Introduction
Bone neoplasm is typical systemic malignant disease,
which mainly leads to common symptoms of bone and joint pain and
fatigue in patients (1,2). Osteosarcoma is the most common
cancer-caused dead and the prognosis remains incompletely
understand due to the paucity of effective therapeutic targets that
significantly influences quality of life and mean survival rate of
the patients with osteosarcoma (3,4).
Clinicopathological and molecular correlations explain the role of
bone marrow microvessel density (MVD) and plasma angiogenic factors
in myeloproliferative neoplasms and highlight the strong
correlation of MVD with plasma angiogenic factors, JAK2 mutant
allele burden, and bone marrow fibrosis in myeloproliferative
neoplasms (5).
Paclitaxel is a secondary metabolite of taxus plant
that has been reported to association with inhibition of cancer
cells growth (6,7). Researches have showed that paclitaxel
leads to generation of tubulin and tubulin dimer, which promotes
the polymerization of tubulin and the assembly of microtubule and
further results in cells cycle arrest for tumor cells (8,9). Previous
studies have investigated the paclitaxel can significantly inhibit
ovarian cancer cells growth through cyclin-dependent kinase 11
(CDK11) and inhibition of insulin-like growth factor (IGF)
signaling in preclinical pancreatic cancer models (10,11).
Notably, paclitaxel shows antitumor activity through the interplay
with apoptosis network in triple-negative breast cancer, indicating
that paclitaxel has therapeutic potential for human endometrial
cancer by targeting of endogenous apoptosis signaling pathway
(12,13).
Elemene is a new drug extracted from the activating
blood circulation herbs, which presents the broad-spectrum
antineoplastic, immune protection and other effects (14). In recent years, evidences have
indicated that β-elemene has the effects to induce cells apoptosis
and differentiation, reverses multiple drug resistance of tumor,
and enhances the sensibility of combined radiotherapy and
chemotherapy (14,15). Interestingly, antineoplastic effects
of β-elemene on prostate cancer cells and other types of solid
tumor cells have been investigated and supported that β-elemene may
act as a new potentially therapeutic drug for castration-resistant
prostate cancer and other solid tumors (16).
Therefore, the purpose of this study investigated
the combined anticancer effects of β-elemene and paclitaxel on bone
neoplasms. We analyzed bone neoplasm cells growth, cells cycle,
apoptosis and in vivo growth after treatment with
β-elemene-paclitaxel. We also focused the important function of
gene G-protein coupled receptor 124 (GPR124) in
β-elemene-paclitaxel-inhibited growth of bone neoplasms.
Materials and methods
Ethics statement
The present study was carried out in strict
accordance with the recommendations in the Guide for the Care and
Use of Laboratory Animals. All experimental protocols and animals
were performed in accordance with National Institutes of Health and
approved by the Ethics Committee of the Nankai Hospital of
Tianjin.
Cell line
U-2OS cells were purchased from American Type
Culture Collection (ATCC, Manassas, VA, USA). The cells were
cultured using RPMI1640 medium (Biosera, Nuaille, France) with 10%
FBS at 37°C in CO2 incubator (5%).
MTT assay
U-2OS cells were incubated with β-elemene (100
mg/ml), paclitaxel (20 mg/ml) or combined treatment in 96-well
plates for 24, 48 and 72 h in triplicate for each condition with
PBS as control in 5% CO2 at 37°C for 24 h. Subsequently,
the control group was added with MTT solution after the removal of
supernatant thereafter incubated for 4 h. In the blank control
group 100 µl DMSO was added after removal of the supernatant after
that shocked for 30 min, the enzyme standard instrument were used
to detect at 570 nm (680 Microplate reader; Bio-Rad Laboratories,
Inc., Hercules, CA, USA).
Flow cytometry assay
U-2OS cells were grown at 37°C with 5%
CO2 until 90% confluence was formatted. Cells were then
incubated with β-elemene (100 mg/ml), paclitaxel (20 mg/ml) or
combined treatment for 24 h. After incubation, the tumor cells were
trypsinized and collected. The cells were then washed in cold PBS,
adjusted to 1×106 cells/ml with PBS, labeled with
Annexin V-FITC and PI (Annexin V-FITC kit), and analyzed with a
FACScan flow cytometer (both BD Biosciences, Franklin Lakes, NJ,
USA). The treatments were performed in triplicate, and the
percentage of labeled cells undergoing apoptosis in each group was
determined and calculated.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay
Total RNA in U-2OS cells was extracted using RNAzol,
and DNase RNase-free was adopted to digest total RNA at 37°C for 15
min, and then RNeasy kit to purify RNA to adjust its concentration
to 1 µg/µl. The 2 µg RNA was used as the template to synthetize
cDNA by reacting with reverse transcriptase at 37°C for 120 min, at
99°C for 4 min, and at 4°C for 3 min respectively. Followed by,
reverse transcription-polymerase chain reaction method was adopted
to amplify the gene expression of GPR124, TIMP metallopeptidase
inhibitor (TIMP)-1, TIMP-2, matrix metallopeptidase (MMP)-2, MMP-9,
vascular endothelial growth factor (VEGF), endostatin, CDK1,
cyclin-B1, P27, MDR1, LRP and TS (Table
I) to determine the transcription level of mRNA, and β-actin
was used as the housekeeping genes of internal control group.
Eventually, agarose electrophoresis with 1% ethidium bromide was
adopted to check PCR amplified products. Relative mRNA expression
changes were calculated by 2-ΔΔCt. The results are expressed as the
n-fold way compared to control.
 | Table I.Sequences of primers were used in this
study. |
Table I.
