Introduction
Lung cancer is the most commonly diagnosed malignant
tumor globally (1), and is currently
the leading cause of cancer-associated mortality in China due to
environmental pollution and a high prevalence of cigarette-smoking
(2). According to histological types,
lung cancers may be generally divided into small cell lung cancer
and non-small cell lung cancer (NSCLC). NSCLC is the largest
histological subtype, accounting for ~80% of all lung cancer cases
(3). Despite advances in diagnosis
and therapy over the last two decades, the overall survival (OS)
rate for NSCLC patients remains at ~16% due to late-stage diagnosis
and unsuccessful treatments (4).
Consequently, there is an urgent demand for the detection of novel
biomarkers that are capable of serving as diagnostic and prognostic
markers for NSCLC.
Notch-regulated ankyrin-repeat protein (NRARP) is a
negative feedback regulator in the Notch signaling pathway and is
regulated by Notch protein (5). The
overexpression of the Notch protein may promote NRARP expression;
however, NRARP exerts feedback inhibition on the activity of Notch
by inducing degradation of the Notch receptor intracellular domain
(6). Previous studies have implicated
Notch signaling and its downstream protein NRARP in the oncogenesis
of various cancer types, including colorectal cancer (CRC)
(7), thyroid (8), breast (9)
and liver cancer (10). However, the
role that NRARP serves in NSCLC remains unclear.
In the present study, the mRNA and protein
expression levels of NRARP in NSCLC patients were investigated, and
the associations of NRARP expression with clinicopathological
characteristics and patient prognosis were explored.
Materials and methods
Tissue specimen selection and
preparation
In the present study, the cancer tissues and paired
adjacent tissues were collected from a total of 108 patients (74
males, 34 females) who underwent surgical resection of a NSCLC at
Ningbo No. 2 Hospital (Ningbo, China) between January 2004 and June
2015. These patients received neither chemotherapy nor radiotherapy
prior to surgery. The age of the selected patients ranged from 32
to 74 years (mean, 54 years). Among these patients, 58 had squamous
cell cancer (SCC) and 50 had adenocarcinoma (AC). The
clinicopathological characteristics of the patients analyzed in the
present study are shown in Table I.
Tumor stage was classified according to the current International
Union Against Cancer Tumor-Node-Metastasis (TNM) classification
guidelines (11). Follow-up was
conducted by telephone interviews until December 2016 (median
follow-up duration, 48.2 months; range, 4–110 months). OS was
defined as the time interval between tumor resection and mortality.
Written consent forms were obtained from each patient prior to
sample collection. Ethical approval for this study was obtained
from the Ethics Committee of Ningbo No. 2 Hospital. Fresh lung
tissues from 40 patients were snap-frozen in liquid nitrogen and
stored at −80°C for reverse-transcription quantitative polymerase
chain reaction (RT-qPCR) analysis. For immunohistochemistry (IHC)
experiments, tissues from all 108 patients were cut into 1×1-cm
cubes and immersed in 4% paraformaldehyde.
 | Table I.Associations between NRARP protein
expression and clinicopathological features in patients with
NSCLC. |
Table I.
Associations between NRARP protein
expression and clinicopathological features in patients with
NSCLC.
