Introduction
Folkman developed the theory that the growth and
metastasis of solid tumors are dependent on angiogenesis (1). In recent years, extensive research has
demonstrated that the mobilization and differentiation of
endothelial progenitor cells (EPCs) from the peripheral blood and
bone marrow serves an important role in tumor angiogenesis
(2).
Tumor cells and their microenvironment secrete
multiple factors that induce EPC activation, including vascular
endothelial growth factor (VEGF), angiopoietin, stromal
cell-derived factor (SDF)-1 and matrix metallopeptidase (MMP)-9.
Mast cells (MCs) accumulate around and within the microenvironments
of numerous types of solid tumor (3).
MC infiltration is associated with microvascular density and prior
to the initiation of angiogenesis, increased numbers of MCs have
been identified in various types of solid tumor, including breast
cancer (4), gastric cancer (5) and lung cancer (6). MCs secrete a variety of angiogenic
factors, including tryptase, chymase, tumor necrosis factor
(TNF)-α, interleukin (IL)-8, fibroblast growth factor (FGF)2 and
VEGF. Therefore, MCs serve a role in tumor angiogenesis, and are
associated with tumor progression, metastasis and prognosis
(7). Tryptase is the most abundant
enzyme in MCs, and is stored as an active tetramer in complex with
heparin in MC secretory granules. It has a variety of biological
activities, including the ability to activate MCs, increase blood
vessel permeability (8), induce the
infiltration of inflammatory cells (9), and stimulate epithelial cell
proliferation and the release of IL-8 (10). Previous studies have identified an
association between the level of tryptase in the tumor
microenvironment and angiogenesis in breast cancer (11).
Proteinase activated receptors (PARs) are members of
the G-protein coupled receptor superfamily, and consist of four
subtypes: PAR-1, PAR-2, PAR-3 and PAR-4. PAR-2 are activated by
trypsin, tryptase, membrane-type serine protease-l, airway
trypsin-like protease or coagulation factors VIIa and Xa (12). PAR-2 is expressed on the cell membrane
of endothelial cells, vascular smooth muscle cells, fibroblasts,
macrophages and MCs; its activation is associated with many
inflammatory, respiratory, gastrointestinal, metabolic,
cardiovascular, and neurological diseases, as well as cancers
(13). Previous studies identified
that PAR-2 expression was significantly higher in tumor tissue
compared with in normal tissue, including in ovarian, colon and
breast cancer, and that this may be associated with tumor
angiogenesis (11,14,15). Liu
and Mueller (16) reported that
MDA-MB-231 breast cancer cells exhibit a high expression of PAR-2,
which, on activation, promotes the expression of VEGF via the
extracellular signal-regulated kinase (ERK) 1/2 and p38 mitogen
activated protein kinase (MAPK) signaling pathways. Using human
umbilical cord blood-derived late-EPC, Smadja et al
(17) identified that PAR-1
expression levels were similar in EPCs and human umbilical vein
endothelial cells (HUVECs), and that treatment with PAR-1 tethered
ligand peptides (SFLLRN), a PAR-1 and −2 activator, induced EPC
proliferation, migration and differentiation. It was concluded from
this data that the PAR-1 signaling pathway is involved in
EPC-mediated angiogenesis, although the role of PAR-2 could not be
excluded (17).
To the best of our knowledge, the expression and
role of PAR-2 in EPC activation has not been previously reported.
Therefore, the present study aimed to detect the effect of tryptase
treatment on the activation of EPCs via PAR-2, which was previously
demonstrated to promote angiogenesis in breast cancer (18).
Materials and methods
Tumor cells and reagents
MB-MDA-231 breast cancer cells, murine mammary
carcinoma cell 4T1 and endothelioma cell bEnd.3 were obtained from
the American Type Culture Collection (Manassas, VA, USA).
Endothelial cell growth medium (EGM) and SingleQuots combinatorial
additive were purchased from Clonetics Corporation (San Diego, CA,
USA). 1,1-dioctadecyl-3,3,3,3-tetramethylindocarbocyanine-labeled
acetylated low density lipoprotein (Dil-Ac-LDL) was purchased from
Thermo Fisher Scientific, Inc. (Molecular Probes; Waltham, MA,
USA). The ReverTra Ace qPCR RT kit and SYBR Green Realtime PCR
master mix were from Toyobo Life Science (Osaka, Japan). TRIzol was
obtained from Thermo Fisher Scientific, Inc. (Invitrogen). High
glucose Dulbecco's modified Eagle's medium (DMEM) and fetal bovine
serum (FBS) were from Thermo Fisher Scientific, Inc. (Gibco).
Fibronectin (Fn) and the PAR-2 agonist, 2-Furoyl LIGRLO-amide
trifluoroacetate salt (2fLI), were from Merck KGaA (Sigma-Aldrich;
Darmstadt, Germany). A selective inhibitor of MC tryptase, APC366
(Ki=7.1 µm; cat. no. 178925-65-0) and a PAR-2-activating
agonist peptide (SLIGRL-NH2; cat. no. 171436-38-7) were obtained
from Tocris Bioscience (Bristol, UK). Unless otherwise indicated,
purified tryptase with heparin (1:1, wt/wt) was diluted with
Minimum Essential medium (MEM) (Gibco; Thermo Fisher Scientific,
Inc.) for use in the study. The western blot
electrophoresis/transmembrane system was obtained from Bio-Rad
Laboratories, Inc. (Hercules, CA, USA).
Culture and identification of
EPCs
The protocol of the present study was approved by
the ethical committee of the School of Basic Medical Sciences,
Fudan University (Shanghai, China) and written informed consent was
obtained from all patients. Blood was collected from 3 patients
(age range, 33–35 years) from the Obstetrics & Gynecology
Hospital of Fudan University from December 2010 to May 2017
respectively. As previously described (19), 20 ml of fresh anticoagulant umbilical
venous blood was collected. Mononuclear cells (MNCs) were isolated
by density gradient centrifugation over Biocoll separating solution
(Biochrom; Merck KGaA) at 500 × g for 20 min at room temperature,
and washed three times in PBS. MNCs were plated and
5×105 cells were seeded onto culture dishes coated with
human Fn and cultured in EGM containing SingleQuots combinatorial
additive at 37°C with 5% CO2 in a humidified atmosphere.
After 3 days, non-adherent EPCs were removed and fresh culture
medium was added. The medium was replaced every third day, and the
cells were passaged on day 14.
Cells from the third and fifth generations were
observed and subsequently examined. In brief, cells were detached,
blocked with 2% fetal calf serum (Invitrogen; Thermo Fisher
Scientific, Inc.) at 4°C for 10 min, washed and then incubated
separately with phycoerythrin (PE)-conjugated VEGF receptor-2
(VEGFR-2; also known as KDR/Flk-1, cat. no. 130-100-308),
FITC-conjugated cluster of differentiation (CD)34 (cat. no.
