Introduction
Prostate cancer (PC) is the second leading cause of
cancer-associated mortality among males in Western countries. The
principal difficulty with PC is its metastasis to the bone, which
results in significant morbidity (1,2). The
5-year survival rate for PC patients with skeletal involvement is
<1% (3). Therefore, there is an
urgent requirement for novel PC treatments.
Tumor metastasis is a complicated process that
involves cellular detachment from the local microenvironment and
degradation of the surrounding extracellular matrix and relocation
to a distal site. All these processes are regulated by multiple
factors and molecular pathways. Epithelial-mesenchymal transition
(EMT) is a differentiation program by which cells switch from an
epithelial to a mesenchymal phenotype; the latter is characterized
by an increased ability to migrate and increased invasiveness and
is associated with metastasis (4).
EMT events have been observed following androgen withdrawal therapy
in PC, leading to increased migration and invasiveness of tumor
cells and ultimately, a significant metastatic burden (5). Therefore, the ability to target primary
tumor cells that are more likely to undergo EMT would benefit
patients at risk of disease progression. A group of transcription
factors, including Snail, Slug, Zinc finger E-box-binding homeobox
1 (ZEB1) and Twist, have been revealed to serve a crucial role in
modulating E-cadherin expression and ultimately in promoting EMT
(6,7).
Furthermore, protein kinase B (Akt) and glycogen synthase kinase-3β
(GSK-3β) have been linked to EMT in cancer. Akt activation leads to
increased phosphorylation of the 9th residue of GSK3β, which
facilitates GSK3β ubiquitination and prevents its participation in
the degradation of Snail (8,9).
Juglone (5-hydroxy-1, 4-naphthoquinone) is a natural
compound that is isolated from plants and has been reported to
possess potent cytotoxic properties in vitro against human
leukemia, cervical carcinoma and pancreatic cancer cell lines
(10–12). In an earlier study, the authors of the
present study reported the effect of juglone on ovarian cancer
cells and concluded that juglone likely induced apoptosis via the
mitochondrial pathway and reduced cell invasiveness by decreasing
matrix metalloproteinase expression (13). In the present study, the effects of
juglone on PC cell invasion and EMT and the underlying mechanisms
of action were investigated.
Materials and methods
Cell culture
The androgen-dependent human LNCaP PC cells, were
obtained from the Institute of Biochemistry and Cell Biology,
Chinese Academy of Sciences (Shanghai, China). The cells were
maintained in phenol red-containing RPMI-1640 medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.).
The castration-resistant PC LNCaP-AI cell line was established by
culturing LNCaP cells in phenol red-free RPMI-1640 medium
supplemented with 10% dextran/charcoal-treated FBS (DCC-FBS;
Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) for 6 months
(14). All cells were cultured at
37°C in a humidified environment of 5% CO2 in air, and
the medium was replenished twice weekly.
MTT assay
Cell viability was assessed by measuring the cells'
ability to metabolize MTT. Cells (2×104/well) were
seeded in 96-well plates and cultured at 37°C with 5%
CO2 for 24 h. Juglone (97% purity; Sigma-Aldrich; Merck
KgaA, Darmstadt, Germany) was added at a final concentration of 0,
6.25, 12.5, 25, 50 or 100 µM. Each sample was assayed in
triplicate. Following incubation for 24 h, 20 µl MTT solution was
added to each well and the cells were incubated for another 4 h.
The resulting formazan crystals were dissolved with dimethyl
sulfoxide and the absorbance was read at 570 nm in an ELISA reader
(Thermo Fisher Scientific, Inc.). The percentage of cell viability
was calculated as follows: Cell viability (%) = (OD of treatment/OD
of control) ×100.
Migration and invasion
Cell migration and invasion assays were performed in
24-well plates with 8-µm pore size filters (Corning Incorporated,
Corning, NY, USA). Following pre-treatment with juglone for 24 h,
the LNCaP and LNCaP-AI cells were suspended in RPMI-1640 medium
containing 0.1% FBS and 0.1% DCC-FBS, respectively. For the
migration assays, the cells (5×104/well; 200 µl) were
seeded with the medium into the upper chamber on top of an uncoated
membrane. For the invasion assays, the cells
(1×105/well, 200 µl) were seeded into the upper chamber
on top of a Matrigel-coated membrane. The bottom chambers were
filled with 600 µl RPMI-1640 medium containing 10% FBS and 10%
DCC-FBS for the LNCaP and LNCaP-AI assays, respectively. Following
incubation for 24 h, the remaining cells in the upper chamber were
gently removed with a cotton swab. The cells that had moved to the
bottom chamber were stained with 0.1% crystal violet for 20 min at
room temperature and observed using light microscopy
(magnification, ×200; Olympus Corporation, Tokyo, Japan).
