Introduction
Prostate cancer is one of the most common male
malignancies, with a mortality rate of about 300,000 people every
year worldwide (1). Although advanced
therapeutic techniques are widely used for patients with prostate
cancer, local recurrence frequently occurs after treatment.
Moreover, dysregulation of oncogene or tumor suppressor factors has
been demonstrated to be closely associated with the development and
progression of human cancers (2).
Therefore, it is necessary to better understand the molecular
mechanism of prostate cancer which may facilitate the development
of new therapeutic techniques for human cancers.
Recently, microRNAs were reported to repress the
expression of corresponding genes through binding with their 3′-UTR
of mRNA which led to mRNA degradation or inhibition of protein
translation (3). Through regulating
the expression of target genes, miRNA plays an important role in
various cell biological activities, including cell survival,
proliferation, differentiation, and apoptosis (4). Moreover, many miRNAs have been reported
to associate with the occurrence and development of human cancers
including prostate cancer (5–8). For example, miR-199a-3p was found to
suppress prostate cancer cell proliferation and invasion by
targeting SMAD1 (9) while miR-483-5p
promoted them by targeting RBM5 (10). Especially, as a tumor suppressor,
miR-205 has been identified in many human cancers. For instance,
miR-205 suppressed proliferation and invasion by targeting IGF1R in
human cervical cancer (11). miR-205
acted as a biological marker in non-small cell lung cancer
(12). More importantly, miR-205 was
demonstrated to be downregulated and regulating Bcl2 expression in
prostate cancer (13). However, the
special role of miR-205-5p is still to be investigated in prostate
cancer.
Additionally, zinc finger E-box binding homeobox 1
(ZEB1) belonging to zinc finger transcription factor superfamily
was found to be involved in regulating transcription (14). Many studies have reported the function
of ZEB1 in the occurrence of human cancers including osteosarcoma
and cervical cancer (15,16). Moreover, ZEB1 was upregulated and had
a promoting role in prostate cancer (17), and it was reported that miR-128
modulated cell chemosensitivity and invasion in prostate cancer by
regulating ZEB1 expression (18).
However, the regulatory mechanism of miR-205/ZEB1 axis in prostate
cancer is still unknown.
Therefore, the purpose of this study was to explore
the regulatory mechanism of miR-205-5p/ZEB1 axis in prostate
cancer, and the findings might provide original biomarkers to
diagnose prostate cancer at early stage.
Materials and methods
Tissue samples
Eighteen pairs of prostate tissues and adjacent
normal prostate tissues were collected from Binzhou City Center
Hospital (Binzhou, China) between January 2015, and May 2017. The
adjacent normal tissues were representative of tissues that were
located 2–5 cm away from tumors that were confirmed to contain no
cancer cells. All tissue specimens were immediately snap-frozen in
liquid nitrogen and then stored at −80°C for a reverse
transcription-quantitative PCR (RT-qPCR) test. Before the surgery,
none of prostate patients received any other treatments. Moreover,
written informed consent was obtained from all patients or
guardians, and this experiment was approved by the Institutional
Ethics Committee of Binzhou City Center Hospital (Binzhou,
China).
Cell culture
Human PCa cell lines LNCaP, DU-145 cell lines and
the normal prostate epithelial cell line RWPE-1 were obtained from
the Cell Bank of Institute of Biochemistry and Cell Biology
(Shanghai, China). In addition, 293T cell line was cultured to
perform the luciferase reporter assay. These cells were cultured in
RPMI-1640 (Gibco: Thermo Fisher Scientific, Inc., Carlsbad, CA,
USA) supplemented with 10% fetal bovine serum (FBS) in an
atmosphere of 5% CO2 at 37°C.
RT-qPCR
TRIzol reagent (Invitrogen: Thermo Fisher
Scientific, Inc., Carlsbad, CA, USA) was applied to extract total
RNA in tissue samples and cell lines. RT-qPCR was conducted using
the SYBR-Green PCR Master Mix on an ABI 7300HT RT-PCR system
(Applied Biosystems: Thermo Fisher Scientific, Inc., Foster City,
CA, USA) to detect expression of miR-205-5p and ZEB1 mRNA. The
reaction conditions were 95°C for 3 min, and 40 cycles of
denaturation at 95°C for 15 sec and annealing/elongation at 58°C
for 30 sec. The miR-205 forward, 5′-TCCTTCATTCCACCGGAGTCTG-3′ and
reverse, 5′-GCGAGCACAGAATTAATACGAC-3′; U6 forward,
5′-ATTGGAACGATACAGAGAAGATT-3′ and reverse,
5′-GGAACGCTTCACGAATTTG-3′; ZEB1 forward, 5′-CAGGCAGATGAAGCAGGATG-3′
and reverse, 5′-CAGCAGTGTCTTGTTGTTGTAG-3′; and GAPDH forward,
5′-TTGGTATCGTGGAAGGACTCA-3′ and reverse,
5′-TGTCATCATATTTGGCAGGTT-3′. U6 or GAPDH served as control for
miR-205-5p or ZEB1. The miR-205-5p or ZEB1 expression levels were
analyzed using the 2−ΔΔCq method (19).