130-098-142) or PE-CD133 (cat. no. 130-098-872) antibodies
(dilution 1:11; Miltenyi Biotec GmbH, Bergisch Gladbach, Germany)
at 4°C for 30 min. Following the incubation, cells were washed with
PBS containing 0.1% bovine serum albumin (Sigma-Aldrich; Merck
KGaA) and analyzed by fluorescence-activated cell sorting with a
FacsCalibur™ flow cytometer (BD Biosciences, Franklin
Lakes, NJ, USA) using CellQuest software (version 5.1, BD
Biosciences).
CD31 (cat. no. 550389; 1:50; BD Biosciences) was
detected by immunocytochemical analysis. Isotype-identical
antibodies (cat. no. 550878; 1:50; BD Biosciences) served as
controls to exclude non-specific binding. The cytoplasm of the
positively stained cells was brown, whereas negative cells remained
colorless. To further verify that the cells were EPCs, the uptake
of Dil-Ac-LDL, a function associated with endothelial cells, was
assessed. Cells were incubated with 4 µg/ml Dil-Ac-LDL at 37°C for
2 h, washed with PBS and fixed with 2% formaldehyde for 10 min at
37°C. The incorporation of DiI-Ac-LDL was evaluated under an
inverted fluorescence microscope (magnification, ×200).
Proliferation, migration and lumen
formation assay of EPCs
The proliferation of EPC was evaluated using a Cell
Counting Kit (CCK)-8 assay (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan), performed according to the manufacturer's
protocol. A total of 2×103 cells/well were incubated
with 100 µl EGM culture medium in 96-multiwell plates. Cells were
cultured for 0, 24, 48 or 72 h prior to the addition of 10 µl CCK-8
(5 mg/ml) to the culture medium of each well. After a 1-h
incubation at 37°C, the absorbance at 450 nm of each well was
measured with a Thermomax microplate reader (Molecular Devices,
LLC, Sunnyvale, CA, USA). Each experiment was repeated three times,
and the mean of the measurements was used.
An in vitro wound-healing assay was performed
to measure cell migration, as previously described (20). Briefly, 5×104 EPCs were
seeded into each well and incubated to form a confluent monolayer.
Following scraping of the cell monolayer in a straight line with a
p200 pipette tip to create a scratch, the debris was removed and
the edge of the scratch was smoothed by washing the cells once with
1 ml growth medium (EGM). Then the growth medium was replaced with
fresh EGM with no treatment, medium containing 1 nmol/l
tryptase/heparin, 1 nmol/l tryptase/heparin with 25 µg/ml APC366,
2.5 µg/ml 2fLI, 1 nmol/l tryptase/heparin with 0.2 µg/ml SAM 11, or
0.4 nmol/l heparin alone.
The tube formation ability of EPCs on the basement
membrane was evaluated by plating cells on Matrigel, as previously
described (21). Cells were divided
into the same six treatment groups as described for the
wound-healing assay.
Collection of conditioned media from
MB-MDA-231 cells
The MB-MDA-231 cells (3×105 cells/well)
were plated in 6-well plates, and the cells were divided into three
groups with serum-free DMEM with or without tryptase: 1 nmol/l
Tryptase with 0.4 nmol/l heparin; 1 nmol/l Tryptase with 0.4 nmol/l
heparin and 0.2 µg/ml SAM11; or 0.4 nmol/l heparin only. After 48
h, the culture medium from each group was centrifuged at 500 × g
for 15 min at 4°C, and the supernatants were filtered to create
MB-MDA-231 cell-conditioned media. The conditioned media were then
suitable for storage at 4°C for up to 3 months. EPCs were cultured
in EGM plus the MB-MDA-231 cell-conditioned media (1:1, v/v) for 48
h before analysis.
Western blotting analysis
Cultured cells were washed with PBS twice and lysed
in RIPA buffer (Beyotime Institute of Biotechnology, Haimen, China)
containing protease inhibitor and phosphatase inhibitor on ice.
Supernatants were collected and the bicinchoninic acid method was
used to determine the protein concentration. A total of 40 µg
protein/lane was subjected to 4–12% SDS-PAGE, and then transferred
onto a polyvinylidene difluoride membrane. Membranes were blocked 2
h at room temperature in 5% non-fat milk solution and then were
incubated with Rabbit anti-PAR-2 (1:1,000; cat. no. 6976, Cell
Signaling Technology, Inc., Danvers, MA, USA), VEGFR-2 (1:1,000;
cat. no. 9698; Cell Signaling Technology, Inc.), phosphorylated
(p)-protein kinase B (AKT; 1:2,000; cat. no. 4060; Cell Signaling
Technology, Inc.) and p-ERK (1:1,000; cat. no. 4370; Cell Signaling
Technology, Inc.) as the primary antibody, overnight at 4°C, with
agitation. Goat anti-rabbit horseradish peroxidase IgG H&L
(1:400, cat. no. ab97051; Abcam, Cambridge, UK) was used as the
secondary antibody and was incubated at room temperature for 1 h.
GAPDH (cat. no. ab9485; 1:2,000) was used as a loading control. The
blots were developed using an enhanced chemiluminescent
autoradiography (Western BrightECL kit; cat. no. k-12045-D50;
Advansta, Inc., Menlo Park, CA, USA). Blotting images were analyzed
using ImageJ software v.1.6 (National Institutes of Health,
Bethesda, MD, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) and semi-quantitative
RT-PCR
TRIzol reagent was used for total RNA extraction
according to the manufacturer's protocol, and 1,000 ng total RNA
was used as a template for cDNA synthesis using the ReverTra Ace
qPCR RT kit. qPCR was performed with a total reaction volume of 10
µl, including 10 ng of cDNA, 0.25 µM forward and reverse primers
and 5 µl SYBR-Green qPCR master mix. The qPCR reaction conditions
included initial denaturing at 94°C for 3 min, 30 sec denaturing at
94°C, 30 sec annealing at 59°C and 30 sec extension at 72°C for 40
cycles, then a final incubation at 65°C for 5 min. The amplified
genes and the primers used were as follows: PAR-2 forward,
TTCATGACCTGCCTCAGTGT and reverse, GTGACCAGCAGAATCAGCAG (Gene ID:
2,150); VEGFR-2 forward, GTGATCGGAAATGACACTGGAG and reverse,
CATGTTGGTCACTAACAGAAGCA; and GAPDH forward, ACAACTTTGGTATCGTGGAAGG
and reverse, GCCATCACGCCACAGTTTC. GAPDH was used as a loading
control. Gene mRNA expression levels were analyzed using the
2−∆∆Cq method (22).
