Introduction
Renal cell carcinoma (RCC), a neoplastic lesion of
the kidney in humans, accounts for ~90% of kidney tumors (1). It is difficult to treat with
conventional treatments including chemical, hormone and radiation
therapy, and cannot be treated without surgery (2,3). A
previous report described metformin may improve the incidence of
cancer-associated diabetes (4). Thus
far, RCC has been treated chemically and immunologically. However,
there is an urgent requirement to identify more efficient
chemo-preventive agents for treating RCC.
Metformin is the most widely used biguanide drug for
treating type 2 diabetes mellitus patients (5). It has been reported that metformin has
anti-diabetic and anticancer effects on colorectal and pancreatic
cancer cells (6,7). It has also been revealed to exert
anti-neoplastic effects in epithelial ovarian cancer (8). Furthermore, metformin has been
demonstrated to reduce the risk of cancer prevalence in diabetic
patients (9,10). Metformin demonstrated a marked
anticancer effect in various cells of different types of human
cancer, including breast cancer, renal cancer, glioblastoma,
insulinoma and cholangiocarcinoma via cell growth inhibition, cell
cycle arrest, apoptosis, adenosine monophosphate-activated protein
kinase (AMPK) signaling and tumor growth inhibition (11–15).
Although the effect of metformin on A498 cells has been reported
(12), the apoptosis-mediated
molecular mechanism of action of metformin remains unclear in human
renal cell carcinoma A498 cells.
The cellular caspase 8 (FLICE)-like inhibitory
protein (c-FLIP) gene makes three isoforms, namely
c-FLIPL, c-FLIPS and c-FLIPR, via
alternative splicing in humans. These proteins are well known as
anti-apoptotic proteins; each exert this effect via different
mechanisms (16). In previous
reports, c-FLIP was demonstrated to be an independent negative
prognostic factor in ovarian, endometrial and colon cancer cells
(17–19). c-FLIPL is known to be
involved in the inhibition of caspase-8 activation-mediated
apoptosis (18,20). The activation of caspase-8 leads to
death-inducing signaling complex (DISC) and augmented apoptosis via
caspase-3 activation. Previous studies have demonstrated that
treatment with metformin suppressed the c-FLIPL protein
expression level in human lung adenocarcinoma and bladder cancer
(21,22).
In the present study, the mechanism of
metformin-mediated apoptosis in human renal cell carcinoma A498
cells was investigated. It was revealed that degradation of
c-FLIPL protein and activation of caspase-8 were
associated with metformin-induced apoptosis.
Materials and methods
Cell culture
A498 human renal carcinoma cells were procured from
the American Type Culture Collection (ATCC; Manassas, VA, USA).
Dulbecco's modified Eagle's medium (DMEM; catalog no. LM 001-05;
Welgene, Inc., Kyungsan, Korea) containing 10% fetal bovine serum
(FBS; catalog no. S001-07; Welgene, Inc.), 20 mM
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES; catalog
no. H0887; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) buffer
and 100 µg/ml gentamicin (catalog no. 15710-072; Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) was used as the culture
medium. The cells were cultured in an incubator at 37°C with
humidified 5% CO2.
Cell morphology
A498 human renal carcinoma cells were treated with
an inhibitor in either the absence or presence of metformin (10
mM). Following 24 h incubation, morphological changes were
visualized with light microscopy (catalog no. DFC495; Leica
Microsystems GmbH, Wetzlar, Germany) at ×200 magnification. The
images were analyzed using the i-Solution program (IMT i-Solution,
Burnaby, BC, Canada).
Flow cytometry analysis
Cell counting was performed using a hemocytometer.
Metformin was immediately added to cell cultures at the indicated
concentrations. Approximately 0.4×106 cells were
resuspended in 100 µl PBS (catalog no. 17-517Q; Lonza,
Walkersville, MD, USA), and 200 µl of 95% ethanol (catalog no.
1.00983.1011; Merck KGaA) was added during vortexing. The cells
were incubated at 4°C for 1 h, washed in PBS, and resuspended in
250 µl 1.12% sodium citrate buffer (pH 8.4) along with 12.5 µl
RNase. Incubation was continued for 30 min at 37°C. Cellular DNA
was stained with 250 µl (1:1 dilution) propidium iodide (50 µg/ml;
catalog no. p4170; Sigma-Aldrich; Merck KGaA) for 30 min at 37°C,
and the relative DNA contents of the stained cells were analyzed
using fluorescence-activated cell sorting (FACS) on the BD FACS
Cato II flow cytometer (BD Biosciences, San Jose, CA, USA).
Western blot analysis
A498 whole-cell lysates were prepared by
resuspending 0.4×106 cells in 50 µl lysis buffer (137 mM
NaCl, 15 mM EGTA, 0.1 mM sodium orthovanadate, 15 mM
MgCl2, 0.1% Triton X-100, 25 mM MOPS, 100 µM
phenylmethylsulfonyl fluoride and 20 µM leupeptin, adjusted to pH
7.2). The cells were disrupted by sonication and protein extracted
at 4°C for 30 min. Protein concentrations were quantified using the
BCA assay kit (catalog no. 23225; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. The proteins (50 µg) were
separated using 10% SDS-PAGE gel and electrotransferred onto
nitrocellulose membranes (catalog no. 23225; GE Healthcare,
Chicago, IL, USA). The membrane was blocked with 5% skim milk in
Tris-buffered saline (TBS) for 30 min at room temperature. The anti
c-FLIPL (dilution, 1:700; catalog no. ALX-804-961)
antibody was obtained from Enzo Life Sciences, Inc. (Farmingdale,
NY, USA). The anti-PARP (dilution, 1:1,000; catalog no. 9542)
antibody was purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). The anti-B-cell lymphoma-2 (Bcl-2); (dilution,
1:700; catalog no. sc-783), anti-B-cell lymphoma-extra-large
(Bcl-xL); (dilution, 1:1,000; catalog no. sc-634), anti-myeloid
cell leukemia-1 (Mcl-1); (dilution, 1:1,000; catalog no. sc-819),
anti-cellular inhibitor of apoptosis 2 (cIAP-2); (dilution,
1:1,000; catalog no. sc-7944) and anti-actin (dilution, 1:2,000;
catalog no. sc-1616) antibodies were procured from Santa Cruz
Biotechnology Inc. (Dallas, TX, USA). The anti-XIAP (dilution,
1:5,000; catalog no. 610762) antibody was supplied by BD
Biosciences (San Jose, CA, USA). Membranes were incubated with the
primary antibodies overnight at 4°C. Following six washes with TBS
(each for 5 min), the membranes were incubated with the indicated
secondary antibody for 1 h at room temperature and washed six times
with TBS. The secondary goat anti-rabbit immunoglobulin G
(IgG)-horseradish peroxidase (HRP) conjugated (dilution 1:1,000;
catalog no. sc-2004) and goat anti-mouse IgG-HRP (dilution 1:1,000;
catalog no. sc-2005) antibodies were procured from Santa Cruz
Biotechnology, Inc. Specific proteins were detected using an ECL
western blotting kit (catalog no. WBKLS0500; Merck KGaA). Proteins
were detected using by ImageQuant LAS 4000 Mini Imaging System (GE
Healthcare).