Sequences of primers were used in this
study.
| Gene name | Sequence |
|---|
| GPR124 | Reverse:
5′-GGTCGTTCTACTGGGCTGATT-3′ |
|
| Forward:
5′-AGCAAGAGGGGATTTCACAAT-3′ |
| TIMP-1 | Forward:
5′-GTCAGTGAGAAGCAAGTCGA-3′ |
|
| Reverse:
5′-ATGTTCTTCTCTGTGACCCA-3′ |
| TIMP-2 | Forward:
5′-TGGGGACACCAGAAGTCAAC-3′ |
|
| Reverse:
5′-TTTTCAGAGCCTTGGAGGAG-3′ |
| MMP-3 | Reverse:
5′-CTTCTTCAAGGACCGGTCA-3′ |
|
| Forward:
5′-GCTGGCTGAGTACCAGTA-3′ |
| MMP-9 | Reverse:
5′-TGGGCTACGTGACCTATGAC-3′ |
|
| Forward:
5′-GCCCAGCCCACCTCCACTCC-3′ |
| VEGF | Reverse:
5′-GCACCCATGGCAGAAGGAGGAG-3′ |
|
| Forward:
5′-GTGCTGACGCTAACTGACC-3′ |
| Endostatin | Forward:
5′-ATGCACAGCCACCGCGACTT-3′ |
|
| Reverse:
5′-CTTCATGACTGCCTCCAAGTAG-3′ |
| Bcl-2 | Forward:
5′-CTCAGCCAGCCAGTGACATA-3′ |
|
| Reverse:
5′-CCGTGCTCCAGATACAT-3′ |
| Bcl-w | Forward:
5′-AGAGTGGACCACACTGCGC-3′ |
|
| Reverse:
5′-ACATCCCAACGGTCATCGTA-3′ |
| Bax | Reverse:
5′-GGAGGGGATCAGTATATACA-3′ |
|
| Forward:
5′-GAAGATGGAGAGATGG-3′ |
| Caspase-3 | Reverse:
5′-CTGTGAGATCACTGGCTTTG-3′ |
|
| Forward:
5′-TTGGAGGGAACAGACGAG-3′ |
| CDK1 | Reverse:
5′-ACAGTGCATCATCGCTGTTC-3′ |
|
| Forward:
5′-CCGGAGAGGAGACTTCACAG-3′ |
| Cyclin-B1 | Reverse:
5′-GAAAGCATCCAGCAATAGGC-3′ |
|
| Forward:
5′-TAAGGAAGCCTGGAGCACAG-3′ |
| P27 | Reverse:
5′-CATGGTGAAACCCCGTCTCTA-3′ |
|
| Forward:
5′-GCCTCAGCCTCCCGAGTAG-3′ |
| MDR1 | Reverse:
5′-GGGCAGAATCTTTCCACCA-3′ |
|
| Forward:
5′-TTAAATGTATACCCAAAGACAA-3′ |
| LRP | Reverse:
5′-CGAATGGGTGTTTTCACATATG-3′ |
|
| Forward:
5′-CTTCAATTGTATTCAGGATGG-3′ |
| TS | Reverse:
5′-CACCCTCAATATTTGGAA-3′ |
|
| Forward:
5′-CCGTTGTTGTAGGACTAATGAA-3′ |
| β-actin | Reverse:
5′-CGGAGTCAACGGATTTGGTC-3′ |
|
| Forward:
5′-AGCCTTCTCCATGGTCGTGA-3′ |
Cells invasion and migration
assays
U-2OS cells were grown at 37°C with 5%
CO2 until 90% confluence was formatted. U-2OS cells were
then incubated with β-elemene (100 mg/ml), paclitaxel (20 mg/ml) or
combined treatment for 24 h. For invasion assay, U-2OS cells were
suspended as a density of 1×105 in 500 µl in serum-free
RPMI 1640. Cells were subjected to the tops of BD BioCoat Matrigel
Invasion Chambers (BD Biosciences) according to the manufacturer's
instructions. For migration assay, cells were subjected to a
control insert (BD Biosciences) instead of a Matrigel Invasion
Chamber. The tumor cells migration and invasion were counted in at
least three randomly stain-field microscope every membrane.
Western blot analysis
U-2OS cells were then incubated with β-elemene (100
mg/ml), paclitaxel (20 mg/ml) or combined treatment for 24 h. Cells
were collected and lysed in RIPA buffer (M-PER reagent for the
cells and T-PER reagent for the tissues, Thermo Scientific)
followed homogenized at 4°C for 10 min. A total of 20 µg protein
extracts was electrophoresed on 12.5% polyacrylamide gradient gels
and then transferred to nitrocellulose membranes. The membranes
were incubated in blocking buffer (5% milk) prior to incubation
with primary antibodies at 4°C overnight. The primary rabbit
anti-mouse antibodies used in the immunoblotting assays were:
metastasis gene metastasis-associated protein (MTA) 3 (1:200;
ab176346), MMP-3 (1:1,000; ab53015), MMP-9 (1:1,000; ab38898), VEGF
(1:500; ab11938), GRP124 (1:500; ab67280), endostatin (1:500;
ab64569), TIMP-1 (1:1,000; ab38978), TIMP-2 (1:500; ab180630), and
β-actin (1:500; ab8226; all Abcam, Cambridge, UK). Horseradish
peroxidase (HRP)-conjugated anti-rabbit IgG (Bio-Rad Laboratories,
Inc.) was used at a 1:5,000 dilution and detected using a Western
Blotting Luminol Reagent.
Animals study
6-8 weeks (n=60) old male BALB/c-nu/nu nude mice
were purchased from Beijing University and housed in a
temperature-controlled facility at 23±1°C and relative humidity of
50±5% with a 12-h light/dark cycle. Cultured U-2OS cells
(5×107) in 20 µl PBS were subcutaneously injected into
the right forelimb of nude mouse under aseptic condition. Mice were
divided into four groups (n=10 in each group) and received
treatment of 1.0 mg/kg β-elemene, 1.0 mg/kg paclitaxel and combined
treatment (1.0 mg/kg β-elemene + 1.0 mg/kg paclitaxel) or PBS by
intravenous injection. Treatments were initiated on day 3 after
tumor implantation (diameter, 5–6 mm). The treatment was continued
7 times once time a day. The tumor volumes were calculated
according to the formula, V=0.5xa2xb, calculate the
relative tumor volume and draw growth curve [(a) short diameter and
(b) the long diameter of tumor with vernier caliper].
Immunohistochemical staining
Paraffin-embedded tumor tissue sections were
prepared and epitope retrieval was performed for further analysis.
The paraffin sections were treated with hydrogen peroxide (3%) for
15 min and subsequently were blocked by a regular blocking solution
for 20 min 37°C. The sections were incubated rabbit anti-mouse
GRP124 (1:500; ab67280), VEGF (1:500; ab11938), and endostatin
(1:500; ab64569; all Abcam) respectively, at 4°C for 12 h after
blocking. All sections were washed three times and incubated with
HRP-conjugated anti-rabbit IgG (Bio-Rad Laboratories, Inc.) was
used at a 1:10,000 dilution for 1 h at 37°C. For apoptotic cells in
tumor tissues, tumor sections were stained with TUNEL according to
previous report (17).
