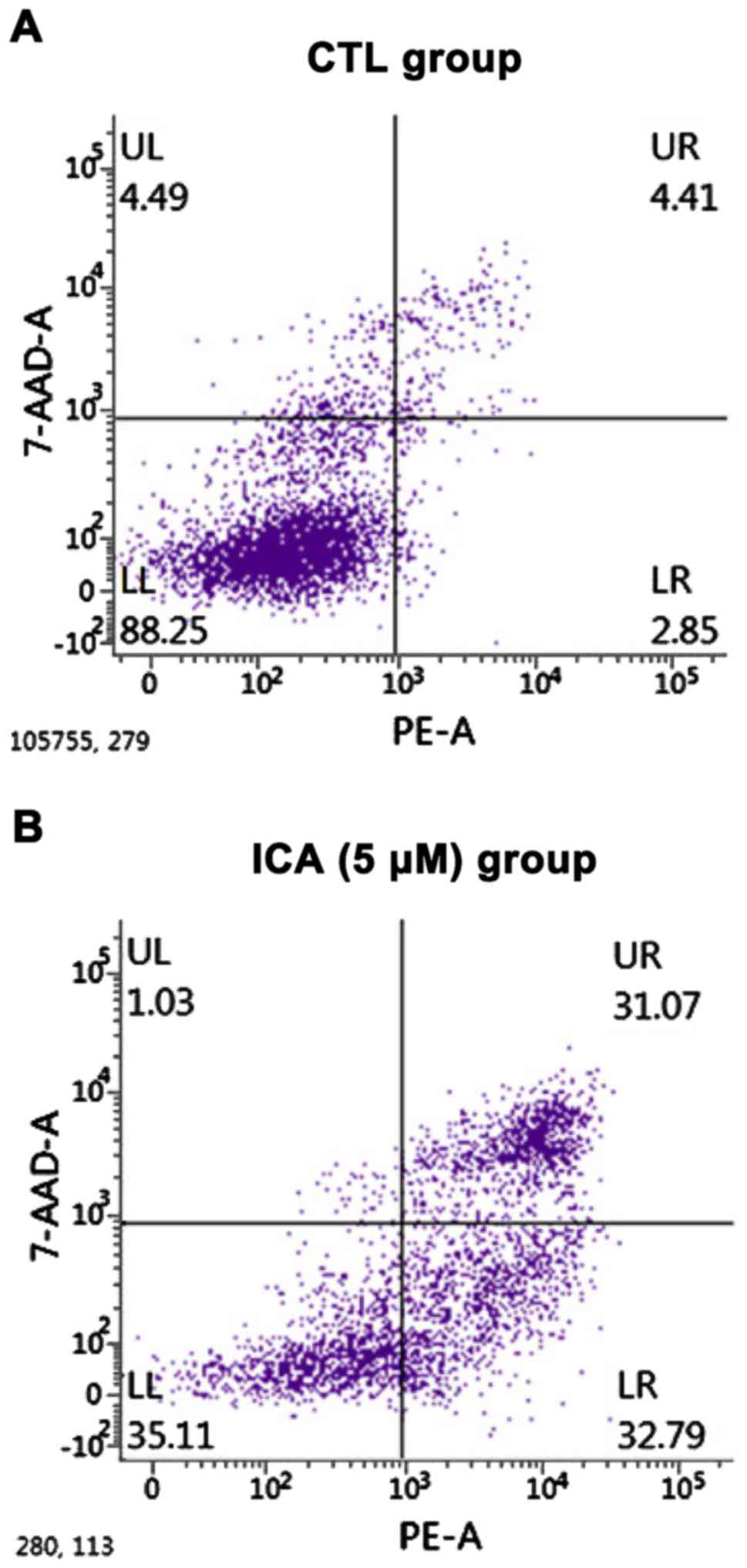
The effects of ICA on the apoptosis of osteosarcoma
cell 143B were detected via a flow cytometer. The results are shown
in Fig. 2. After 5 µM ICA was added
to the osteosarcoma cell 143B, the number of apoptotic cells in the
ICA group was significantly increased compared with that in the
blank control group, and the difference was statistically
significant (p<0.01).
Detection of related gene expressions
via semi-quantitative PCR
After the RNA of osteosarcoma cell 143B treated with
ICA was extracted, the relative expression quantities of related
genes were detected via semi-quantitative PCR with GAPDH as the
internal reference. The results are shown in Figs. 3–5.
