Introduction
Garlic has traditionally been used both for culinary
reasons and for the treatment of diseases in many cultures for
thousands of years, as evidenced in written histories (1). Studies have shown that garlic can reduce
the risk of heart disease (2), and
also exerts anticarcinogenic activity against several types of
cancer through similar mechanisms (3,4). Garlic is
predominantly rich in organosulfur compounds, which are known to
contribute to its aroma and potential biological activities
(5). The organosulfur compounds
derived from garlic have been under investigation for many years,
and accumulated evidence has indicated that they have health
benefits. However, the composition of raw garlic is variable, and
garlic extracts prepared by different methods have different
properties (6). Therefore, it is
still unclear which components are key to its potential medical
use. For example, alliin is one of the main organosulfur compounds
found in whole garlic cloves, and accounts for the majority of
cysteine sulfoxides in garlic; however, although alliin is known to
have antioxidant properties and many bioactivities, there exists
very little direct evidence to support its beneficial effect in
vivo. More in-depth studies are still required in order to
reveal the components of garlic that are key contributors to its
medical effects.
N-acetyl-L-cysteine (NAC), a thiol-containing
antioxidant, is known to prevent cell damage in disorders caused by
oxidative stress (7). In our previous
study, it was demonstrated that NAC is the most abundant compound
in the water-soluble extract of garlic (8). The antioxidative property of NAC enables
it to directly interact with reactive oxygen species (ROS) and
nitrogen species. ROS play a major role in the pathogenesis of many
diseases (9,10). Under normal conditions, cells express
a low level of ROS to regulate intracellular signaling pathways and
organismal homeostasis (11,12). ROS regulation of signaling pathways
contributes beneficial results to normal cellular functions.
However, excessively high levels of ROS are detected in almost all
types of cancer, suggesting that ROS play a major role in cancer
development (13).
Antioxidants could prevent certain reactive species
in cells from damaging cellular components, including proteins,
lipids and DNA (14). As DNA damage
has been linked to cancer, it is reasonable to assume that reducing
DNA damage might prevent or slow the progression of cancer.
Decreasing the ROS level by administering an antioxidant, such as
NAC, to cancer cells has been shown to cause cancer cell damage and
reduce invasion and invadopodia formation (15,16),
indicating that antioxidants play an important role in alleviating
metastasis. However, several recent studies have demonstrated that
administration of high dosages of antioxidants such as NAC or
vitamin E in a mouse model or in cell culture promoted tumor growth
(17–19). This evidence raises the possibility
that an optimal level of antioxidant that allows maintenance of
homeostasis of oxidative stress is critical for cancer treatment or
prevention. Regulating cellular antioxidant activity is therefore
thought to represent an important approach for cancer prevention,
and is of critical clinical importance for the treatment of cancers
such as acute myelocytic leukemia (AML).
AML is the most common type of leukemia in adults
(20), and the second most common
type of leukemia affecting children (21). Chemotherapy is still the first-line
treatment for AML, though the treatment outcome is still poor. With
an increasing understanding of AML biology and genetics in recent
years, several emerging therapies have been proposed (22,23).
Progress reports suggest that development of novel agents against
AML is still necessary, especially novel agents with less
toxicity.
No study has evaluated the leukemia-preventive
effect of NAC. As NAC is the major compound of the water-soluble
extract of garlic, we investigated whether NAC has a free-radical
scavenging effect in leukemia, and whether it can prevent leukemia
in vivo. The present study was therefore designed to
determine the antioxidant activity of NAC in human leukemia cells
and to examine its role in the cells under oxidative stress. The
effect of NAC on initial occurrence of leukemiaprevention in an AML
mouse model was also examined.
Materials and methods
Cell culture
A human acute promyelocytic leukemia HL-60 cell line
was kindly provided by Dr Tzou-Chi Huang (National Pingtung
University of Science and Technology, Pingtung, Taiwan). HL-60
cells were grown in Iscove's modified Dulbecco's medium (Hyclone,
Logan, UT, USA) supplemented with 4 mM L-glutamine (Gibco;
Invitrogen, Carlsbad, CA, USA), 15% fetal bovine serum (Hyclone),
100 U/ml penicillin and 100 µg/ml streptomycin (Gibco; Invitrogen).
HL-60 cells from passages 20–40 were used in the experiments. A
WEHI-3 mouse myelomonocytic, macrophage-like leukemia cell line
purchased from the Bioresource Collection and Research Center
(Hsinchu, Taiwan) was grown in Iscove's modified Dulbecco's medium
with 4 mM L-glutamine, supplemented with 0.05 mM 2-mercaptoethanol
(Sigma-Aldrich, St. Louis, MO, USA) and 10% fetal bovine serum.
Both cell lines were cultured in a 95% humidity atmosphere under 5%
CO2 in air at 37°C.
Determination of 10% cytotoxic
concentration
Cultured HL-60 cells were treated with different
concentrations of H2O2 (ranging from 0–100
mM) or NAC (ranging from 0–50 mM) for 24 h. The cytotoxicities of
H2O2 and NAC towards HL-60 cells were
determined by trypan blue exclusion. This method is based on the
principle that live cells possess intact plasma membranes that
exclude the trypan blue dye. The CC10 indicated the
cytotoxic concentration that kills 10% of treated cells (24,25). The
10% cytotoxic concentration (CC10) after 24 h of
treatment was calculated using Microsoft Excel software.
Cellular reactive oxygen species
detection assay
In order to evaluate the radical scavenging effect
of NAC, the intracellular ROS level was analyzed. A DCF-DA assay
was performed to detect the intracellular production of hydroxyl,
peroxyl and other ROS within the cells. HL-60 cells were incubated
for 30 min with 10 µM DCF-DA and washed with PBS, followed by
treatment with H2O2 at the CC10
concentration alone or in combination with various concentrations
of NAC for 2 h. The fluorescence intensities of DCF were quantified
using a microplate reader (Turner Biosystems, Sunnyvale, CA, USA),
with an excitation wavelength of 485 nm and an emission wavelength
of 525 nm.