Statistical analysis
The experiments data were expressed as mean ±
standard (SD) deviation. The significant difference (P<0.05) of
data of different groups were calculated using Duncan's multiple
range test using SAS version 9.2 (SAS Institute Inc., Cary, NC,
USA).
Results
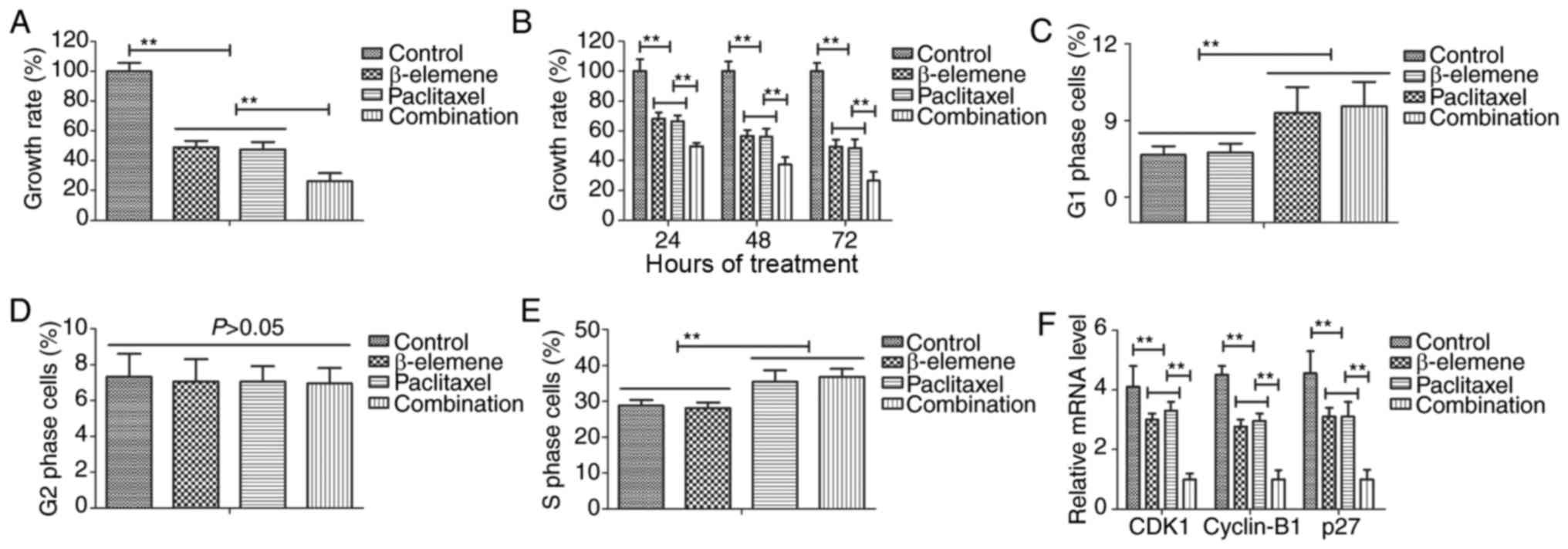
Treatment of β-elemene-paclitaxel on
growth and cell cycle of U-2OS cells
The efficacy of β-elemene-paclitaxel on growth and
cell cycle was investigated in U-2OS cells. Results demonstrated
that combined treatment of β-elemene (100 mg/ml) and paclitaxel (20
mg/ml) significantly inhibited growth of U-2OS cells (Fig. 1A). Results also showed that inhibitory
effects of β-elemene-paclitaxel for growth of U-2OS cells presented
time-dependent manner (Fig. 1B). As
shown in Fig. 1C-E, Treatment of
β-elemene-paclitaxel, β-elemene and paclitaxel arrested U-2OS cells
cycle at G1 and S, G1, S phase respectively. We found that
decreased gene CDK1, cyclin-B1 and P27 expression levels in U-2OS
cells (Fig. 1F). Collectively, these
results suggest that treatment of β-elemene-paclitaxel could
inhibit growth and arrests cell cycle of U-2OS cells compared to
single treatment.
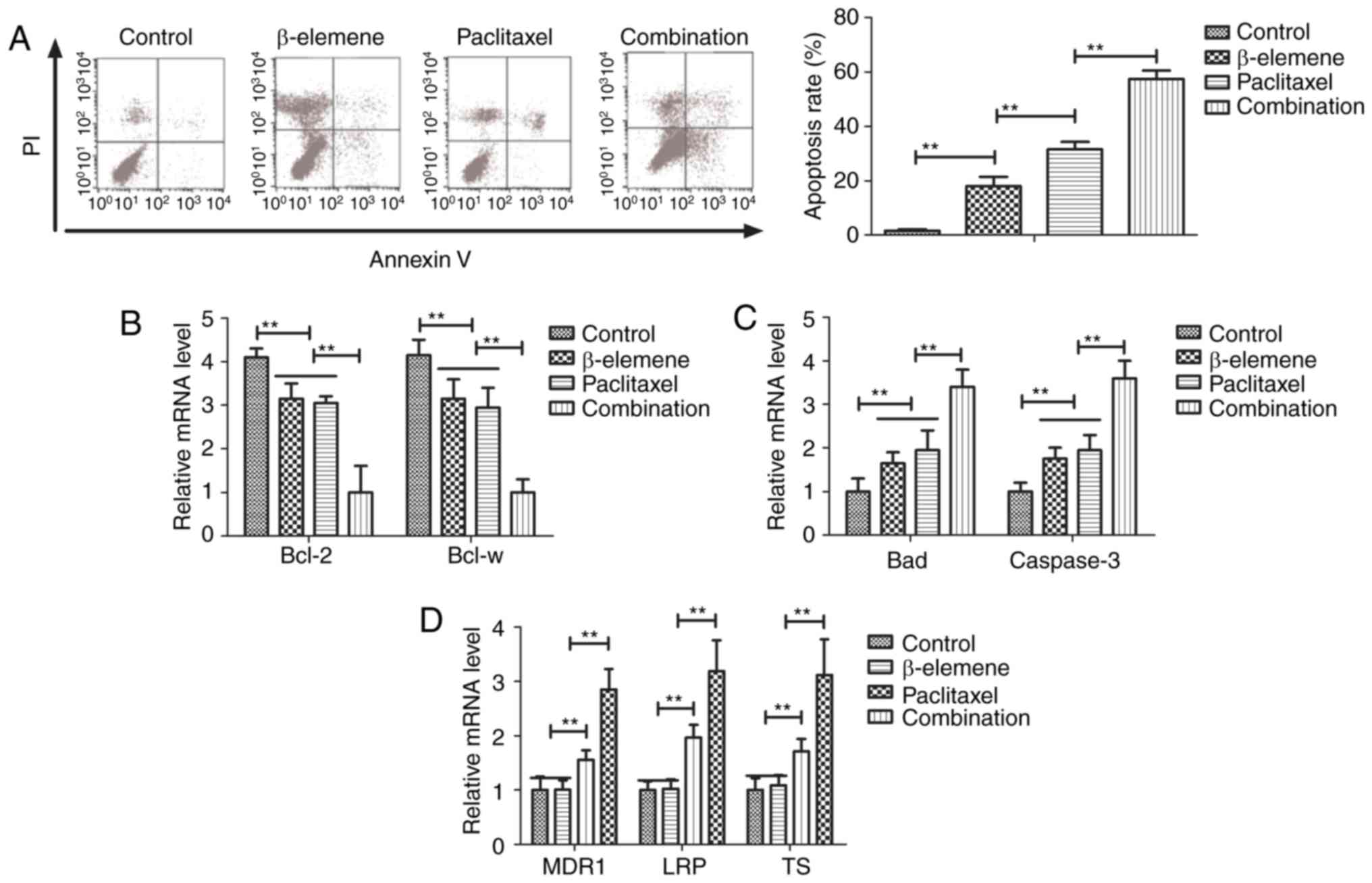
Treatment of β-elemene-paclitaxel on