Transfection
A498 cells were seeded onto 6-well plates at a
concentration of 0.2×106 cells/well and incubated
overnight at 37°C. The pcDNA 3.1 vector and pcDNA 3.1
c-FLIPL plasmid were provided by Professor Tae-Jin Lee
(Yeungnam University, South Korea). They were then transfected with
control plasmid pcDNA 3.1 vector or pcDNA 3.1-c-FLIPL
plasmid for 5 h using lipofectamine 2000 (catalog no. 11668-019;
Invitrogen; Thermo Fisher Scientific, Inc.) in Opti-MEM medium
(catalog no. 31985-070; Invitrogen; Thermo Fisher Scientific,
Inc.). Following transfection, the cells were cultured in DMEM
supplemented with 10% FBS for 12 h. Next, the cells were treated
with metformin for 24 h. Finally, the cells were analyzed for
c-FLIPL expression using western blotting.
RNA isolation and reverse
transcription-polymerase chain reaction (RT-PCR)
c-FLIPL mRNA expression was determined by
RT-PCR. Total RNA was extracted from A498 cells using the EasyBlue
reagent (catalog no. 17061; Thermo Fisher Scientific, Inc.). cDNA
was prepared using M-MLV reverse transcriptase (catalog no.
18057018; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. In addition, the total cellular RNA was
reverse-transcribed using a random primer and subsequently
amplified using PCR. PCR primers were purchased from GenoTech
(Daejeon, Korea). GAPDH was used as the internal control. The PCR
cycling conditions used were as follows: For c-FLIPL:
95°C for 45 sec, 54°C for 45 sec, 72°C for 45 sec (33 cycles) and
for GAPDH: 94°C for 30 sec, 58°C for 45 sec, 72°C for 42 sec (25
cycles). The following primer sequences were used to amplify
c-FLIPL and GAPDH: For c-FLIPL:
5′-CGGACTATAGAGTGCTGATGG-3′ (forward) and
5′-GATTATCAGGCAGATTCCTAG-3′ (reverse); and for GAPDH:
5′-AGGTCGGAGTCAACGGATTTG-3′ (forward) and
5′-GTGATGGCATGGACTGTGGT-3′ (reverse). PCR products were analyzed by
electrophoresis using 1.5% agarose gels and visualized by ethidium
bromide using UV light gel (catalog no. WGD30; DAIHAN Scientific,
Seoul, Korea).
Measurement of reactive oxygen
species
A498 cells were plated in 6 well plates at a density
of 0.4×106 cells/well and incubated for 24 h. The cells
were incubated with metformin for 1 h and loaded with 10 µM
2′,7′-dichlorofluorescin diacetate (H2DCFDA;
Sigma-Aldrich; Merck KGaA) for 30 min at 37°C. Next, they were
washed three times with PBS. Fluorescence was measured using flow
cytometry. ROS generation was assessed by the dichlorofluorescence
in fluorescence intensity (FL-1, 530 nm) of 10,000 cells using the
BD FACS Cato II flow cytometer (BD Biosciences).
Statistical analysis
Data were analyzed using one-way ANOVA followed by
post hoc comparisons (Student-Newman-Keuls) using the Statistical
Package for Social Sciences 8.0 (SPSS, Inc., Chicago, IL, USA). At
least three independent experiments were performed. The data were