PAR-2 expression was also observed by
semi-quantitative PCR in MB-MDA-231 breast cancer cells, murine
mammary carcinoma cell 4T1, endothelioma cell bEnd.3 and EPC. The
PCR reaction contained 1 µM each of the forward and reverse
primers, 10 µl of 2X PCR master mix (Thermo Fisher Scientific,
Inc.) and 1 µl of cDNA in a total volume of 20 µl. The PCR products
were visualized on a 2% agarose gel containing 5 µg/ml ethidium
bromide.
Statistical analysis
Mean values were calculated from the data obtained
from three or more separate experiments, and are presented as the
mean ± standard error of the mean. The significance of the
differences between groups was estimated by one-way analysis of
variance followed by a Student-Newman-Keuls test. SPSS version 19.0
(IBM Corp., Armonk, NY, USA) was used for analysis, and P<0.05
was considered to indicate a statistically significant
difference.
Results
Culture and identification of
EPCs
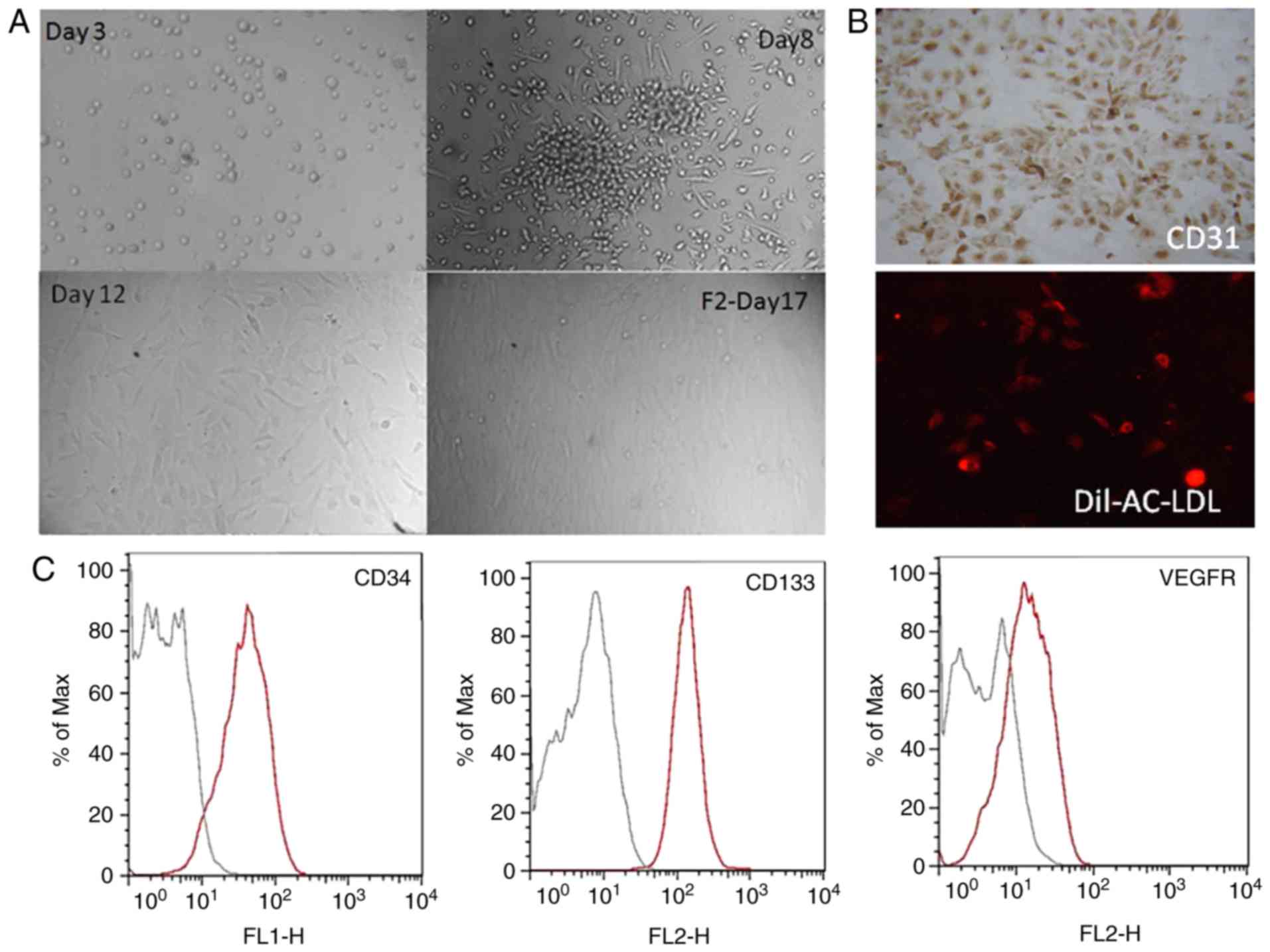
Consistent with previous literature (20), freshly separated MNCs appeared small
and rounded by 72 h. When cultured on Fn-coated culture plates,
from the third day, spindle-like cell morphology was visible at the
edge of the cell clusters. By day 12, the adherent cells presented
a typical cobblestone morphology (Fig.
1A). As presented in Fig. 1B, the
EPCs expressed CD31 and had the ability to uptake Dil-Ac-LDL. CD133
is a marker for hematopoietic stem cells and EPCs, and its
expression is gradually lost during the differentiation of EPCs
into mature cells. As CD34, CD133 and VEGFR-2 are specific markers
for EPCs, the expression of these markers was assessed by flow
cytometry to further confirm the identity of the EPC. Approximately
90% of the cells were CD34-, CD133- and VEGFR-2-positive (Fig. 1C).
