Introduction
Mammary tumors remain the most common cancer and one
of the most fatal diseases in females globally, accounting for 14%
of the total cancer mortalities in 2018 (1–3). With
improved breast screening procedures, mammary tumors can be
diagnosed at an early stage. Consequently, less extensive treatment
may be required (3).
Photodynamic therapy (PDT) is a minimally invasive
cancer treatment. The key agent used in PDT is a photosensitizer,
which is a compound that is excited by light and is converted to a
triplet-excited state (4,5). Osaki et al, Takahashi et
al and Sakata et al developed three novel
photosensitizers, TONS501Na (6),
TONS501 (7), and TONS504 (6–8). TONS501Na
(C37H38N4Na2O7;
molecular weight, 696.8) is a hydrophilic and anionic porphyrin
salt, and is a chlorin derivative that can be synthesized from the
protoporphyrin IX dimethyl ester via a four-step process (8). In a previous study, TONS501Na-mediated
PDT induced the death of mouse mammary tumor EMT6 cells in a
concentration-dependent manner; however, dark cytotoxicity was also
observed with TONS501Na (6).
Therefore, photosensitizers that are ideal for PDT against tumors
are required.
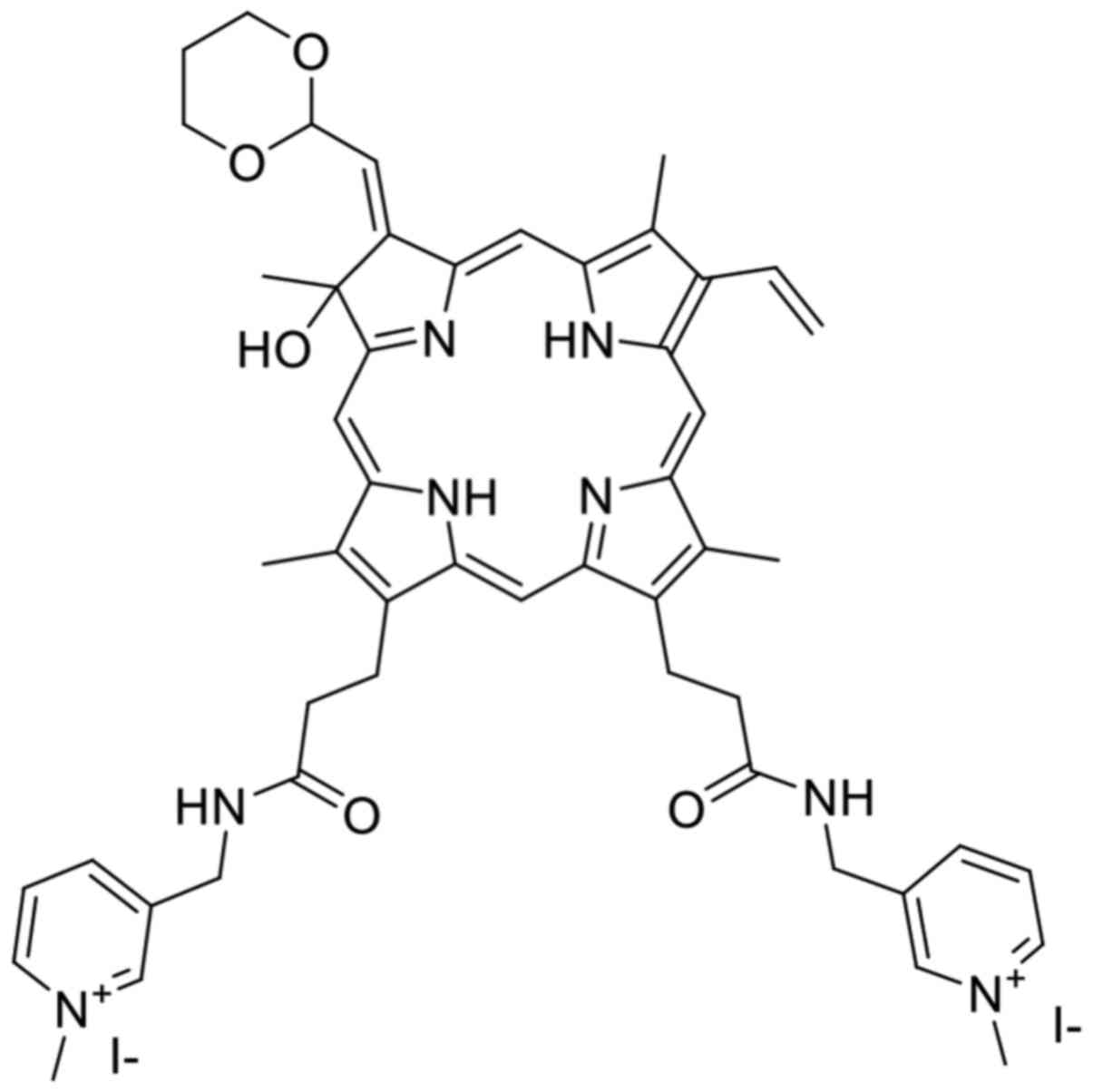
TONS504 (Fig. 1) is a
novel cationic hydrophilic photosensitizer
(C51H58N8O5I2;
molecular weight, 1,116.9) that is synthesized from protoporphyrin
IX dimethyl ester via a five-step process (9,10). PDT
mediated by TONS504 ointment was reported to produce potent
antitumor effects in mouse skin papilloma in an in vivo
study (7).
To the best of our knowledge, the effectiveness of
TONS504-mediated PDT has not been previously reported in an in
vitro study. In the present study, the subcellular localization
of TONS504 and the cytotoxic effects of TONS504-mediated PDT in
EMT6 cells were investigated.
Materials and methods
Cell line and culture conditions
Mouse mammary tumor EMT6 cells were supplied by Dr
Shinichiro Masunaga of Kyoto University (Kyoto, Japan). The cells
were maintained as an adherent monolayer culture in RPMI-1640
medium (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% heat-inactivated fetal bovine serum
(FBS; Nichirei Biosciences Inc., Tokyo, Japan) and antibiotics (5
mg/ml penicillin, 5 mg/ml streptomycin and 10 mg/ml neomycin;
Invitrogen; Thermo Fisher Scientific, Inc.) in an atmosphere
containing 5% CO2 at 37°C. All subsequent incubations
were also performed at 37°C.
The cells were harvested from near-confluent
cultures by brief exposure to a solution containing 0.25% trypsin
and 1 mmol/l ethylenediaminetetraacetic acid (tetrasodium salt)
solution with phenol red (Invitrogen; Thermo Fisher Scientific,
Inc.). Trypsinization was stopped using RPMI-1640 containing 10%
FBS. The cells were concentrated via centrifugation at 300 × g for
5 min at room temperature and resuspended in RPMI-1640. The cell
suspension was mixed at 1:1 with 0.4% Trypan blue at room
temperature and immediately loaded into the counting chamber slide
(Watson Bio Lab; Fukae-Kasei Co., Ltd., Kobe, Japan). Within 5 min,
the Trypan blue exclusion assay was performed to assess cell
viability using an inverted microscope (Nikon Eclipse TS100; Nikon
Corporation, Tokyo, Japan) at a magnification of ×100.