|
|
| NRARP expression, n
(%) |
|
|---|
|
|
|
|
|
|---|
| Clinical
features | Cases, n | Low | High | P-value |
|---|
| Tissue type |
|
|
| <0.001 |
| Normal
lung | 108 | 102 (94.4) | 6 (5.6) |
|
|
NSCLC | 108 | 51 (47.2) | 57 (52.8) |
|
| Sex |
|
|
| 0.420 |
| Male | 74 | 33 (44.6) | 41 (55.4) |
|
|
Female | 34 | 18 (52.9) | 16 (47.1) |
|
| Age, years |
|
|
| 0.607 |
| ≤50 | 43 | 19 (44.2) | 24 (55.8) |
|
|
>50 | 65 | 32 (49.2) | 33 (50.8) |
|
| Histological
type |
|
|
| 0.803 |
| SCC | 58 | 28 (48.3) | 30 (51.7) |
|
| AC | 50 | 23 (46.0) | 27 (54.0) |
|
| Differentiation |
|
|
| 0.001 |
| Well | 42 | 29 (69.0) | 13 (31.0) |
|
|
Moderate | 42 | 13 (31.0) | 29 (69.0) |
|
| Poor | 24 | 9 (37.5) | 15 (62.5) |
|
| TNM stage |
|
|
| 0.004 |
| I | 40 | 27 (67.5) | 13 (32.5) |
|
| II | 42 | 16 (38.1) | 26 (61.9) |
|
| III | 26 | 8 (30.8) | 18 (69.2) |
|
| T classification |
|
|
| 0.847 |
| T1,
T2 | 54 | 26 (48.1) | 28 (51.9) |
|
| T3,
T4 | 54 | 25 (46.3) | 29 (53.7) |
|
| N classification |
|
|
| 0.974 |
| N0 | 57 | 27 (47.4) | 30 (52.6) |
|
| N1, N2,
N3 | 51 | 24 (47.1) | 27 (52.9) |
|
| Cigarette
smoker |
|
|
| <0.001 |
|
Yes | 73 | 26 (35.6) | 47 (64.4) |
|
| No | 35 | 25 (71.4) | 10 (28.6) |
|
RNA extraction and RT-qPCR
Total RNA samples from fresh primary NSCLC tissues
from 40 patients (20 SCC, 20 AC) and their corresponding normal
tissues were extracted using RNAiso Plus® (Takara
Biotechnology, Co. Ltd., Dalian, China). The concentrations of RNA
in the samples were determined by reading the absorbance at 260 nm.
RT of total RNA (1 µg) was performed with a PrimeScript®
RT Master Mix (Perfect Real-Time) kit (Takara Biotechnology, Co.
Ltd.) according to the manufacturer's protocol (37°C for 15 min,
85°C for 5 sec and then maintained at 4°C). The resulting cDNA was
then amplified using a SYBR® Premix Ex Taq™
II (Perfect Real-Time) kit (Takara Biotechnology, Co., Ltd.) with
an ABI 7500 Real-Time PCR system (Applied Biosystems, Thermo Fisher
Scientific, Inc., Waltham, MA, USA). The primers used were as
follows: NRARP forward, 5′-ATCTTCCAGGAGGCTGTGC-3′; NRARP reverse,
5′-CTTCGCCTTGGTGATGAGAT-3′; GAPDH (internal control) forward,
5′-CCCTTCATTGACCTCAACTAC-3′; and GAPDH reverse,
5′-CCACCTTCTTGATGTCATCAT-3′. The conditions used for PCR were as
follows: 30 sec incubation at 95°C; followed by 40 cycles of 95°C
for 5 sec and 64°C for 34 sec. The 2−ΔΔCq method was
applied to analyze gene expression (12). For each gene, three independent
experiments were performed.
IHC analysis
The paraformaldehyde-fixed, paraffin-embedded tissue
blocks were cut into 4-µm thick sections. Hematoxylin and eosin
staining was performed to ensure the inclusion of normal or tumor
cells. Subsequently, IHC staining was performed to detect the NRARP
protein expression in lung tissues. To summarize, sections were
initially baked at 65°C for 30 min and then deparaffinized in
xylene and rehydrated in a series of ethanol solutions with
increasing concentration. Next, the sections underwent antigen
retrieval in a microwave for 10 min, and 3%
H2O2-methanol was used to block endogenous
peroxidase activity. Following blocking of nonspecific staining
with normal goat serum, sections were incubated overnight at 4°C
with rabbit anti-human NRARP polyclonal antibodies (dilution,
1:100; cat. no. HPA025729; Sigma-Aldrich, Merck KGaA, Darmstadt,
Germany); then, sections were washed in phosphate-buffered saline
(PBS) three times and incubated with a horseradish
peroxidase-labeled goat anti-rabbit IgG (H+L) secondary antibody
(dilution, 1:500; cat. no. A0208, Beyotime Institute of
Biotechnology, Haimen, China) for a further 30 min at room
temperature. Finally, sections were developed with
3,3-diaminobenzidine solution (cat. no. D3939; Sigma-Aldrich, Merck
KGaA) according to the manufacturer's protocol at room temperature
for 20 sec, and were then counterstained with 0.1% hematoxylin at
room temperature for 30 sec. For the negative control, the tissues
were incubated with PBS instead of the primary antibody. Breast
cancer tissues known to have a high expression of NRARP were used
as the positive control. The breast cancer tissues were collected
from 12 patients who underwent surgical resection of a breast
cancer at Ningbo No. 2 Hospital. Written consent forms were
obtained from all patients.