apoptosis of U-2OS cells
Efficacy of β-elemene-paclitaxel-induced apoptosis
of U-2OS cells was analyzed in this study. Paclitaxel (20
mg/ml)-induced apoptosis of U-2OS cells was promoted by β-elemene
(100 mg/ml) after 24-h incubation (Fig.
2A). Gene analysis demonstrated that β-elemene-paclitaxel
treatment showed lower expression of B-cell lymphoma (Bcl)-2 and
Bcl-w anti-apoptosis genes compared to β-elemene and paclitaxel
group (Fig. 2B). However,
pro-apoptosis Bad and caspase-3 genes were markedly up-regulated in
U-2OS cells by β-elemene-paclitaxel treatment (Fig. 2C). We also investigated the effects of
β-elemene on drug resistant genes for paclitaxel in U-2OS cells. As
shown in Fig. 2D, β-elemene decreased
drug resistant genes expression levels of MDR1, LRP and TS in U-2OS
cells (Fig. 2D). These results
indicate that β-elemene enhances apoptosis of bone neoplasms cells
induced by paclitaxel via decreasing of drug resistant genes
expression.
Effects of β-elemene on drug resistant
genes for paclitaxel Treatment of β-elemene-paclitaxel on migration
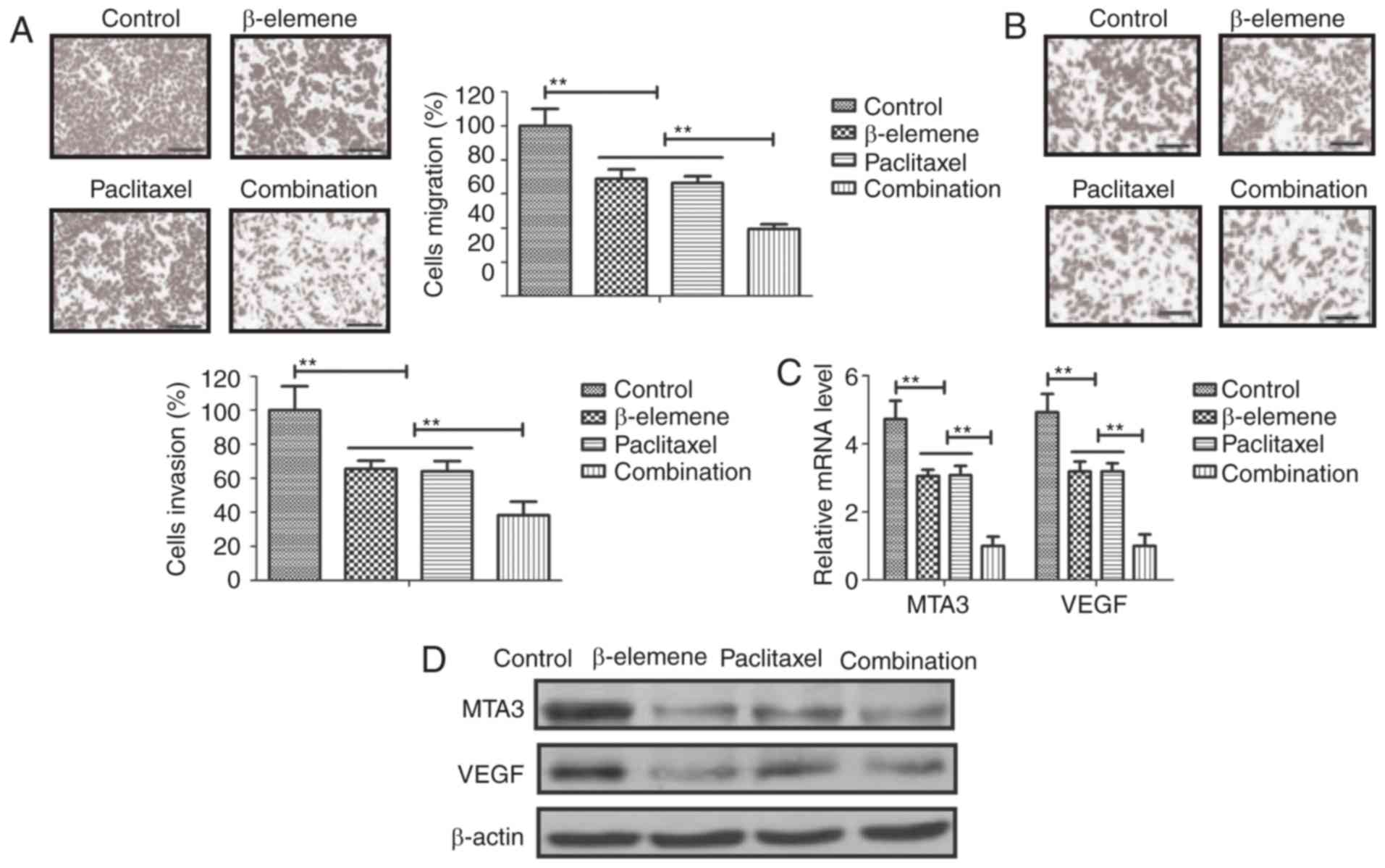
and invasion of U-2OS cells
Anti-metastasis effects of β-elemene-paclitaxel were
analyzed in U-2OS cells. As shown in Fig.