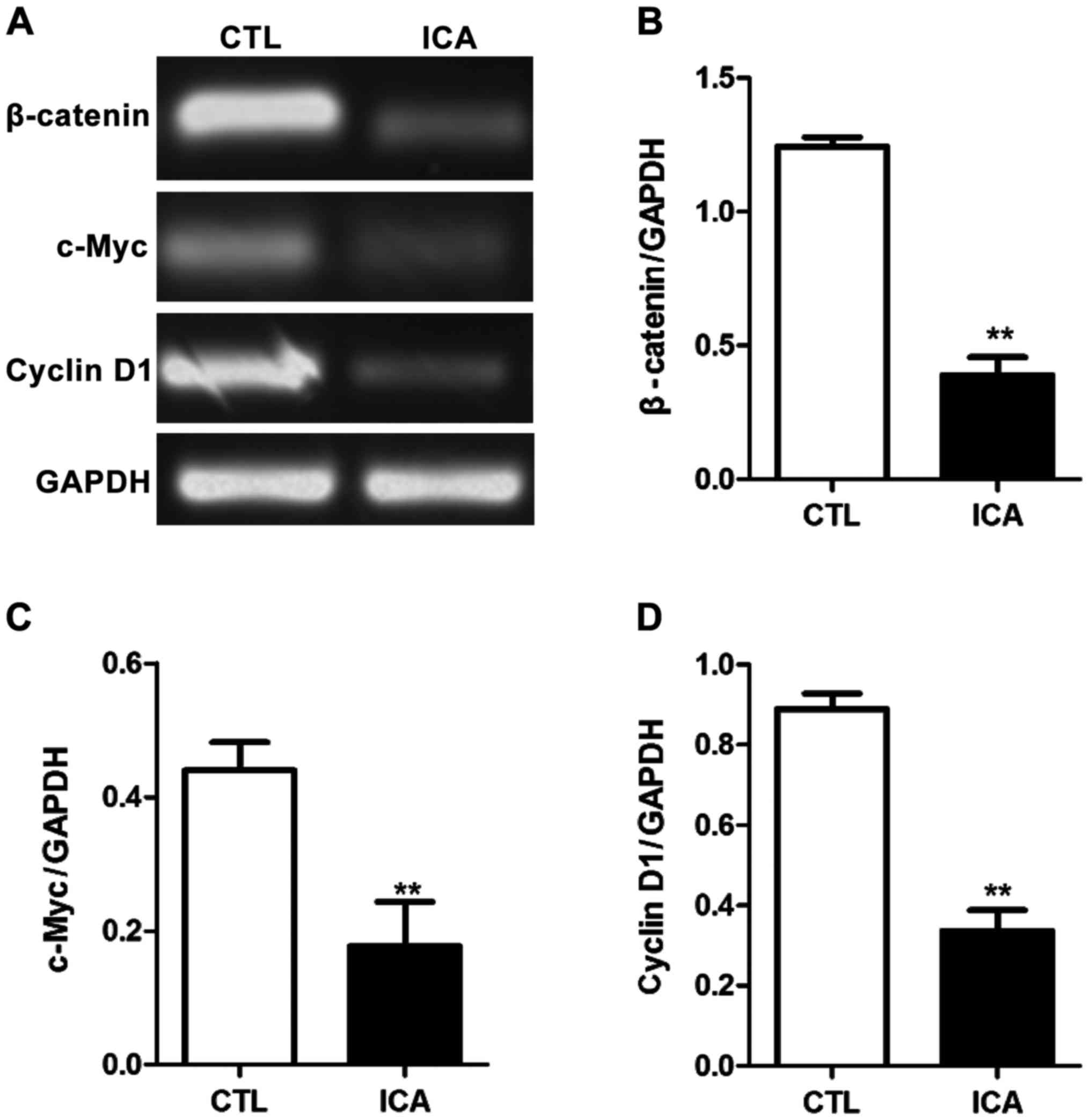
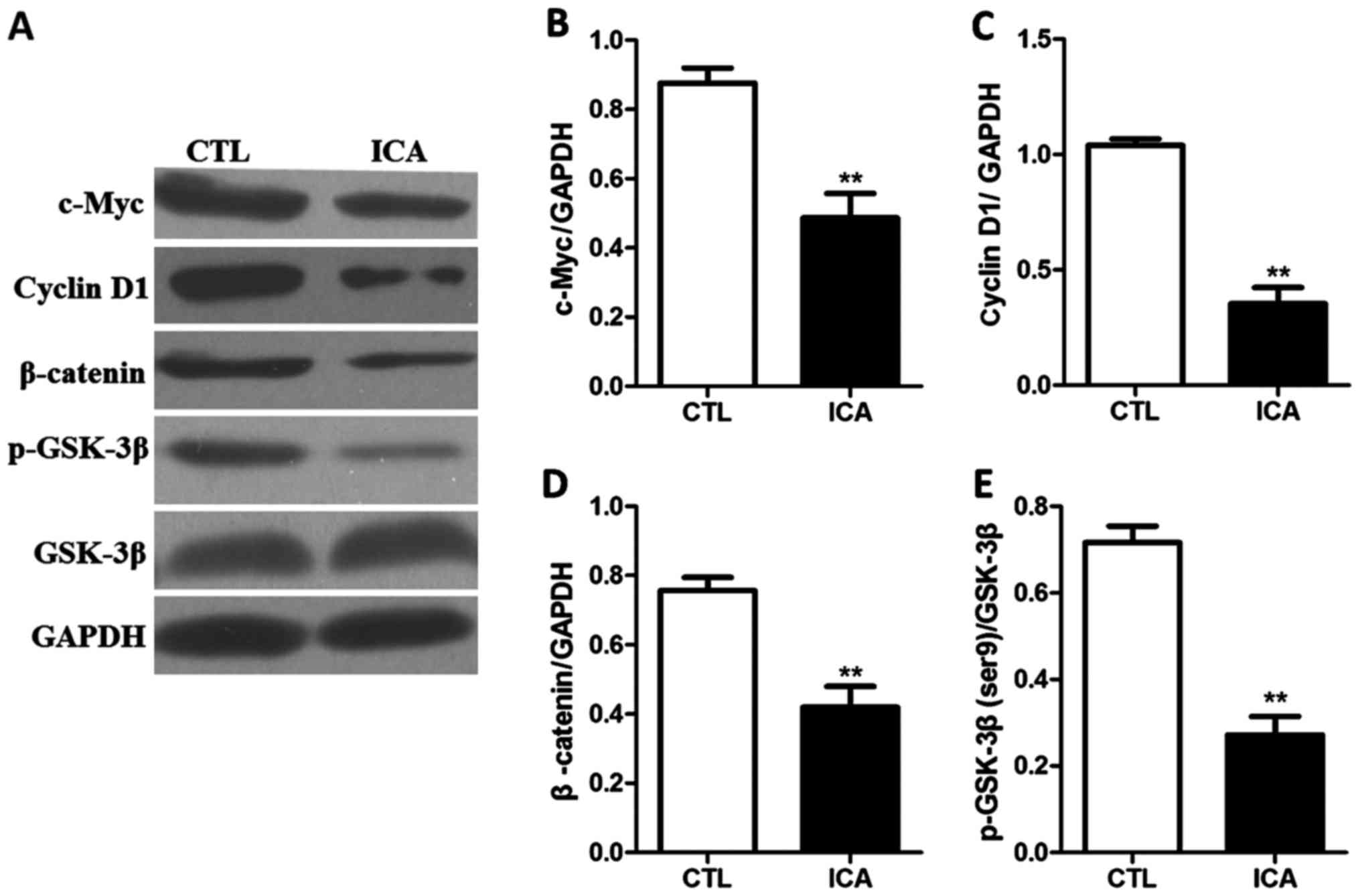
Fig. 3 shows that compared with those
in the blank control group, the expression levels of β-catenin were