Cell transfection
The miR-205-5p mimic (HMI0359-5NMOL) and miR-205-5p
inhibitor (HLTUD0359) were obtained from Sigma-Aldrich: Merck KGaA
(Shanghai, China). The on-target plus siRNA that targets human ZEB1
was purchased from GE Healthcare Dharmacon, Inc. (J-006564-10-0005;
Lafayette, CO, USA). Then they were transferred into prostate cells
respectively with Lipofectamine 2000 (Invitrogen: Thermo Fisher
Scientific, Inc.) according to manufacturers' instructions.
Western blotting
The protein samples were obtained using RIPA buffer.
Proteins were separated through 10% SDS-PAGE and then incubated
with 5% non-fat milk blocked polyvinylidene fluoride (PVDF)
membranes (EMD Millipore, Billerica, MA, USA). Next we incubated
the membranes overnight at 4°C with anti-ZEB1 (dilution 1:1,000;
rabbit monoclonal; ab203829; Abcam, Cambridge, MA, USA), anti-GAPDH
(dilution 1:1,000; mouse monoclonal; 60004-1-Ig; Wuhan Sanying
Biotechnology, Wuhan, China) and subsequently incubated with goat
anti-rabbit IgG H&L (HRP) (dilution 1:3,000; ab6721; Abcam)
secondary antibodies. Then, protein expression levels were measured
by increasing the chemiluminescence and exposure to
chemiluminescent film (Pierce: Thermo Fisher Scientific, Inc.,
Shanghai, China).
Luciferase reporter assay
TargetScan (http://www.targetscan.org/) predicts biological
targets of miRNAs by searching for the presence of conserved 8mer,
7mer, and 6mer sites that match the seed region of each miRNA
(20). The ZEB1 was predicted as a
taret gene of miR-205-5p. Then the wild-type and mutated 3′-UTR
sequences of ZEB1 mRNA containing the miR-205-5p targeting sequence
were inserted into the pGL3 basic plasmid (GenScript, Nanjing,
China) for luciferase reporter experiments. Then, the plasmid and
miR-205-5p mimic were transfected into cells using Lipofectamine
2000 (Invitrogen: Thermo Fisher Scientific, Inc.). Finally,
Dual-Luciferase Reporter Assay system (Beyotime Institute of
Biotechnology, Beijing, China) was applied to measure luciferase
activity.
Transwell assay
Cell migration was assessed using uncoated Transwell
membrane (BD Biosciences, Franklin Lakes, NJ, USA) with a pore size