IHC scoring
The NRARP expression levels in the sections were
examined by light microscopy. NRARP staining in tumor and normal
tissues was scored semi-quantitatively according to the intensity
of staining and the percentage of positively stained cells. The
overall immunoreactivity score (IRS) was calculated as the sum of
the staining intensity score (0, no staining; 1, mild staining; 2,
moderate staining; 3, strong staining) and the score for the
percentage of positively stained cells (0, <10%; 1, 11–25%; 2,
26–50%; 3, 51–75%; 4, >75%). According to the IRS, each tissue
was defined as having low (score 0–3) or high (score 4–7) NRARP
expression. Two independent histopathologists, who had no knowledge
of the clinicopathological information, evaluated the sections.
Statistical analysis
The data were presented at the mean ± standard
deviation. A one-way ANOVA (with Student-Newman-Keuls as a post hoc
test) or Student's t-test was used to compare continuous variables.
The Pearson χ2 test was used to assess the association
between NRARP expression and pathological parameters. The
Kaplan-Meier method was used to estimate the OS, and significance
was assessed using log-rank test. The Cox proportional hazards
regression model was used for univariate and multivariate analyses.
All P-values corresponded to two-sided tests and P<0.05 was
considered to indicate a statistically significant difference. All
statistical calculations were performed by using SPSS 17.0 (SPSS,
Inc., Chicago, IL, USA) statistical software.
Results
NRARP expression in NSCLC
patients
In order to investigate oncogenic properties of
NRARP in NSCLC, the expression of NRARP was assessed by IHC in 108
tumor tissues and adjacent normal tissues from NSCLC patients.
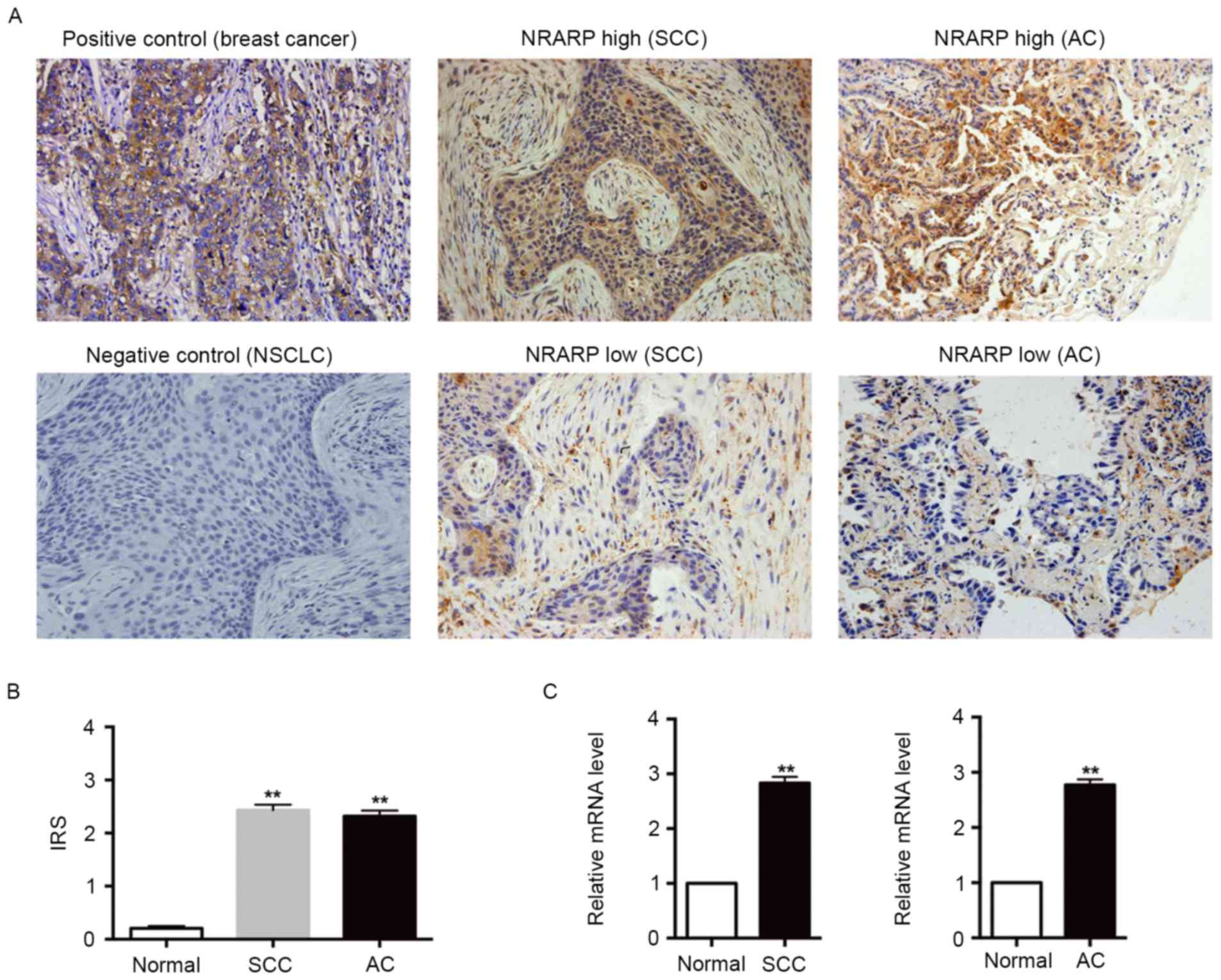