Western blotting
Cells were washed twice with phosphate-buffered
saline and then lysed with radioimmunoprecipitation assay lysis
buffer (Beyotime Institute of Biotechnology, Nanjing, Jiangsu,
China) containing a cocktail of protease inhibitors (Roche
Diagnostics GmbH, Mannheim, Germany). To extract specific protein
compartments, a Compartmental Protein Extraction kit (EMD
Millipore, Billerica, MA, USA) was used according to the
manufacturer's protocol. Protein concentration was determined using
a bicinchoninic acid protein assay kit (Beyotime Institute of
Biotechnology, Nanjin, Jiangsu, China). Equal amounts of protein
(30 µg) were separated on 10% sodium dodecyl sulfate-polyacrylamide
gels and transferred to polyvinylidene fluoride membranes (EMD
Millipore) and then blocked with 5% fat-free milk in PBS containing
0.05% Tween-20 at room temperature for 2 h. The membranes were
incubated with rabbit monoclonal antibodies against human p-Akt
(Ser 473; cat. no. 4060), p-GSK-3β (Ser 9; cat. no. 9322),
E-cadherin (cat. no. 3195), N-cadherin (cat. no. 13116), Vimentin
(cat. no. 5741) and Snail (cat. no. 3879) all at a dilution of
1:1,000 (Cell Signaling Technology, Inc., Danvers, MA, USA)
overnight at 4°C. β-actin was used as a loading control (1:2,000;
cat. no. 4970; Cell Signaling Technology, Inc.). Primary antibody
binding was detected using a horseradish peroxidase-conjugated
anti-rabbit IgG antibody (1:3,000; cat. no. A0208; Beyotime
Institute of Biotechnology) and was visualized using an enhanced
chemiluminescence detection system (Pierce; Thermo Fisher
Scientific, Inc.).
Statistical analysis
The data are presented as the mean ± standard
deviation. One-way analysis of variance, followed by Holm's method
was used to compare significant differences in the means among all
treatment groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
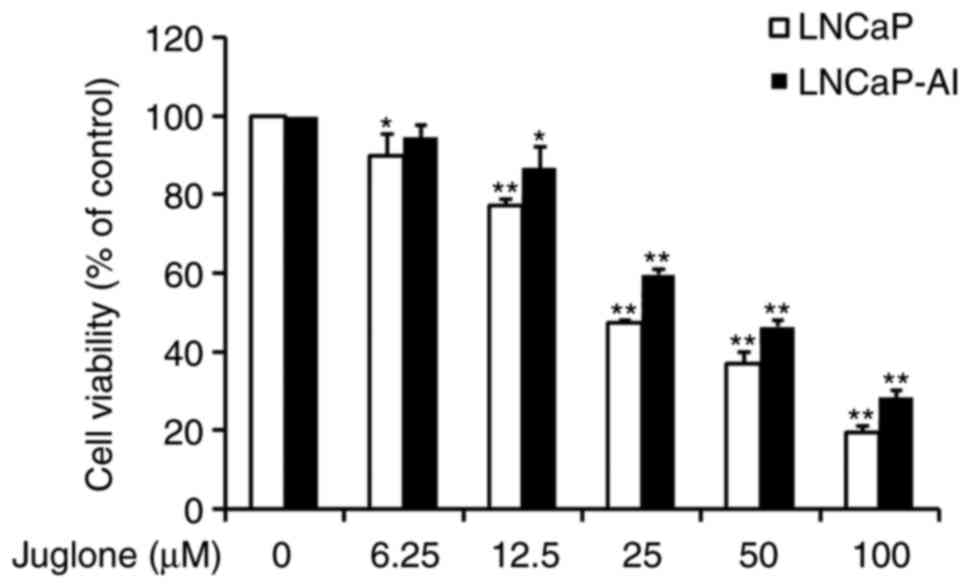
Suppressive effect of juglone on
viability of LNCaP and LNCaP-AI cells
LNCaP and LNCaP-AI cells were treated with
increasing doses of juglone for 24 h and examined for cell
viability using the MTT assay. As revealed in Fig. 1, juglone treatment markedly decreased
cell viability in a dose-dependent manner. The IC50 dose
of juglone at 24 h was 32.2 µM for LNCaP cells and 43.1 µM for
LNCaP-AI cells (data not shown). Based on the IC50 of
juglone, subsequent experiments were performed using 12.5 or 25 µM
of juglone in order to reduce the influence of drug-induced
apoptosis on the experimental results.