Role of tryptase in promoting EPC
migration
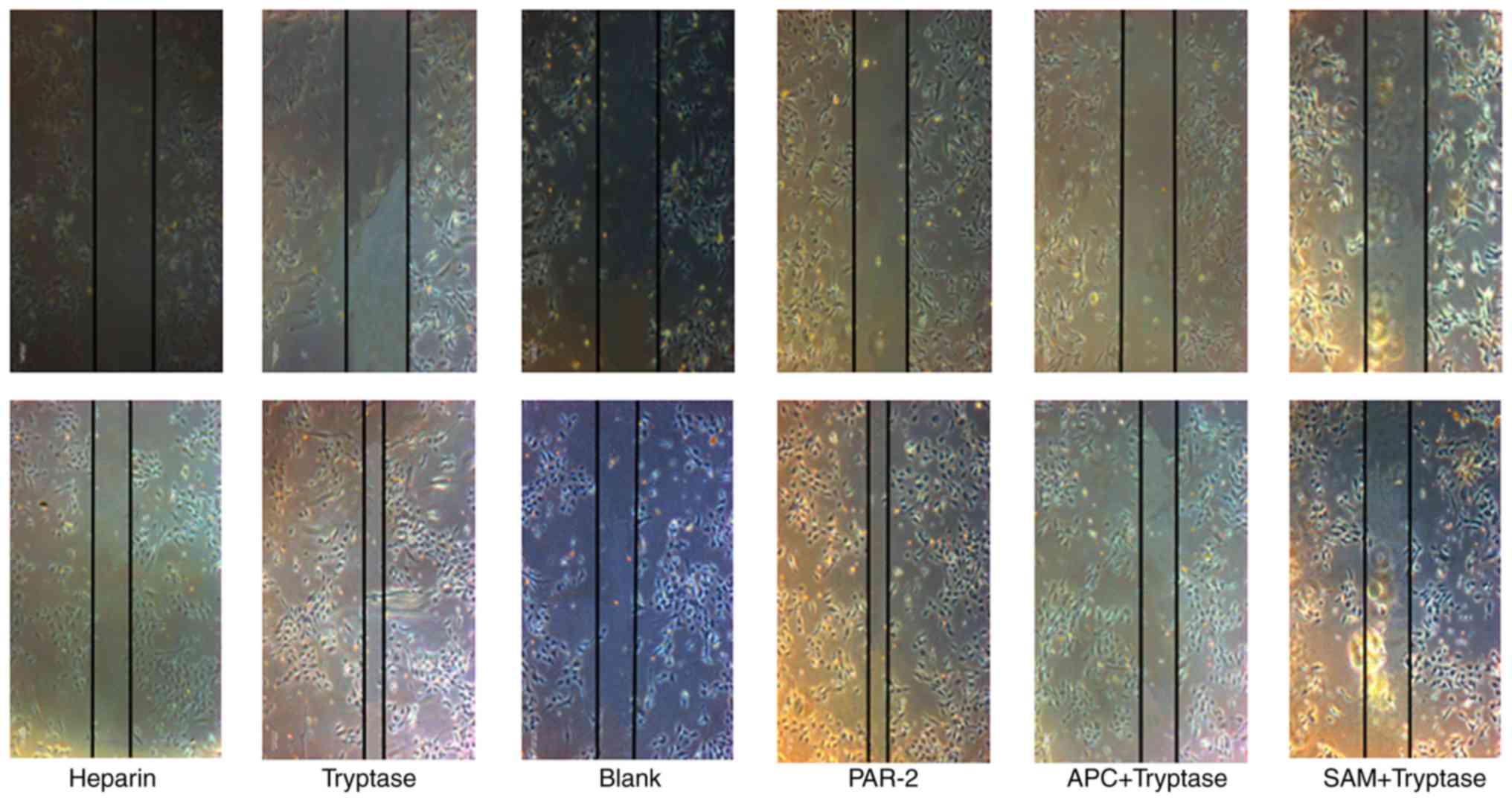
As demonstrated in Fig.
2, tryptase promoted EPC migration, and treatment with a PAR-2
agonist had a similar effect to tryptase. In addition, treatment
with the tryptase inhibitor APC366 or the PAR-2 inhibitor SAM 11
reversed the effect of tryptase on EPC migration.
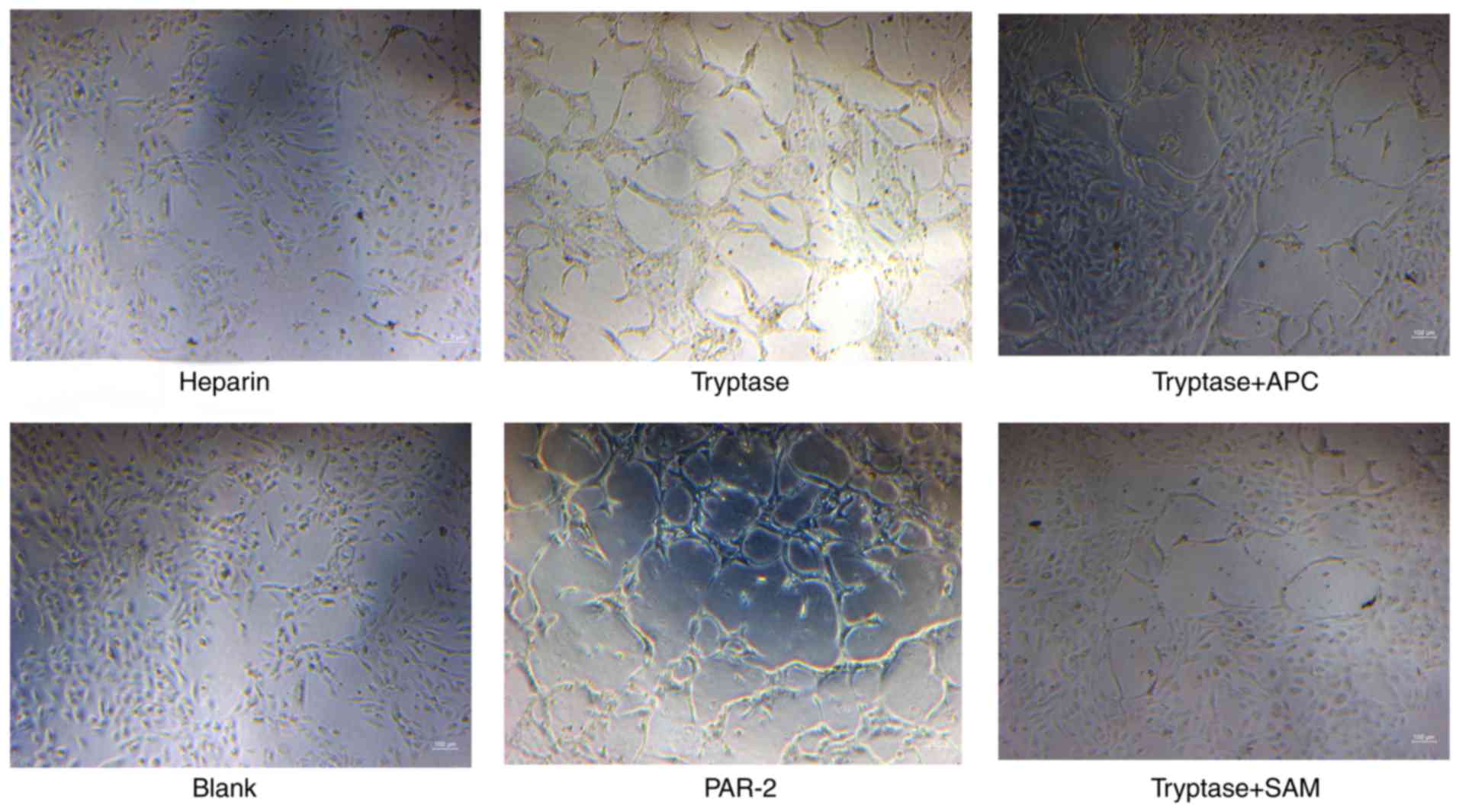
Tryptase promotes tube formation in
EPCs
The formation of tube-like structures was more
prominent in tryptase- or PAR-2 agonist-treated EPCs, whereas
treatment with APC366 or SAM 11 decreased the tube formation
ability of EPCs (Fig. 3).