Fig. 1A demonstrates that NRARP
expression was significantly increased in the tumor tissues, and
the staining was predominantly located in the cytoplasm of tumor
cells. The percentage of tumor tissues exhibiting high expression
of NRARP was 52.78% (57/108) among all patients, 51.72% (30/58) in
patients with SCC, and 54.00% (27/50) in patients with AC. By
contrast, only 5.56% (6/108) of normal tissues exhibited high NRARP
expression. In addition, compared with their respective normal
tissues, SCC and AC tissues each had a significantly higher IRS
(Fig. 1B). Subsequently RT-qPCR was
used to assess NRARP mRNA expression in fresh tumor and normal
tissues collected from 40 patients with NSCLC. Fig. 1C demonstrates that the NRARP mRNA
levels in SCC and AC tissues were markedly increased compared with
their adjacent normal tissues. Taken together, these results
suggest that the mRNA and protein expression levels of NRARP are
elevated in NSCLC tissues and the overexpression of NRARP was
observed in all NSCLC cells.
Association between NRARP expression
and pathological features of NSCLC
The associations between NRARP expression levels and
the clinicopathological features (including sex, age, histological
type, differentiation, TNM stage, T classification, N
classification and smoking status) of patients with NSCLC were
analyzed (Table I). The results
demonstrated that increased NRARP protein expression was
significantly associated with differentiation (P=0.001), TNM stage
(P=0.004) and the smoking status of the patient (P<0.001). By
contrast, no significant association was identified between high
NRARP protein expression and sex (P=0.420), age (P=0.607),
histological type (P=0.803), T classification (P=0.847) or N
classification (P=0.974).