decreased after ICA treatment, and the expression levels of its
downstream genes c-Myc and cyclin D1 were also significantly
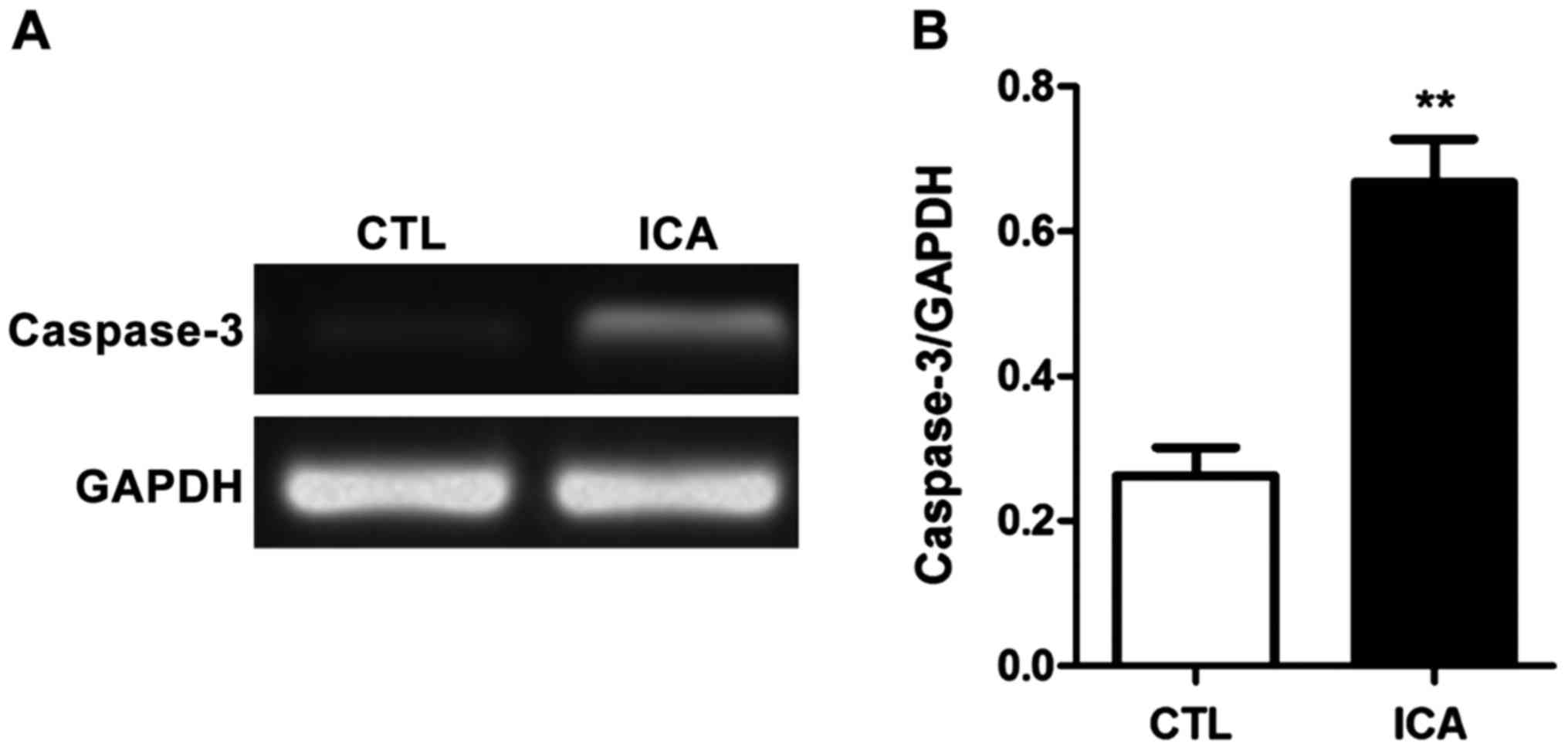