Chemicals
MitoTracker Green FM and LysoTracker Yellow HCK-123
were obtained from Thermo Fisher Scientific Inc. Cell Counting
Kit-8 and the PromoKine Apoptotic/Necrotic Cells Detection kit were
purchased from Dojindo Molecular Technologies, Inc., (Kumamoto,
Japan) and PromoCell GmbH (Heidelberg, Germany), respectively. The
Muse® Oxidative Stress kit and the Muse Annexin V and
Dead Cell assay kit were purchased from EMD Millipore (Billerica,
MA, USA).
Subcellular localization of
TONS504
The intracellular distribution of TONS504 was
monitored using an Olympus Fluoview FV1000 (Olympus Corporation,
Tokyo, Japan) confocal laser scanning microscope (CLSM). The EMT6
cells were seeded in 8-well cell culture slides (SPL Life Sciences,
Pocheon, Korea) and incubated in RPMI-1640 medium for 24 h at 37°C.
Following this, the cells were incubated with TONS504 at a final
concentration of 30 µg/ml for 4 h, followed by co-incubation with
100 nM MitoTracker Green FM and 50 nM LysoTracker Yellow HCK-123
for an additional 30 min in the culture medium prior to a CLSM
being used. The fluorescence of TONS504 was detected at an
excitation wavelength of 543 nm using a helium-neon (G) laser and a
560-nm long-pass filter. The fluorescent signals of MitoTracker
Green FM and LysoTracker Yellow HCK-123 were detected by excitation
at 488 nm using an argon laser, a 560 nm dichroic mirror and a
505–525 nm band-pass barrier filter.
Uptake kinetics of TONS504
The EMT6 cells were seeded at 1–2×104
cells/well in 96-well plates (Corning Incorporated, Corning, NY,
USA) in RPMI-1640 medium supplemented with 10% FBS and incubated
for 24 h in an atmosphere of 5% CO2 at 37°C. Next, the
cells were incubated with TONS504 at a final concentration of 30
µg/ml for 12 h at 37°C. Images were automatically captured every 1
h for 12 h using the IncuCyte® S3 system (Essen
BioScience, Ann Arbor, MI, USA) in phase-contrast and fluorescence
modes. Phase contrast and red-phase images were obtained from the
system. The total fluorescence intensity of objects in an image was
determined by object counting using the image analysis software
IncuCyte (IncuCyte® S3 Software; Essen Bioscience, Ann
Arbor, MI, USA).
Evaluation of the cytotoxic effects of
TONS504 and light in EMT6 cells
EMT6 cells were seeded at 1–2×104
cells/well in 96-well plates (Corning Incorporated) and incubated
for 24 h. The cells were then incubated with various concentrations
of TONS504 (0, 1, 3, 10, 30 or 100 µg/ml) for 24 h at 37°C.
Following replacing with fresh RPMI-1640 medium supplemented with
10% FBS, the cells were irradiated by a semiconductor laser at a
wavelength of 677 nm. PDT was performed at 11 mW/cm2 in
cells exposed to TONS504 (0, 1, 3, 10, 30 or 100 µg/ml), using four
light doses (0, 1, 5 or 10 J/cm2). The cells were then
incubated for 24 h in the dark prior to assessing their viability
using a WST8 assay (Cell Counting Kit-8; Dojindo Molecular
Technologies, Inc.), according to the manufacturer's protocols. The
cytotoxic effects of PDT were evaluated to calculate the
appropriate concentration of TONS504 and light dose for the
following experiment. The concentrations of TONS504 or light doses
that were too low were ineffective; however, the concentrations of
TONS504 or light doses that were too high were too effective. The
optimal concentrations of TONS504 and light dose were 10 µg/ml and
5 J/cm2 respectively, which results in the best
cytotoxic effect.
Kinetics experiment to assess
apoptosis
EMT6 cells were seeded at 1–2×104
cells/well in 96-well plates (Corning Incorporated) and incubated
for 24 h. The cells were then incubated with various concentrations
of TONS504 (0, 1, 3, 10, 30 or 100 µg/ml) for 24 h at 37°C.
Following replacing with fresh RPMI-1640 medium supplemented with
10% FBS, the cells were irradiated with 677-nm light emitted by a
semiconductor laser. PDT was performed at 5 J/cm2, which
was determined as optimal light dose by taking into consideration
the results of the cytotoxicity effects of TONS504 and light on
EMT6 cells. Then, the cells were stained with Annexin V-FITC
immediately following PDT and incubated in an atmosphere containing
5% CO2 at 37°C for 24 h. Images were automatically
captured every 1 h for 24 h in phase-contrast and fluorescence
modes using the IncuCyte S3 system. Phase contrast and green-phase
images were obtained from the system. The Total Green Object
Integrated Intensity in an image was determined by object counting
with IncuCyte® S3 Software according to the
manufacturer's protocol. In the present study, object counting
means the total sum of intensity of Annexin V Green fluorescence in
the image.
Analysis of cell death
EMT6 cells were seeded at 4–5×104
cells/well in 35-mm petri dishes (Nalge Nunc International,
Penfield, NY, USA) containing 2 ml RPMI-1640 medium supplemented
with 10% FBS. Following 24 h of incubation, the cells were divided
into the TONS504 groups (treated with 10 or 30 µg/ml TONS504) and
the PDT groups (treated with 10 or 30 µg/ml TONS504 and then
irradiated with a light dose of 1, 5 or 10 J/cm2). The
concentration of used TONS504 was selected by taking into
consideration the results of the cytotoxicity effects of TONS504
and light on EMT6 cells.
The cells were incubated with 10 or 30 µg/ml TONS504
for 24 h. Following washing with fresh media, the cells were
irradiated with a laser light of wavelength 677 nm (11
mW/cm2; 0, 1, 5 or 10 J/cm2). Following 24 h
of PDT, the cells were stained using the PromoKine
Apoptotic/Necrotic Cells Detection kit, according to the
manufacturer's protocols. To assess apoptosis and necrosis, the
cells were stained with Annexin V-fluorescein isothiocyanate (FITC)
and ethidium homodimer III (EthD-III) at 24 h after laser
irradiation. Cell morphology was examined using a CLSM. The cells
were analyzed by fluorescence microscopy using an FITC and Texas
Red filter set.
Analysis of apoptosis and reactive
oxygen species (ROS) generation
EMT6 cells were seeded at 4–5×104
cells/well in 35-mm petri dishes containing 2 ml culture medium.
Following 24 h of incubation, the cells were divided into the
following groups: Control (no treatment); laser (irradiated with a
light dose of 10 J/cm2); TONS504 (treated with 10 or 30
µg/ml TONS504); and PDT (treated with 10 or 30 µg/ml TONS504, and
then irradiated with a light dose of 1, 5 or 10 J/cm2).
Following this, the cells were incubated with 10 or 30 µg/ml
TONS504 for 24 h.
Apoptosis
Following the treatment with 10 or 30 µg/ml TONS504,
the cells were washed with fresh media and irradiated with 677-nm
light (1, 5 or 10 J/cm2). Apoptosis was assessed at 24 h
after laser irradiation using the Muse Annexin V and Dead Cell
Assay kit, according to the manufacturer's protocols. Annexin V was
used to detect phosphatidylserine on the external membrane of
apoptotic cells.
ROS assay
Following treatment with 10 or 30 µg/ml TONS504, the
cells were washed with fresh media and irradiated with 677-nm light
(1, 5 or 10 J/cm2). ROS generation was assessed at 24 h
after laser irradiation using the Muse Oxidative Stress kit,
according to the manufacturer's protocols. The kit was used to
determine the percentages of cells that were ROS(−) (healthy cells)
and ROS(+).