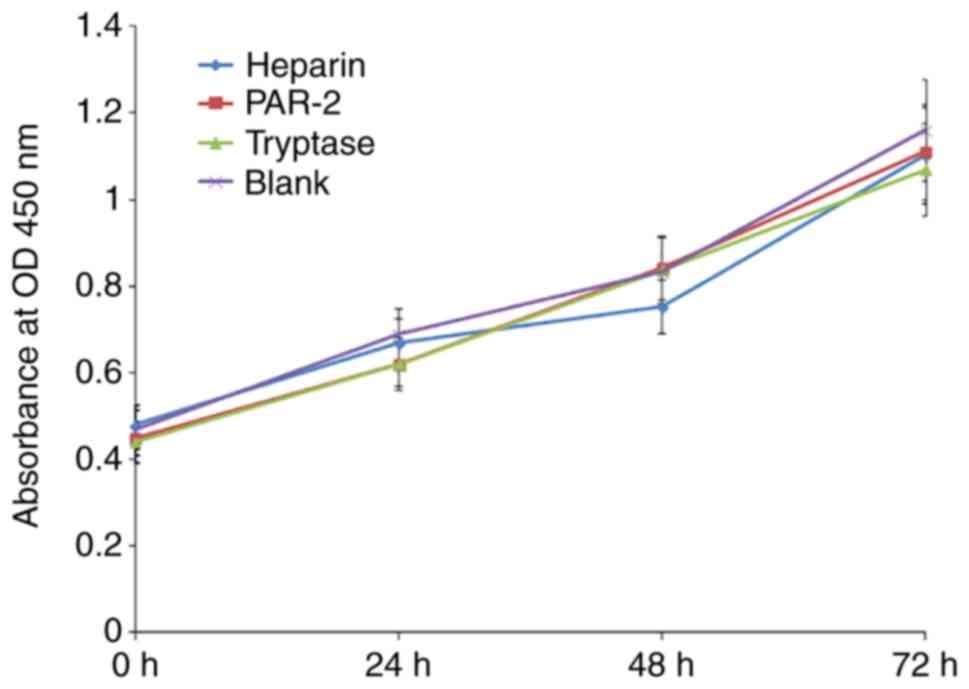
Tryptase and PAR-2 agonists exhibit no
effect on the proliferation of EPCs
A CCK-8 assay was used to analyze the proliferation
rate of EPCs. It was identified that tryptase and PAR-2 agonists
had no significant effect on the proliferation of EPCs at 0, 24, 48
or 72 h (Fig. 4).
Effect of tryptase on the expression
of PAR-2, p-AKT, p-ERK and VEGFR-2 in EPCs
PAR-2 was expressed in EPCs and MB-MDA-231 cells, as
determined by RT-PCR detection (Fig.
5A). Similarly to positive control cells, PAR-2 expression was
observed in murine mammary carcinoma cell 4T1 and endothelioma cell
bEnd.3. The effect of tryptase on the mRNA expression of VEGFR-2 in
EPCs was then analyzed using RT-qPCR. The data suggested that
treatment with tryptase or a PAR-2 agonist significantly increased
the VEGFR-2 mRNA level in EPCs compared with the blank control and
herapin groups (all P<0.05); furthermore, treatment with APC 366
or SAM 11 reversed the effect of tryptase (P<0.05; Fig. 5B). In addition, the expression of
PAR-2, p-AKT, p-ERK and VEGFR-2 in EPCs was increased following
treatment with tryptase or tryptase pretreated MB-MDA-231
cell-conditioned medium, as demonstrated with western blotting.
These results suggest that tryptase may act directly on EPC through
PAR-2/ERK signaling pathways (Fig.
5C), and on EPC indirectly through breast cancer cells
MB-MDA-231 (Fig. 5D), but further
studies are required to confirm this association. Thus, MC tryptase
may serve an important role in breast cancer angiogenesis by
affecting the tumor microenvironment.
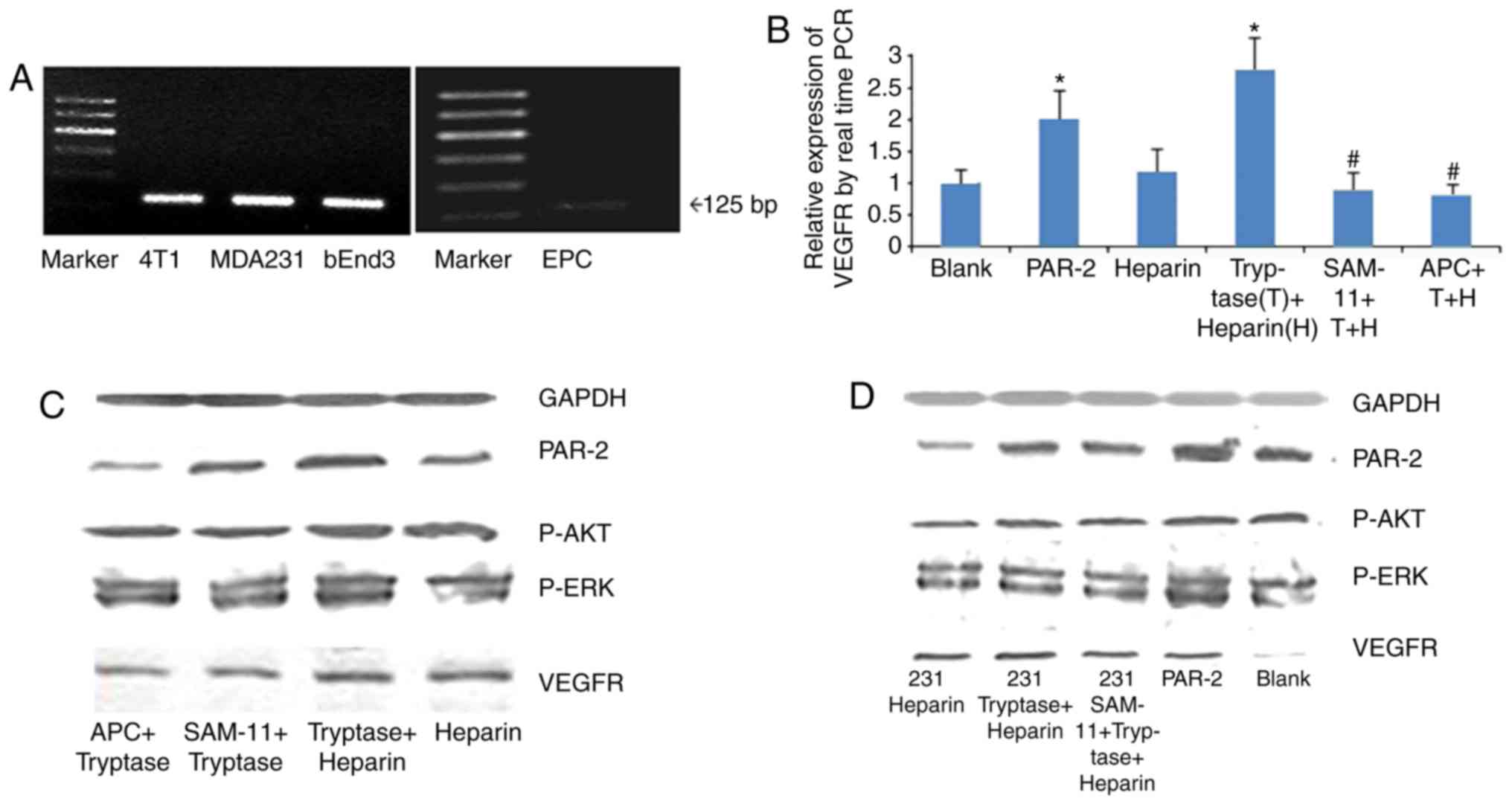 | Figure 5.Effect of T on the expression of
PAR-2, p-AKT, p-ERK and VEGFR-2 in EPCs. (A) EPCs and MB-MDA231
cells were confirmed to express PAR-2, as well as murine mammary
carcinoma cell 4T1 and endothelioma cell bEnd.3. (B) mRNA level of
VEGFR-2 in EPCs as determined by reverse transcription-quantitative
PCR. Treatment with PAR2 or T increased the VEGFR-2 mRNA level,
while the PAR-2 inhibitor SAM 11 or the tryptase inhibitor APC366
inhibited the effect of PAR2 and T, respectively. (C) T increased
the expression of PAR-2, p-AKT, p-ERK and VEGFR-2 in EPCs, as
determined by western blot analysis, whereas treatment with APC366
or SAM 11 inhibited the effect of tryptase. (D) Conditioned medium
had the same effect as T. *P<0.05 vs. con, heparin, SAM and APC
group, #P<0.05 vs. PAR2 and T groups. PAR-2,
proteinase activated receptor-2; p-, phosphorylated; ERK,
extracellular signal-regulated kinase; VEGFR-2, vascular
endothelial growth factor-2; EPCs, endothelial progenitor cells;
PAR2, PAR-2 agonist; T, tryptase; APC, APC366; con, control; AKT,
protein kinase B; PCR, polymerase chain reaction. |
Discussion
There is increasing evidence to demonstrate that
EPCs are essential in the initial stages of carcinogenesis.
Vajkoczy et al (23) reported
that embryonic endothelial progenitor cells (eEPCs) isolated from
stage E7.5 in mouse development at the onset of vasculogenesis
retained their ability to contribute to tumor angiogenesis in the
adult environment when systemically injected. eEPC homing was
mediated by E- and P-selectin, and P-selectin glycoprotein ligand
1. In a previous study in patients with tumor, the number and