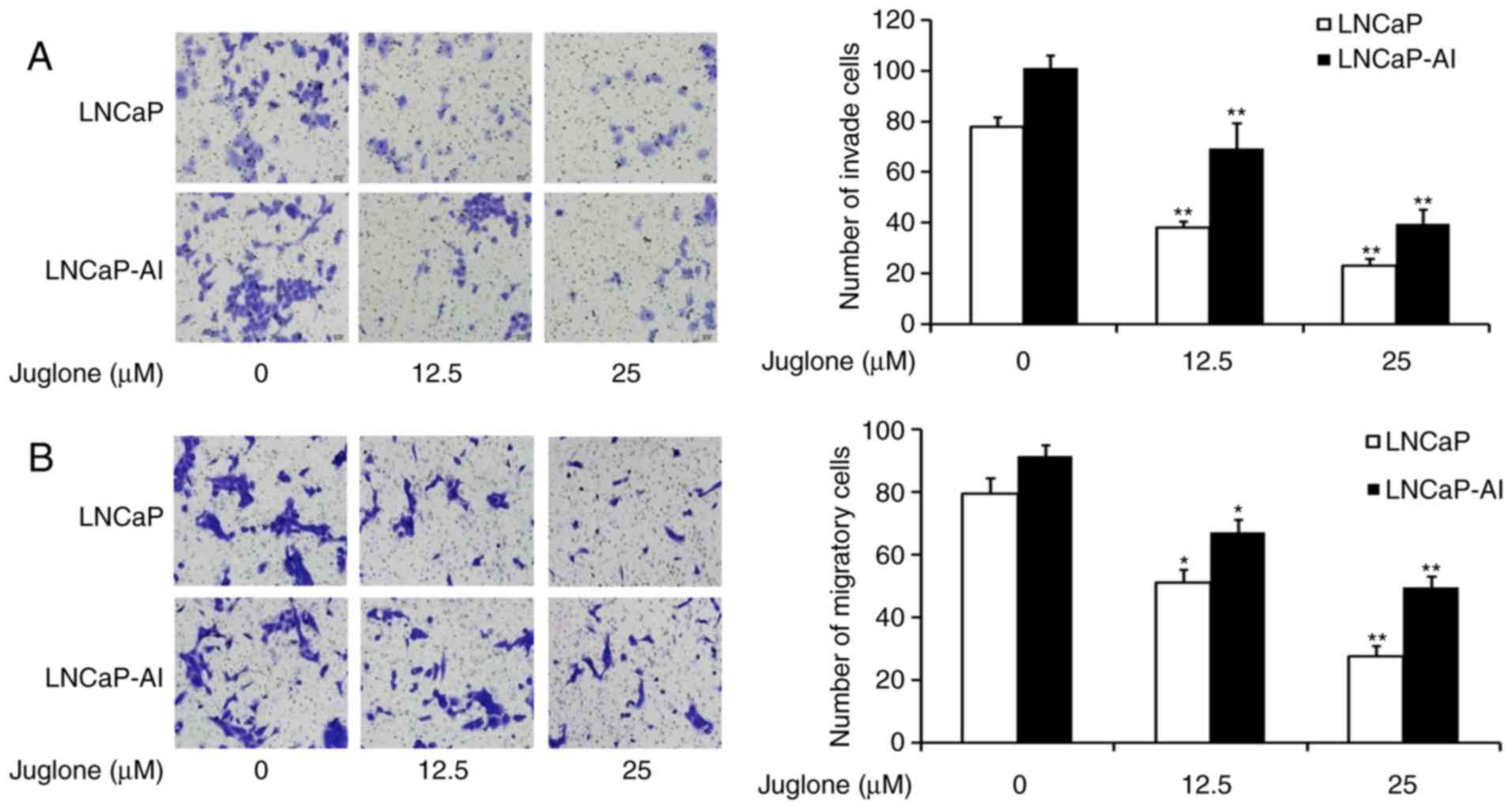
Juglone inhibits the migration and
invasion of LNCaP and LNCaP-AI cells
To investigate whether juglone inhibits the motility
of LNCaP and LNCaP-AI cells, Transwell assays were performed with
un-coated and Matrigel-coated membranes to characterize juglone's
effect on cell migration and invasion, respectively. The cells were
treated with 0, 12.5 or 25 µM juglone for 24 h and then assessed
for their ability to migrate through the membranes. The Transwell
chamber migration assays revealed that juglone inhibited LNCaP and
LNCaP-AI cell migration (Fig. 2A).