of 8 µm. The lower chamber was filled with complete medium while
the upper chamber was filled with serum-free medium containing
1×105 prostate cells. These cells were cultured at 37°C
for 48 h and then fixed with methanol. By using cotton swabs,
non-migrating cells on the upper membranes were carefully removed.
At the same time, the migration cells on the lower membrane were
stained by 0.1% crystal violet and photographed under an inverted
light microscope (Zeiss, Oberkochen, Germany). The invasion test
was carried out according to the method of migration assay except
for the pre-coated membrane.
Statistical analysis
All statistical analysis was conducted using SPSS
17.0 (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 6.0 software
(GraphPad Software, Inc., La Jolla, CA, USA). Data are presented as
mean ± SD. The differences between groups were examined by analysis
of variance (ANOVA) with Tukey-Kramer post hoc test and Student's
t-tests. Pearson's correlation test was employed to evaluate the
association between miR-205-5p and ZEB1 mRNA expression. It was
considered significant at P<0.05.
Results
miR-205-5p is downregulated in
prostate tissues and cell lines
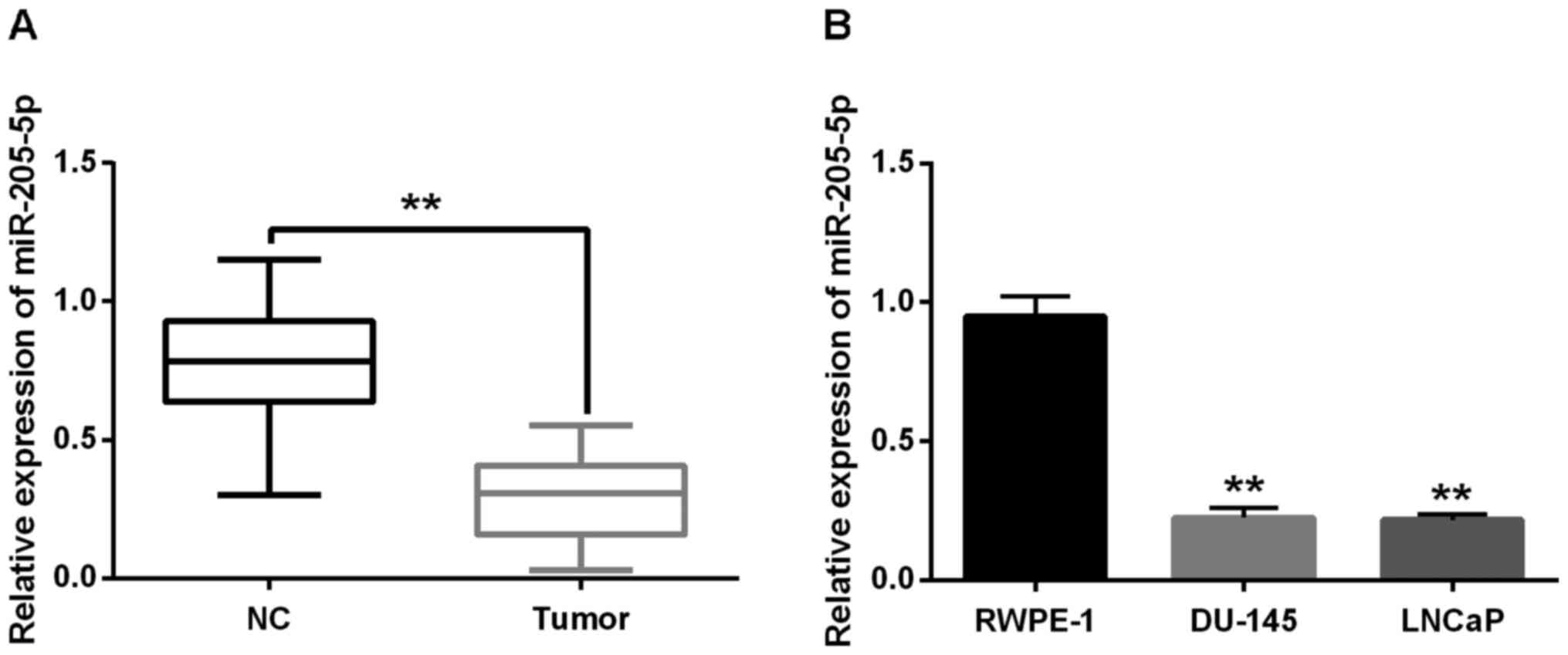
Firstly, miR-205-5p expression level was examined in
prostate tissues and cell lines using RT-qPCR assay. The results
showed that miR-205-5p expression was lower in prostate cancer
tissue than the normal prostate tissues (P<0.01, Fig. 1A). Moreover, the expression levels of
miR-205-5p in the DU-145 and LNCaP cells were significantly reduced
compared with the normal prostate cells RWPE-1 (P<0.01, Fig. 1B). All these results indicated that
miR-205-5p was downregulated in prostate cancer.
Cell migration and invasion are
inhibited by miR-205-5p overexpression
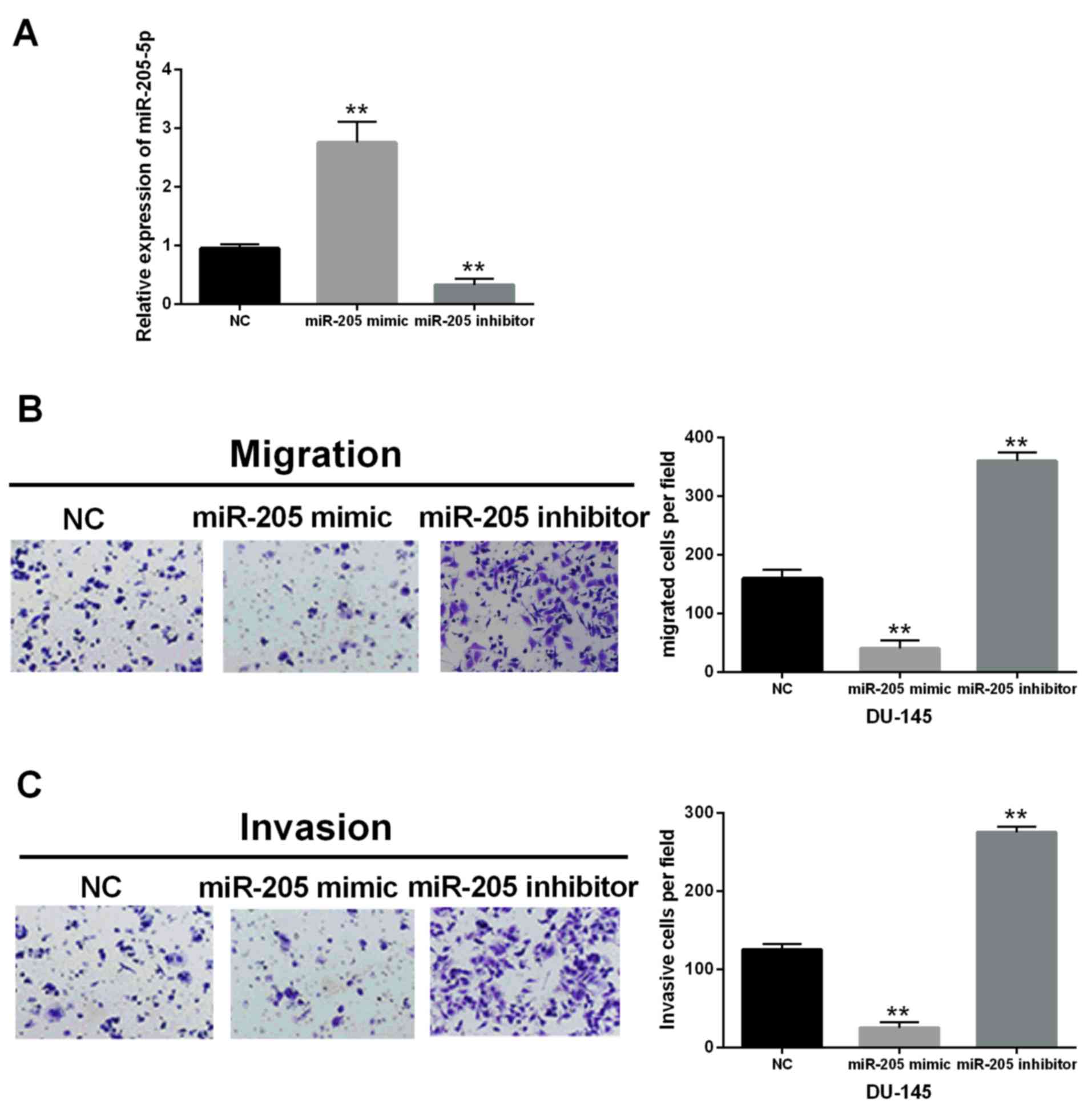
In order to explore the function of miR-205-5p in
human prostate cells, the miR-205-5p mimic or inhibitor was
transfected into DU-145 cells, respectively. The RT-qPCR analysis
suggested that the expression level of miR-205-5p in DU-145 cells
with miR-205-5p mimic was obviously increased while decreased by
miR-205-5p inhibitor (P<0.01, Fig.