Statistical analysis
Data were analyzed using Sidak's multiple comparison
test following two-way analysis of variance. P<0.05 was
considered to indicate a statistically significant difference.
Statistical analyses were performed using GraphPad Prism version 6
(GraphPad Software Inc., La Jolla, CA, USA). Results are presented
as the mean ± standard deviation (n=3).
Results
Subcellular localization of
TONS504
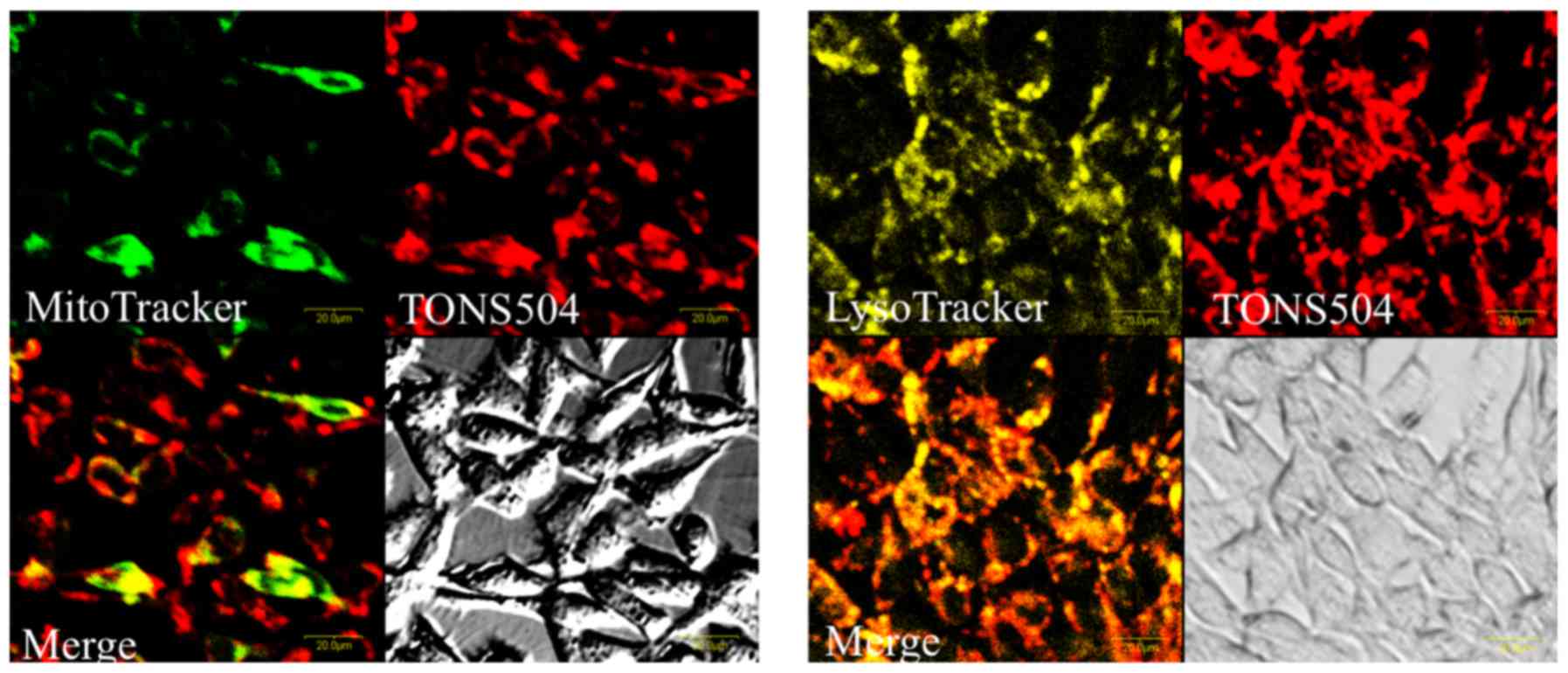
Confocal micrographs of EMT6 cells exposed to
TONS504 and fluorescent molecular probes are depicted in Fig. 2. TONS504 was mainly localized in the
lysosomes and partially localized in the mitochondria. No
fluorescence was detected in the nuclei.
Uptake kinetics of TONS504
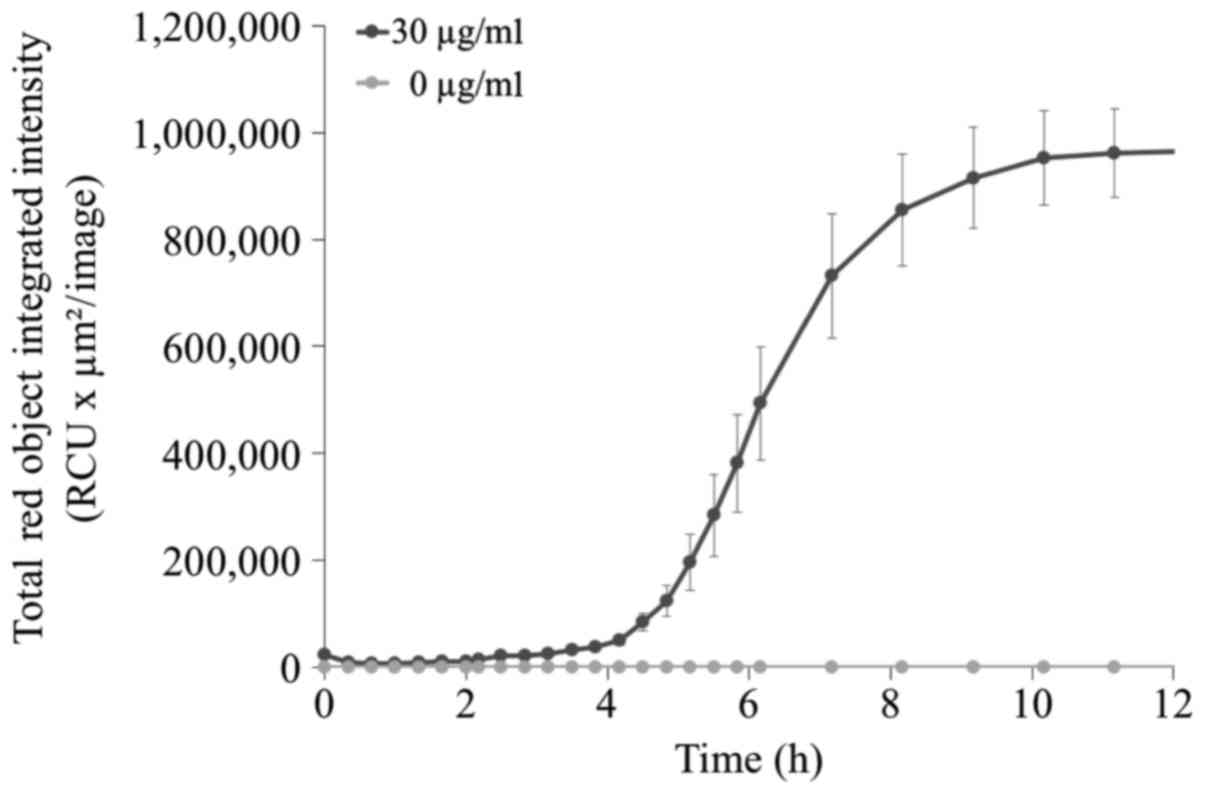
The uptake of TONS504 was investigated by studying
its fluorescence emission using a live-cell analysis system. It was
observed that fluorescence intensity increased slowly within 5 h,
followed by a rapid increase up to 8 h post-incubation.