decreased (p<0.01). Fig. 4 shows
that the expression level of caspase-3 was significantly increased
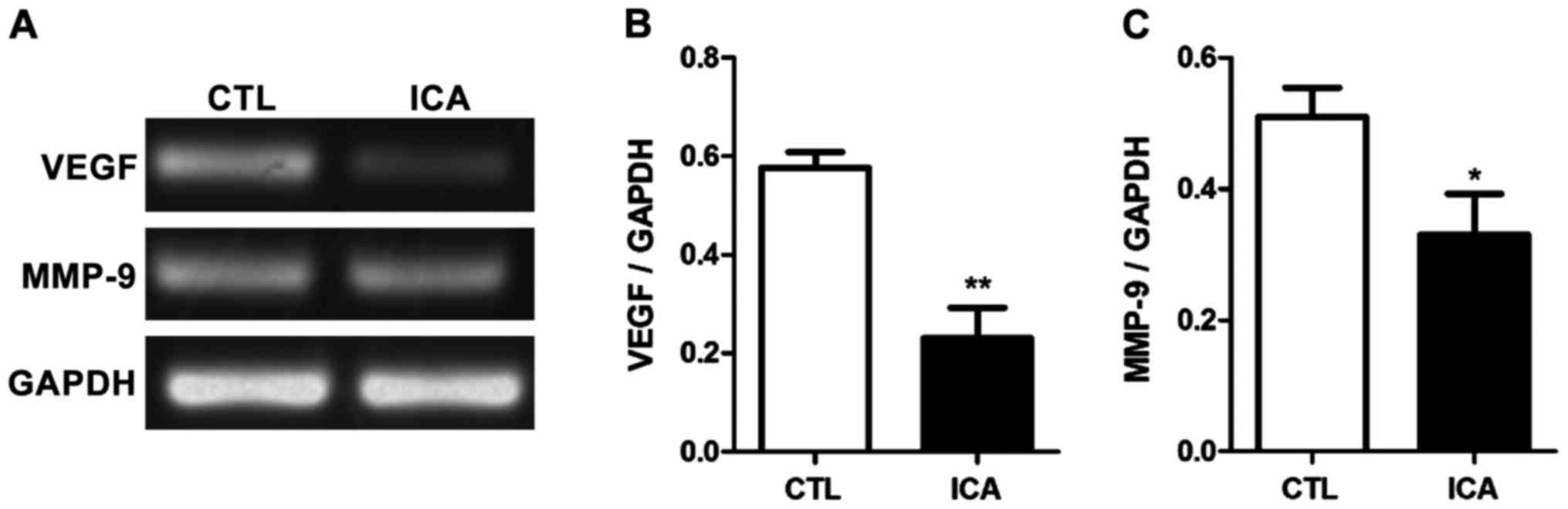
after ICA treatment (p<0.01). Fig.