Survival analysis of NRARP expression
in NSCLC
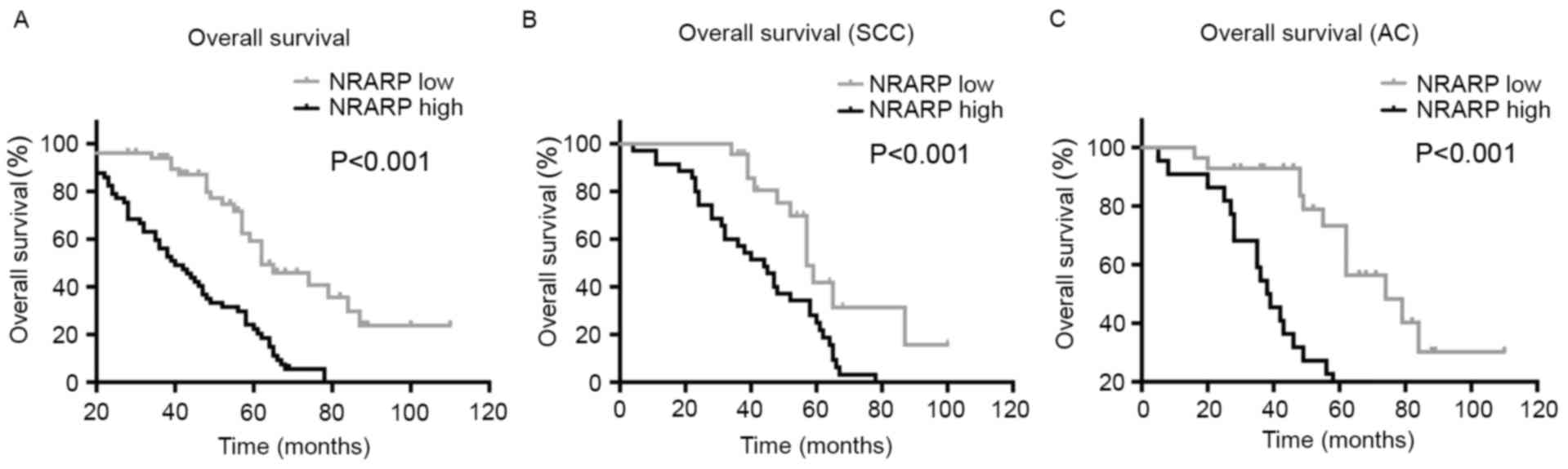
The prognostic significance of high NRARP expression
and the survival curves in NSCLC patients were determined by using
the Kaplan-Meier method. The results showed that aberrant high
expression of NRARP in NSCLC patients was significantly associated
with shorter OS time (P<0.001, Fig.
2A). Additionally, the same association was identified in SCC
and AC patients (P<0.001, Fig. 2B and
C). Univariate analysis demonstrated that NRARP expression,
differentiation, TNM stage, T classification, N classification and
cigarette smoking were associated with the prognosis of patients
with NSCLC (Table II). Multivariate
analysis identified that NRARP expression (P<0.001),
differentiation (P=0.006) and TNM stage (P=0.001) were all
independent prognostic markers for OS in NSCLC patients (Table II). Taken together, these data
suggest that NRARP may be applied as a valuable biomarker for
predicting prognosis in NSCLC patients.
 | Table II.Univariate and multivariate
statistical analyses for various prognostic parameters in patients
in NSCLC. |
Table II.