2A). Furthermore, the migration and invasion of prostate cancer
cells were detected also in our study. The Transwell assay
indicated that compared with the control group, miR-205-5p mimic
reduced the migrated or invasive cell number to ~25 or 20%,
respectively. On the contrary, the migrated or invasive cell number
in transfected cells with miR-205-5p inhibitor was 2.25 or 2.2
times that of the control group. The results implied that the
migration and invasion of DU-145 cells were significantly inhibited
by miR-205-5p overexpression (P<0.01) while markedly promoted by
miR-205-5p silence compared with the control group (P<0.01)
(Fig. 2B and C). Therefore, we
deduced that miR-205-5p inhibited cell migration and invasion in
prostate cancer.
ZEB1 is a direct target gene of
miR-205-5p in prostatic carcinoma
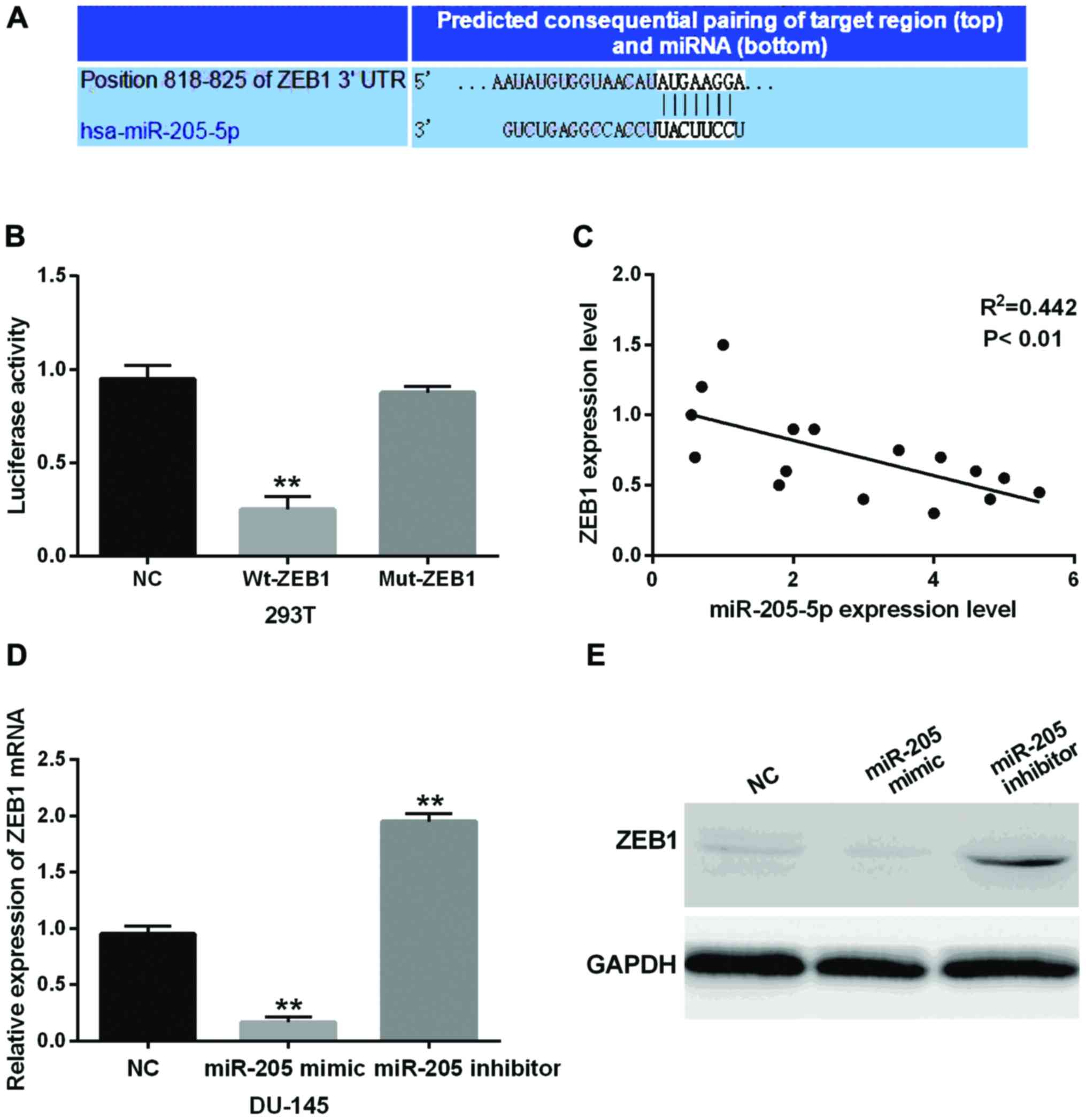
The prediction of TargetScan (http://www.targetscan.org/) is shown in Fig. 3A indicating the binding site of
miR-205-5p bound with the 3′-UTR of the ZEB1 mRNA. In order to
confirm the above prediction, the ZEB1-wt or ZEB1-mut vector with
miR-205-5p mimic was transfected into 293T cells. Then the
luciferase activity was detected to confirm the above result. As