Fluorescence reached a plateau at 10 h (Fig. 3).
TONS504- and light-induced
cytotoxicities to EMT6 cells
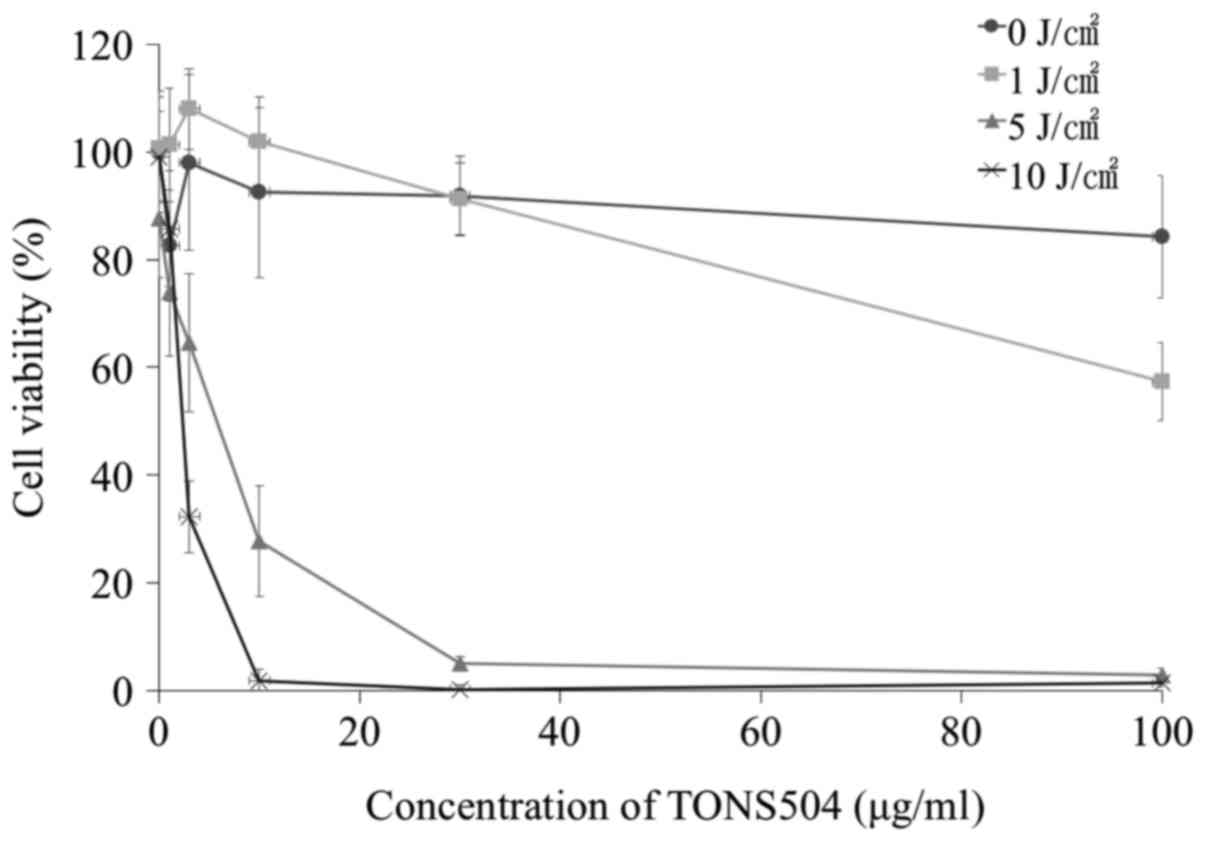
The viability of EMT6 cells at 24 h post-PDT is
illustrated in Fig. 4. Cell survival
was dependent on TONS504 concentration or light dose, and was
demonstrated to be markedly lower at high TONS504 concentrations
compared with that at lower concentrations. No cytotoxicity was
observed in cells treated with 0 J/cm2 of light. At 1
µg/ml TONS504, the cell viability in 1 J/cm2 of light
was significantly lower compared with that in 0 J/cm2 of
light (P=0.0046). Additionally, the cell viability in 5
J/cm2 of light was significantly lower compared with
that in 1 J/cm2 of light (P<0.0001). Furthermore, the
cell viability in 10 J/cm2 of light was significantly
lower compared with that in 1 J/cm2 of light (P=0.029).
At 3 µg/ml TONS504, the cell viability in 5 J/cm2 of
light was significantly lower compared with that in 0 and 1
J/cm2 of light (both P<0.0001), and the cell
viability in 10 J/cm2 of light was significantly lower
compared with that in 0, 1 and 5 J/cm2 of light (all
P<0.0001). At 10 µg/ml TONS504, the cell viability in 5
J/cm2 of light was significantly lower compared with
that in 0 and 1 J/cm2 of light (both P<0.0001), and
the cell viability in 10 J/cm2 of light was
significantly lower compared with that in 0, 1 and 5
J/cm2 of light (all P<0.0001). At 30 µg/ml TONS504,
the cell viability in 5 J/cm2 of light was significantly
lower compared with that in 0 and 1 J/cm2 of light (both
P<0.0001), and the cell viability in 10 J/cm2 of
light was significantly lower compared with that in 0 and 1
J/cm2 of light (both P<0.0001). At 100 µg/ml TONS504,
the cell viability in 1 J/cm2 of light was significantly
lower compared with that in 0 J/cm2 of light
(P<0.0001). The cell viability in 5 J/cm2 of light
was significantly lower compared with that in 0 and 1
J/cm2 of light (both P<0.0001), and the cell
viability in 10 J/cm2 of light was significantly lower
compared with that in 0 and 1 J/cm2 of light (both
P<0.0001).
The half maximal inhibitory concentration
(IC50) was determined. It was determined that the
IC50 values of TONS504 against EMT6 cells exposed to
light doses of 5 and 10 J/cm2 were 5.9 and 1.9 µg/ml,
respectively.