Univariate and multivariate
statistical analyses for various prognostic parameters in patients
in NSCLC.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Characteristic | No. | P-value | Regression
coefficient (SE) | P-value | Relative risk | 95% CI |
|---|
| Sex |
| 0.132 |
| – | – | – |
|
Male | 74 |
|
|
|
|
|
|
Female | 34 |
|
|
|
|
|
| Age, years |
| 0.465 |
| – | – | – |
|
≤50 | 43 |
|
|
|
|
|
|
>50 | 65 |
|
|
|
|
|
| Histological
type |
| 0.066 |
| – | – | – |
|
SCC | 58 |
|
|
|
|
|
| AC | 50 |
|
|
|
|
|
|
Differentiation |
| <0.001 | 1.012 (0.179) | 0.006 | 1.780 | 1.176–2.694 |
|
Well | 42 |
|
|
|
|
|
|
Moderate | 42 |
|
|
|
|
|
|
Poor | 24 |
|
|
|
|
|
| TNM stage |
| <0.001 | 0.974 (0.138) | 0.001 | 1.832 | 1.272–2.640 |
| I | 40 |
|
|
|
|
|
| II | 42 |
|
|
|
|
|
|
III | 26 |
|
|
|
|
|
| T
classification |
| 0.018 | 0.551 (0.233) | 0.151 | n.s. | n.s. |
| T1,
T2 | 54 |
|
|
|
|
|
| T3,
T4 | 54 |
|
|
|
|
|
| N
classification |
| 0.029 | 0.507 (0.232) | 0.471 | n.s. | n.s. |
| N0 | 57 |
|
|
|
|
|
| N1, N2,
N3 | 51 |
|
|
|
|
|
| Cigarette
smoker |
| 0.012 | 0.653 (0.261) | 0.539 | n.s. | n.s. |
|
Yes | 73 |
|
|
|
|
|
| No | 35 |
|
|
|
|
|
| NRARP
expression |
| <0.001 | 1.337 (0.262) | <0.001 | 2.975 | 1.779–4.975 |
|
Low | 51 |
|
|
|
|
|
|
High | 57 |
|
|
|
|
|
Discussion
In the present study, the results demonstrated that
NRARP expression was increased in human NSCLC tissues at the mRNA
and protein levels. In addition, high NRARP expression was
associated with tumor differentiation, TNM stage and whether the
patients where cigarette smokers. Furthermore, it was identified
that patients with NSCLC with high NRARP expression had a
significantly shorter OS time compared with patients with low NRARP
expression. Multivariate analysis revealed that NRARP was an
independent prognostic factor for OS in NSCLC patients.
Collectively, these results suggested the potential value of NRARP
as a novel biomarker for the prediction of NSCLC prognosis.
The Notch signaling pathway participates in a
plethora of cell activities, including cell proliferation,
differentiation and apoptosis. Although its specific role in
tumorigenesis remains controversial, it has been reported that
dysregulation of Notch signaling is associated with various types
of cancer, including CRC and breast, prostate, bladder and thyroid
cancers (13–17). NRARP, a downstream effector of Notch
signaling, is a small, evolutionarily conserved protein containing
two ankyrin-repeat motifs (5).
Previous studies have suggested a close and complex association
between Notch signaling and NRARP (5,18). Because
NRARP acts as a downstream target of Notch signaling, its
expression may be regulated by Notch signaling. Conversely, NRARP
may also function as a negative feedback regulator of Notch
signaling and suppress the activation of Notch signaling by
promoting the degradation of Notch intracellular domain (19). A previously study demonstrated that
NRARP was upregulated in thyroid cancer in response to
over-activated Notch signaling and that downregulation of NRARP may
inhibit thyroid cancer cell proliferation, induce G1
arrest, inhibit cell invasion, and promote apoptosis (8). In breast cancer, the Notch pathway was
revealed to be dysregulated, and silencing of NRARP in human breast
cancer cell lines led to reduced cell growth (9). These studies supported the hypothesis
that NRARP serves a direct oncogenic role in connecting Notch
signals to cancer progression.
At present, the majority of patients with NSCLC are
diagnosed at advanced stages, which leads to unsatisfactory
prognosis. The traditional factors for evaluating prognosis, such
as TNM stage, lymph node status and histological differentiation,
at present, do not meet the developing demands for accurate and
individualized prognostic evaluation in patients with NSCLC. Thus,
there is an urgent requirement to identify novel biomarkers to
better predict the prognosis these patients.
Several studies have revealed that NRARP is
associated with prognosis in different types of cancer. For
example, Chu et al (8)
demonstrated that NRARP was highly expressed in thyroid carcinoma
compared with normal thyroid tissues, and also identified that
NRARP protein level was negatively associated with patient
prognosis. Similar results were obtained in breast cancer, wherein
high NRARP expression was found to be associated with shorter
survival time (9). However, decreased
NRARP expression in CRC tissues compared with normal intestinal
epithelium has been reported, and significantly longer survival
times were observed in such patients with high NRARP expression
(7).