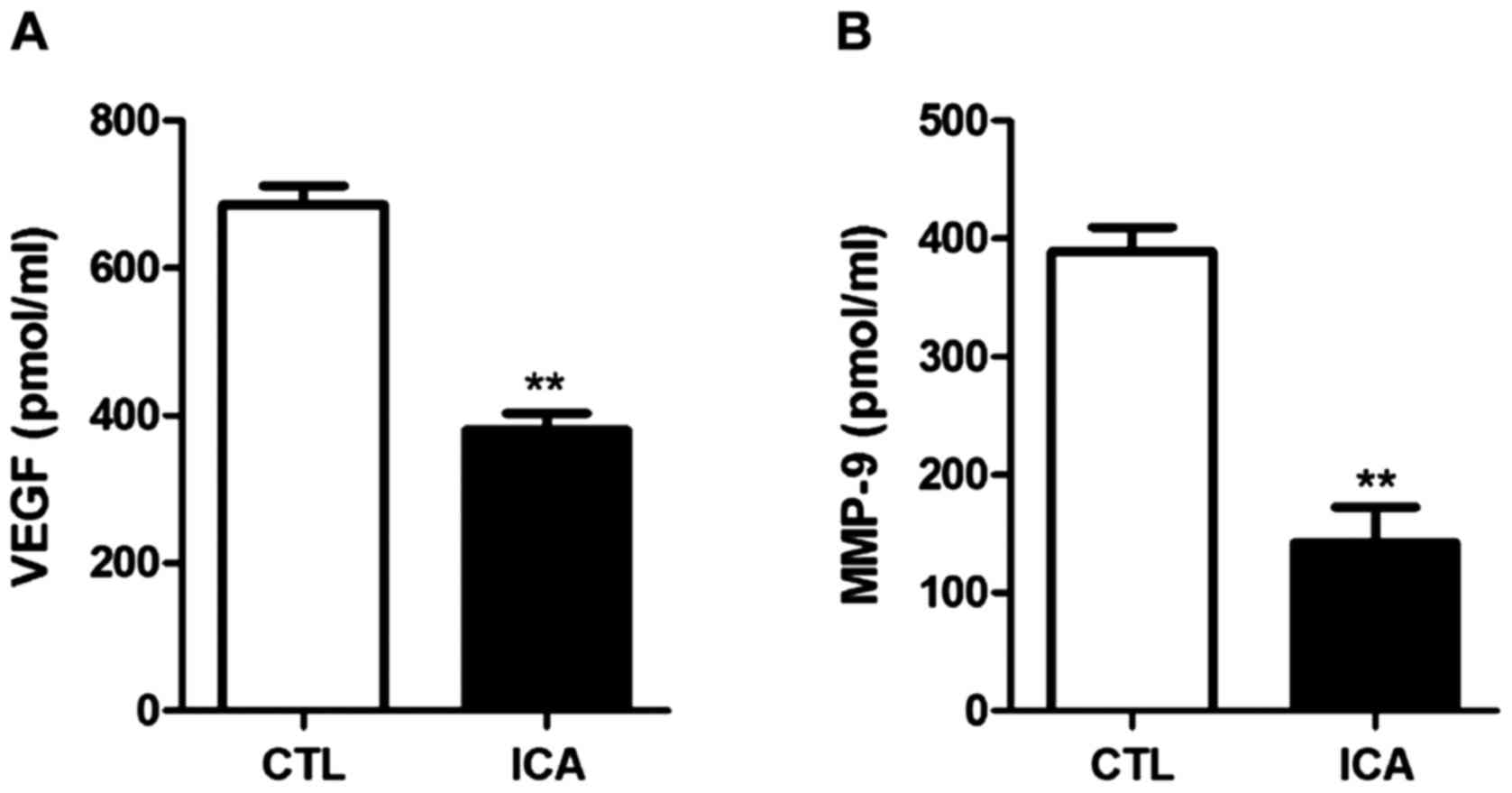
5 shows that the expression levels of MMP-9 and VEGF were
decreased after ICA treatment (p<0.01).
Detection of expression levels of
related proteins via western blot analysis
The expression levels of related proteins in
osteosarcoma cell 143B treated with ICA (5 µM) were detected via
western blot analysis, and the results are shown in Figs. 6 and 7.