shown in Fig. 3B, miR-205-5p mimic
reduced the luciferase activity of wild-type of ZEB1 by binding
with its 3′-UTR (P<0.01) while almost no difference was found in
cells co-transfected with ZEB1-mut and miR-205-5p mimic. In order
to further confirm the relationship between miR-205-5p and ZEB1, we
analyzed the association between miR-205-5p and ZEB1 mRNA
expression using Pearson's correlation test, and we found that ZEB1
was negatively correlated with miR-205-5p (P<0.01, Fig. 3C). Besides, the expression of mRNA and
protein of ZEB1 was clearly decreased by the miR-205-5p mimics
(P<0.01) while increased by the miR-205-5p inhibitor compared
with the control group (P<0.01, Fig.
3D and E). It indicated that miR-205-5p could inhibit the
expression of ZEB1 by targeting its 3′-UTR. In conclusion, these
results mainly revealed that ZEB1 was a direct target gene of
miR-205-5p in prostatic carcinoma and had negative association with
miR-205-5p.
ZEB1 knockdown inhibits cell migration
and invasion
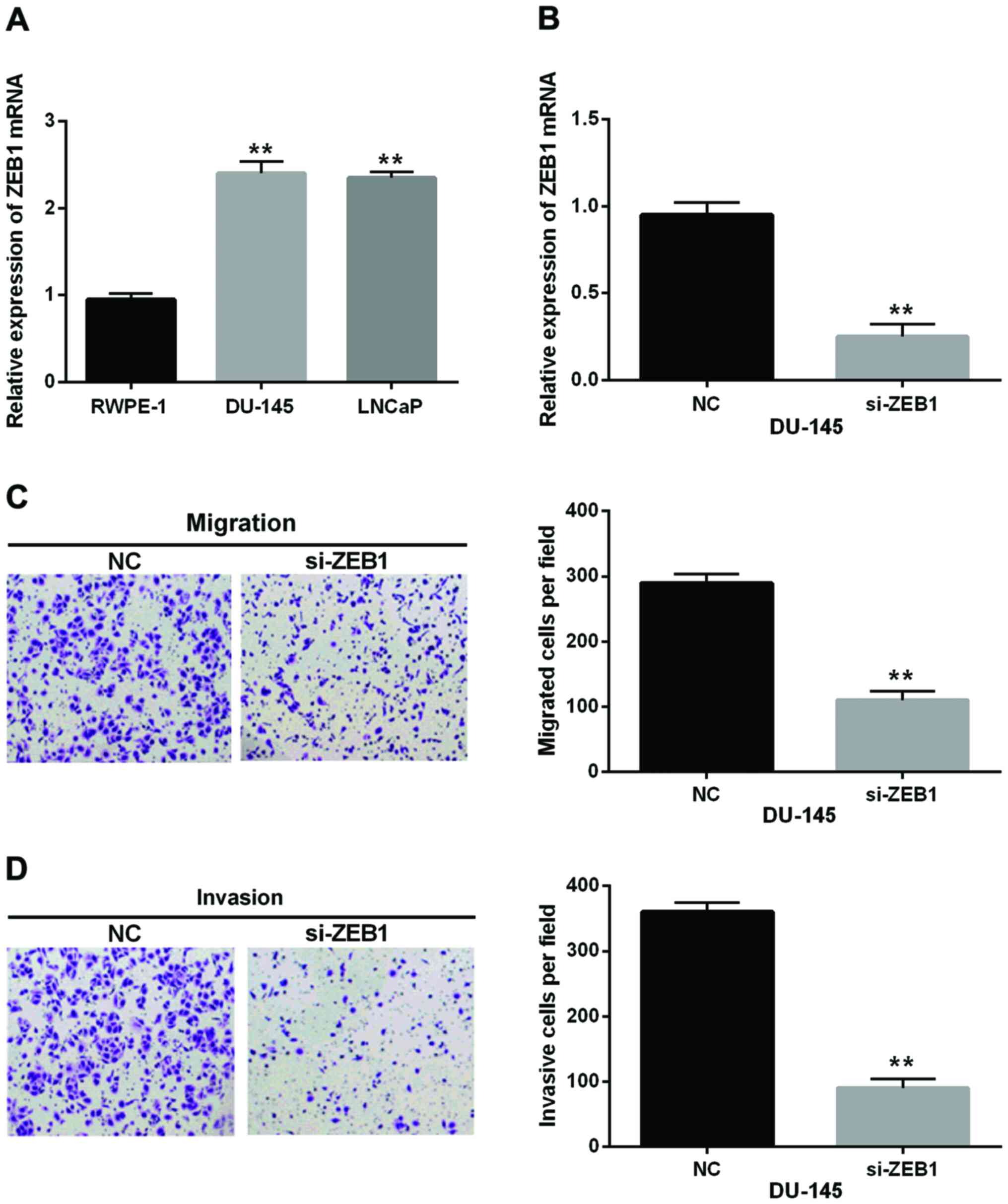
ZEB1 was found to be upregulated in DU-145 and LNCaP
cell lines (Fig. 4A). Then,
siRNA-ZEB1 was used to inhibit ZEB1 expression to investigate its
effect in prostate cancer cells (Fig.