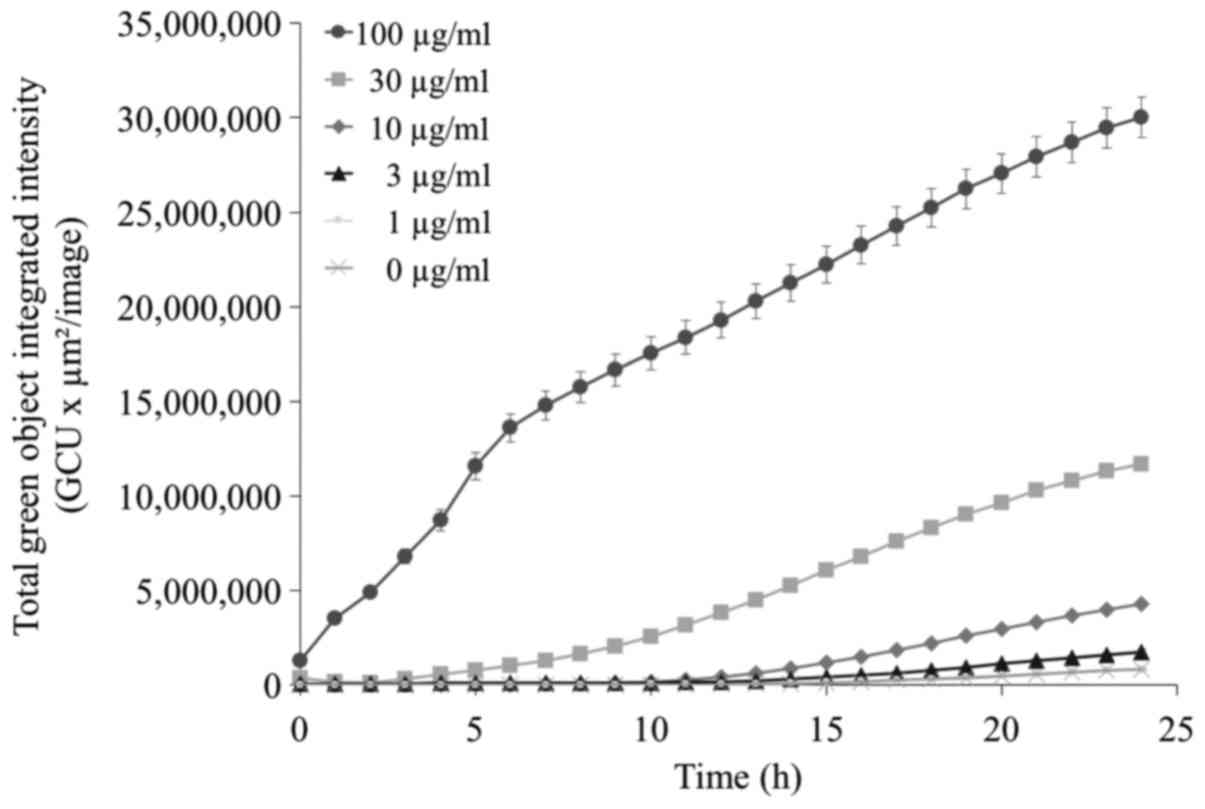
Kinetics experiment to assess
apoptosis
Apoptosis induced by TONS504-mediated PDT was
determined by Annexin V-FITC fluorescence using a live-cell
analysis system. Apoptosis was dependent on the concentration of
TONS504 (Fig. 5). At a low TONS504
concentration (<10 µg/ml), apoptosis occurred from ~10 h after
PDT; however, treatment with 30 µg/ml TONS504 (middle dose)
resulted in apoptosis induction at 1–3 h after PDT. Furthermore,
when TONS504 was used at a high dose (100 µg/ml), apoptosis
occurred immediately after PDT. The Total Green Object Integrated
Intensity [green calibrated unit (GCU) × µm2/image] in 3
µg/ml TONS504 was significantly higher compared with that in 0
µg/ml TONS504 (P=0.0459). The GCU in 10 µg/ml TONS504 was
significantly higher compared with that in 0, 1 and 3 µg/ml TONS504
(all P<0.0001). The GCU in 30 µg/ml TONS504 was significantly
higher compared with that in 0, 1, 3 and 10 µg/ml TONS504 (all
P<0.0001). The GCU in 100 µg/ml TONS504 was significantly higher
compared with that in 0, 1, 3, 10 and 30 µg/ml TONS504 (all
P<0.0001).
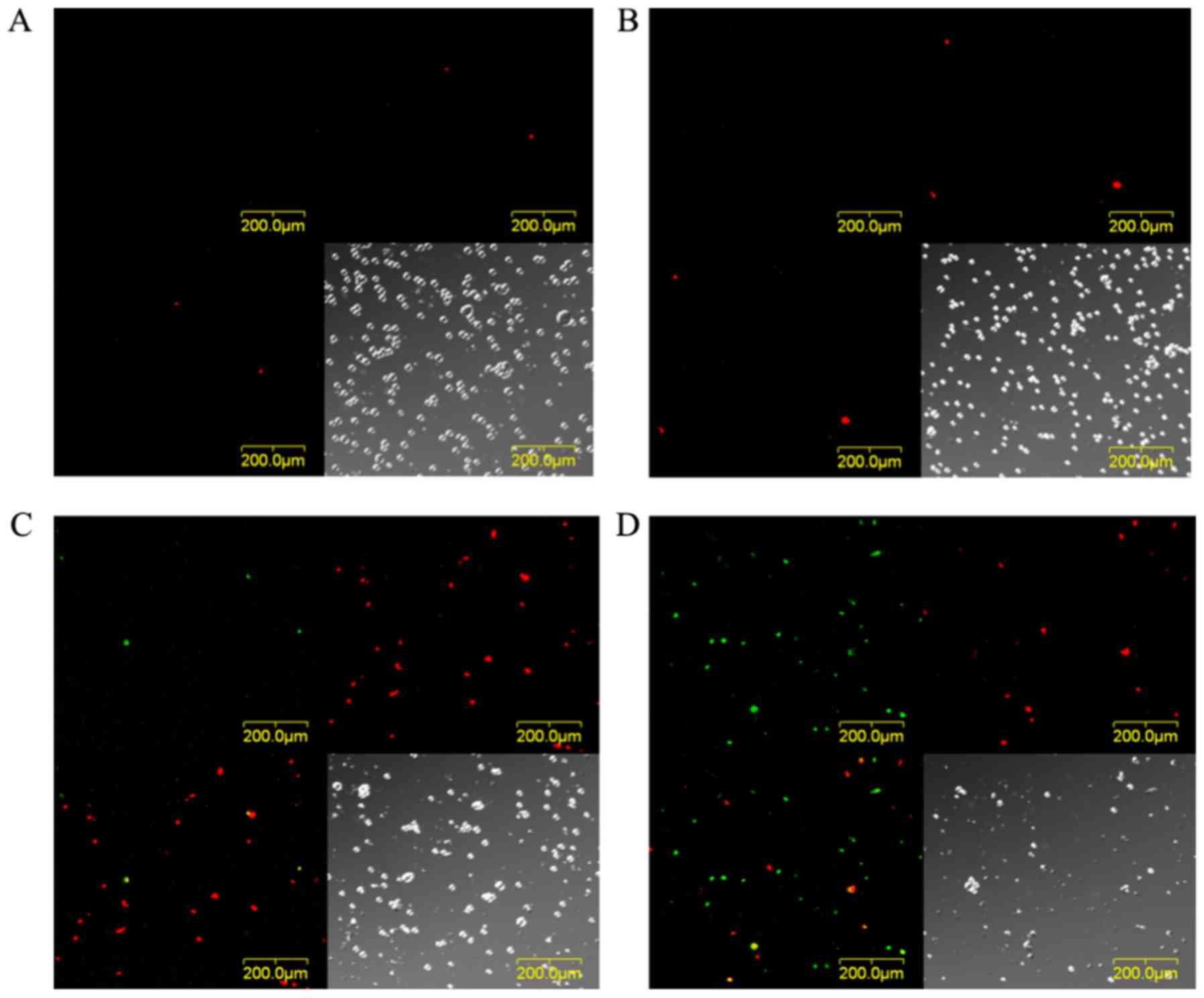
Analysis of cell death using a
CLSM
The images of EMT6 cells stained with Annexin V-FITC
and EthD-III at 24 h after PDT with 10 µg/ml TONS504 are depicted
in Fig. 6. The cells subjected to PDT
with 5 J/cm2 laser energy were stained with EthD-III,
which indicated that they were necrotic (Fig. 6C). Furthermore, following PDT with 10
J/cm2 laser energy, different cells were positively
stained with Annexin V or EthD-III, which indicated that they were
apoptotic or necrotic (Fig. 6D).