activation of EPCs were increased, and the number of circulating
EPCs was demonstrated to be associated with the tumor volume
(24). EPC activation, including
migration, proliferation, homing and tube formation, controls the
‘angiogenic switch’ and contributes to the angiogenesis-mediated
progression of micrometastases into potentially deadly
macrometastases (25). The activation
and recruitment of EPCs may be induced by angiogenic factors in the
tumor microenvironment, including VEGF, angiopoietin, SDF-1, MMP-9
and platelet-derived growth factor (26). These factors are secreted by tumor
cells and/or other cells, including tumor-associated macrophages or
MCs, into the microenvironment.
Starkey et al (27) used genetically MC-deficient W/Wv mice
to investigate the role of MC in tumor angiogenesis. It was
reported that W/Wv mice exhibited a lower tumor angiogenesis
response and fewer lung metastases, while bone-marrow repair of the
mast-cell deficiency restored the angiogenic response of W/Wv mice,
and restored the incidence of hematogenous metastases to approach
that of +/+ mice. These results indicate a role for MCs during
tumor angiogenesis. In Kaposi's sarcoma, endometrial carcinoma,
B-cell non-Hodgkin's lymphomas and breast cancer, the MC count was
identified to be increased compared with normal tissue, and the
microvascular density was positively associated with the MC number
(18).
Tryptase is the most abundant enzyme in MCs, and
serves an important role in a variety of biological activities,
including inflammation and angiogenesis, in tumors and other
diseases (28). Tryptase promotes
angiogenesis, as demonstrated by Marech et al (29), induces lumen formation of endothelial
cells (30), and degrades connective
tissue matrix to provide adequate space for the formation of tumor
blood vessels (31). In the present
study, it was demonstrated that tryptase facilitated EPC migration
and tube formation, but not proliferation, suggesting that tryptase
and MCs may participate in tumor angiogenesis by activating
EPCs.
PAR-2 is a receptor for thrombin, trypsin and
tryptase that is expressed on cell membranes; it is associated with
tumor cell adhesion, invasion and metastasis (32). Notably, these studies indicated that
PAR-2 serves and essential role in cancer development, and it has
indirect effects on angiogenesis in human vascular endothelial and
tumor cell proliferation (33). Ge
et al (34) observed that
PAR-2 aggregated in the pseudopodia of metastatic breast cancer
cells, and that its activation promoted tumor cell cytoskeletal
remodeling and migration. PAR-2 silencing may inhibit the migration
and invasion of the breast cancer cell lines MDA-MB-231 and BT549
(35). The EPCs derived from the bone
marrow of mice were demonstrated to express PAR-2 (36), which is consistent with the data in
the present study. The present study also demonstrated that the
EPCs from human umbilical cord blood and MB-MDA-231 cells expressed
PAR-2. The PAR-2 agonist mimicked the effect of tryptase on the
proliferation, migration and tube formation of EPCs. In addition,
tryptase and conditioned medium from tryptase-pretreated MBA-MD-231
cells increased the expression of PAR-2 in EPCs. The results of the
current study indicate that tryptase may activate EPCs through
PAR-2.