4B). The Transwell assay indicated that compared with the
control group, siRNA-ZEB1 reduced the migrated or invasive cells
number to ~38 or 25%, respectively. It indicated that the cell
migration and invasion were remarkably decreased by ZEB1 silence in
prostate cancer (Fig. 4C and D). We
inferred that ZEB1 knockdown inhibited cell migration and invasion
in prostate cancer.
Cell migration and invasion are
affected by dysregulation of miR-205-5p via targeting ZEB1
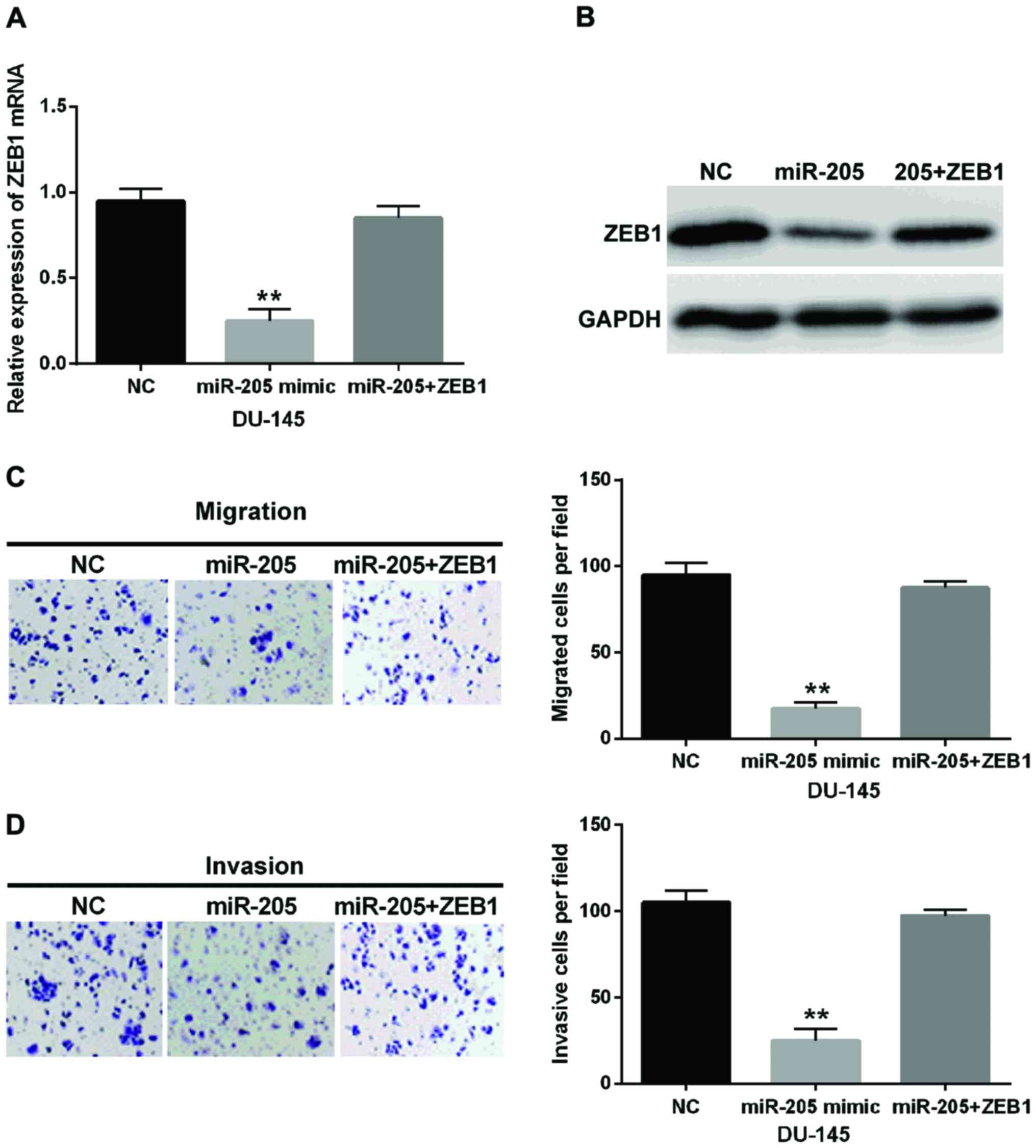
In order to further confirm the relationship between
the miR-205-5p and ZEB1 in prostate cancer, ZEB1 plasmid and
miR-205-5p mimic were transfected into DU-145 cells. Then the
expression level of ZEB1 in these transfected cells was examined by
RT-qPCR and western blotting assay. Simultaneously, the cell
migration and invasion were detected. We found that ZEB1 expression
was declined in cells containing miR-205-5p mimic, whereas little
change for ZEB1 expression was found in cells with ZEB1 plasmid and
miR-205-5p mimic compared with the control group (Fig. 5A). Moreover, western blotting assay
confirmed the above result again (Fig.