Similarly, juglone inhibited cell invasion compared with that of
control cells without juglone treatment (Fig. 2B). The results of these Transwell
assays demonstrated that juglone suppresses the migration and
invasion of LNCaP and LNCaP-AI cells.
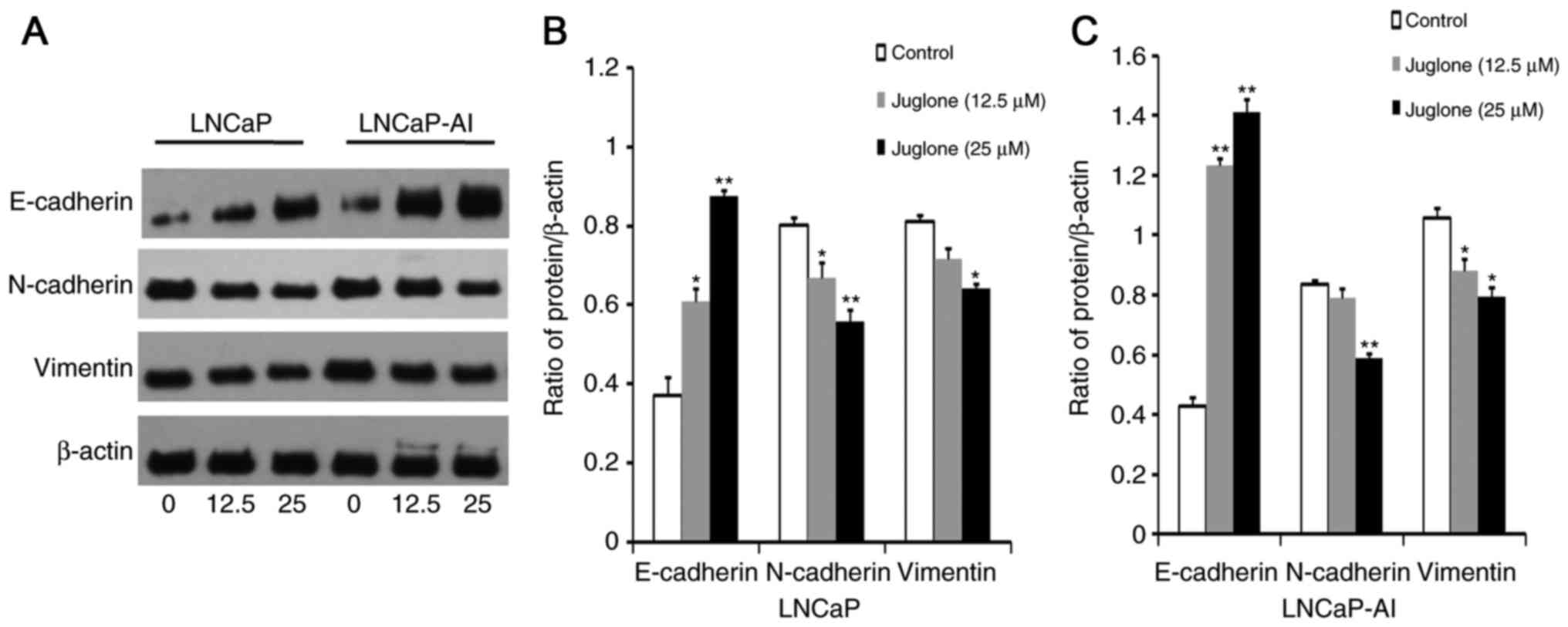
Juglone alters the expression of EMT
markers in LNCaP and LNCaP-AI cells
To determine whether juglone inhibits EMT, western
blot analysis was used to investigate the effect of 24 h treatment
with juglone (0, 12.5 or 25 µM) on the expression of the EMT
markers E-cadherin, N-cadherin and Vimentin in LNCaP and LNCaP-AI
cells. As demonstrated in Fig. 3,
E-cadherin expression increased significantly, whereas the
expression of N-cadherin and vimentin decreased in juglone-treated
LNCaP and LNCaP-AI cells. Collectively, these observations
suggested that inhibition of migration and invasion by juglone in
LNCaP and LNCaP-AI cells may be associated with inhibition of
EMT.