Determination of malondialdehyde
(MDA), TAC, SOD and GSH/GSSG levels in cultured HL-60 cells
To further understand the antioxidant effects of
NAC, the levels of MDA, TAC, SOD and GSH/GSSG in cultured HL-60
cells treated with H2O2 at the
CC10 concentration were measured. Upregulation of the
MDA level indicates oxidative degradation of cellular major
components; however, enhancement of the total antioxidation
capacity (TAC) represents a cellular protective response to
oxidative stress (26). HL-60 cells
were treated with H2O2 alone or in
combination with various concentrations of NAC for 8 h and then
subjected to MDA assay. For measurement of the TAC, SOD and
GSH/GSSG levels, well-grown HL-60 cells were incubated with various
concentrations of compounds for 12 h. Cells were washed twice with
PBS, harvested and analyzed following the instructions of the kit
manufacturers. The OD value was determined using a
spectrophotometer (Bio-Rad Model 680).
Quantification of oxidative DNA
damage
HL-60 cells were treated with 20 mM
H2O2 alone or in combination with various
concentrations of NAC for 16 h. The assay protocol followed the
instruction manual provided with the kit. Briefly, cellular DNA was
extracted and converted to single-stranded DNA at 95°C for 5 min,
and rapidly chilled on ice, then DNA nucleosides were digested by
incubating the denatured DNA with nuclease P1. The unknown sample
or 8-OHdG standard was incubated with a 8-OHdG conjugate-coated
plate followed by reaction with anti-8-OHdG antibody. After washing
and substrate reaction, the absorbance of the microwells was
determined using a spectrophotometer (Bio-Rad Model 680) at a
wavelength of 450 nm.
Animal experiment
The animal test procedure examined and approved by
the Institutional Animal Care and Use Committee (IACUC) of
our University (NPUST-103-052). Co-author Ching-Dong Chang is a
veterinary pathologists and in charge of the animal monitoring and
minimize the animal distress. A leukemia mice model was established
in our laboratory (27). Thirty male
BALB/c mice, 6 weeks of age, were obtained from BioLASCO (Taipei,
Taiwan). After accommodation for one week, the mice were randomly
divided into 5 groups: Group 1 was the negative control with no
treatment; group 2 received intraperitoneal (i.p.) injection with
NAC 200 mg/kg for 7 days; group 3 was treated with vehicle PBS
through the i.p. route for 7 days then injected with WEHI-3 cells
via the tail vein; groups 4 and 5 received NAC 50 or 200 mg/kg
through i.p. injection for 7 days, following which leukemia was
induced by injection of WEHI-3 cells into the tail vein on day 8.
The NAC administration dose was cousulted and referred to the
literatures (28,29). Following death, blood and major organs
were collected. All resting living mice were euthanized by carbon
dioxide on day 7 post WEHI-3 injection. The mortality rate and
organ weights were recorded. Blood samples were analyzed to assess
liver function markers aspartate aminotransferase (AST) and alanine
aminotransferase (ALT), as well as antioxidant parameters,
including total antioxidant capacity (TAC) and levels of SOD,
glutathione and lysozyme. Furthermore, blood samples were also
subjected to total acid phosphatase (ACP) analysis, a general
diagnostic marker of disease condition. Considering the animal
welfare, animals inoculated with tumor cells should be euthanized
if following conditions is observed and diagnosed by the co-author
Ching-Dong Chang veterinarian (1)
Animal is unable to present normal activities due to the tumor
(2) Animal abdomen appears
dark-gray/green or ascites exceed 20% of the animal weight
(3). Lethargy, anorexia, dehydration,
or other sign of obvious stress or pain (6). Animal is unable to feed or drink
normally due to the tumor.
Reagents and kits
2′,7′-Dichlorofluorescin diacetate (DCF-DA),
N-acetyl-L-cysteine (NAC) and H2O2 were
purchased from Sigma-Aldrich. A Lipid Peroxidation (MDA)
Colorimetric Assay Kit, Total Antioxidant Capacity (TAC)
Colorimetric Assay Kit, Superoxide Dismutase (SOD) Activity Assay
Kit, Glutathione (GSH/GSSG/Total) Fluorometric Assay Kit, Aspartate
aminotransferase (AST) Assay Kit, Alanine aminotransferase (ALT)
Assay Kit, Total Acid Phosphatase (ACP) and Lysozyme Activity Assay
Kit were purchased from Biovision (Mountain View, CA, USA). An
8-OHdG DNA Damage ELISA kit was obtained from Cell Biolabs, Inc.
(San Diego, CA, USA).
Statistical analysis
All cell-based experiments were performed at least 3
times, and data are presented as mean ± standard deviation (SD).
Statistical significance was determined between groups using
Student's t-test. In the animal experiment, the data were analyzed
by one-way analysis of variance (ANOVA), with the post-hoc t-test.
A value of P<0.05 was considered to be significant.
Results
Cytotoxicity response
First of all, the non-toxic maximum concentration
based on our experimental treatment duration needed to be
determined. In order to determine the cytotoxicity response, the
initial testing concentration range of NAC from 0–50 mM. As the
duration of compound treatment in all cell culture assays did not
exceed 16 h, we needed to identify the concentration that did not
cause the death of more than 10% of cells after 24 h of incubation.
Our data indicated that the CC10 values of
H2O2 and NAC were 11.16 and 12.34 mM,
respectively. The growth curve showed the higher NAC concentration,
the cell viability decreased gradually (data not shown). The
working concentration of the next experiments will not over the
CC10 dose. Thus, we can then investigate the benefit
effects under the enough safety prerequisites.