3A, B, β-elemene-paclitaxel treatment significantly inhibited
migration and invasion of U-2OS cells. We showed that
tumor-metastasis gene MTA3 and VEGF expression after treatment with
β-elemene-paclitaxel (Fig. 3C).
Western blot also confirmed the efficacy of β-elemene-paclitaxel
treatment on inhibition of MTA3 and VEGF expression in U-2OS cells
(Fig. 3D). These results indicate
that Treatment of β-elemene-paclitaxel can efficiently inhibit
migration and invasion of U-2OS cells compared to either β-elemene
or paclitaxel treatment.
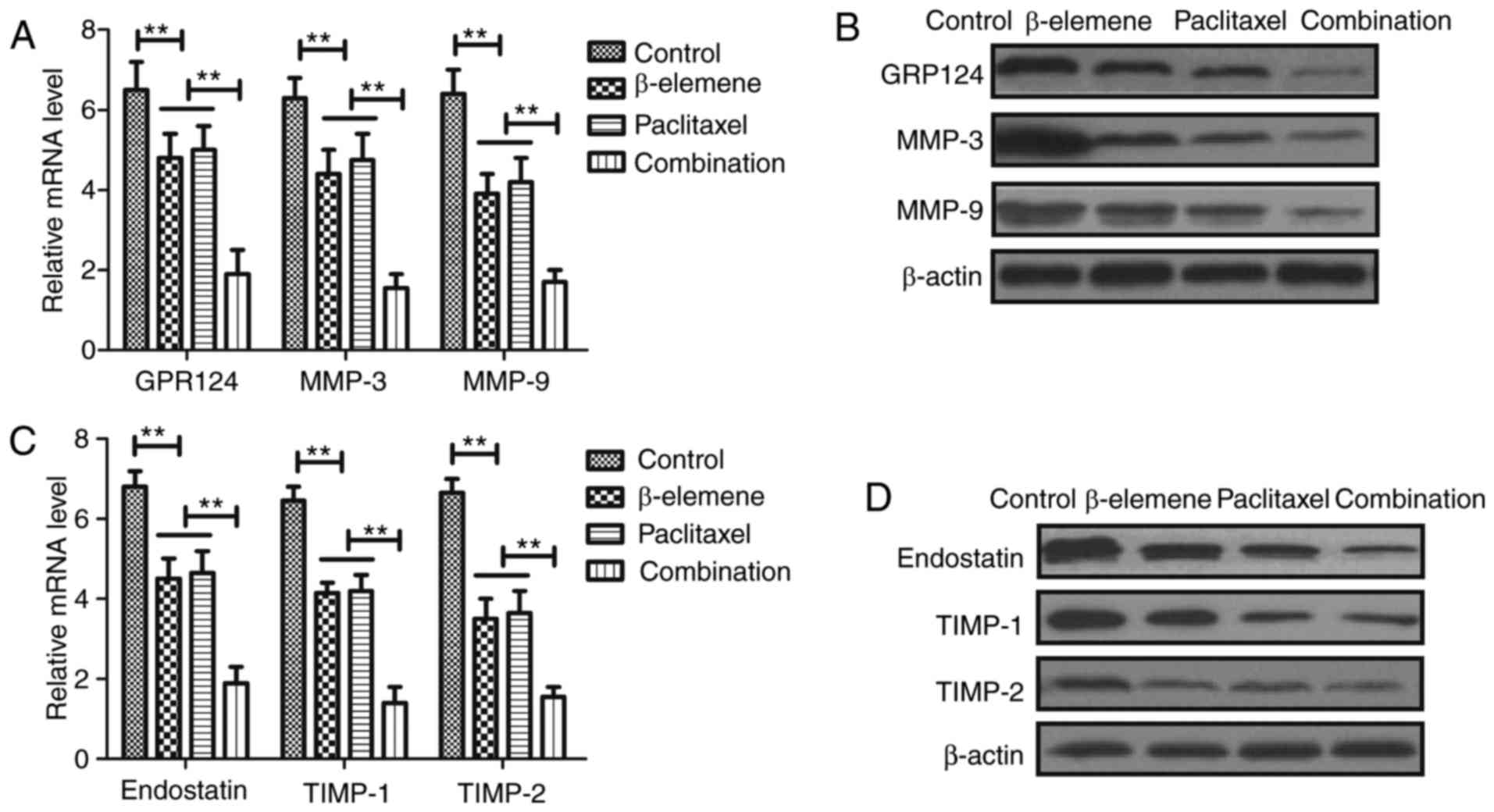
Treatment of β-elemene-paclitaxel on
tumor angiogenesis-related gene expression in U-2OS cells
In order to analyze the inhibitory effects of
treatment of β-elemene-paclitaxel, apoptosis tumor
angiogenesis-related gene expression levels were detected in U-2OS
cells. Results demonstrated that β-elemene-paclitaxel combination
significantly decreased GPR124, MMP-3 and MMP-9 gene and protein
expression levels in U-2OS cells (Fig.
4A, B). However, we showed that β-elemene-paclitaxel
combination significantly increased endostatin, TIMP-1 and TIMP-2
expression in U-2OS cells compared single treatment of β-elemene
and paclitaxel (Fig. 4C, D).