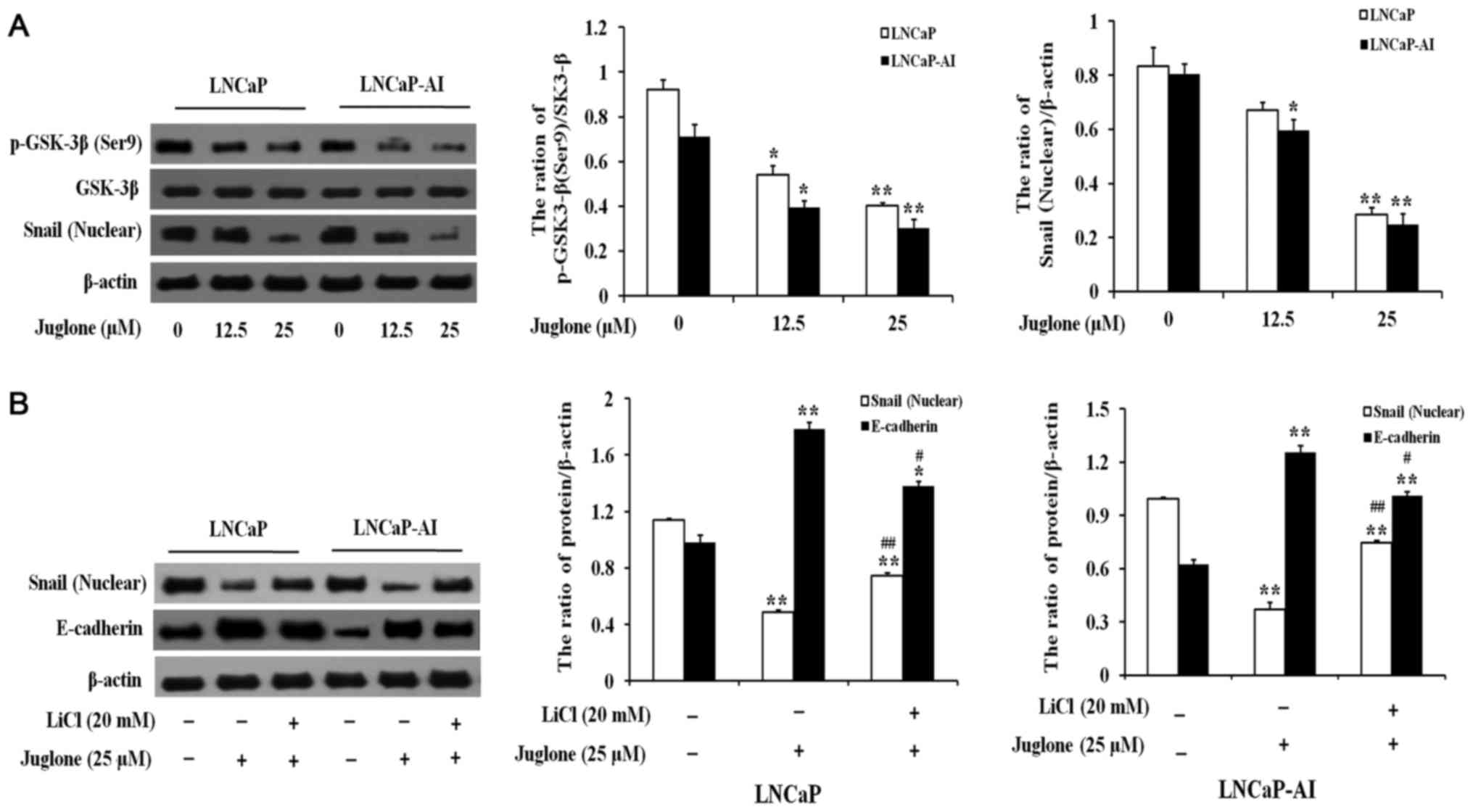
Juglone induces downregulation of
Snail via GSK-3β activation in LNCaP and LNCaP-AI cells
LNCaP and LNCaP-AI cells were treated with various
concentrations of juglone (0, 12.5 or 25 µM) for 24 h and the
expression of Snail and phosphorylated (p-)GSK-3β was assessed. As
demonstrated in Fig. 4A, juglone
significantly inhibited the expression of Snail and p-GSK-3β in a
dose-dependent manner. Therefore, the effects of juglone on the
association between GSK-3β and Snail were further investigated.
LNCaP and LNCaP-AI cells were pretreated with LiCl, a GSK-3β
inhibitor, for 2 h prior to the addition of juglone. As
demonstrated in Fig. 4B, treatment
with lithium chloride (LiCl) significantly attenuated the
inhibitory effect of juglone on Snail expression, leading to
downregulation of E-cadherin. Taken together, these data suggested
that treating LNCaP and LNCaP-AI cells with juglone causes
activation of GSK-3β, leading to the degradation of Snail and
upregulation of E-cadherin protein expression.