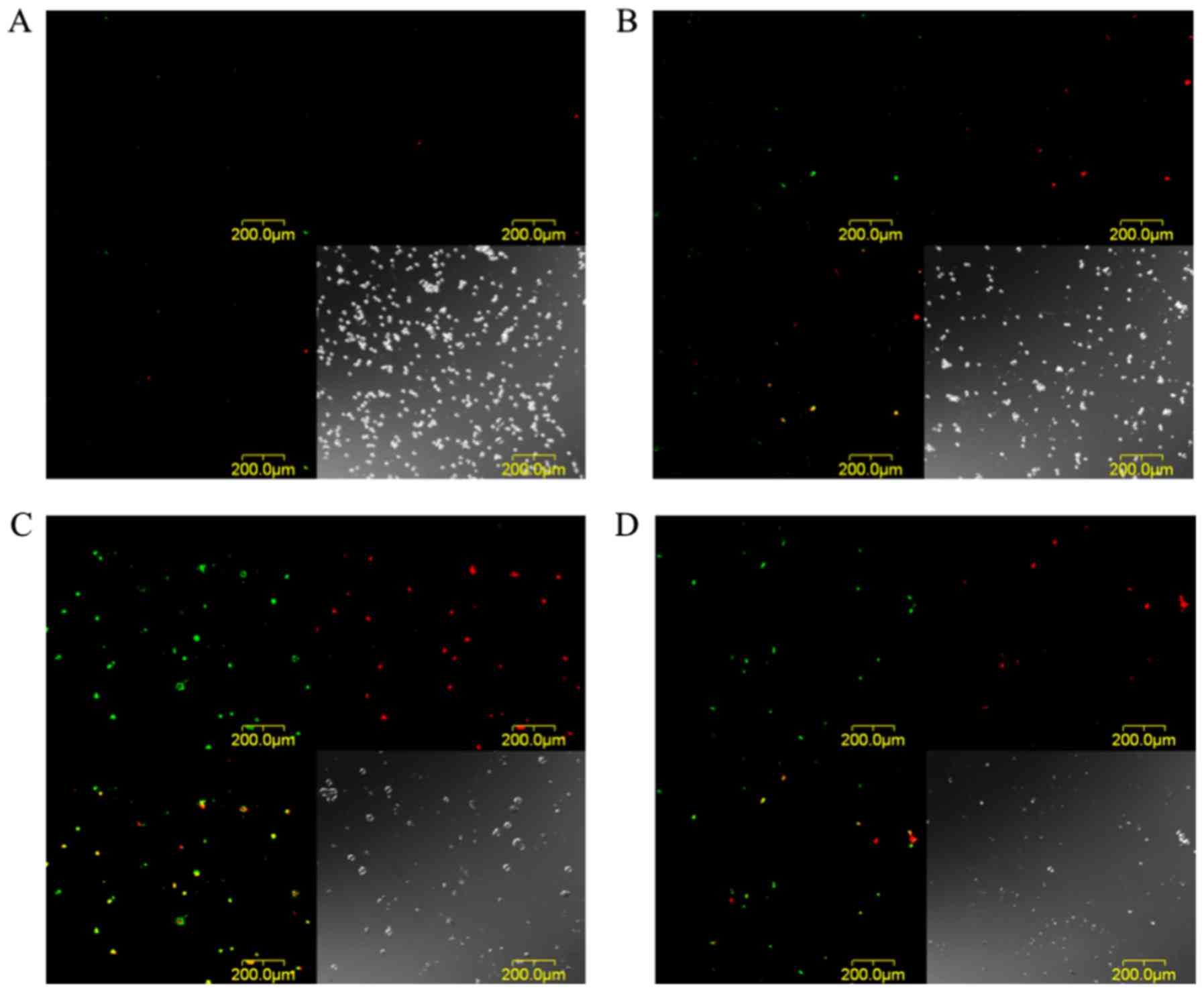
The images of EMT6 cells stained with Annexin V-FITC
and EthD-III at 24 h after PDT with 30 µg/ml TONS504 are depicted
in Fig. 7. The cells that were
subjected to PDT with 5 J/cm2 laser energy were stained
with Annexin V and EthD-III, which indicated that they were in
either in the late apoptosis or early necrosis stage (Fig. 7C); however, a decrease in the number
of EMT6 cells was observed following PDT with 10 J/cm2
laser energy. In addition, different cells were stained with
Annexin V or EthD-III, which indicated that the cells were
apoptotic or necrotic (Fig. 7D).
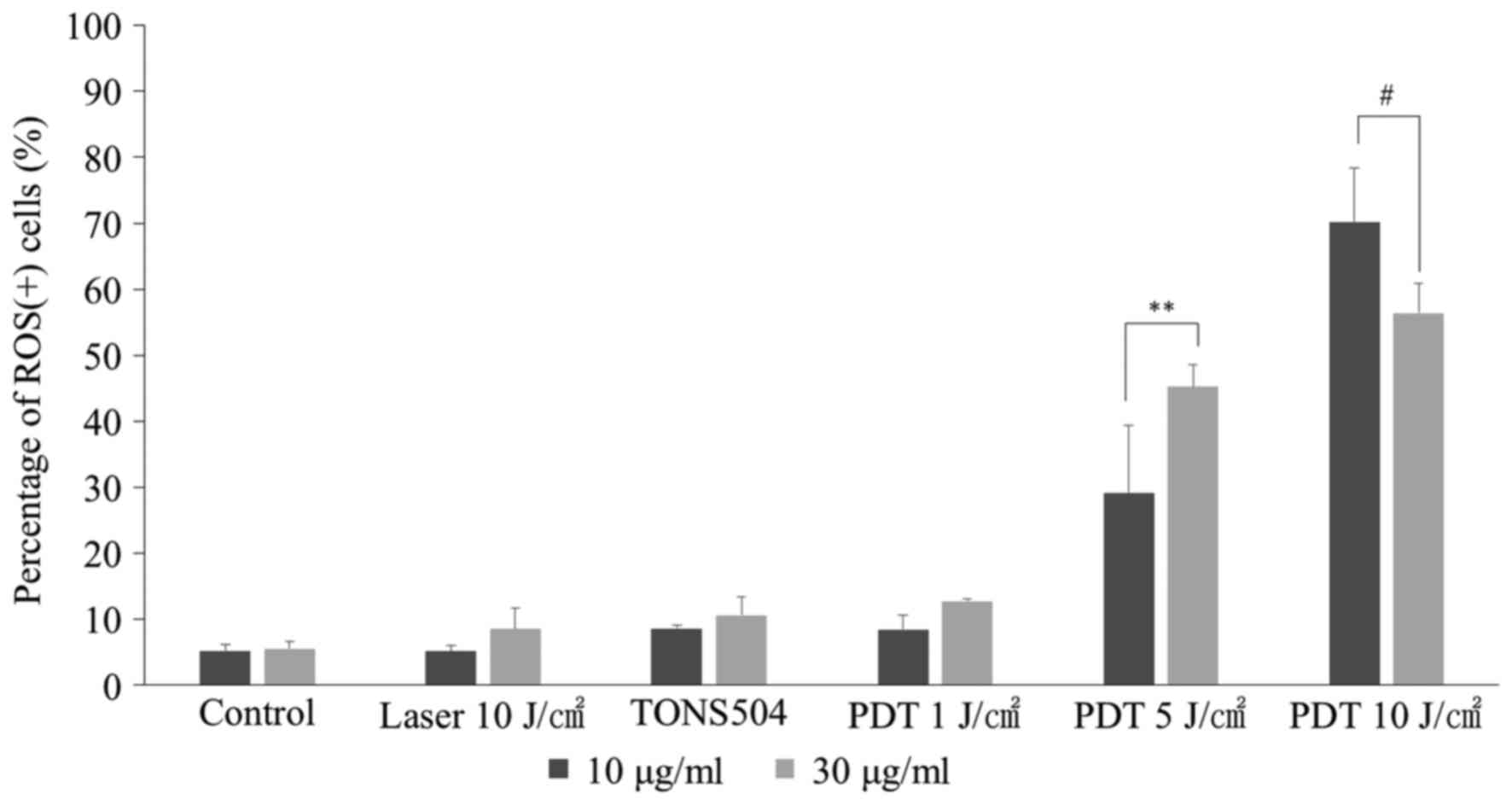
ROS assay
The percentage of ROS(+) cells at 24 h after PDT is
depicted in Fig. 8. For cells
subjected to PDT with 5 J/cm2 laser energy, the
percentage of ROS(+) cells following treatment with 30 µg/ml
TONS504 was significantly higher compared with that of cells
treated with 10 µg/ml TONS504 (P=0.001). By contrast, for cells
subjected to PDT with 10 J/cm2 laser energy, the
percentage of ROS(+) cells following treatment with 10 µg/ml
TONS504 was significantly higher compared with that of cells
treated with 30 µg/ml TONS504 (P=0.0048). The percentage of ROS(+)
cells in the PDT group increased in a light- and dose-dependent
manner for each TONS504 dose.