To the best of our knowledge, the present study is
the first to explore the prognostic value of NRARP in NSCLC. The
data demonstrated that NRARP protein expression was associated with
tumor differentiation, TNM stage and smoking status. In addition,
patients with NSCLC who had a higher NRARP expression had a worse
prognosis, which was consistent with the aforementioned results in
other types of tumor. All of these findings suggest that NRARP has
the potential to serve as an independent biomarker for the
prognosis of NSCLC.
Presently, there are only a small number of studies
that have been published regarding the functions of NRARP in
tumorigenesis. Several genetic observations suggest that functional
crosstalk exists between WNT and Notch signaling (20) and NRARP may participate in
tumorigenesis through this crosstalk. In human breast cancer,
NRARP may serve a functional role in the ‘rewiring’ of Notch
and WNT pathways, in which incoming Notch signals from neighboring
cells lead to expression of WNT target genes in breast cancer cells
(9). Furthermore, it has been
demonstrated that Notch signaling may directly target NRARP and
suppress the expression of WNT target genes in human CRC cells
through epigenetic modification (7).
Additionally, in a previous study, treatment with Lenti-NRARP-shRNA
in thyroid cancer cells was able to significantly suppress matrix
metallopeptidase 9 expression and inhibit thyroid cancer cell
invasion, which suggested that downregulation of NRARP promotes the
activation of Notch, subsequently inhibiting WNT signaling and cell
invasion (8).
Epithelial-mesenchymal transition (EMT) is a vital
pathological mechanism in the progression of the majority of
tumors. The research conducted by Zhu et al (10) verified that NRARP knockout leads to
attenuation of cancer cell stemness, which is linked with EMT.
Furthermore, two EMT-associated transcription factors were also
revealed to be highly associated with NRARP (21,22).
Additionally, Fazio et al (23) demonstrated that increased NRARP was
involved in NOTCH-induced EMT in CRC, and also enhanced the
functional association with NRARP and EMT. However, no research
regarding the mechanism of NRARP in NSCLC has been reported and
this consequently requires elucidation by further studies.
In conclusion, the present study demonstrated that
NRARP is aberrantly expressed in NSCLC and that high NRARP protein
expression is associated with tumor progression and OS. These
results indicated the potential value of NRARP as a novel
therapeutic target for the treatment of NSCLC. However, further
studies are required in order to determine the definitive role that
NRARP serves in NSCLC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Zhejiang
Provincial Plan for Medical and Health Science and Technology
(grant no. 2014KYB233) and the Natural Science Foundation of Ningbo
City (grant no. 2012A610194).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL and JC performed the molecular genetic studies,
participated in the sequence alignment and drafted the manuscript.
JM conducted the human studies. QM performed the statistical
analysis. RW and JZ conceived the study and participated in its
design and coordination and helped to draft the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Written consent forms were obtained from each
patient prior to sample collection. Ethical approval for this study
was obtained from the Ethics Committee of Ningbo No. 2
Hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
She J, Yang P, Hong Q and Bai C: Lung
cancer in China: Challenges and interventions. Chest.
143:1117–1126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reck M: What future opportunities may
immuno-oncology provide for improving the treatment of patients
with lung cancer? Ann Oncol. 23(Suppl 8): viii28–viii34. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Krebs LT, Deftos ML, Bevan MJ and Gridley
T: The Nrarp gene encodes an ankyrin-repeat protein that is
transcriptionally regulated by the notch signaling pathway. Dev
Biol. 238:110–119. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yun TJ and Bevan MJ: Notch-regulated
ankyrin-repeat protein inhibits Notch1 signaling: Multiple Notch1
signaling pathways involved in T cell development. J Immunol.