Previous studies have suggested that the effect of
tryptase on the activation of endothelial cells may not be produced
by the enzymatic cleavage of PAR-2, and that PAR-2 was genetically
polymorphic (37–39). To determine whether PAR-2 mediates the
role of tryptase in regulating the activation of EPCs, SAM 11 was
used as an inhibitor of PAR-2 in the current study. SAM 11 is a
monoclonal antibody against amino acids 37–50 in human PAR-2, which
is the region at which PAR-2 activates peptides. Koo et al
(40) reported that SAM 11 acts as an
effective PAR-2 inhibitor. The results of the present study
revealed that SAM 11 inhibited the effect of tryptase on EPC
activation. In addition, treatment with tryptase or a PAR-2 agonist
significantly promoted the expression of VEGFR-2 in EPCs, whereas
treatment with SAM 11 or APC366, a tryptase inhibitor,
significantly attenuated the effects of tryptase treatment. These
data demonstrated that tryptase promoted EPC activation and
angiogenesis via PAR-2.
The signaling pathways associated with the
tryptase-mediated EPC activation in breast cancer angiogenesis by
PAR-2 were further investigated. The activation of PAR-2 may
mediate several types of selective signal transduction in cells,
particularly the amplifying cascade of the MAPK signaling pathway.
Through the induction of the phosphorylation of MEK (41) and ERK ½ (42), PAR-2 activation promotes cell
hyperplasia, and subsequent structural changes at the tissue and
organ levels. In human peripheral eosinophils, tryptase activates
the MAPK/AP1 pathway, and promotes the synthesis and release of
cytokines. Previous studies have identified that increased MC
density is associated with the expression of p-AKT in human colon
cancer tissue, indicating that the PI3K/AKT signaling pathway may
be activated by tryptase (43). The
PI3K/AKT signaling pathway activates a positive feedback loop to
maintain the recruitment of inflammatory cells. In certain
inflammatory and tumor environments, EPCs may be activated by the
AKT and ERK signaling pathways (44).
In the present study, treatment with tryptase, PAR-2 agonists and
conditioned medium from tryptase-pretreated MBA-MD-231 human breast
cancer cells promoted the expression of PAR-2, p-AKT, p-ERK and
VEGFR-2 in EPCs, whereas treatment with APC366 and SAM 11 inhibited
the effects of tryptase. These results confirmed that tryptase
activated EPCs and promoted breast cancer angiogenesis via
PAR-2-mediated AKT and ERK pathway activation.
In conclusion, tryptase may not only act directly on
EPC activation, but also indirectly through breast tumor cells, to
promote angiogenesis in breast cancer. This research provides a
novel theoretical and molecular basis for anti-angiogenesis drug
development.
Acknowledgements
The authors would like to thank Dr. Xianxian Sui and
Ms. Fengdi Zhao for technical assistance.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81001170) and the
Feed Fund of Shanghai University of Medicine and Health Sciences
(grant no. HMSF-16-22-011).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
NQ performed PCR and Western blotting; XL, XW and CW
performed the cell culture, wound healing and lumen formation
assay; and LY and XZ performed EPC identification and data analysis
and wrote the manuscript.
Ethics approval and consent to
participate
The protocol of this study was approved by the
Ethical Committee of The School of Basic Medical Sciences, Fudan
University (Shanghai, China). Written informed consent was obtained
from all patients.
Consent for publication
All authors have reviewed the manuscript and
approved its submission for publication.
Competing interests
The authors declare no competing interests.
References
|
1
|
Folkman J: Antiangiogenesis in cancer
therapy-endostatin and its mechanisms of action. Exp Cell Res.
312:594–607. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roberts N, Jahangiri M and Xu Q:
Progenitor cells in vascular disease. J Cell Mol Med. 9:583–591.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ribatti D, Ennas MG, Vacca A, Ferreli F,
Nico B, Orru S and Sirigu P: Tumor vascularity and
tryptase-positive mast cells correlate with a poor prognosis in
melanoma. Eur J Clin Invest. 33:420–425. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ribatti D, Finato N, Crivellato E,
Guidolin D, Longo V, Mangieri D, Nico B, Vacca A and Beltrami CA:
Angiogenesis and mast cells in human breast cancer sentinel lymph
nodes with and without micrometastases. Histopathology. 51:837–842.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mauro LV, Bellido M, Morandi A, Bonadeo F,
Vaccaro C, Quintana GO, Pallotta MG, Lastiri J, Puricelli LI and de
Cidre LL: Association between mast cells of different phenotypes
and angiogenesis in colorectal cancer. Mol Med Rep. 1:895–902.
2008.PubMed/NCBI
|
|
6
|
Micu GV, Stăniceanu F, Sticlaru LC, Popp
CG, Bastian AE, Gramada E, Pop G, Mateescu RB, Rimba M, Archip B
and Bleotu C: correlations between the density of tryptase positive
mast cells (DMCT) and that of new blood vessels (CD105+) in
patients with gastric cancer. Rom J Intern Med. 54:113–120.
2016.PubMed/NCBI
|
|
7
|
Norrby K: Mast cells and angiogenesis.
APMIS. 110:355–371. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
He S and Walls AF: Human mast cell
tryptase: A stimulus of microvascular leakage and mast cell
activation. Eur J Pharmacol. 328:89–97. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He S, Peng Q and Walls AF: Potent
induction of a neutrophil and eosinophil-rich infiltrate in vivo by
human mast cell tryptase: Selective enhancement of eosinophil
recruitment by histamine. J Immunol. 159:6216–6225. 1997.PubMed/NCBI
|
|
10
|
Cairns JA and Walls AF: Mast cell tryptase
is a mitogen for epithelial cells. Stimulation of IL-8 production
and intercellular adhesion molecule-1 expression. J Immunol.
156:275–283. 1996.PubMed/NCBI
|
|
11
|
Xiang M, Gu Y, Zhao F, Lu H, Chen S and
Yin L: Mast cell tryptase promotes breast cancer migration and
invasion. Oncol Rep. 23:615–619. 2010.PubMed/NCBI
|
|
12
|
Ossovskaya VS and Bunnett NW:
Protease-activated receptors: Contribution to physiology and
disease. Physiol Rev. 84:579–621. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yau MK, Liu L and Fairlie DP: Toward drugs
for protease-activated receptor 2 (PAR2). J Med Chem. 56:7477–7497.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jahan I, Fujimoto J, Alam SM, Sato E,
Sakaguchi H and Tamaya T: Role of protease activated receptor-2 in
tumor advancement of ovarian cancers. Ann Oncol. 18:1506–1512.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Darmoul D, Gratio V, Devaud H and Laburthe
M: Protease-activated receptor 2 in colon cancer: trypsin-induced
MAPK phosphorylation and cell proliferation are mediated by
epidermal growth factor receptor transactivation. J Biol Chem.
279:20927–20934. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Y and Mueller BM: Protease-activated
receptor-2 regulates vascular endothelial growth factor expression
in MDA-MB-231 cells via MAPK pathways. Biochem Biophys Res Commun.