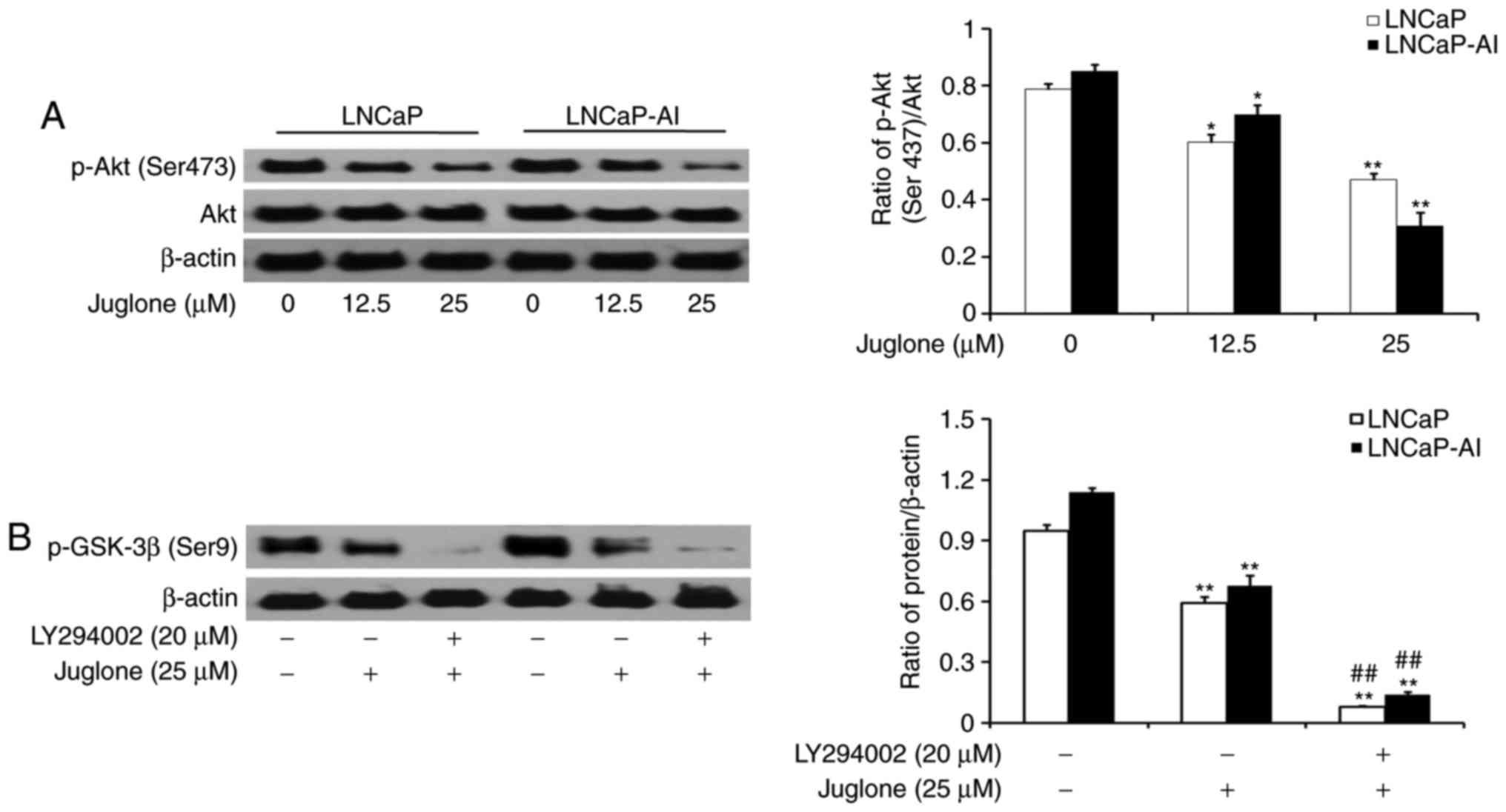
Juglone modulates GSK-3β
phosphorylation via the PI3K/Akt pathway in LNCaP and LNCaP-AI
cells
LNCaP and LNCaP-AI cells were treated with various
concentrations of juglone (0, 12.5 or 25 µM) for 24 h and the
phosphorylation of Akt was assessed by western bolt analysis. The
results revealed that juglone inhibited the expression of p-Akt in
a dose-dependent manner (Fig. 5A). To
further investigate the role of the PI3K/Akt pathway in
juglone-mediated regulation of p-GSK-3β expression, LY294002, a
specific inhibitor of PI3K, was applied to LNCaP and LNCaP-AI
cells. As revealed in Fig. 5B,
LY294002 further enhanced the juglone-induced inhibition of GSK3-β
phosphorylation. The results demonstrated that juglone inhibits
p-Akt expression, thereby enhancing GSK-3β activity.