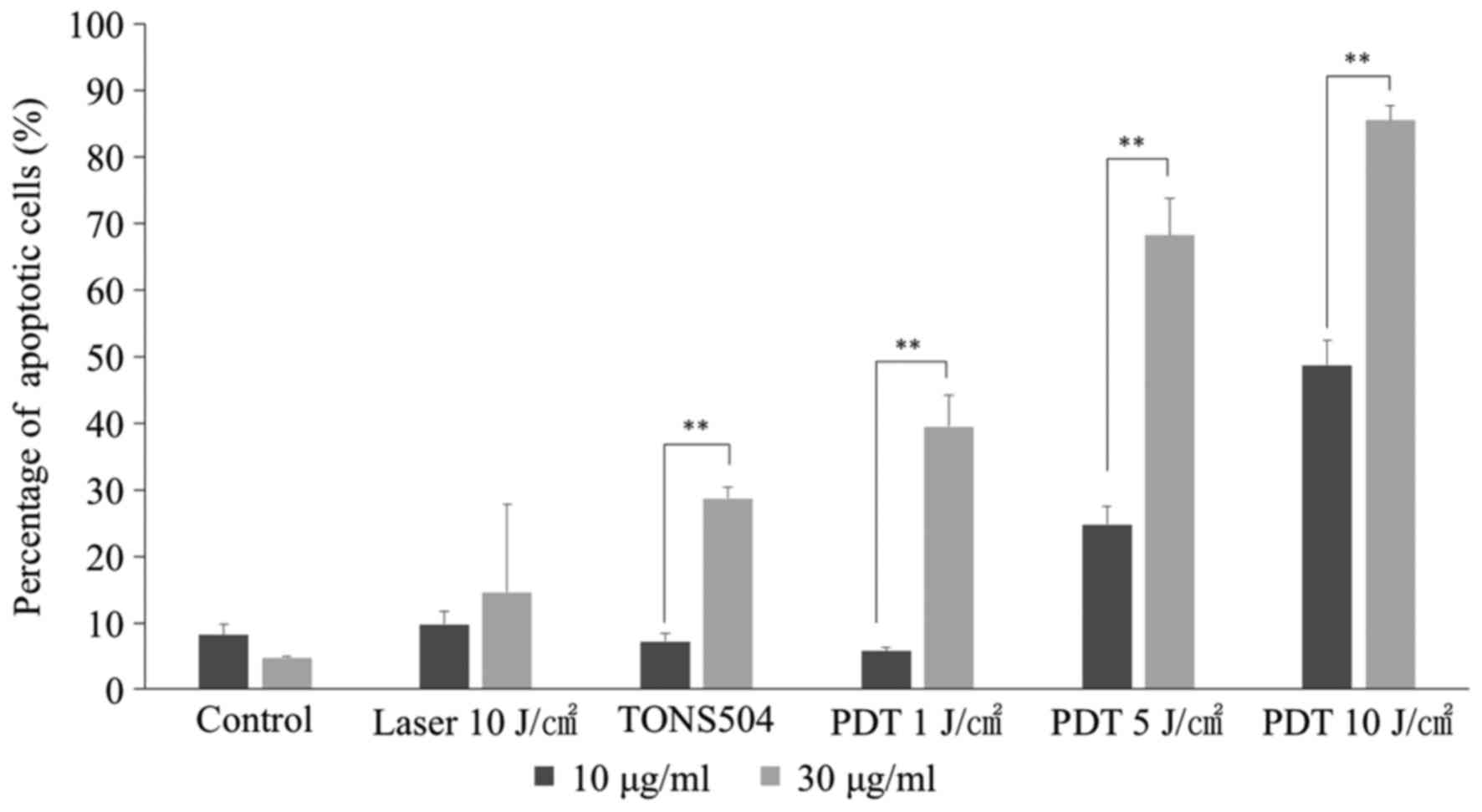
Analysis of apoptosis
At 24 h after PDT with 1, 5 or 10 J/cm2
laser energy, the apoptotic rates in groups treated with 10 µg/ml
TONS504 were significantly higher compared with those in groups
treated with 30 µg/ml TONS504 in the respective groups (all
P<0.0001; Fig. 9). Furthermore,
the percentage of apoptotic cells following PDT increased in a
light- and dose-dependent manner for each TONS504 dose.
Discussion
In our previous study, CLSM analysis revealed that
TONS501Na accumulates in the mitochondria and lysosomes (6). It was also reported that meso-tetra
(hydroxyphenyl) chlorin and hypericin exhibit similar uptake
kinetics, with a continuous increase in fluorescence within the
first 20 h (11). In the present
study, TONS504 accumulated primarily in the lysosomes. In addition,
its uptake reached a plateau at 10 h. It was considered that
TONS504, being cationic, may strongly interact with the
cytomembrane, which possesses a negative charge, and enter the
cells rapidly (12); therefore,
TONS504 may partially move to the mitochondria following
accumulation in the lysosomes.
In our previous study, TONS501Na-mediated
PDT-induced cell death in EMT6 cells in a manner dependent on
photosensitizer dose and light energy. The IC50 values
of TONS501Na against EMT6 cells were 8.2 and 2.2 µg/ml at a laser
power of 6 and 13 J/cm2, respectively (6). In the present study, it was determined
that TONS504-mediated PDT-induced EMT6 cell death in a
dose-dependent manner. The IC50 values of TONS504
against EMT6 cells were 5.9 and 1.9 µg/ml at light doses of 5 and
10 J/cm2, respectively. Although the experimental
conditions were slightly different between the two studies, the
IC50 of TONS504 was similar to that of TONS501Na. In
addition, dark cytotoxicity was observed with TONS501Na in our
previous study (6); however, this was
not observed with TONS504 in the present study. Therefore, TONS504
is a photosensitizer more suitable for tumor therapy than
TONS501Na.