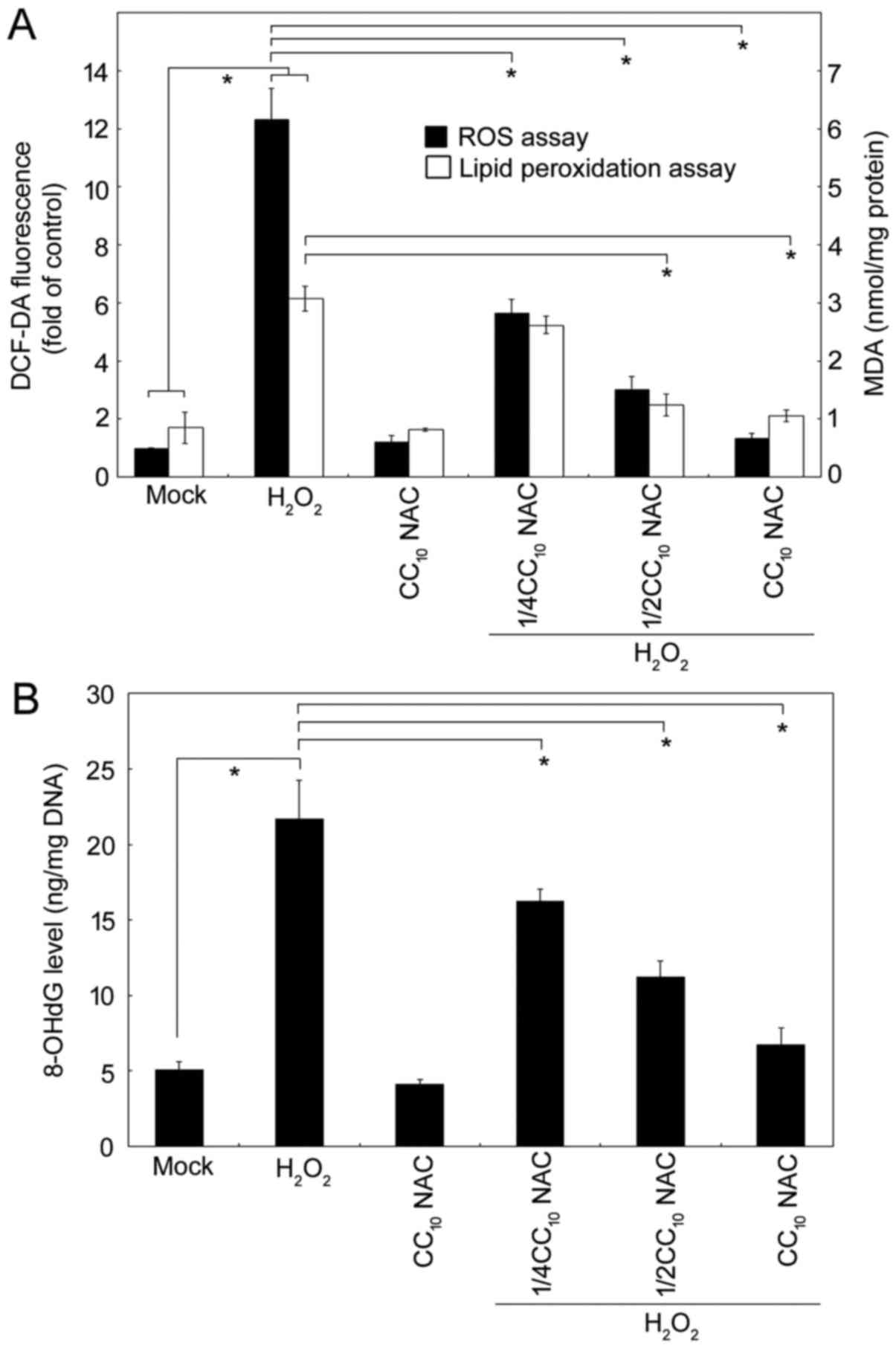
NAC alleviatesthe cellular ROS, lipid
peroxidation and oxidative DNA damage induced by
H2O2
Next, we analyzed the oxidative response induced by
H2O2 in our system, and characterized the
protective dose-response in the presence of NAC. Several
experiments were performed. A fluorescent probe, DCF-DA, has been
widely-used to measure intracellular oxidant levels. As shown in
Fig. 1A, H2O2
produced significantly higher levels of ROS. Critically, at the
CC10, NAC efficiently blocked the
H2O2-generated ROS; lower dosages of NAC also
exerted significant activity in terms of reducing the ROS level in
a dose-response manner. Additionally, MDA is one of the most
critical byproducts of lipid peroxidation during oxidative stress,
and therefore analysis of lipid peroxidation is essential in the
study of pathophysiological processes. The results for ROS and MDA
showed an identical trend with very similar response patterns.
Furthermore, in order to demonstrate that
H2O2-elicited ROS affected DNA integrity, we
measured the concentration of 8-hydroxydeoxyguanosine (8-OHdG), a
critical oxidative DNA damage byproduct. As shown in Fig. 1B, NAC exerted an antioxidant
protective activity against H2O2-mediated
oxidative stress in HL-60 cells.
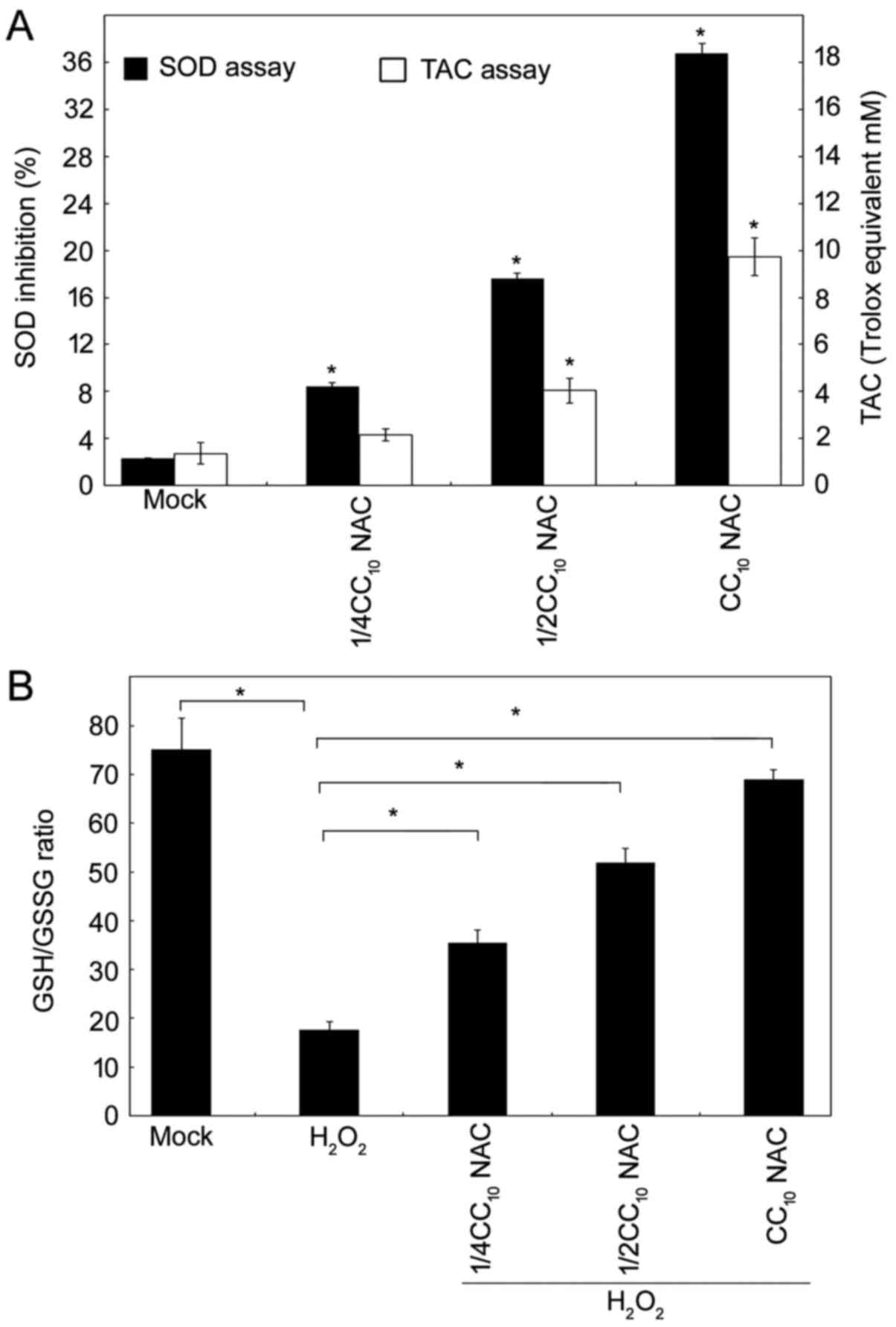
NAC possesses antioxidant
activity
To further confirm the comprehensive antioxidant
function of NAC in a HL-60 leukemia cell line, we examined whether
NAC elevated cellular endogenous antioxidant mechanisms. There are
three different types of antioxidant species, including enzyme
systems, small molecules and proteins. The total antioxidant