Discussion
Metastasis is the leading cause of cancer-associated
morbidity and mortality (15).
Therefore, it is important to seek effective drugs to suppress
cancer metastasis. EMT is one of the early steps in the metastatic
process and is an important mechanism contributing toward the
increased invasive and metastatic potential of cancer cells. An
increasing number of studies have reported that EMT serves a role
in metastatic progression of PC and treatment resistance (16,17).
Previous data have indicated that juglone may be a useful
anti-proliferative and anti-invasive agent for certain types of
cancer (10–12). In this study, it was demonstrated that
juglone can modulate the migration and invasion of PC cells. It was
further revealed that juglone suppresses mesenchymal markers
(vimentin and N-cadherin) and promotes the expression of the
epithelial marker E-cadherin in LNCaP and LNCaP-AI cells. Notably,
juglone-induced inhibition of E-cadherin expression was increased
in LNCaP-AI cells. LNCaP-AI cells have been revealed to have a more
aggressive phenotype than LNCaP cells (18), and androgen independence has been
linked to cell growth and EMT (5);
therefore, it is imperative for therapeutic agents to be active in
androgen-dependent and independent PC cells. The results of the
present study suggested that juglone inhibits EMT in prostate
cancer cells.
Snail, a zinc finger protein, is a crucial
transcription factor in the regulation of EMT through its role in
downregulating E-cadherin, a hallmark of the EMT process (6). The data from the present study
demonstrated that juglone treatment results in downregulation of
Snail in LNCaP and LNCaP-AI cells. In particular, low
concentrations of juglone (12.5 µM) could inhibit Snail expression
in LNCaP-AI cells with concomitant upregulation of E-cadherin. It
is known that Snail is phosphorylated by GSK-3β, which induces its
degradation (8,9). The present study revealed significant
changes in p-GSK-3β and Snail protein levels following treatment of
LNCaP and LNCaP-AI cells with juglone. To confirm that the GSK-3β
signaling pathway was involved in juglone-induced inhibition of EMT
in LNCaP and LNCaP-AI cells, GSK-3β activity was blocked using
LiCl. It was revealed that the suppression of GSK-3β activity by
LiCl prevented juglone-induced E-cadherin expression and promoted
Snail expression. Taken together, these data indicated that GSK-3β
activation serves an important role in the progression of EMT and
that the effect of juglone is GSK-3β/Snail-dependent.
Previous studies have reported the role of
Akt/GSK-3β in regulating metastasis of various types of tumors via
modulation of EMT, including PC (8,9,19). It is known that the PI3K/Akt pathway
is involved in the phosphorylation of the Ser9 in GSK-3β,
inhibiting its activity (8,9). The present study therefore investigated
whether this pathway is involved in the juglone-mediated effects on
p-GSK-3β expression. It was revealed that the expression of p-Akt
and p-GSK-3β decreased with juglone treatment. It was also
demonstrated that inhibition of the PI3K/Akt pathway with LY294002
further decreased p-GSK-3β expression, suggesting that juglone
acted on GSK-3β via the PI3K/Akt pathway.
In summary, the present study described a novel role
for juglone in the inhibition of metastasis in PC cells. The
results of the present study demonstrated that juglone inhibits the
migration and invasion of PC cells by suppressing EMT. In addition,
in LNCaP and LNCaP-AI cells, juglone affected the expression of
proteins involved in EMT regulation through the Akt/GSK-3β/Snail
signaling pathway. Taken together, the results of the present study
demonstrated that juglone is a promising agent for PC therapy.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
China National Natural Science Foundation (grant no. 81202031), the
Scientific Research Project of Jilin Province Science and
Technology Department (grant no. 20160204033YY), the Technical
Innovation Project of Jilin Province Health Department (grant no.
2016J103), the Technical Innovation Project of Jilin Science and
Technology Bureau (grant no. 20163306) and the National Training
Programs of Innovation and Entrepreneurship for Undergraduates
(grant no. 201713743006).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LW conceived and designed the study. FF and SC
contributed toward the data acquisition and analysis and drafted
the manuscript. JM and JC were involved in data acquisition. QL and
GM contributed toward data acquisition and revised the manuscript.
All authors read and approved the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EMT
|
epithelial-mesenchymal transition
|
|
GSK-3β
|
glycogen synthase kinase-3β
|
|
PC
|
prostate cancer
|
|
FBS
|
fetal bovine serum
|
|
PI3K
|
phosphatidylinositol-3-kinase
|
References
|
1
|
Ibrahim T, Flamini E, Mercatali L, Sacanna
E, Serra P and Amadori D: Pathogenesis of osteoblastic bone
metastases from prostate cancer. Cancer. 116:1406–1418. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roodman GD: Mechanisms of bone metastasis.