expressed as the mean ± standard deviation and P<0.05 was
considered to indicate a statistically significant difference.
Results
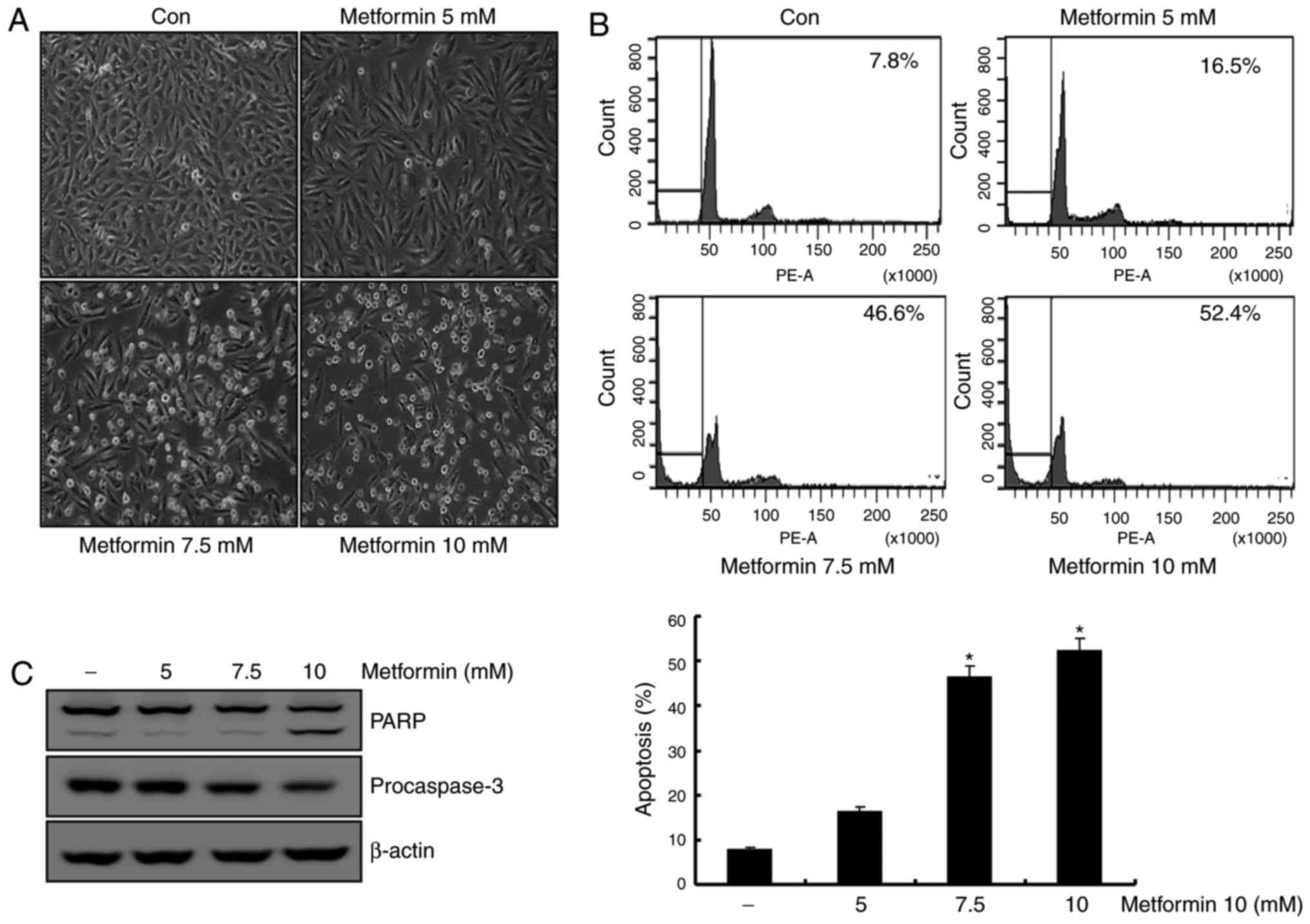
Metformin induces apoptosis in human
renal cell carcinoma A498 cells
Previous studies reported that metformin induces
apoptosis in renal cancer and breast cancer cell lines (12,23). To
determine the apoptotic effects of metformin on A498 cells, these
cells were treated with various concentrations of metformin (0, 5,
7.5 and 10 mM) for 24 h. In a dose-dependent manner, the
metformin-stimulated A498 cells demonstrated features of apoptosis,
including cell contraction and rounding and segregation of cells
from the well (Fig. 1A). As presented
in Fig. 1B, treatment of A498 cells
with metformin resulted in a dose-dependent increase in sub-G1
populations. Additionally, treatment of A498 cells with metformin
stimulated a reduction in the protein levels of the 32-kDa
precursor (procaspase-3), along with the concomitant cleavage of
PARP, a protein substrate for caspases (Fig. 1C). These results suggested that
metformin-treated A498 cells demonstrated an increase in the Sub-G1
population in a dose-dependent manner.
Metformin-induced apoptosis is
modulated through degradation of the c-FLIPL protein in
A498 cells
Caspases are important regulators of apoptotic cell
death associated with apoptotic signaling pathways in various
cancer cells (24). The present study
examined whether activation of the caspase signaling pathway served
a key role in metformin-mediated apoptosis. Treatment of A498 cells
with metformin did not affect the activation of caspase-2. However,
treatment with metformin for 24 h resulted in the appearance of
p43/41-kDa fragments of caspase-8 (Fig.
2A). These results demonstrated that caspase-8 activation was
involved in metformin-induced apoptosis in A498 cells. The
association between metformin-induced apoptosis and regulation of
other apoptotic modulators was investigated. As presented in
Fig. 2B, protein expression levels of
anti-apoptotic molecules including Bcl-2, Bcl-xL, Mcl-1, XIAP and
c-IAP2 were not altered by metformin treatment. c-FLIP
is a major regulator of the activity of caspase-8 (20). The protein level of c-FLIPL
decreased following metformin treatment in A498 cells in a
dose-dependent manner. Taken together, degradation of the
c-FLIPL protein was involved in metformin-induced
apoptosis via activation of caspase-8.
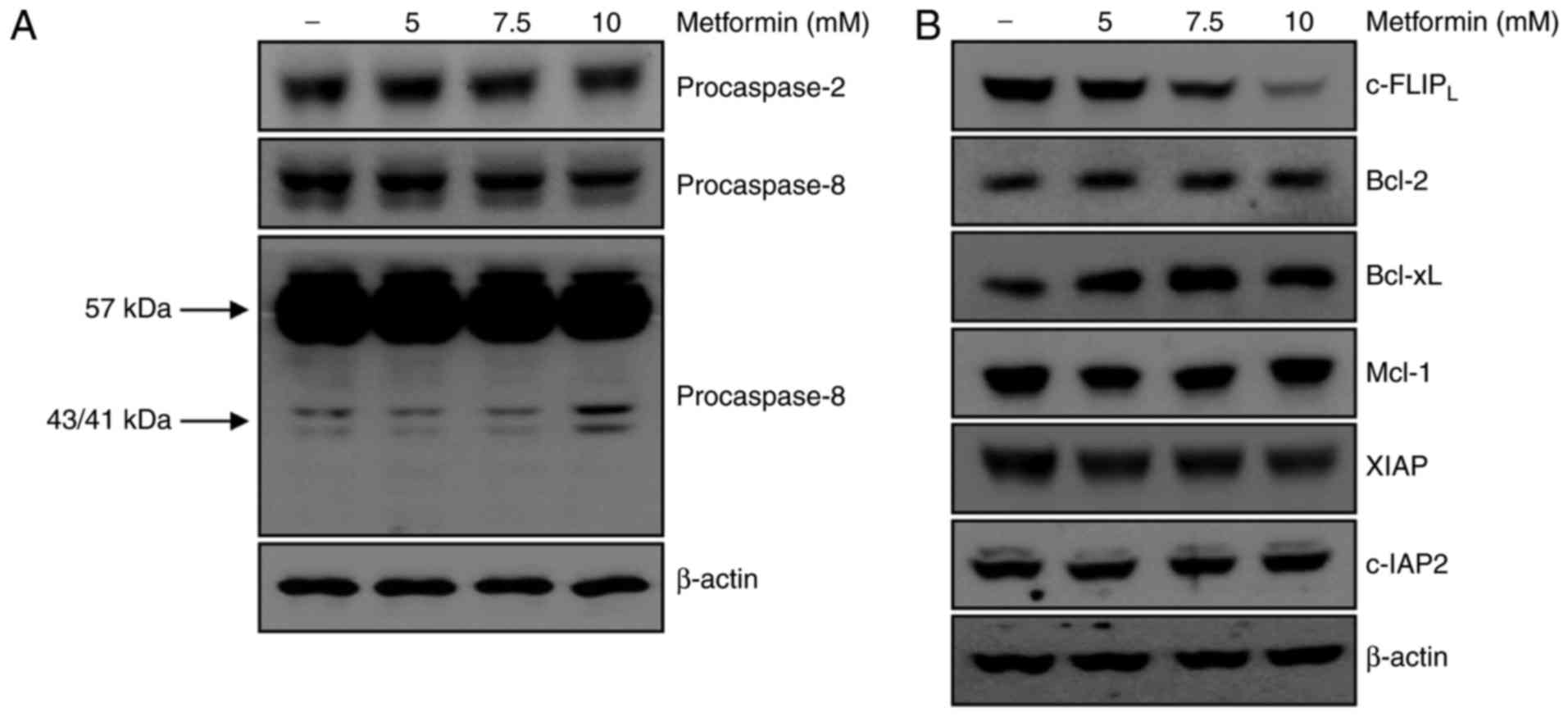 | Figure 2.Metformin-induced apoptosis is
associated with activation of procaspase-8 and degradation of
c-FLIPL. (A) A498 cells were treated with metformin for
24 h, and procaspase-2 and procaspase-8 expression was analyzed
using western blotting. β-actin was used as the loading control.