capacity (TAC) assay kit used in this study provided a method by
which to measure the combined nonenzymatic antioxidant capacity
from culture medium. Thus, both small-molecule antioxidants and
protein antioxidants were determined in the NAC-treated HL-60 cell
system. Another antioxidant enzyme, superoxide dismutase (SOD),
which is considered one of the body's most powerful enzymes, was
also evaluated. As shown in Fig. 2A,
NAC upregulated the endogenous cellular antioxidant activity, and
increased TAC and SOD in a dose-response manner. Furthermore,
glutathione (GSH) is critical in terms of protecting cells against
free-radical damage, and an increased ratio of GSSG to GSH
indicates oxidative stress. As clearly demonstrated in Fig. 2B, H2O2 reduced
the GSH/GSSG ratio, and NAC upregulated the GSH/GSSG ratio by
itself; furthermore, NAC reversed the
H2O2-mediated oxidative effects in HL-60
cells dramatically. Taken together, these results demonstrated that
NAC is a good antioxidant for use in this leukemia cell line, not
only upregulating the endogenous cellular antioxidant machinery,
but also scavenging free-radicals in cells facing oxidative
stress.
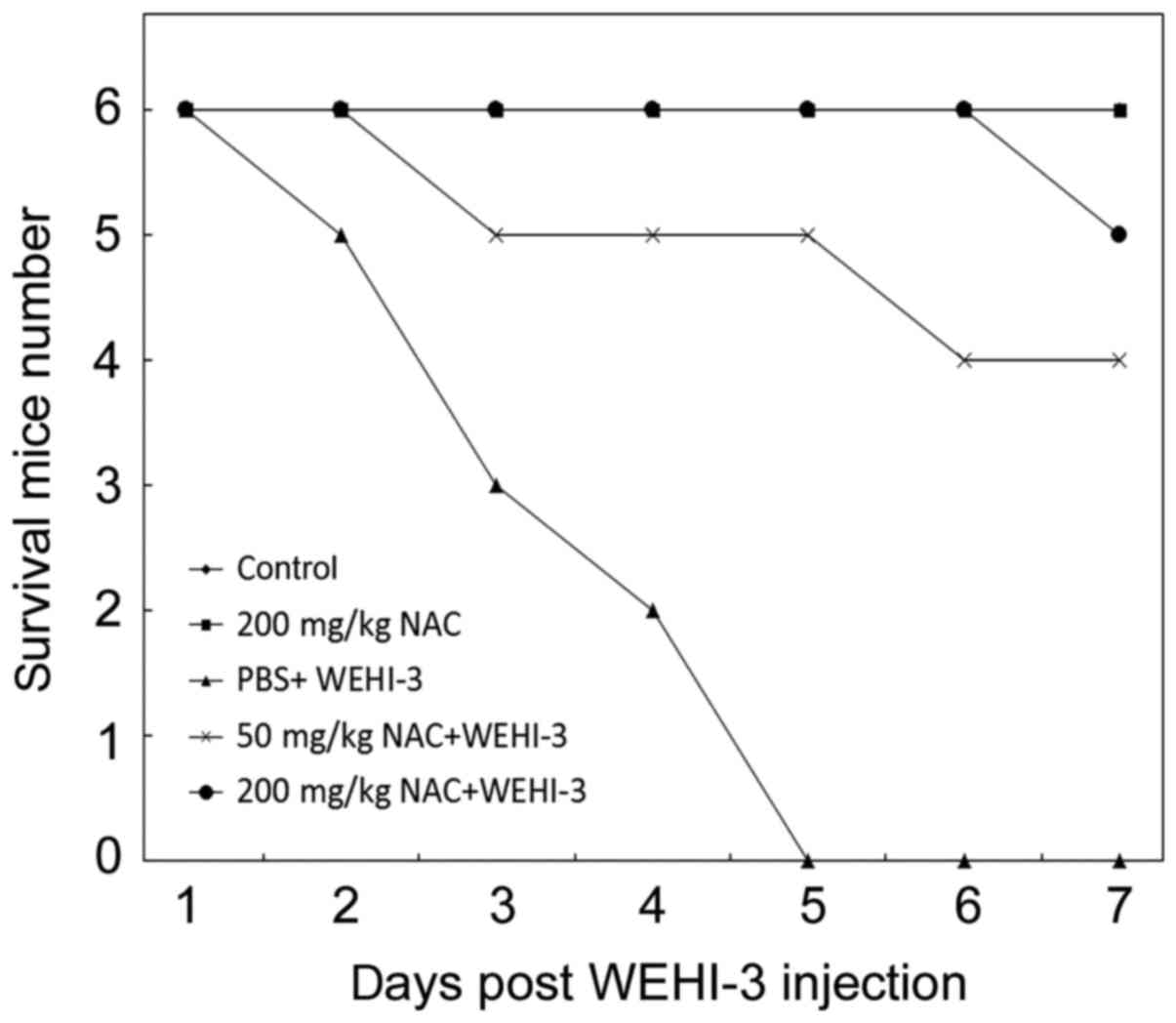
Leukemia chemoprevention activity of
NAC in BALB/c mice
To compare and confirm the antioxidant protective
activity of NAC against leukemia in vivo, a circulated
leukemia mouse model was utilized, created by WEHI-3 injection via
the tail vein and resulting in mouse death within one week
(30,31). Based on our previous publication, the
non-treated-mice died of leukemia by histopathological examination
(27). The mortality rates of the
individual mouse groups are shown in Fig.