N Engl J Med. 350:1655–1664. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gutierrez Uzquiza A, Lopez Haber C,
Jernigan DL, Fatatis A and Kazanietz MG: PKCε is an essential
mediator of prostate cancer bone metastasis. Mol Cancer Res.
13:1336–1346. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shiota M, Itsumi M, Takeuchi A, Imada K,
Yokomizo A, Kuruma H, Inokuchi J, Tatsugami K, Uchiumi T, Oda Y and
Naito S: Crosstalk between epithelial-mesenchymal transition and
castration resistance mediated by Twist1/AR signaling in prostate
cancer. Endocr Relat Cancer. 22:889–900. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kaufhold K and Bonavida B: Central role of
Snail1 in the regulation of EMT and resistance in cancer: A target
for therapeutic intervention. J Exp Clin Cancer Res. 33:622014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Banyard J and Bielenberg DR: The role of
EMT and MET in cancer dissemination. Connect Tissue Res.
56:403–413. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheng Z, Guo Y, Yang Y, Kan J, Dai S,
Helian M, Li B, Xu J and Liu C: Nitidine chloride suppresses
epithelial-to-mesenchymal transition in osteosarcoma cell migration
and invasion through Akt/GSK-3β/Snail signaling pathway. Oncol Rep.
36:1023–1029. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang H, Wang HS, Zhou BH, Li CL, Zhang F,
Wang XF, Zhang G, Bu XZ, Cai SH and Du J: Epithelial-mesenchymal
transition (EMT) induced by TNF-α requires AKT/GSK-3β-mediated
stabilization of snail in colorectal cancer. PLoS One.
8:e566642013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu HL, Yu XF, Qu SC, Qu XR, Jiang YF and
Sui da Y: Juglone, from Juglans mandshruica Maxim, inhibits growth
and induces apoptosis in human leukemia cell HL-60 through a
reactive oxygen species dependent mechanism. Food Chem Toxicol.
50:590–596. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang W, Liu A, Li Y, Zhao X, Lv S, Zhu W
and Jin Y: Anticancer activity and mechanism of juglone on human
cervical carcinoma Hela cells. Can J Physiol Pharmacol.
90:1553–1558. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Avci E, Arikoğiu H and Erkoç Kaya D:
Investigation of juglone effects on metastasis and angiogenesis in
pancreatic cancer cells. Gene. 588:74–78. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fang F, Qin Y, Qi L, Fang Q, Zhao L, Chen
S, Li Q, Zhang D and Wang L: Juglone exerts antitumor effect in
ovarian cancer cells. Iran J Basic Med Sci. 18:544–548.
2015.PubMed/NCBI
|
|
14
|
Kong DY, Yang DM, Chen YY, Wang Y, Zhang
JQ, Meng G and Fang F: Establishment of androgen independent human
prostate cancer cell line model LNCaP-AI. J Jilin Med College.
33:361–363. 2012.(In Chinese).
|
|
15
|
Hurst DR and Welch DR: Metastasis
suppressor genes: At the interface between the environment and
tumor cell growth. Int Rev Cell Mol Biol. 286:107–180. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bitting RL, Schaeffer D, Somarelli JA,
Garcia-Blanco MA and Armstrong AJ: The role of epithelial
plasticity in prostate cancer dissemination and treatment
resistance. Cancer Metastasis Rev. 33:441–468. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Marín-Aguilera M, Codony-Servat J, Reig Ò,
Lozano JJ, Fernández PL, Pereira MV, Jiménez N, Donovan M, Puig P,
Mengual L, et al: Epith elial-to-mesenchymal transition mediates
docetaxel resistance and high risk of relapse in prostate cancer.
Mol Cancer Ther. 13:1270–1284. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu P, Duan X, Cheng Y, Liu C, Chen Y, Liu
W, Yin B, Wang X and Tao Z: Androgen-independent LNCaP cells are a
subline of LNCaP cells with a more aggressive phenotype and
androgen suppresses their growth by inducing cell cycle arrest at
the G1 phase. Int J Mol Med. 40:1426–1434. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu ZC, Wang HS, Zhang G, Liu H, Chen XH,
Zhang F, Chen DY, Cai SH and Du J: AKT/GSK-3β regulates stability
and transcription of Snail which is crucial for bFGF-induced
epithelial-mesenchymal transition of prostate cancer cells. Biochim
Biophys Acta. 1840:3096–3105. 2014. View Article : Google Scholar : PubMed/NCBI
|