(B) A498 cells were treated with various concentrations of
metformin for 24 h. c-FLIPL, Bcl-2, Bcl-xL, Mcl-1, XIAP
and c-IAP2 expression was determined using western blot
analysis. β-actin served as the loading control. c-FLIP, cellular
FLICE (FADD-like IL-1β-converting enzyme)-inhibitory protein;
Bcl-2, B-cell lymphoma 2; Bcl-xL, B-cell lymphoma-extra-large;
Mcl-1, myeloid cell leukemia-1, XIAP, X-linked inhibitor of
apoptosis protein; c-IAP, cellular inhibitor of apoptosis protein,
baculoviral IAP repeat containing 3. |
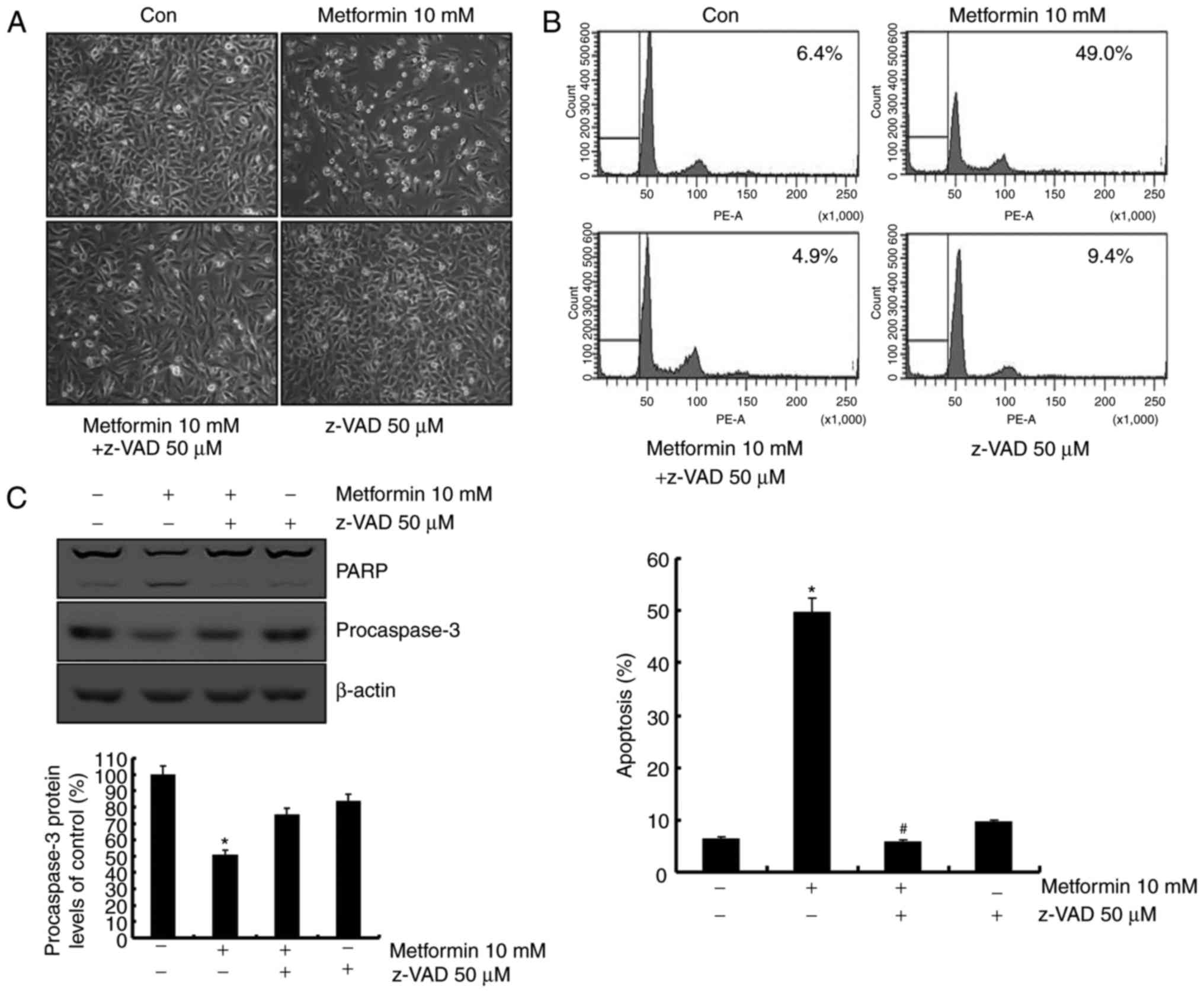
Metformin-mediated apoptosis is
associated with activation of the caspase signaling pathway
The role of the caspase signaling pathway in
metformin-mediated apoptosis was investigated. As presented in
Fig. 3A, metformin-induced apoptosis
was blocked following pretreatment with a general caspase
inhibitor, z-VAD-fmk. Sub-G1 population was markedly decreased by
treatment with z-VAD-fmk in the presence of metformin (Fig. 3B). In addition, treatment with
z-VAD-fmk prevented the cleavage of PARP and caspase-3 activation
(Fig. 3B). These data indicated that
metformin-induced apoptosis was mediated by caspase-dependent
apoptosis in the presence of z-VAD-fmk.