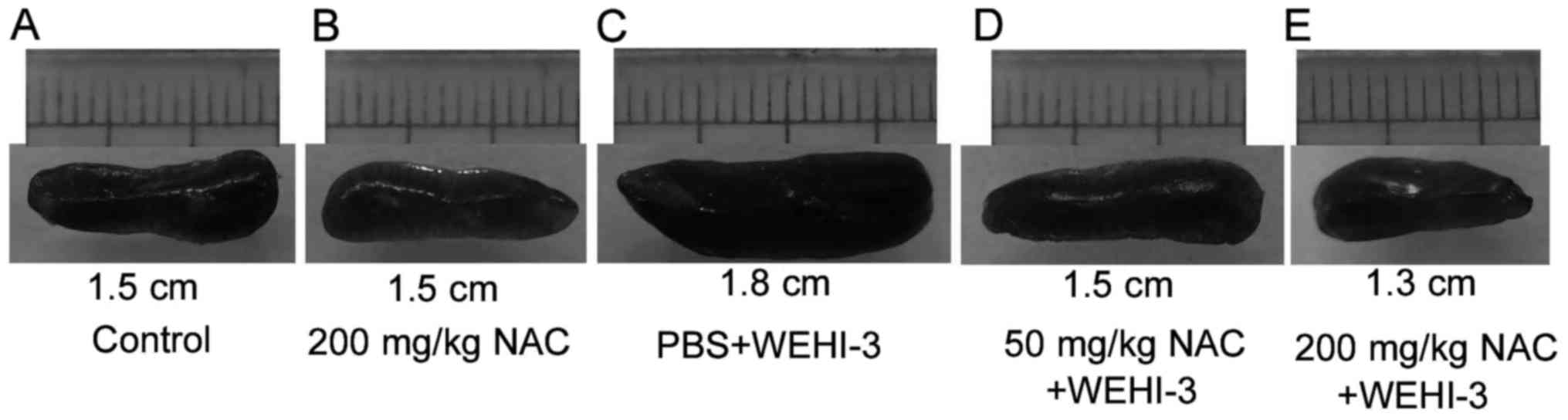
3. Additionally, the spleen size of each group was showed in
Fig. 4. The result clearly
illustrated the larger spleen in mice received WEHI-3 cells. All
mice had died by day 5 after WEHI-3 cell injection; however, in
mice that received 50 mg/kg NAC, survival was significantly
increased, and in mice receiving 200 mg/kg NAC, all mice were alive
on day 7 after WEHI-3 administration (Fig. 3). There are nine mice died in our
total thirty mice. These nine died mice occurs during the daytime
and we isolate the blood and organs immediately.
NAC does not cause organ damage,
increases serum antioxidant marker activity and protects mouse
organs
Mouse sera and organs were collected and analyzed.
First, we evaluated the safety of the dosage of NAC we used. Based
on organ weights/10 g body weight (Table
I) and serum levels of AST, ALT and ACP markers (Table II), a comparison of group 2 with corn
oil-treated healthy mice was performed, and no significant
difference was observed in any of these parameters. Thus, 200 mg/kg
NAC was sufficiently safe for use in our mouse model. In the group
3 mice, WEHI-3 cells were used to establish leukemia, and the
liver, spleen and lung weights were significantly higher than those
of the group 1 mice. The food consumption of the WEHI-3-injected
mice was significantly lower than that of the control mice. The
leukemia mice in group 3 also exhibited higher levels of AST, ALT
and ACP, as well as lower antioxidant serum parameters of TAC, SOD,
and GSH/GSSG. In the 50 mg/kg NAC administration group, some
parameter reversed as compared with group 3, indicated the in
vivo protective effects of NAC. In the 200 mg/kg NAC treatment
groups, the levels of AST, ALT and ACP were not significantly
different to those of the control mice. The levels of TAC, SOD and
GSH/GSSG indicated that NAC promoted good health. Finally,
administration of both 50 and 200 mg/kg NAC reduced mouse death
(Fig. 3).
 | Table I.Major organ weight and food and water
consumption of the BALB/c mice under various treatment
protocols. |
Table I.
Major organ weight and food and water
consumption of the BALB/c mice under various treatment
protocols.
|
| Groups |
|---|
|
|
|
|---|
| Parameters | Control | 200 mg/kg NAC | PBS + WEHI-3 | 50 mg/kg NAC +
WEHI-3 | 200 mg/kg NAC +
WEHI-3 |
|---|
| Heart (g/10g
bw) | 0.090±0.007 | 0.093±0.010 | 0.103±0.023 | 0.086±0.013 | 0.093±0.006 |
| Liver (g/10g
bw) | 0.687±0.074 | 0.647±0.136 |
1.608±0.154a |
1.005±0.079a | 0.755±0.132 |
| Spleen (g/10g
bw) | 0.051±0.006 | 0.078±0.023 |
0.178±0.032a |
0.097±0.027b | 0.064±0.013 |
| Lungs (g/10g
bw) | 0.101±0.025 | 0.129±0.030 | 0.252±0.019 | 0.168±0.035 | 0.129±0.027 |
| Kidneys (g/10g
bw) | 0.381±0.062 | 0.450±0.085 | 0.488±0.154 | 0.408050 | 0.379±0.060 |
| Food (g/days) | 3.098±0.166 | 3.085±0.149 |
2.222±0.165a |
2.750±0.130b | 2.909±0.193 |
| Water
(ml/days) | 4.508±0.353 | 4.366±0.542 | 4.445±0.464 | 4.656±0.560 | 4.550±0.383 |
 | Table II.Serum marker analysis of the BALB/c
mice under various treatment protocols. |
Table II.