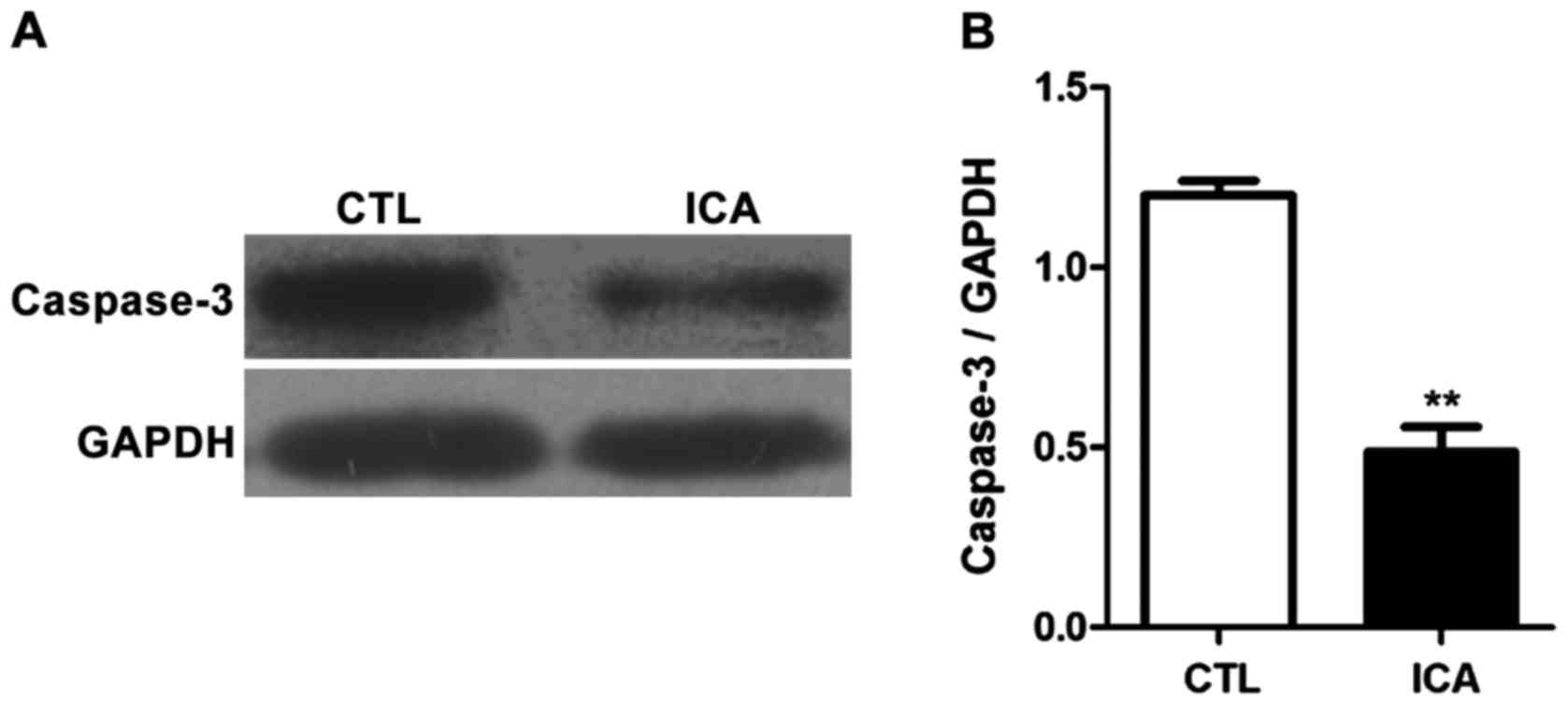
Fig. 6 shows that the protein
expression levels in p-GSK3β, β-catenin and its downstream genes
c-Myc and cyclin D1 were significantly decreased
after ICA treatment (p<0.01). Fig.
7 shows that the protein expression level in caspase-3 was
significantly increased after ICA treatment (p<0.01).
Detection of the expression levels of
VEGF and MMP-9 via ELISA
The protein expression levels in VEGF and MMP-9 in
osteosarcoma cell 143B treated with ICA (5 µM) were detected via
ELISA. The results are shown in Fig.
8. The protein expression levels in VEGF and MMP-9 after ICA
treatment were significantly decreased compared with those in the
blank control group (p<0.01).
Discussion
Osteosarcoma is a clinically common malignant bone
tumor occurring in young individuals, and its main clinical
manifestations are progressively aggravated bone pain and local
swelling (14,15). The 5-year survival rate of
osteosarcoma patients is low, placing great economic and mental
burdens on the society and family (16,17). The
effective constituents in natural medicine and traditional Chinese
medicine have been regarded as the ideal source of antitumor drugs
with high efficiency and low toxicity. Previous studies have shown
that herb Epimedium has a significant development potential
for antitumor drugs. ICA is one of the most studied effective
constituents in the studies of herb Epimedium. Li et
al (18) studied and found that
ICA can cause the death of lung cancer cells in vivo, which
has an inhibitory effect on lung cancer cell migration and
infiltration. Zhang et al (19) found that ICA can promote the
proliferation and differentiation of osteoblasts, which may be
realized via the BMP2/Smads/Runx2/Osterix signaling pathways.
Hartemayer et al (20) studied
and proved that the Wnt/β-catenin signaling pathway in chondrocytes
can lead to hypertrophy and matrix mineralization of chondrocytes,
induce the expressions of MMP-9 and VEGF and promote cell
proliferation and migration. In addition, non-steroidal
anti-inflammatory drugs can inhibit the proliferation of tumor
cells by reducing β-catenin/TCF transcriptional activity and
disturbing the signal transduction of the Wnt pathway (21).