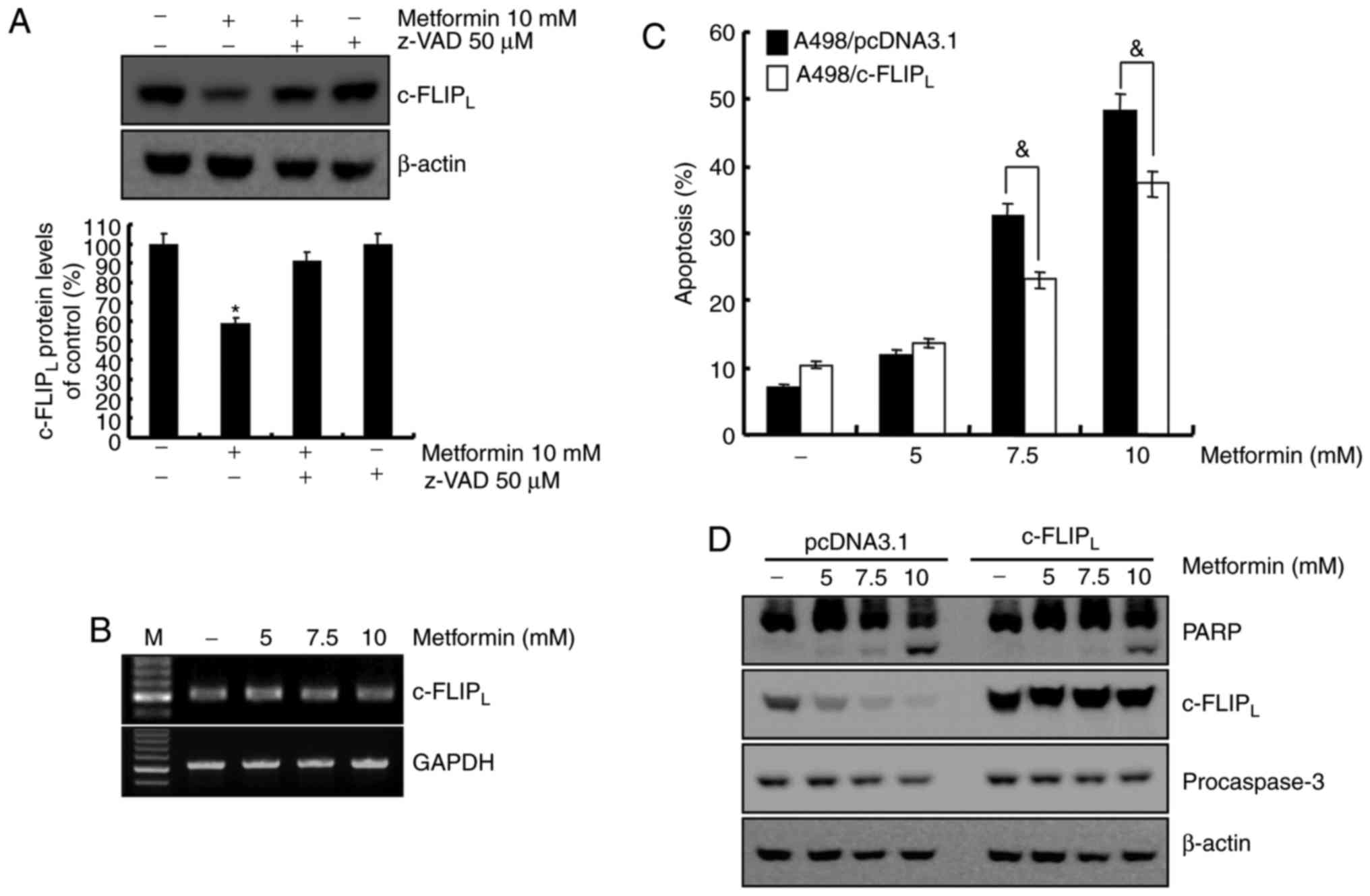
Metformin-mediated apoptosis is
dependent on the degradation of c-FLIPLprotein in A498
cells
The association between caspase-8 activation and
metformin-mediated apoptosis was investigated. Treatment with
metformin led to c-FLIPL degradation, which was
recovered by z-VAD-fmk (Fig. 4A). To
determine whether the mRNA level of c-FLIPL was
associated with protein level in A498 human renal cell carcinoma
cells, the c-FLIPL mRNA level was examined using RT-RCR.
As demonstrated in Fig. 4B,
c-FLIPL mRNA level remained constant following metformin
treatment in A498 cells at indicated concentrations. A previous
study reported that c-FLIP functions as an anti-apoptotic regulator
and is overexpressed in various cancer cell lines (25). To determine whether the reduced level
of c-FLIPL was involved in the induction of apoptosis in
metformin-treated A498 cells, c-FLIPL overexpressing
cells were established. Overexpression of c-FLIPL
attenuated apoptosis (Fig. 4C) and
PARP cleavage (Fig. 4D) induced by
metformin. Taken together, metformin-induced apoptosis was
associated with the degradation of c-FLIPL through
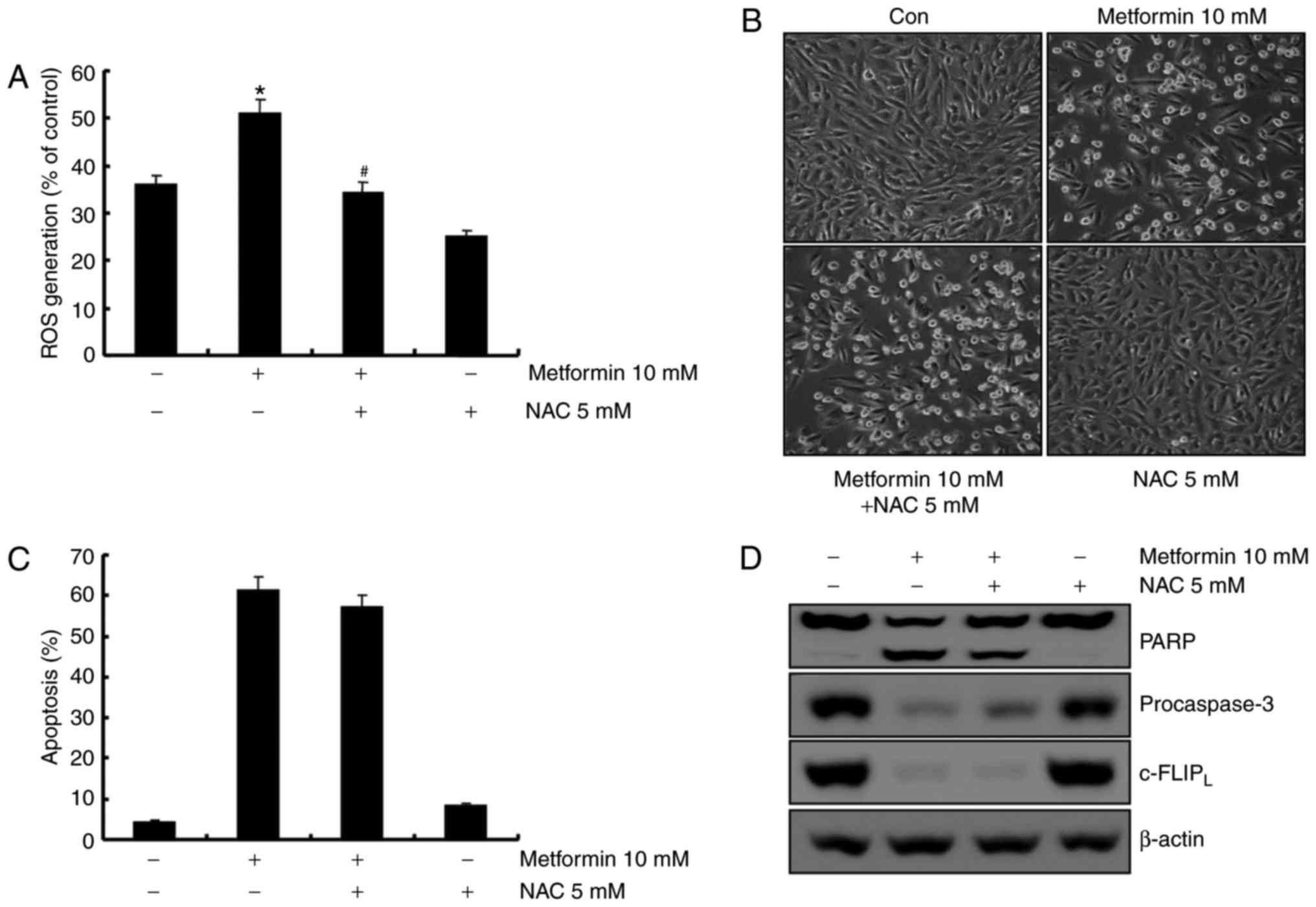
activation of caspase-8. ROS is not involved in
metformin-mediated apoptosis in A498 cells. ROS is an important
regulator of apoptosis (26). A
previous report demonstrated that metformin induces ROS production
in breast cancer cells (23).