Serum marker analysis of the BALB/c
mice under various treatment protocols.
| Parameters | Control | 200 mg/kg NAC | PBS + WEHI-3 | 50 mg/kg NAC +
WEHI-3 | 200 mg/kg NAC +
WEHI-3 |
|---|
| AST (mU/ml) | 63.83±22.06 | 61.69±16.59 |
585.4±113.25a |
210.23±76.61a | 77.52±26.35 |
| ALT (mU/ml) | 70.12±27.52 | 69.41±28.21 |
989.50±165.12a |
378.73±106.69a | 80.37±21.36 |
| ACP (U/ml) | 0.40±0.20 | 0.37±0.15 |
0.85±0.23b |
0.62±0.11c | 0.41±0.14 |
| TAC (Trolox
mM) | 14.48±3.18 |
19.67±2.98a |
7.34±0.63a |
16.53±3.35b |
21.47±4.04a |
| SOD (U/ml) | 40.95±8.73 | 43.09±9.6 |
19.03±5.42a | 33.95±4.68 | 47.58±7.40 |
| GSH/GSSG | 7.09±1.88 | 7.36±1.99 |
3.17±1.22b |
3.90±1.07b | 8.82±2.04 |
| Lysozyme
(µg/ml) | 1.78±0.91 | 1.66±0.62 | 1.65±0.31 | 2.37±0.55 | 2.19±0.58 |
Discussion
Several organosulfur compounds of garlic have been
shown to exert multiple pharmacological activities (32–38), and
studies have demonstrated that aqueous garlic extract has
antioxidant properties (39,40). It is well-known and well-published
that garlic extract possessed the strong antioxidant activities
in vitro and in vivo (41–43).
However, there have been no further studies of the active
components of garlic extract in terms of the antioxidant effect. In
comparison with garlic oil, garlic water-soluble extract has a
higher antioxidant activity, and among the identified constituents
of garlic water-soluble extract, NAC has been found to be the most
abundant (8). In this study, we
explored whether NAC has the potential to prevent damage caused by
leukemia cells owing to its antioxidant capacity. Further efforts
will trying to establish the standard extraction method of garlic
containing high amount of NAC and evaluate its possible
broad-spectrum cancer-preventive ability.
H2O2 is an extremely strong
reagent that causes oxidizing damage in cells, as treatment with
H2O2 leads to high levels of ROS in cells
(44). To evaluate the protective
effect of NAC against H2O2-induced HL-60 cell
damage, we used 11.16 µM of H2O2 in
subsequent assays, as this concentration only caused a ~10%
reduction in cell viability after 24 h of incubation; this
treatment also caused an increase in the ROS content of ~12-fold
and in the MDA content of 3.5-fold.
In the past few decades, many bioactive natural
products with potential therapeutic applications have been
widely-studied as sources for new drug development (45). Although numerous compounds derived
from plants have been consumed as food for centuries, cytotoxicity
is still an important issue in relation to their medical use. Our
results showed that at a concentration of 12.34 mM, NAC only caused
a decrease in cell viability of 10%, while at this concentration
NAC efficiently diminished the number of free-radicals, lowered
lipid peroxidation, and reduced the DNA oxidative damage induced by
H2O2 in an HL-60 leukemia cell line. The
results showed that even at low, non-toxic concentrations, NAC
significantly reduced ROS and MDA, signs of
H2O2-induced oxidative stress (Fig. 1).
Increased oxidative stress and ROS levels have been
found to be associated with many aspects of tumor development and
progression (46). The GSH precursor,
NAC, can increase the GSH/GSSG ratio. NAC is an antioxidant that
act as ROS-scavenger during stressful conditions (47). To confirm that NAC induced antioxidant
activity in HL-60 cells, we investigated whether NAC induced
cellular endogenous antioxidant mechanisms. We found that
administration of NAC resulted in a significant increase in SOD
activity in HL-60 cells, and reversed the reduction in the GSH/GSSG
ratio caused by H2O2 treatment. The dose
response to NAC showed the effects of NAC treatment on the TAC and
SOD levels in the cells, indicating upregulation of endogenous
cellular antioxidant activity. In addition, incubation of cells
with H2O2 reduced the GSH/GSSG ratio, while
NAC treatment reversed the reduction in the GSH/GSSG level in HL-60
cells. These results also indicated that NAC has a good antioxidant
activity in leukemia cells, and also acts as a scavenger of
free-radicals when cells face oxidative stress. In order to
evaluate whether the antioxidant capacity of NAC has a protective
effect in vivo, we investigated whether administration of
NAC inhibited WEHI-3 leukemia cells in a BALB/c mouse model. The
current study did not perform antioxidant experiments on WEHI-3
cells, there is still some procedures points are not totally
covered. Peritoneal inoculation of BALB/c mice with WEHI-3 leukemia
cells has been reported (48) and
widely-used as an in vivo mouse leukemia model in many
studies examining the anti-leukemia activities of several agents
(30,49). In our laboratory, we developed an
acute promyelocytic leukemia mouse model by intravenous injection
of BALB/c mice with WEHI-3 leukemia cells, and employed this model
to study the anti-proliferation and preventive effects of NAC on
leukemia development in mice (27).