5B). Importantly, ZEB1 remarkably weakened the suppressive
effect of miR-205-5p on cell migration and invasion in prostate
cancer (Fig. 5C and D). We considered
that ZEB1 could reverse the effect of miR-205-5p in prostate
cancer.
Discussion
In the present study, the downregulation of
miR-205-5p was identified in the human prostate cancer, and
miR-205-5p inhibited cell migration and invasion in prostatic
carcinoma by targeting ZEB1. It was demonstrated that miR-205 has a
different effect in different human cancers. Some studies
demonstrated that miR-205 had a carcinogenic effect in he
occurrence of tumors (21, 22). On the contrary, downregulation of
miR-205 was also detected in many cancers acting as a tumor
suppressor in previous studies (11,13,23). These
results were consistent with our results that miR-205 expression
was decreased in prostate cancer. Thus, miR-205 may act as an
indicator for the diagnosis of prostate cancer patients.
Moreover, miR-205 was reported to regulate cell
proliferation, migration and invasion in all kinds of human cancers
(24,25). In this research, miR-205-5p
overexpression suppressed the migration and invasion of prostate
cancer cells. On the contrary, Nie et al demonstrated that
miR-205 overexpression promoted the proliferation, migration and
invasion in nasopharyngeal carcinoma (26). These findings suggested that different
effects of miR-205 on tumor pathogenesis depend on different human
cancers.
Additionally, miR-205 was found to bind to the
3′-UTR of ZEB1 to degrade its mRNA and inhibit the translation of
proteins. Increased evidence indicated that ZEB1 was strongly
associated with the biological behavior of the tumor, such as
migration and invasion, which greatly facilitated the formation and
development of tumors (27,28). Moreover, many miRNAs have been
reported to be involved in ZEB1 regulation. For instance, ZEB1
promoted cell metastasis and invasion in ESCC regulatd by
miR-125a/miR-99b/let-7e (29).
miR-429 suppressed ZEB1 expression to regulate the progression and
metastasis of osteosarcoma (30). In
current research, miR-205-5p was also found to repress ZEB1
expression, and ZEB1 was found to promote the migration and
invasion of prostate cancer cells. Importantly, ZEB1 was also
identified to weaken the suppressive effect of miR-205-5p in
prostate cancer. Thus, we considered that miR-205-5p inhibited cell
migration and invasion in prostatic carcinoma by suppressing ZEB1
expression.
In summary, miR-205-5p downregulation and ZEB1
upregulation was identified in prostate cancer. Moreover,
miR-205-5p suppressed cell migration and invasion of prostatic
carcinoma through inhibiting ZEB1 expression. All the results
suggested that the miR-205-5p/ZEB1 axis has potential to contribute
to the treatment of prostate cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LL contributed to the design and conception of the
study, the data collection and analysis, and wrote the manuscript.
SL also contributed to the conception of the study. Both authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Binzhou City Center Hospital (Binzhou, China). Signed informed
consents were obtained from the patients or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tao ZQ, Shi AM, Wang KX and Zhang WD:
Epidemiology of prostate cancer: Current status. Eur Rev Med
Pharmacol Sci. 19:805–812. 2015.PubMed/NCBI
|
|
2
|
Debebe Z and Rathmell WK: Ror2 as a
therapeutic target in cancer. Pharmacol Ther. 150:143–148. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Croce CM and Calin GA: miRNAs, cancer, and
stem cell division. Cell. 122:6–7. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu X, Fortin K and Mourelatos Z:
MicroRNAs: Biogenesis and molecular functions. Brain Pathol.
18:113–121. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao N, Lin T, Zhao C, Zhao S, Zhou S and
Li Y: MicroRNA-588 is upregulated in human prostate cancer with
prognostic and functional implications. J Cell Biochem. Oct 5.
2017, (Epub ahead of print). View Article : Google Scholar
|
|
6
|
Zhou YJ, Yang HQ, Xia W, Cui L, Xu RF, Lu
H, Xue Z, Zhang B, Tian ZN, Cao YJ, et al: Down-regulation of
miR-605 promotes the proliferation and invasion of prostate cancer
cells by up-regulating EN2. Life Sci. 190:7–14. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Song XL, Tang Y, Lei XH, Zhao SC and Wu
ZQ: miR-618 inhibits prostate cancer migration and invasion by
targeting FOXP2. J Cancer. 8:2501–2510. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Luo B, Kang N, Chen Y, Liu L and Zhang Y:
Oncogene miR-106a promotes proliferation and metastasis of prostate
cancer cells by directly targeting PTEN in vivo and in vitro.