Therefore, the capacity of metformin to induce ROS production in
A498 human renal carcinoma cells was investigated using flow
cytometry. As demonstrated in Fig.
5A, metformin treatment increased ROS production 1 h
post-treatment, and pretreatment with the anti-oxidant
N-acetyl-L-cysteine (NAC, a ROS scavenger) inhibited
metformin-induced ROS production. To confirm whether ROS generation
served a key role in metformin-mediated apoptosis, A498 cells were
pretreated with NAC for 30 min. Pretreatment with NAC did not
inhibit metformin-induced morphological changes and apoptosis in
A498 cells (Fig. 5B and C).
Furthermore, NAC did not prevent PARP cleavage, caspase activation
and degradation of c-FLIPL protein in metformin-treated
cells (Fig. 5D). These results
suggested that ROS generation was not critical for the induction of
apoptosis by metformin.
Discussion
Metformin has been used as a therapeutic agent for
diabetic patients (27). It was
reported to possess various effects, including anti-proliferation,
anti-inflammation, anti-angiogenesis and anti-invasion (12,28–30).
Metformin also has anti-apoptotic effects on prostate,
hepatocellular carcinoma, gall bladder and breast cancer cell lines
(31–34). In addition, the insulin receptor may
serve a major role in facilitating cancer development via increases
in insulin levels (35,36). However, the specific apoptotic
mechanism of action of metformin in human renal cancer cells has
not been reported. The present study investigated whether metformin
had an anti-cancer effect on human renal cell carcinoma A498 cells.
It was revealed that metformin-mediated apoptosis led to the
activation of caspase-8 through the downregulation of
c-FLIPL protein expression level in A498 cells.
Apoptosis is the process of programmed cell death
that is closely associated with caspase activation. Caspases can be
divided into two main groups, namely initiator caspases (caspase-2,
−8, −9 and −10) and executioner caspases (caspase-3, −6 and −7)
(37). Caspase-8 enhances apoptosis
through caspase-3 activation. Caspase-3 is a primary caspase
because it is associated with the intrinsic and extrinsic signaling
pathways of apoptosis (38). In the
present study, the involvement of caspases in metformin-mediated
apoptosis was examined. Caspase-8 was activated by metformin,
resulting in the emergence of p43/41 kDa fragments (Fig. 2A). Activation of the executioner
caspase, caspase-3, was increased via metformin-induced apoptosis
(Fig. 1C). In addition, z-VAD-fmk (a
pan-caspase inhibitor) completely blocked metformin-induced
apoptosis (Fig. 3). These results
suggested that metformin-induced apoptosis was associated with
caspase activation. Activation of the caspase cascade is modulated
via upregulation of pro-apoptotic proteins and/or downregulation of
anti-apoptotic proteins (39). In
addition, activation of caspase-8 at DISC is blocked via c-FLIP,
which inhibits the death receptor-mediated apoptotic pathways
(40). In the present study,
metformin also decreased c-FLIPL protein level in A498
cells in a dose-dependent manner (Fig.
2B). However, treatment with metformin did not affect
c-FLIPL mRNA levels (Fig.
4B) and proteasomal degradation (data not shown). Previous
reports also indicated that metformin had no effect on
c-FLIPL mRNA level and the proteasomal pathway in
non-muscle invasive bladder cancer (NMIBC) (22). It was also revealed that
c-FLIPL-overexpressing cells partly prevented
metformin-induced apoptosis in A498 cells (Fig. 4). These results suggested that
metformin-induced apoptosis occurred via degradation of
c-FLIPL protein. Metformin has also been reported to
suppress the expression levels of anti-apoptotic proteins,
including Bcl-2, Bcl-XL, Mcl-1, c-IAP2 and XIAP in primary ovarian
cell lines, p53-deficient cells, colorectal and breast cancer cell
lines, respectively (41–44). It was demonstrated that Bcl-2, Bcl-XL,
Mcl-1, c-IAP2 and XIAP did not affect metformin-treated A498 cells
(Fig. 2B). These results demonstrated
that suppression of the anti-apoptotic protein, c-FLIPL,
was associated with metformin-induced apoptosis in A498 cells.
Reactive oxygen species (ROS) are important
mediators of apoptosis in several cancer cell lines (45–48).
Previous reports demonstrated that metformin modulated apoptosis
through ROS production in hepatoma, ovary and breast cancer cell
lines (49,50). Therefore, the association between
metformin-mediated apoptosis and ROS generation was investigated.
In the present study, metformin was observed to stimulate ROS
generation in A498 cells. Pretreatment with N-acetylcysteine did
not prevent metformin-induced apoptosis (Fig. 5). These results suggested that
metformin-induced ROS generation was not associated with
metformin-mediated apoptosis in A498 cells.
Taken together, these results demonstrated that
metformin-induced apoptosis was mediated by the degradation of
c-FLIPL protein via activation of caspase-8 in A498
human renal cell carcinoma cells. This suggested that metformin can
serve the role of a chemotherapeutic agent for diabetes, as well as
an anti-cancer agent.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education (grant no.
2017R1D1A1B3030961).
Availability of data and materials
All data generated or analyzed during present study
are included within the article.
Authors' contributions
JJ and JK designed the study, collected and analyzed
the data. TL, IS and ES advised on the morphological images and
performed the data analysis. JJ and JK drafted and wrote the
manuscript. TL and JK revised the manuscript critically for
intellectual content. All authors gave intellectual input to the
study and approved the final version of the manuscript.