We first tested the safety of NAC by i.p. injection with 50 or 200
mg/kg body weight for 7 days, and no organ abnormalities resulted
at either dosage. We then used these doses to study the effect of
NAC on WEHI-3-induced ALM mice. Based on the results of the present
study, we demonstrated that NAC significantly reduced
WEHI-3-induced organ damage in the liver, spleen and lungs
(Table I). In addition, mice treated
with WEHI-3 alone exhibited increased AST, ALT and ACP serum
levels, while in mice pre-treated with 200 mg/kg NCA, these
increases were prevented, and in addition, the levels of SOD and
GSH/GSSG were returned to the same ranges as those of the control
animals (Table II). Although WEHI-3
inoculated mice showed reduced average food intake ~28% as compared
to control group mice and reduced body weight ~14% (27), not yet reached the euthanasia
criteria. Additionally, WEHI-3 inoculated mice died very soon when
symptoms appear, there was no detectable and noticeable suffering
which diagnosis by a veterinarian. Taken together, the results
indicated that NAC provided a good health promotion function that
protected the mice from damage caused by WEHI-3 leukemia cell
injection. Lysozyme is present in secretions and body fluid, and is
associated with immunity; additionally, it exerts antioxidant
activity in terms of clearing free-radicals and hydroxyl molecules.
In our mouse model, the role of lysozyme was not evident. On the
other hand, as the majority of cancer clinic drugs are oxidative
agents, NAC might be a potential cancer treatment drug candidate,
however, NAC might also prompt chemoresistance.
Taken together, the results of the in vivo
mouse model revealed NAC to be a good antioxidant agent for use in
the initial occurrence prevention of leukemia. The longer
observation and complete pathological examination togerther with
the involved molecular mechanisms regarding the NAC-mediated
effects will be investigated in our next coming study.
In summary, NAC efficiently scavenged free-radicals,
lowered lipid peroxidation and reduced DNA damage induced by HL-60
leukemia cells under oxidative stress. NAC prevented death from
WEHI-3 leukemia cell-induced AML, and was associated with reduced
organ damage, and may be mediated by its activation of antioxidant
mechanisms. The results of the present study suggested that NAC is
a potential agent for development as a new drug for the prevention
of leukemia initiation.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data are available from the
corresponding author on reasonable request.
Authors' contributions
CDC, HTC and KKF were involved in data collection.
WLS, CDC, HTC and KKF performed analysis and interpretation of
data. WLS was involved in study conception and design, drafting of
the manuscript, and was the project leader. WLS, CDC, HTC and KKF
critically revised the manuscript.
Ethics approval and consent to
participate
The Institutional Animal Care and Use Committee
(IACUC) of National Pingtung University of Science and Technology
approved the animal experiments, which was conducted in accordance
with the highest standards of animal welfare and care
(NPUST-103-052). All contributed authors were fully informed of the
procedures.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rivlin RS: Historical perspective on the
use of garlic. J Nutr. 131(Suppl 3): 951S–954S. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Orekhov AN and Grünwald J: Effects of
garlic on atherosclerosis. Nutrition. 13:656–663. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Milner JA: A historical perspective on
garlic and cancer. J Nutr. 131(Suppl 3): 1027S–1031S. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
El-Bayoumy K, Sinha R, Pinto JT and Rivlin
RS: Cancer chemoprevention by garlic and garlic-containing sulfur
and selenium compounds. J Nutr. 136(Suppl 3): 864S–869S. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Amagase H: Clarifying the real bioactive
constituents of garlic. J Nutr. 136(3 Suppl): 716S–725S. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rahman K and Lowe GM: Garlic and
cardiovascular disease: A critical review. J Nutr. 136(Suppl 3):
736S–740S. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cotgreave IA: N-acetylcysteine:
Pharmacological considerations and experimental and clinical
applications. Adv Pharmacol. 38:205–227. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dewi ADR, Kusnad J and Shih WL: Comparison
of the main bioactive compounds and antioxidant activity from
garlic water-soluble and garlic oil. In:. NRLS Conference
Proceedings, International Conference on Natural Resources and Life
Sciences (2016). KnE Life Sciences. pp. 20–34. 2017
|
|
9
|
Alfadda AA and Sallam RM: Reactive oxygen
species in health and disease. J Biomed Biotechnol.
2012:9364862012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Görlach A, Dimova EY, Petry A,
Martínez-Ruiz A, Hernansanz-Agustín P, Rolo AP, Palmeira CM and
Kietzmann T: Reactive oxygen species, nutrition, hypoxia and
diseases: Problems solved? Redox Biol. 6:372–385. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
D'Autréaux B and Toledano MB: ROS as
signalling molecules: Mechanisms that generate specificity in ROS
homeostasis. Nat Rev Mol Cell Biol. 8:813–824. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shadel GS and Horvath TL: Mitochondrial
ROS signaling in organismal homeostasis. Cell. 163:560–569. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Prasad S, Gupta SC and Tyagi AK: Reactive
oxygen species (ROS) and cancer: Role of antioxidative
nutraceuticals. Cancer Lett. 387:95–105. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Young IS and Woodside JV: Antioxidants in
health and disease. J Clin Pathol. 54:176–186. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vaquero EC, Edderkaoui M, Pandol SJ,
Gukovsky I and Gukovskaya AS: Reactive oxygen species produced by
NAD(P)H oxidase inhibit apoptosis in pancreatic cancer cells. J
Biol Chem. 279:34643–34654. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Diaz B, Shani G, Pass I, Anderson D,
Quintavalle M and Courtneidge SA: Tks5-dependent, nox-mediated
generation of reactive oxygen species is necessary for invadopodia
formation. Sci Signal. 2:ra532009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sayin VI, Ibrahim MX, Larsson E, Nilsson
JA, Lindahl P and Bergo MO: Antioxidants accelerate lung cancer
progression in mice. Sci Transl Med. 6:221ra152014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Le Gal K, Ibrahim MX, Wiel C, Sayin VI,
Akula MK, Karlsson C, Dalin MG, Akyürek LM, Lindahl P3, Nilsson J
and Bergo MO: Antioxidants can increase melanoma metastasis in
mice. Sci Transl Med. 7:308re82015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Diao QX, Zhang JZ, Zhao T, Xue F, Gao F,
Ma SM and Wang Y: Vitamin E promotes breast cancer cell
proliferation by reducing ROS production and p53 expression. Eur
Rev Med Pharmacol Sci. 20:2710–2717. 2016.PubMed/NCBI
|
|
20
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bhatia S and Neglia JP: Epidemiology of
childhood acute myelogenous leukemia. J Pediatr Hematol Oncol.