Minerva Med. 109:24–30. 2018.PubMed/NCBI
|
|
9
|
Qu F, Zheng J, Gan W, Lian H, He H, Li W,
Yuan T, Yang Y, Li X, Ji C, et al: MiR-199a-3p suppresses
proliferation and invasion of prostate cancer cells by targeting
Smad1. Oncotarget. 8:52465–52473. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang ZG, Ma XD, He ZH and Guo YX:
miR-483-5p promotes prostate cancer cell proliferation and invasion
by targeting RBM5. Int Braz J Urol. 43:1060–1067. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pang H and Yue X: MiR-205 serves as a
prognostic factor and suppresses proliferation and invasion by
targeting insulin-like growth factor receptor 1 in human cervical
cancer. Tumour Biol. 39:10104283177013082017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Duan B, Guo T, Sun H, Cai R, Rui Q and Xi
Z: miR-205 as a biological marker in non-small cell lung cancer.
Biomed Pharmacother. 91:823–830. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Verdoodt B, Neid M, Vogt M, Kuhn V,
Liffers ST, Palisaar RJ, Noldus J, Tannapfel A and
Mirmohammadsadegh A: MicroRNA-205, a novel regulator of the
anti-apoptotic protein Bcl2, is downregulated in prostate cancer.
Int J Oncol. 43:307–314. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pazos MC, Abramovich D, Bechis A,
Accialini P, Parborell F, Tesone M and Irusta G: Gamma secretase
inhibitor impairs epithelial-to-mesenchymal transition induced by
TGF-β in ovarian tumor cell lines. Mol Cell Endocrinol.
440:125–137. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang R, Zhang C, Liu G, Gu R and Wu H:
MicroRNA-126 inhibits proliferation, migration, invasion and EMT in
osteosarcoma by targeting ZEB1. J Cell Biochem. 118:3765–3774.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu Y, Xie H, Liu Y, Liu W, Liu M and Tang
H: miR-484 suppresses proliferation and epithelial-mesenchymal
transition by targeting ZEB1 and SMAD2 in cervical cancer cells.
Cancer Cell Int. 17:362017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cha YJ, Lee JH, Han HH, Kim BG, Kang S,
Choi YD and Cho NH: MicroRNA alteration and putative target genes
in high-grade prostatic intraepithelial neoplasia and prostate
cancer: STAT3 and ZEB1 are upregulated during prostate
carcinogenesis. Prostate. 76:937–947. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun X, Li Y, Yu J, Pei H, Luo P and Zhang
J: miR-128 modulates chemosensitivity and invasion of prostate
cancer cells through targeting ZEB1. Jpn J Clin Oncol. 45:474–482.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Volinia S, Visone R, Galasso M, Rossi E
and Croce CM: Identification of microRNA activity by Targets'
Reverse EXpression. Bioinformatics. 26:91–97. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wei J, Zhang L, Li J, Zhu S, Tai M, Mason
CW, Chapman JA, Reynolds EA, Weiner CP and Zhou HH: MicroRNA-205
promotes cell invasion by repressing TCF21 in human ovarian cancer.
J Ovarian Res. 10:332017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Niu K, Shen W, Zhang Y, Zhao Y and Lu Y:
MiR-205 promotes motility of ovarian cancer cells via targeting
ZEB1. Gene. 574:330–336. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang C, Long F, Wan J, Hu Y and He H:
MicroRNA-205 acts as a tumor suppressor in osteosarcoma via
targeting RUNX2. Oncol Rep. 35:3275–3284. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen S, Jin L, Nie S, Han L, Lu N and Zhou
Y: MiR-205 inhibits growth and invasion of neuroblastoma by
targeting cAMP responsive element binding protein 1. Oncol Res. Jun
26. 2017, (Epub ahead of print). View Article : Google Scholar
|
|
25
|
Zhuo Z and Yu H: miR-205 inhibits cell
growth by targeting AKT-mTOR signaling in progesterone-resistant
endometrial cancer Ishikawa cells. Oncotarget. 8:28042–28051. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nie G, Duan H, Li X, Yu Z, Luo L, Lu R, Ji
Z and Zhang W: MicroRNA-205 promotes the tumorigenesis of
nasopharyngeal carcinoma through targeting tumor protein
p53-inducible nuclear protein 1. Mol Med Rep. 12:5715–5722. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dou J, He X, Liu Y, Wang Y, Zhao F, Wang
X, Chen D, Shi F and Wang J: Effect of downregulation of ZEB1 on
vimentin expression, tumour migration and tumourigenicity of
melanoma B16F10 cells and CSCs. Cell Biol Int. 38:452–461. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Y, Yan S, Liu X, Zhang W, Li Y, Dong
R, Zhang Q, Yang Q, Yuan C, Shen K, et al: miR-1236-3p represses
the cell migration and invasion abilities by targeting ZEB1 in
high-grade serous ovarian carcinoma. Oncol Rep. 31:1905–1910. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ma J, Zhan Y, Xu Z, Li Y, Luo A, Ding F,
Cao X, Chen H and Liu Z: ZEB1 induced miR-99b/let-7e/miR-125a
cluster promotes invasion and metastasis in esophageal squamous
cell carcinoma. Cancer Lett. 398:37–45. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Deng Y, Luan F, Zeng L, Zhang Y and Ma K:
MiR-429 suppresses the progression and metastasis of osteosarcoma
by targeting ZEB1. EXCLI J. 16:618–627. 2017.PubMed/NCBI
|