Ethics approval and consent to
participate
This research was approved by the Ethics Committee
of Yeungnam University (Daegu, South Korea). All procedures were
performed according to the ethical standards of Ethics Committee of
Yeungnam University and with the 1964 Helsinki declaration and its
later amendments or comparable ethical standards.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Low G, Huang G, Fu W, Moloo Z and Girgis
S: Review of renal cell carcinoma and its common subtypes in
radiology. World J Radiol. 8:484–500. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Atzpodien J, Kirchner H and Poliwoda H:
Interleukin 2 based ambulatory therapy of metastatic renal cell
carcinoma. Med Klin (Munich). 91(Suppl. (3): S38–S43. 1996.(In
German).
|
|
3
|
Rabinovitch RA, Zelefsky MJ, Gaynor JJ and
Fuks Z: Patterns of failure following surgical resection of renal
cell carcinoma: Implications for adjuvant local and systemic
therapy. J Clin Oncol. 12:206–212. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Malek M, Aghili R, Emami Z and Khamseh ME:
Risk of cancer in diabetes: The effect of metformin. ISRN
Endocrinol. 2013:6369272013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Algire C, Amrein L, Zakikhani M, Panasci L
and Pollak M: Metformin blocks the stimulative effect of a
high-energy diet on colon carcinoma growth in vivo and is
associated with reduced expression of fatty acid synthase. Endocr
Relat Cancer. 17:351–360. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang ZJ, Zheng ZJ, Kan H, Song Y, Cui W,
Zhao G and Kip KE: Reduced risk of colorectal cancer with metformin
therapy in patients with type 2 diabetes: A meta-analysis. Diabetes
Care. 34:2323–2328. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nair V, Pathi S, Jutooru I, Sreevalsan S,
Basha R, Abdelrahim M, Samudio I and Safe S: Metformin inhibits
pancreatic cancer cell and tumor growth and downregulates Sp
transcription factors. Carcinogenesis. 34:2870–2879. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gotlieb WH, Saumet J, Beauchamp MC, Gu J,
Lau S, Pollak MN and Bruchim I: In vitro metformin anti-neoplastic
activity in epithelial ovarian cancer. Gynecol Oncol. 110:246–250.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Emami Riedmaier A, Fisel P, Nies AT,
Schaeffeler E and Schwab M: Metformin and cancer: From the old
medicine cabinet to pharmacological pitfalls and prospects. Trends
Pharmacol Sci. 34:126–135. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang ZJ, Zheng ZJ, Shi R, Su Q, Jiang Q
and Kip KE: Metformin for liver cancer prevention in patients with
type 2 diabetes: A systematic review and meta-analysis. J Clin
Endocrinol Metab. 97:2347–2353. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rice S, Pellat L, Ahmetaga A, Bano G,
Mason HD and Whitehead SA: Dual effect of metformin on growth
inhibition and oestradiol production in breast cancer cells. Int J
Mol Med. 35:1088–1094. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fang Z, Xu X, Zhou Z, Xu Z and Liu Z:
Effect of metformin on apoptosis, cell cycle arrest migration and
invasion of A498 cells. Mol Med Rep. 9:2251–2256. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ucbek A, Ozunal ZG, Uzun O and Gepdiremen
A: Effect of metformin on the human T98G glioblastoma multiforme
cell line. Exp Ther Med. 7:1285–1290. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jung TW, Lee MW, Lee YJ and Kim SM:
Metformin prevents endoplasmic reticulum stress-induced apoptosis
through AMPK-PI3K-c-Jun NH2 pathway. Biochem Biophys Res Commun.
417:147–152. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fujimori T, Kato K, Fujihara S, Iwama H,
Yamashita T, Kobayashi K, Kamada H, Morishita A, Kobara H, Mori H,
et al: Antitumor effect of metformin on cholangiocarcinoma: In
vitro and in vivo studies. Oncol Rep. 34:2987–2996. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He MX and He YW: A role for c-FLIP(L) in
the regulation of apoptosis, autophagy, and necroptosis in T
lymphocytes. Cell Death Differ. 20:188–197. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bagnoli M, Ambrogi F, Pilotti S, Alberti
P, Ditto A, Barbareschi M, Galligioni E, Biganzoli E, Canevari S
and Mezzanzanica D: c-FLIPL expression defines two ovarian cancer
patient subsets and is a prognostic factor of adverse outcome.
Endocr Relat Cancer. 16:443–453. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Safa AR, Day TW and Wu CH: Cellular
FLICE-like inhibitory protein (C-FLIP): A novel target for cancer
therapy. Current Cancer Drug Targets. 8:37–46. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bagnoli M, Canevari S and Mezzanzanica D:
Cellular FLICE-inhibitory protein (c-FLIP) signalling: A key
regulator of receptor-mediated apoptosis in physiologic context and
in cancer. Int J Biochem Cell Biol. 42:210–213. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koenig A, Buskiewicz IA, Fortner KA,
Russell JQ, Asaoka T, He YW, Hakem R, Eriksson JE and Budd RC: The
c-FLIPL cleavage product p43FLIP promotes activation of
extracellular signal-regulated kinase (ERK), nuclear factor kappaB
(NF-κB), and caspase-8 and T cell survival. J Biol Chem.
289:1183–1191. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nazim UM, Moon JH, Lee JH, Lee YJ, Seol
JW, Eo SK, Lee JH and Park SY: Activation of autophagy flux by
metformin downregulates cellular FLICE-like inhibitory protein and
enhances TRAIL-induced apoptosis. Oncotarget. 7:23468–23481. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang T, Wang X, He D, Jin X and Guo P:
Metformin sensitizes human bladder cancer cells to TRAIL-induced
apoptosis through mTOR/S6K1-mediated downregulation of c-FLIP.
Anticancer Drugs. 25:887–897. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gao ZY, Liu Z, Bi MH, Zhang JJ, Han ZQ,
Han X, Wang HY, Sun GP and Liu H: Metformin induces apoptosis via a
mitochondria-mediated pathway in human breast cancer cells in
vitro. Exp Ther Med. 11:1700–1706. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Riedl SJ and Shi Y: Molecular mechanisms
of caspase regulation during apoptosis. Nat Rev Mol Cell Biol.