Collectively, these results indicate that Treatment of
β-elemene-paclitaxel is beneficial for controlling U-2OS cells
growth by regulation of apoptosis tumor angiogenesis-related gene
expression in U-2OS cells.
In vivo efficacy of
β-elemene-paclitaxel in tumor-bearing mice
Finally, the in vivo efficacy of
β-elemene-paclitaxel treatment was investigated in U-2OS-bearing
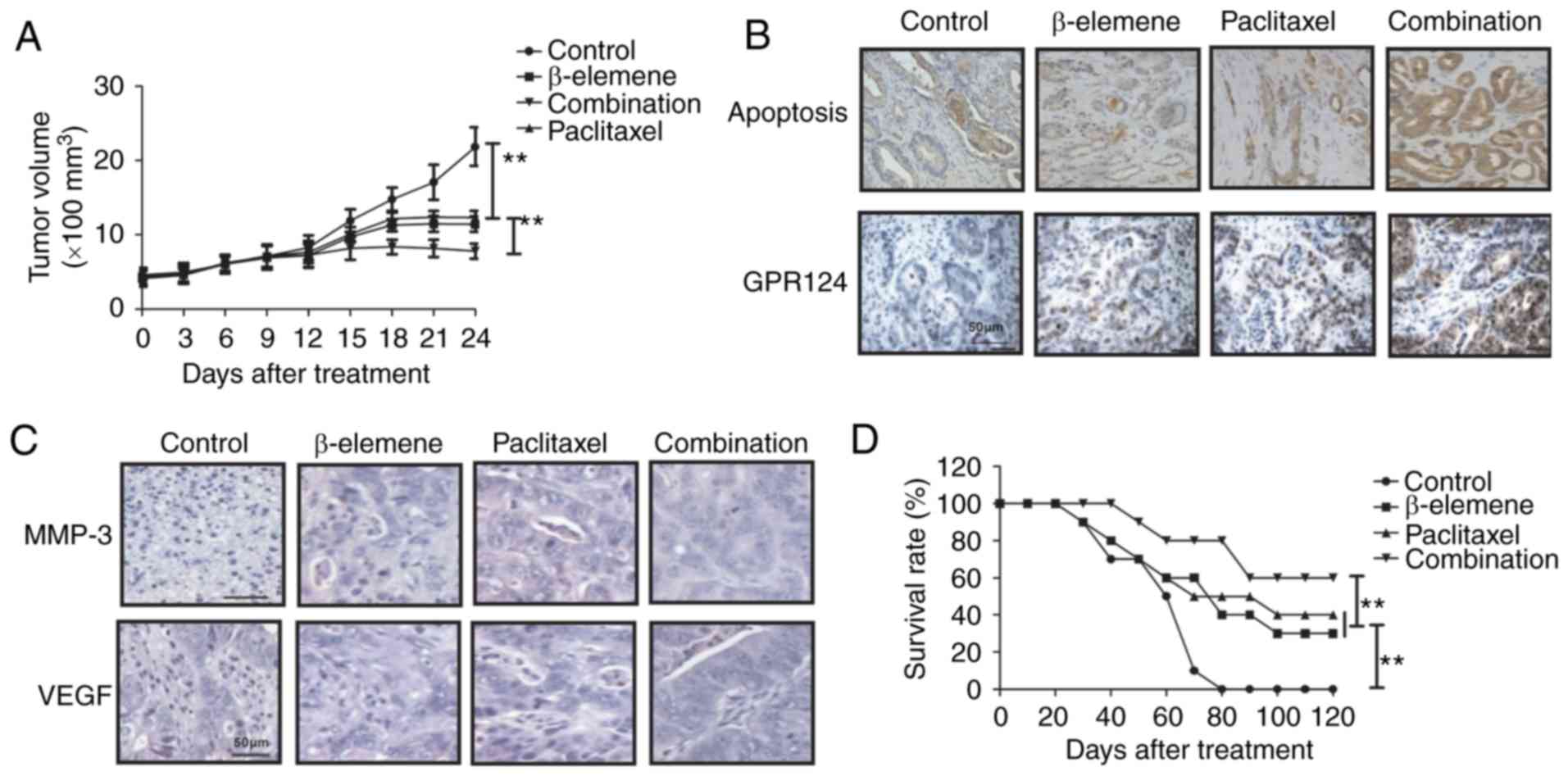
mouse model. As shown in Fig. 5A, we
showed that β-elemene-paclitaxel treatment significantly inhibited
tumor growth compared to either β-elemene or paclitaxel treatment
in a 24-day observation. Immunostaining demonstrated that
β-elemene-paclitaxel treatment increased numbers of apoptotic body
and GPR124 expression in tumor tissue compared to β-elemene or
paclitaxel treatment group (Fig. 5B).
Immunohistochemistry assays demonstrated that MMP-3 and VEGF
expression levels were significantly increased in tumor tissue
after β-elemene-paclitaxel treatment compared to β-elemene or
paclitaxel treatment groups (Fig.
5C). 120-day observation indicated that β-elemene-paclitaxel
treatment promoted survival rate of tumor-bearing mice (Fig. 5D). These results suggest that
β-elemene-paclitaxel treatment is more effective in inhibition of
U-2OS cells growth in vivo.
Discussion
Bone neoplasm is one kind of malignant tumors that
cells occur in skeleton and its affiliates, which has been reported
to present aberrant growth and migration in osseous tissues
(18,19). Evidences have indicated that systemic
administration of paclitaxel can be regarded as efficient therapy
for human prostate cancer metastasis in bone of nude mice by
simultaneous blockade of platelet-derived growth factor-receptor
and epidermal growth factor-receptor signaling (20). In addition, antitumor effect of
β-elemene for cancer cells is mediated by induction of cell cycle
arrest and apoptotic cell death (21). In this study, we analyzed combined
treatment of β-elemene and paclitaxel on bone neoplasm both in
vitro and in vivo. Findings in this study showed that
combined treatment of β-elemene and paclitaxel significantly
inhibited growth and aggressiveness of U-2OS cells. In vitro
and in vivo assays have demonstrated that
β-elemene-paclitaxel treatment induced apoptosis and increased
numbers of apoptotic body in tumor tissue compared to control
groups.