170:5834–5841. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim HA, Koo BK, Cho JH, Kim YY, Seong J,
Chang HJ, Oh YM, Stange DE, Park JG, Hwang D and Kong YY: Notch1
counteracts WNT/β-catenin signaling through chromatin modification
in colorectal cancer. J Clin Invest. 122:3248–3259. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chu BF, Qin YY, Zhang SL, Quan ZW, Zhang
MD and Bi JW: Downregulation of notch-regulated ankyrin repeat
protein exerts antitumor activities against growth of thyroid
cancer. Chin Med J (Engl). 129:1544–1552. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Imaoka T, Okutani T, Daino K, Iizuka D,
Nishimura M and Shimada Y: Overexpression of NOTCH-regulated
ankyrin repeat protein is associated with breast cancer cell
proliferation. Anticancer Res. 34:2165–2171. 2014.PubMed/NCBI
|
|
10
|
Zhu P, Wang Y, Du Y, He L, Huang G, Zhang
G, Yan X and Fan Z: C8orf4 negatively regulates self-renewal of
liver cancer stem cells via suppression of NOTCH2 signalling. Nat
Commun. 6:71222015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goldstraw P, Crowley J, Chansky K, Giroux
DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V, Sobin L;
International Association for the Study of Lung Cancer
International Staging Committee; Participating Institutions; et al:
The IASLC lung cancer staging project: Proposals for the revision
of the TNM stage groupings in the forthcoming (seventh) edition of
the TNM Classification of malignant tumours. J Thorac Oncol.
2:706–714. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Geers C, Colin IM and Gerard AC:
Delta-like 4/Notch pathway is differentially regulated in benign
and malignant thyroid tissues. Thyroid. 21:1323–1330. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo S, Liu M and Gonzalez-Perez RR: Role
of Notch and its oncogenic signaling crosstalk in breast cancer.
Biochim Biophys Acta. 1815:197–213. 2011.PubMed/NCBI
|
|
15
|
Leong KG and Gao WQ: The Notch pathway in
prostate development and cancer. Differentiation. 76:699–716. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rampias T, Vgenopoulou P, Avgeris M,
Polyzos A, Stravodimos K, Valavanis C, Scorilas A and Klinakis A: A
new tumor suppressor role for the Notch pathway in bladder cancer.
Nat Med. 20:1199–1205. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang J, Wang Y, Li D and Jing S: Notch
and TGF-β/Smad3 pathways are involved in the interaction between
cancer cells and cancer-associated fibroblasts in papillary thyroid
carcinoma. Tumour Biol. 35:379–385. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Phng LK, Potente M, Leslie JD, Babbage J,
Nyqvist D, Lobov I, Ondr JK, Rao S, Lang RA, Thurston G and
Gerhardt H: Nrarp coordinates endothelial Notch and Wnt signaling
to control vessel density in angiogenesis. Dev Cell. 16:70–82.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lamar E, Deblandre G, Wettstein D,
Gawantka V, Pollet N, Niehrs C and Kintner C: Nrarp is a novel
intracellular component of the Notch signaling pathway. Genes Dev.
15:1885–1899. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hayward P, Kalmar T and Arias AM:
Wnt/Notch signalling and information processing during development.
Development. 135:411–424. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brabletz T: EMT and MET in metastasis:
Where are the cancer stem cells? Cancer Cell. 22:699–701. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Preca BT, Bajdak K, Mock K, Sundararajan
V, Pfannstiel J, Maurer J, Wellner U, Hopt UT, Brummer T, Brabletz
S, et al: A self-enforcing CD44s/ZEB1 feedback loop maintains EMT
and stemness properties in cancer cells. Int J Cancer.
137:2566–2577. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fazio C, Piazzi G, Vitaglione P, Fogliano
V, Munarini A, Prossomariti A, Milazzo M, D'Angelo L, Napolitano M,
Chieco P, et al: Inflammation increases NOTCH1 activity via MMP9
and is counteracted by Eicosapentaenoic Acid-free fatty acid in
colon cancer cells. Sci Rep. 6:206702016. View Article : Google Scholar : PubMed/NCBI
|