5:897–907. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ili CG, Brebi P, Tapia O, Sandoval A,
Lopez J, Garcia P, Leal P, Sidransky D, Guerrero-Preston R and Roa
JC: Cellular FLICE-like inhibitory protein long form (c-FLIPL)
overexpression is related to cervical cancer progression. Int J
Gynecol Pathol. 32:316–322. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ding H, Han C, Guo D, Chin YW, Ding Y,
Kinghorn AD and D'Ambrosio SM: Selective induction of apoptosis of
human oral cancer cell lines by avocado extracts via a ROS-mediated
mechanism. Nutr Cancer. 61:348–356. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Maruthur NM, Tseng E, Hutfless S, Wilson
LM, Suarez-Cuervo C, Berger Z, Chu Y, Iyoha E, Segal JB and Bolen
S: Diabetes medications as monotherapy or metformin-based
combination therapy for type 2 diabetes: A systematic review and
Meta-analysis. Ann Intern Med. 164:740–751. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu HY, Fang W, Huang ZW, Lu JC, Wang YQ,
Tang QL, Song GH, Kang Y, Zhu XJ, Zou CY, et al: Metformin reduces
SATB2-mediated osteosarcoma stem cell-like phenotype and tumor
growth via inhibition of N-cadherin/NF-κB signaling. Eur Rev Med
Pharmacol Sci. 21:4516–4528. 2017.PubMed/NCBI
|
|
29
|
Figueroa-González G, Garcia-Castillo V,
Coronel-Hernández J, López-Urrutia E, León-Cabrera S, Arias-Romero
LE, Terrazas LI, Rodríguez-Sosa M, Campos-Parra AD, Zúñiga-Calzada
E, et al: Anti-inflammatory and antitumor activity of a triple
therapy for a colitis-related colorectal cancer. J Cancer.
7:1632–1644. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang Y, Jin G, Liu H, Liu K, Zhao J, Chen
X, Wang D, Bai R, Li X, Jang Y, et al: Metformin inhibits
esophageal squamous cell carcinoma-induced angiogenesis by
suppressing JAK/STAT3 signaling pathway. Oncotarget. 8:74673–74687.
2017.PubMed/NCBI
|
|
31
|
Tran LNK, Kichenadasse G, Butler LM,
Centenera MM, Morel KL, Ormsby RJ, Michael MZ, Lower KM and Sykes
PJ: The combination of metformin and valproic acid induces
synergistic apoptosis in the presence of p53 and androgen signaling
in prostate cancer. Mol Cancer Ther. 16:2689–2700. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tsai HH, Lai HY, Chen YC, Li CF, Huang HS,
Liu HS, Tsai YS and Wang JM: Metformin promotes apoptosis in
hepatocellular carcinoma through the CEBPD-induced autophagy
pathway. Oncotarget. 8:13832–13845. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bi T, Zhu A, Yang X, Qiao H, Tang J, Liu Y
and Lv R: Metformin synergistically enhances antitumor activity of
cisplatin in gallbladder cancer via the PI3K/AKT/ERK pathway.
Cytotechnology. 70:439–448. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Al-Zaidan L, El Ruz RA and Malki AM:
Screening novel molecular targets of metformin in breast cancer by
proteomic approach. Front Public Health. 5:2772017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Belfiore A and Malaguarnera R: Insulin
receptor and cancer. Endocr Relat Cancer. 18:R125–R147. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rizos CV and Elisaf MS: Metformin and
cancer. Eur J Pharmacol. 705:96–108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Galluzzi L, Lopez-Soto A, Kumar S and
Kroemer G: Caspases connect cell-death signaling to organismal
homeostasis. Immunity. 44:221–231. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Walters J, Pop C, Scott FL, Drag M, Swartz
P, Mattos C, Salvesen GS and Clark AC: A constitutively active and
uninhibitable caspase-3 zymogen efficiently induces apoptosis.
Biochem J. 424:335–345. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Spagnuolo C, Cerella C, Russo M,
Chateauvieux S, Diederich M and Russo GL: Quercetin downregulates
Mcl-1 by acting on mRNA stability and protein degradation. Br J
Cancer. 105:221–230. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kruidering M and Evan GI: Caspase-8 in
apoptosis: The beginning of ‘the end’? IUBMB Life. 50:85–90. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Patel S, Singh N and Kumar L: Evaluation
of effects of metformin in primary ovarian cancer cells. Asian Pac
J Cancer Prev. 16:6973–6979. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li X, Li B, Ni Z, Zhou P, Wang B, He J,
Xiong H, Yang F, Wu Y, Lyu X, et al: Metformin synergizes with
BCL-XL/BCL-2 inhibitor ABT-263 to induce apoptosis specifically in
p53-defective cancer cells. Mol Cancer Ther. 16:1806–1818. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Park SH, Lee DH, Kim JL, Kim BR, Na YJ, Jo
MJ, Jeong YA, Lee SY, Lee SI, Lee YY and Oh SC: Metformin enhances
TRAIL-induced apoptosis by Mcl-1 degradation via Mule in colorectal
cancer cells. Oncotarget. 7:59503–59518. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Strekalova E, Malin D, Rajanala H and
Cryns VL: Metformin sensitizes triple-negative breast cancer to
proapoptotic TRAIL receptor agonists by suppressing XIAP
expression. Breast Cancer Res Treat. 163:435–447. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jang JH, Iqbal T, Min KJ, Kim S, Park JW,
Son EI, Lee TJ and Kwon TK: Helenalin-induced apoptosis is
dependent on production of reactive oxygen species and independent
of induction of endoplasmic reticulum stress in renal cell
carcinoma. Toxicol In Vitro. 27:588–596. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Woo SM, Min KJ and Kwon TK: Calyculin A
causes sensitization to tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL)-induced apoptosis by ROS-mediated
down-regulation of cellular FLICE-inhibiting protein (c-FLIP) and
by enhancing death receptor 4 mRNA stabilization. Apoptosis.
17:1223–1234. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang Y, Yu X, Song H, Feng D, Jiang Y, Wu
S and Geng J: The STAT-ROS cycle extends IFN-induced cancer cell
apoptosis. Int J Oncol. 52:305–313. 2018.PubMed/NCBI
|
|
48
|
Sheikh BY, Sarker MMR, Kamarudin MNA and
Mohan G: Antiproliferative and apoptosis inducing effects of citral
via p53 and ROS-induced mitochondrial-mediated apoptosis in human
colorectal HCT116 and HT29 cell lines. Biomed Pharmacother.
96:834–846. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Rogalska A, Bukowska B and Marczak A:
Metformin and epothilone A treatment up regulate pro-apoptotic
PARP-1, Casp-3 and H2AX genes and decrease of AKT kinase level to
control cell death of human hepatocellular carcinoma and ovary
adenocarcinoma cells. Toxicol In Vitro. 47:48–62. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Haugrud AB, Zhuang Y, Coppock JD and
Miskimins WK: Dichloroacetate enhances apoptotic cell death via
oxidative damage and attenuates lactate production in
metformin-treated breast cancer cells. Breast Cancer Res Treat.
147:539–550. 2014. View Article : Google Scholar : PubMed/NCBI
|