Currently, β-elemene in combination with cisplatin
has been regarded as a regimen for prostate cancer chemotherapy
through regulation of apoptosis-related gene in tumor cells
(22). Liu et al have
suggested that β-elemene induces apoptosis as well as protective
autophagy in human non-small-cell lung cancer A549 cells by
inhibiting the activity of the PI3K/Akt/mTOR/p70S6K1 signalling
pathway (23). Results in this study
demonstrated that β-elemene could induce apoptosis of U-2OS cells
by reduction of anti-apoptosis gene and increasing pro-apoptosis
gene expression levels. Zhang et al also indicated that
β-elemene blocks epithelial-mesenchymal transition in human breast
cancer cell line MCF-7 through Smad3-mediated down-regulation of
nuclear transcription factors (24).
This study found that β-elemene inhibited tumor
angiogenesis-related gene GPR124, VEGFR, MMP-3 and MMP-9 expression
in U-2OS cells, while increased endostatin, TIMP-1 and TIMP-2 gene
and protein expression in U-2OS cells. Reports have showed that
GPR124 could affect migration and differentiation in endothelial
cells in the generation and growth process of blood vessel. The
role of GPR124 in endothelial cells regulates VEGF-induced tumor
angiogenesis has been reported determined by the growth and the
metastasis of in some tumors (25).
Our results showed that β-elemene treatment led to decreasing of
GPR124 and VEGF in U-2OS cells.
The combined treatments for human cancers could
efficiently inhibit growth and prolong survival of cancer patients,
including chemotherapeutic and antiangiogenic drugs, as well as
targeting moieties (26). Previous
studies have indicated that antiangiogenic antitumor activity of
paclitaxel is efficiently for inhibiting breast cancer bone
metastasis mouse model (27,28). Although the emergence of adjuvant and
neoadjuvant chemotherapy has been greatly improved the survival
rate of patients with bone cancer, the morbidity and mortality rate
of osteosarcoma is still keeping a steady increase (29). This research found that paclitaxel can
inhibit the hematogenous metastasis and lymphatic metastasis of
tumor via down-regulation for the protein level of GPR124, VEGF,
MMP-3 and MMP-9 in U-2OS cells, which indicated that paclitaxel
could inhibit the hematogenous metastasis and tumor growth through
reducing vascular growth factor and its receptors. Importantly, we
found that paclitaxel induced apoptosis of U-2OS cells and combined
β-elemene and paclitaxel promoted apoptosis of U-2OS cells both
in vitro and in vivo. Previous reports have showed
that gene expression levels of MDR1, LRP and TS played important
role in increasing the risk of drug-resistance in the treatment of
human cancer (30–32). Interestingly, our results found that
β-elemene decreased drug resistant genes expression levels of MDR1,
LRP and TS in U-2OS cells, which contributed to apoptosis of U-2OS
cells.
In conclusion, the combined treatment of β-elemene
and paclitaxel enhanced the inhibition of U-2OS cells growth and
aggressiveness, as well as increasing apoptosis both in
vitro and in vivo. Especially, it is proved through the
molecular biology experiment that the combined effect of β-elemene
and paclitaxel can stimulate apoptosis and decrease the expression
of GPR124, which further led to inhibition of growth and metastasis
and arresting of cells cycle. Notably, tumor growth can be
effectively inhibited through regulating the expression of GPR124
in bone cancer cells that contributed to long survival of
tumor-bearing mice.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
ZW and YL constructed the experiments and organized
the data, FZ, ZP, JH assisted in the analysis of data and ZW wrote
the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Nankai Hospital of Tianjin.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Maeyama I: Review of bone tumor. Iryo.
24:(Suppl):. S2271970.(In Japanese).
|
|
2
|
Sanchez-Pareja A, Larousserie F,
Boudabbous S, Beaulieu JY, Mach N, Saiji E and Rougemont AL: Giant
cell tumor of bone with pseudosarcomatous changes leading to
premature denosumab therapy interruption: A case report with review
of the literature. Int J Surg Pathol. 24:366–372. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Huang L, Garcia-Manero G, Jabbour E,
Goswami M, Routbort MJ, Medeiros LJ, Jorgensen JL and Wang SA:
Persistence of immunophenotypically aberrant CD34+ myeloid
progenitors is frequent in bone marrow of patients with
myelodysplastic syndromes and myelodysplastic/myeloproliferative
neoplasms treated with hypomethylating agents. J Clin Pathol.
pii:jclinpath-2016-203715. 2016.
|
|
4
|
Sever C, Abbott CL, de Baca ME, Khoury JD,
Perkins SL, Reichard KK, Taylor A, Terebelo HR, Colasacco C, Rumble
RB and Thomas NE: Bone marrow synoptic reporting for hematologic
neoplasms: Guideline from the college of American pathologists
pathology and laboratory quality center. Arch Pathol Lab Med.
140:932–949. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lekovic D, Gotic M, Skoda R,
Beleslin-Cokic B, Milic N, Mitrovic-Ajtic O, Nienhold R, Sefer D,
Suboticki T and Buac M: et alBone marrow microvessel density
and plasma angiogenic factors in myeloproliferative neoplasms:
Clinicopathological and molecular correlations. Ann Hematol.
96:393–404. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Song T, Zhang X, Fang M, Zhao R and Wu S:
Long-term results of definitive concurrent chemoradiotherapy using
paclitaxel plus oxaliplatin in unresectable locally advanced
esophageal cancer: A prospective phase II trial. Cancer Med.
5:3371–3377. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fukuchi M, Mochiki E, Ishiguro T, Ogura T,
Sobajima J, Kumagai Y, Ishibashi K and Ishida H: Efficacy of
Nab-Paclitaxel as second-line chemotherapy for unresectable or
recurrent gastric cancer. Anticancer Res. 36:6699–6703. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Garon EB, Neidhart JD, Gabrail NY, de
Oliveira MR, Balkissoon J and Kabbinavar F: A randomized Phase II
trial of the tumor vascular disrupting agent CA4P (fosbretabulin
tromethamine) with carboplatin, paclitaxel, and bevacizumab in
advanced nonsquamous non-small-cell lung cancer. Onco Targets Ther.