344:1263–1270. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Smadja DM, Bièche I, Uzan G, Bompais H,
Muller L, Boisson-Vidal C, Vidaud M, Aiach M and Gaussem P: PAR-1
activation on human late endothelial progenitor cells enhances
angiogenesis in vitro with upregulation of the SDF-1/CXCR4 system.
Arterioscler Thromb Vasc Biol. 25:2321–2327. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ranieri G, Ammendola M, Patruno R, Celano
G, Zito FA, Montemurro S, Rella A, Di Lecce V, Gadaleta CD,
Battista De Sarro G and Ribatti D: Tryptase-positive mast cells
correlate with angiogenesis in early breast cancer patients. Int J
Oncol. 35:115–120. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eggermann J, Kliche S, Jarmy G, Hoffmann
K, Mayr-Beyrle U, Debatin KM, Waltenberger J and Beltinger C:
Endothelial progenitor cell culture and differentiation in vitro: A
methodological comparison using human umbilical cord blood.
Cardiovasc Res. 58:478–486. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liang CC, Park AY and Guan JL: In vitro
scratch assay: A convenient and inexpensive method for analysis of
cell migration in vitro. Nat Protoc. 2:329–333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Arnaoutova I, George J, Kleinman HK and
Benton G: The endothelial cell tube formation assay on basement
membrane turns 20: State of the science and the art. Angiogenesis.
12:267–274. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vajkoczy P, Blum S, Lamparter M,
Mailhammer R, Erber R, Engelhardt B, Vestweber D and Hatzopoulos
AK: Multistep nature of microvascular recruitment of ex
vivo-expanded embryonic endothelial progenitor cells during tumor
angiogenesis. J Exp Med. 197:1755–1765. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mancuso P, Burlini A, Pruneri G,
Goldhirsch A, Martinelli G and Bertolini F: Resting and activated
endothelial cells are increased in the peripheral blood of cancer
patients. Blood. 97:3658–3661. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gao D, Nolan DJ, Mellick AS, Bambino K,
McDonnell K and Mittal V: Endothelial progenitor cells control the
angiogenic switch in mouse lung metastasis. Science. 319:195–198.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li B, Sharpe EE, Maupin AB, Teleron AA,
Pyle AL, Carmeliet P and Young PP: VEGF and PlGF promote adult
vasculogenesis by enhancing EPC recruitment and vessel formation at
the site of tumor neovascularization. FASEB J. 20:1495–1497. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Starkey JR, Crowle PK and Taubenberger S:
Mast-cell-deficient W/Wv mice exhibit a decreased rate of tumor
angiogenesis. Int J Cancer. 42:48–52. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Caughey GH: Mast cell tryptases and
chymases in inflammation and host defense. Immunol Rev.
217:141–154. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Marech I, Ammendola M, Sacco R, Capriuolo
GS, Patruno R, Rubini R, Luposella M, Zuccalà V, Savino E, Gadaleta
CD, et al: Serum tryptase, mast cells positive to tryptase and
microvascular density evaluation in early breast cancer patients:
Possible translational significance. BMC Cancer. 14:5342014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Blair RJ, Meng H, Marchese MJ, Ren S,
Schwartz LB, Tonnesen MG and Gruber BL: Human mast cells stimulate
vascular tube formation. Tryptase is a novel, potent angiogenic
factor. J Clin Invest. 99:2691–2700. 1997.
|
|
31
|
Hiromatsu Y and Toda S: Mast cells and
angiogenesis. Microsc Res Tech. 60:64–69. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kawabata A: Gastrointestinal functions of
proteinase-activated receptors. Life Sci. 74:247–254. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ammendola M, Leporini C, Marech I,
Gadaleta CD, Scognamillo G, Sacco R, Sammarco G, de Sarro G, Russo
E and Ranieri G: Targeting mast cells tryptase in tumor
microenvironment: A potential antiangiogenetic strategy. Biomed Res
Int. 2014:1547022014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ge L, Shenoy SK, Lefkowitz RJ and DeFea K:
Constitutive protease-activated receptor-2-mediated migration of
MDA MB-231 breast cancer cells requires both beta-arrestin-1 and
−2. J Biol Chem. 279:55419–55424. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wilson SR, Gallagher S, Warpeha K and
Hawthorne SJ: Amplification of MMP-2 and MMP-9 production by
prostate cancer cell lines via activation of protease-activated
receptors. Prostate. 60:168–174. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ma Y, Zhang B, Qian R, Lu C, Zhao F and
Yin L: Tryptase activates PKB in inflammatory reaction in ECV304
cells. Biochim Biophys Acta. 1763:313–321. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nonaka M, Pawankar R, Fukumoto A, Ogihara
N, Sakanushi A and Yagi T: Induction of eotaxin production by
interleukin-4, interleukin-13 and lipopolysaccharide by nasal
fibroblasts. Clin Exp Allergy. 34:804–811. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kohri K, Ueki IF and Nadel JA: Neutrophil
elastase induces mucin production by ligand-dependent epidermal
growth factor receptor activation. Am J Physiol Lung Cell Mol
Physiol. 283:L531–L540. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Compton SJ, McGuire JJ, Saifeddine M and
Hollenberg MD: Restricted ability of human mast cell tryptase to
activate proteinase-activated receptor-2 in rat aorta. Can J
Physiol Pharmacol. 80:987–992. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Koo BH, Chung KH, Hwang KC and Kim DS:
Factor Xa induces mitogenesis of coronary artery smooth muscle cell
via activation of PAR-2. FEBS Lett. 523:85–89. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yoshii M, Jikuhara A, Mori S, Iwagaki H,
Takahashi HK, Nishibori M and Tanaka N: Mast cell tryptase
stimulates DLD-1 carcinoma through prostaglandin- and MAP
kinase-dependent manners. J Pharmacol Sci. 98:450–458. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Weidinger S, Mayerhofer A, Kunz L,
Albrecht M, Sbornik M, Wunn E, Hollweck R, Ring J and Kohn FM:
Tryptase inhibits motility of human spermatozoa mainly by
activation of the mitogen-activated protein kinase pathway. Hum
Reprod. 20:456–461. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Khan MW, Keshavarzian A, Gounaris E,
Melson JE, Cheon EC, Blatner NR, Chen ZE, Tsai FN, Lee G, Ryu H, et
al: PI3K/AKT signaling is essential for communication between
tissue-infiltrating mast cells, macrophages, and epithelial cells
in colitis-induced cancer. Clin Cancer Res. 19:2342–2354. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang JY, Lee YT, Chang PF and Chau LY:
Hemin promotes proliferation and differentiation of endothelial
progenitor cells via activation of AKT and ERK. J Cell Physiol.
219:617–625. 2009. View Article : Google Scholar : PubMed/NCBI
|