Fig. 5 depicted that
apoptotic cell death induced by TONS504-mediated PDT increased in a
time-dependent manner and was initiated at 1–3 h after PDT. The
cells treated with 10 µg/ml TONS504 and a light dose of 5
J/cm2 were mainly necrotic (Fig. 6C). Furthermore, the cells treated with
30 µg/ml TONS504 and a light dose of 5 J/cm2 underwent
either late apoptosis or early-stage necrosis (Fig. 7C). The percentages of ROS(+) cells and
apoptotic cells in the group subjected to TONS504-mediated PDT
increased in a light-energy-dependent manner. When the cells were
treated with 10 or 30 µg/ml TONS504 and with a light dose of 10
J/cm2, the percentage of ROS(+) cells treated with 30
µg/ml TONS504 (56.1%) was lower than that of cells treated with 10
µg/ml TONS504 (70.1%). The results demonstrated that PDT with a
high dose of TONS504 may result in a rapid decrease in the number
of ROS(+) cells. This is due to the notable decrease in cell
viability and the rapid induction of apoptosis by TONS504 at a
concentration of 30 µg/ml. The results also indicated that
TONS504-mediated PDT may induce apoptosis, but not ROS-induced
damage. It was considered that the differences in cell death
induced by TONS504-PDT may be associated with the intracellular
accumulation of TONS504, mainly in the lysosomes.
We hypothesized that apoptosis induced by
TONS504-mediated PDT may be dependent on lysosomal damage. A
previous report indicated that apoptosis induced by
ATX-S10-mediated PDT was dependent on lysosomal damage (13). In addition, ATX-S10 was localized to
the mitochondria and lysosomes. It has been hypothesized that
ATX-S10-mediated PDT initiates an apoptotic response through direct
damage to lysosomes. Furthermore, it regulates cell death through
photodamage to B-cell lymphoma-2 (Bcl-2) by inducing direct and
indirect damage to the mitochondria (13). Talaporfin sodium (NPe6) is also
localized in lysosomes. It was reported that following treatment of
murine hepatoma 1c1c7 cells with NPe6-PDT, there was a delayed
apoptotic response, which was marked by rapid destruction of
lysosomes, BH3-interacting domain death agonist (Bid) cleavage to
generate the proapoptotic Bcl-2 family member t-Bid, and activation
of caspase-3 and −9. Bid activation decreased mitochondrial
membrane potential and cytochrome-c release (14,15).
Additionally, photodamaged lysosomes triggered the mitochondrial
apoptotic pathway by releasing proteases that activate Bid
(14). It was considered that Bid may
be activated by high-dose PDT, but not by low-dose PDT. Depending
on the extent of photodamage, either apoptosis or necrosis was
considered to be initiated; therefore, in the present study, it was
considered that apoptosis induced by TONS504-mediated PDT may be
dependent on the extent of lysosomal damage, as well as direct and
indirect mitochondrial damage. However, these results are only
based on in vitro studies, and further in vivo
studies are necessary.
In conclusion, TONS504-mediated PDT induced the
death of mouse mammary tumor cells in a manner dependent on the
TONS504 dose and light energy. In addition, TONS504 did not induce
dark cytotoxicity. Therefore, TONS504 could be an ideal
photosensitizer for PDT against tumors. However, future studies are
warranted to evaluate the in vivo pharmacokinetics, tissue
distribution and photodynamic effects of TONS504 in a mouse model
of EMT6 mammary tumors.
Acknowledgements
The authors would like to thank Mr Shohei Shimonishi
(Essen BioScience, Inc., Tokyo, Japan) for providing technical
assistance with the experiments.
Funding
The present study was partly funded by Saisei Mirai
Clinic (Osaka, Japan).
Availability of data and materials
All data generated and analyzed during the present
study are included in this published article.
Authors' contributions
TO, IS and YU conceived, designed, and performed the
experiments and wrote the paper. MY, YM, KA, TT, NI, TI and YO
interpreted the data and were involved in drafting the manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
George BP and Abrahamse H: A review on
novel breast cancer therapies: Photodynamic therapy and plant
derived agent induced cell death mechanisms. Anticancer Agents Med
Chem. 16:793–801. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Banerjee SM, MacRobert AJ, Mosse CA,
Periera B, Bown SG and Keshtgar MRS: Photodynamic therapy:
Inception to application in breast cancer. Breast. 31:105–113.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bacellar IO, Tsubone TM, Pavani C and
Baptista MS: Photodynamic efficiency: From molecular photochemistry
to cell death. Int J Mol Sci. 16:20523–20559. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Triesscheijn M, Baas P, Schellens JH and
Stewart FA: Photodynamic therapy in oncology. Oncologist.
11:1034–1044. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Osaki T, Sakata I, Uto Y, Azuma K,
Murahata Y, Tsuka T, Itoh N, Imagawa T and Okamoto Y: Photodynamic
therapy mediated by a novel chlorin derivative, TONS 501-Na, in
EMT6 cells. Anticancer Res. 37:1723–1728. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takahashi H, Nakajima S, Asano R, Nakae Y,
Sakata I and Iizuka H: Photodynamic therapy using a novel
photosensitizer, TONS501, is similarly effective to ALA and EC036
photodynamic therapy on DMBA-and TPA-induced mouse skin papilloma.
J Dermatol Sci. 66:221–224. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sakata I: Chlorin derivatives. Japan
patent JP5651426. B2:2015-01-14.
|
|
9
|
Latief MA, Chikama T, Shibasaki M, Sasaki
T, Ko JA, Kiuchi Y, Sakaguchi T and Obana A: Antimicrobial action
from a novel porphyrin derivative in photodynamic antimicrobial
chemotherapy in vitro. Lasers Med Sci. 30:383–387. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Latief MA, Chikama T, Ko JA, Kiuchi Y,
Sakaguchi T and Obana A: Inactivation of acyclovir-sensitive and
-resistant strains of herpes simplex virus type 1 in vitro by
photodynamic antimicrobial chemotherapy. Mol Vis. 21:532–537.
2015.PubMed/NCBI
|
|
11
|
Berlanda J, Kiesslich T, Engelhardt V,
Krammer B and Plaetzer K: Comparative in vitro study on the
characteristics of different photosensitizers employed in PDT. J
Photochem Photobiol B. 100:173–180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kou L, Sun J, Zhai y and He Z: The
endocytosis and intracellular fate of nanomedicines: Implication
for rational design. AJPS. 8:1–10. 2013.
|
|
13
|
Ichinose S, Usuda J, Hirata T, Inoue T,
Ohtani K, Maehara S, Kubota M, Imai K, Tsunoda Y, Kuroiwa Y, et al:
Lysosomal cathepsin initiates apoptosis, which is regulated by
photodamage to Bcl-2 at mitochondria in photodynamic therapy using
a novel photosensitizer, ATX-s10 (Na). Int J Oncol. 29:349–355.
2006.PubMed/NCBI
|
|
14
|
Reiners JJ Jr, Caruso JA, Mathieu P,
Chelladurai B, Yin XM and Kessel D: Release of cytochrome c and
activation of pro-caspase-9 following lysosomal photodamage
involves Bid cleavage. Cell Death Differ. 9:934–944. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ribeiro JN, da Silva AR and Jorge RA:
Involvement of mitochondria in apoptosis of cancer cells induced by
photodynamic therapy. J Bras Patol Med Lab. 40:383–390. 2004.
View Article : Google Scholar
|