9:7275–7283. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kreger BT, Johansen ER, Cerione RA and
Antonyak MA: The enrichment of survivin in exosomes from breast
cancer cells treated with paclitaxel promotes cell survival and
chemoresistance. Cancers. 8(pii): E1112016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu X, Gao Y, Shen J, Yang W, Choy E,
Mankin H, Hornicek FJ and Duan Z: Cyclin-dependent kinase 11
(CDK11) is required for ovarian cancer cell growth in vitro and in
vivo, and its inhibition causes apoptosis and sensitizes cells to
paclitaxel. Mol Cancer Ther. 15:1691–1701. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Awasthi N, Scire E, Monahan S, Grojean M,
Zhang E, Schwarz MA and Schwarz RE: Augmentation of response to
nab-paclitaxel by inhibition of insulin-like growth factor (IGF)
signaling in preclinical pancreatic cancer models. Oncotarget.
7:46988–47001. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang H, Li D, Li X, Ou X, Liu S, Zhang Y,
Ding J and Xie B: Mammalian target of rapamycin inhibitor RAD001
sensitizes endometrial cancer cells to paclitaxel-induced apoptosis
via the induction of autophagy. Oncol Lett. 12:5029–5035. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Şakalar Ç, İzgi K, İskender B, Sezen S,
Aksu H, Çakır M, Kurt B, Turan A and Canatan H: The combination of
thymoquinone and paclitaxel shows anti-tumor activity through the
interplay with apoptosis network in triple-negative breast cancer.
Tumour Biol. 37:4467–4477. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang B, Peng XX, Sun R, Li J, Zhan XR, Wu
LJ, Wang SL and Xie T: Systematic review of β-elemene injection as
adjunctive treatment for lung cancer. Chin J Integr Med.
18:813–823. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu J, Zhang Y, Qu J, Xu L, Hou K, Zhang
J, Qu X and Liu Y: β-Elemene-induced autophagy protects human
gastric cancer cells from undergoing apoptosis. BMC Cancer.
11:1832011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li QQ, Wang G, Huang F, Banda M and Reed
E: Antineoplastic effect of beta-elemene on prostate cancer cells
and other types of solid tumour cells. J Pharm Pharmacol.
62:1018–1027. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oberhaus SM: TUNEL and immunofluorescence
double-labeling assay for apoptotic cells with specific antigen(s).
Methods Mol Biol. 218:85–96. 2003.PubMed/NCBI
|
|
18
|
Li YF, Cha TL, Jin JS and Yu CP:
Chromophobe renal cell carcinoma with osteosarcoma differentiation:
Case report and literature review. Urol Int. 85:470–474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kager L, Pötschger U and Bielack S: Review
of mifamurtide in the treatment of patients with osteosarcoma. Ther
Clin Risk Manag. 6:279–286. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim SJ, Uehara H, Yazici S, Langley RR, He
J, Tsan R, Fan D, Killion JJ and Fidler IJ: Simultaneous blockade
of platelet-derived growth factor-receptor and epidermal growth
factor-receptor signaling and systemic administration of paclitaxel
as therapy for human prostate cancer metastasis in bone of nude
mice. Cancer Res. 64:4201–4208. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang G, Li X, Huang F, Zhao J, Ding H,
Cunningham C, Coad JE, Flynn DC, Reed E and Li QQ: Antitumor effect
of beta-elemene in non-small-cell lung cancer cells is mediated via
induction of cell cycle arrest and apoptotic cell death. Cell Mol
Life Sci. 62:881–893. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li QQ, Wang G, Reed E, Huang L and Cuff
CF: Evaluation of cisplatin in combination with β-elemene as a
regimen for prostate cancer chemotherapy. Basic Clin Pharmacol
Toxicol. 107:868–876. 2010.PubMed/NCBI
|
|
23
|
Liu J, Hu XJ, Jin B, Qu XJ, Hou KZ and Liu
YP: β-Elemene induces apoptosis as well as protective autophagy in
human non-small-cell lung cancer A549 cells. J Pharm Pharmacol.
64:146–153. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang X, Li Y, Zhang Y, Song J, Wang Q,
Zheng L and Liu D: Beta-elemene blocks epithelial-mesenchymal
transition in human breast cancer cell line MCF-7 through
Smad3-mediated down-regulation of nuclear transcription factors.
PLoS One. 8:e587192013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, Cho SG, Wu X, Siwko S and Liu M:
G-protein coupled receptor 124 (GPR124) in endothelial cells
regulates vascular endothelial growth factor (VEGF)-induced tumor
angiogenesis. Curr Mol Med. 14:543–554. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Airley R: Lab reports and cat scans: Can
veterinary oncology guide our way to new treatments for human
cancers? Future Med Chem. 4:1391–1394. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ding D and Kong WM: Analysis of relative
factors of bone marrow suppression after chemotherapy with
carboplatin and paclitaxel on the patients with ovarian cancer.
Zhonghua fu chan ke za zhi. 46:188–192. 2011.(In Chinese).
PubMed/NCBI
|
|
28
|
Miller K, Eldar-Boock A, Polyak D, Segal
E, Benayoun L, Shaked Y and Satchi-Fainaro R: Antiangiogenic
antitumor activity of HPMA copolymer-paclitaxel-alendronate
conjugate on breast cancer bone metastasis mouse model. Mol Pharm.
8:1052–1062. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
He H, Ni J and Huang J: Molecular
mechanisms of chemoresistance in osteosarcoma (Review). Oncol Lett.
7:1352–1362. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen JR, Jia XH, Wang H, Yi YJ, Wang JY
and Li YJ: Timosaponin A-III reverses multi-drug resistance in
human chronic myelogenous leukemia K562/ADM cells via
downregulation of MDR1 and MRP1 expression by inhibiting PI3K/Akt
signaling pathway. Int J Oncol. 48:2063–2070. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kurata M, Hasegawa M, Nakagawa Y, Abe S,
Yamamoto K, Suzuki K and Kitagawa M: Expression dynamics of drug
resistance genes, multidrug resistance 1 (MDR1) and lung resistance
protein (LRP) during the evolution of overt leukemia in
myelodysplastic syndromes. Exp Mol Pathol. 81:249–254. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ijichi K, Adachi M, Ogawa T, Hasegawa Y
and Murakami S: Cell-cycle distribution and Thymidilate Synthatase
(TS) expression correlate with 5-FU resistance in head and neck
carcinoma cells. Anticancer Res. 34:2907–2911. 2014.PubMed/NCBI
|