17:94–100. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kavanagh S, Murphy T, Law A, Yehudai D, Ho
JM, Chan S and Schimmer AD: Emerging therapies for acute myeloid
leukemia: translating biology into the clinic. JCI Insight. 2(pii):
956792017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Saygin C and Carraway HE: Emerging
therapies for acute myeloid leukemia. J Hematol Oncol. 10:932017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen M, Hough AM and Lawrence TS: The role
of p53 in gemcitabine-mediated cytotoxicity and radiosensitization.
Cancer Chemother Pharmacol. 45:369–374. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Monge-Fuentes V, Muehlmann LA, Longo JP,
Silva JR, Fascineli ML, de Souza P, Faria F, Degterev IA, Rodriguez
A, Carneiro FP, et al: Photodynamic therapy mediated by acai oil
(Euterpe oleracea Martius) in nanoemulsion: A potential treatment
for melanoma. J Photochem Photobiol B. 166:301–310. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dringen R: Metabolism and functions of
glutathione in brain. Prog Neurobiol. 62:649–671. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rawendra RD, Lin PY, Chang CD, Hsu JL,
Huang TC and Shih WL: Potentiation of acute promyelocytic leukemia
cell differentiation and prevention of leukemia development in mice
by oleanolic acid. Anticancer Res. 35:6583–6590. 2015.PubMed/NCBI
|
|
28
|
Rocksén D, Lilliehöök B, Larsson R,
Johansson T and Bucht A: Differential anti-inflammatory and
anti-oxidative effects of dexamethasone and N-acetylcysteine in
endotoxin-induced lung inflammation. Clin Exp Immunol. 122:249–256.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hacimuftuoglu A, Handy CR, Goettl VM, Lin
CG, Dane S and Stephens RL Jr: Antioxidants attenuate multiple
phases of formalin-induced nociceptive response in mice. Behav
Brain Res. 173:211–216. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Su CC, Yang JS, Lin SY, Lu HF, Lin SS,
Chang YH, Huang WW, Li YC, Chang SJ and Chung JG: Curcumin inhibits
WEHI-3 leukemia cells in BALB/c mice in vivo. In Vivo. 22:63–68.
2008.PubMed/NCBI
|
|
31
|
Chang YH, Yang JS, Yang JL, Wu CL, Chang
SJ, Lu KW, Lin JJ, Hsia TC, Lin YT, Ho CC, et al: Ganoderma lucidum
extracts inhibited leukemia WEHI-3 cells in BALB/c mice and
promoted an immune response in vivo. Biosci Biotechnol Biochem.
73:2589–2594. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang CS, Wang ZY and Hong JY: Inhibition
of tumorigenesis by chemicals from garlic and tea. Adv Exp Med
Biol. 354:113–122. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Salman H, Bergman M, Bessler H, Punsky I
and Djaldetti M: Effect of a garlic derivative (alliin) on
peripheral blood cell immune responses. Int J Immunopharmacol.
21:589–597. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bordia A, Verma SK and Srivastava KC:
Effect of garlic on platelet aggregation in humans: A study in
healthy subjects and patients with coronary artery disease.
Prostaglandins Leukot Essent Fatty Acids. 55:201–205. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hall A, Troupin A, Londono-Renteria B and
Colpitts TM: Garlic organosulfur compounds reduce inflammation and
oxidative stress during dengue virus infection. Viruses. 9(pii):
E1592017. View Article : Google Scholar
|
|
36
|
Ho SC and Su MS: Evaluating the
anti-neuroinflammatory capacity of raw and steamed garlic as well
as five organosulfur compounds. Molecules. 19:17697–17714. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Trio PZ, You S, He X, He J, Sakao K and
Hou DX: Chemopreventive functions and molecular mechanisms of
garlic organosulfur compounds. Food Funct. 5:833–844. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
You S, Nakanishi E, Kuwata H, Chen J,
Nakasone Y, He X, He J, Liu X, Zhang S, Zhang B and Hou DX:
Inhibitory effects and molecular mechanisms of garlic organosulfur
compounds on the production of inflammatory mediators. Mol Nutr
Food Res. 57:2049–2060. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Boonpeng S, Siripongvutikorn S, Sae-Wong C
and Sutthirak P: The antioxidant and anti-cadmium toxicity
properties of garlic extracts. Food Sci Nutr. 2:792–801. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rasul Suleria HA, Sadiq Butt M, Muhammad
Anjum F, Saeed F, Batool R and Nisar Ahmad A: Aqueous garlic
extract and its phytochemical profile; special reference to
antioxidant status. Int J Food Sci Nutr. 63:431–439. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bravi E, Marconi O, Sileoni V, Rollo MR
and Perretti G: Antioxidant effects of supercritical fluid garlic
extracts in canned artichokes. J Food Sci Technol. 53:3744–3751.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Naji KM, Al-Shaibani ES, Alhadi FA,
Al-Soudi SA and D'Souza MR: Hepatoprotective and antioxidant
effects of single clove garlic against CCl4-induced hepatic damage
in rabbits. BMC Complement Altern Med. 17:4112017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Petropoulos S, Fernandes Â, Barros L,
Ciric A, Sokovic M and Ferreira ICFR: Antimicrobial and antioxidant
properties of various Greek garlic genotypes. Food Chem. 245:7–12.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Giorgio M, Trinei M, Migliaccio E and
Pelicci PG: Hydrogen peroxide: A metabolic by-product or a common
mediator of ageing signals? Nat Rev Mol Cell Biol. 8:722–728. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs from 1981 to 2014. J Nat Prod. 79:629–661.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liou GY and Storz P: Reactive oxygen
species in cancer. Free Radic Res. 44:479–496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sun SY: N-acetylcysteine, reactive oxygen
species and beyond. Cancer Biol Ther. 9:109–110. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
He Q and Na X: The effects and mechanisms
of a novel 2-aminosteroid on murine WEHI-3B leukemia cells in vitro
and in vivo. Leuk Res. 25:455–461. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lin JP, Yang JS, Lu CC, Chiang JH, Wu CL,
Lin JJ, Lin HL, Yang MD, Liu KC, Chiu TH and Chung JG: Rutin
inhibits the proliferation of murine leukemia WEHI-3 cells in vivo
and promotes immune response in vivo. Leuk Res. 33:823–828. 2009.
View Article : Google Scholar : PubMed/NCBI
|