In the present study, the effect of ICA on the
proliferation of osteosarcoma cell 143B was investigated and it was
found that the proliferation of 143B was significantly inhibited
when the concentration of ICA reached 5 µM. At the same time, the
flow cytometry showed that the cell apoptosis after ICA treatment
was significantly increased. The aforementioned results indicated
that ICA has a good inhibitory effect on the proliferation of
osteosarcoma cells with a low onset concentration and high
activity. The expression levels of related genes were detected via
semi-quantitative PCR, and the results showed that the expression
level of β-catenin was significantly decreased after ICA treatment,
while the expression levels of its downstream genes c-Myc
and cyclin D1 were also significantly decreased. Previous
findings have shown that multiple ligands and receptors in the
Wnt/β-catenin signaling pathway are highly expressed in
osteosarcoma cells, suggesting that ICA may inhibit the
proliferation of osteosarcoma cells by inhibiting the
transcriptional level of β-catenin (22). Additionally, the protein expression
levels in p-GSK3β and β-catenin were significantly decreased after
ICA treatment. During typical Wnt/β-catenin signal transduction,
the intracellular accumulation of β-catenin and its transfer to the
nucleus were dominated in regulating the signal activity. GSK3β is
an important negative regulator in the signal transduction process.
Activation of GSK3β can promote the phosphorylation of β-catenin
and eventually lead to the degradation of β-catenin and
inactivation of the Wnt/β-catenin signal (23). ICA can increase the catalytic activity
of GSK3β by inhibiting the phosphorylation of 9-serine of GSK3β,
thus promoting β-catenin degradation and downregulating the protein
level of β-catenin in cells. It was also found that the expression
levels of downstream proteins of β-catenin, c-Myc and cyclin D1,
were also inhibited. Therefore, it was inferred that ICA inhibits
the Wnt/β-catenin signal and the expression of related target
proteins in the pathway through inhibition of the phosphorylation
of p-GSK3β, thus regulating the proliferation and invasion of
osteosarcoma cells. At the same time, the expression level of
caspase-3 in osteosarcoma cells after ICA treatment was increased,
suggesting that ICA can also inhibit the proliferation of
osteosarcoma cells by inhibiting their apoptosis.
In conclusion, ICA has a significant effect of
inhibiting the proliferation and inducing the apoptosis of
osteosarcoma cell 143B, which may be realized by activating GSK3β
and inhibiting the activity of the Wnt/β-catenin signaling pathway.
However, there were still some shortcomings in this experiment. The
inhibitory effect of ICA on osteosarcoma cell proliferation and its
mechanism were investigated only through in vitro
experiments, and the abovementioned results were not verified in
in vivo experiments. In the follow-up studies, the author
aims to verify the efficacy of ICA and the role of Wnt/β-catenin
signaling pathway from in vivo experiments again in the hope
to provide new ideas for the efficacious development of ICA and the
clinical treatment of osteosarcoma.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shimizu T, Kido A, Honoki K, Murata K,
Fujii H, Higuchi B, Ishihara T, Takeshita Y, Shima M, Yajima H, et
al: A successful reconstruction using a frozen autograft and a
pedicled latissimus dorsi flap after a S12345B shoulder girdle
resection in a patient with osteosarcoma. J Reconstr Microsurg.
28:155–159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qiu Q, Jiang J, Lin L, Cheng S, Xin D,
Jiang W, Shen J and Hu Z: Downregulation of RSK2 influences the
biological activities of human osteosarcoma cells through
inactivating AKT/mTOR signaling pathways. Int J Oncol.
48:2508–2520. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hurley C, McCarville MB, Shulkin BL, Mao
S, Wu J, Navid F, Daw NC, Pappo AS and Bishop MW: Comparison of
(18) F-FDG-PET-CT and bone scintigraphy for evaluation of osseous
metastases in newly diagnosed and recurrent osteosarcoma. Pediatr
Blood Cancer. 63:1381–1386. 2016. View Article : Google Scholar : PubMed/NCBIPubMed/NCBI
|
|
4
|
Waresijiang N, Sun J, Abuduaini R, Jiang
T, Zhou W and Yuan H: The downregulation of miR 125a 5p functions
as a tumor suppressor by directly targeting MMP 11 in osteosarcoma.
Mol Med Rep. 13:4859–4864. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ma Q, Zhang Y, Liu T, Jiang K, Wen Y, Fan
Q and Qiu X: Hypoxia promotes chemotherapy resistance by
down-regulating SKA1 gene expression in human osteosarcoma. Cancer
Biol Ther. 18:177–185. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li H, Yuan Y, Zhang Y, Zhang X, Gao L and
Xu R: Icariin inhibits AMPK-dependent autophagy and adipogenesis in
adipocytes in vitro and in a model of Graves' orbitopathy. Front
Physiol. 8:452017.doi: 10.3389/fphys.2017.00045. PubMed/NCBI
|
|
7
|
Su YS, Fan ZX, Xiao SE, Lin BJ, Miao Y, Hu
ZQ and Liu H: Icariin promotes mouse hair follicle growth by
increasing insulin-like growth factor 1 expression in dermal
papillary cells. Clin Exp Dermatol. 42:287–294. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xiao HB, Sui GG and Lu XY: Icariin
improves eNOS/NO-pathway to prohibit the atherogenesis of
apolipoprotein E null mice. Can J Physiol Pharmacol. 95:625–633.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qian ZQ, Wang YW, Li YL, Ling-Zhu Li YQ
and Yang DL: Icariin prevents hypertension-induced cardiomyocyte
apoptosis through the mitochondrial apoptotic pathway. Biomed
Pharmacother. 88:823–831. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang Y, Yin L, Zheng N, Zhang L, Liu J,
Liang W and Wang Q: Icariin enhances remyelination process after
acute demyelination induced by cuprizone exposure. Brain Res Bull.
34:713–725. 2016.
|
|
11
|
Zhang S, Feng P, Mo G, Li D, Li Y, Mo L,
Yang Z and Liang D: Icariin influences adipogenic differentiation
of stem cells affected by osteoblast-osteoclast co-culture and
clinical research adipogenic. Biomed Pharmacother. 88:436–442.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brennecke P1, Arlt MJ, Muff R, Campanile
C, Gvozdenovic A, Husmann K, Holzwarth N, Cameroni E, Ehrensperger
F, Thelen M, et al: Expression of the chemokine receptor CXCR7 in
CXCR4-expressing human 143B osteosarcoma cells enhances lung
metastasis of intratibial xenografts in SCID mice. PLoS One.
8:e740452013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mamie Yu, Selvaraj Suresh K..Liang-Chu MM,
Aghajani S, Busse M, Yuan J, Lee G, Peale F, Klijn C, et al: A
resource for cell line authentication, annotation and quality
control. Nature. 520:307–3112015.
|
|
14
|
Dang H, Wu W, Wang B, Cui C, Niu J, Chen
J, Chen Z and Liu Y: CXCL5 plays a promoting role in osteosarcoma
cell migration and invasion in autocrine- and paracrine-dependent
manners. Oncol Res. 25:177–186. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang S, Liu L, Lv Z, Li Q, Gong W and Wu
H: MicroRNA-342-3p inhibits the proliferation, migration, and
invasion of osteosarcoma cells by targeting astrocyte-elevated
gene-1 (AEG-1). Oncol Res. 8:435–447. 2017.
|
|
16
|
Xu M, Zhang YY, Wang HF and Yang GS: The
expression and function of miRNA-106 in pediatric osteosarcoma. Eur
Rev Med Pharmacol Sci. 21:715–722. 2017.PubMed/NCBI
|
|
17
|
Rushing CJ, Rogers DE, Spinner SM and
Gajzer DC: A case report of heel pain mimicking plantar fasciitis
and osteosarcoma: A unique presentation of a Nora's lesion. J Foot
Ankle Surg. 56:670–673. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li W, Wang L, Chu X, Cui H and Bian Y:
Icariin combined with human umbilical cord mesenchymal stem cells
significantly improve the impaired kidney function in chronic renal
failure. Mol Cell Biochem. 428:203–212. 2017.
|
|
19
|
Zhang L, Wang XZ, Li YS, Zhang L and Hao
LR: Icariin ameliorates IgA nephropathy by inhibition of nuclear
factor kappa b/Nlrp3 pathway. FEBS Open Bio. 7:54–63. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hartemayer R, Kuo C and Kent P:
Osteosarcoma metastases with direct cardiac invasion: A case report
and review of the pediatric literature. J Pediatr Hematol Oncol.
39:188–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sareddy GR, Kesanakurti D, Kirti PB and
Babu PP: Nonsteroidalanti-inflammatory drugs diclofenac and
celecoxib attenuatesWnt/β-catenin/Tcf signaling pathway in human
glioblastomacells. Neurochem Res. 38:2313–2322. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen G, Wang C, Wang J, Yin S, Gao H,
Xiang LU, Liu H, Xiong Y, Wang P, Zhu X, Yang LI and Zhang R:
Antiosteoporoticeffect of icariin in ovariectomized rats is
mediated via the Wnt/β-catenin pathway. Exp Ther Med. 12:279–287.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li R, Dong T, Hu C, Lu J, Dai J and Liu P:
Salinomycinrepressed the epithelial-mesenchymal transition of
epithelialovarian cancer cells via downregulating
Wnt/β-cateninpathway. Onco Targets Ther. 28:328–335. 2017.
|