Introduction
Colorectal cancer (CRC), a cancer that arises from
uncontrolled cell growth in the colon, rectum or the appendix, has
become the third most common malignant disease worldwide with a
yearly increasing incidence and mortality rate, and it is among the
leading causes of cancer-associated mortality (1). It is the fourth leading cause of
cancer-associated mortality in China (2). The incidence of CRC is increasing
rapidly with ~1 million new CRC cases reported annually (3). In developed countries, the mortality of
CRC increased to 33% between 2010 to 2013 (3). Although the 5-year survival rate of
patients with CRC has been improved from 22–47% in the last 30
years with advancements in early diagnosis and therapeutic
interventions, the overall survival rate remains pessimistic
(3). The pathogenesis of CRC is not
yet completely understood. It is currently known that colorectal
carcinogenesis involves numerous molecular processes, including the
activation of oncogenes, the mutation of mismatch repair genes or
the inactivation of tumor suppressor genes, which affect the
proliferation, migration, invasion and apoptosis of cancer cells,
among other features (2).
Inhibitor of growth protein 4 (ING4), encoded by the
ING4 gene, serves an essential function in cancer-associated
cellular progression. It can interact with p53 and act as a tumor
suppressor, thus affecting cell proliferation, migration and
apoptosis, angiogenesis, contact inhibition and the DNA damage
response (4,5). Previous studies have demonstrated that
ING4 expression is decreased in various types of cancer, including
lung cancer, gastric carcinoma, colon cancer, breast cancer,
melanoma and hepatocellular carcinoma, suggesting that the function
of ING4 is to suppress tumor growth, angiogenesis and invasion in a
number of types of cancer (6).
Furthermore, overexpression of ING4 has been demonstrated to impair
colony-forming efficiency, reduce the population of cells in the S
phase and to induce cancer cell migration, invasion and apoptosis
(7,8).
In a previous study, it was identified that ING4 protein expression
was downregulated in adenoma relative to in the normal mucosa, and
was further decreased in CRC tissues (9). Furthermore, the suppression of ING4
expression was also associated with a more advanced Dukes stage
(9). Therefore, it was hypothesized
that ING4 serves important functions in colorectal carcinoma
progression; however, the underlying molecular mechanism remains
unresolved.
MicroRNAs (miR) are small noncoding RNA strands of
19–25 nucleotides in length. MiRs anneal inexactly to complementary
sequences in the 3′-untranslated region (3′-UTR) of the target
mRNAs of protein-coding genes and trigger cleavage of these target
mRNAs, or they inhibit protein translation as key
post-transcriptional regulators (10). In addition to gene inactivation,
increasing evidence has identified that miRs are involved in a
number of biological processes, including cell proliferation,
differentiation, metastasis, apoptosis and immune responses
(11). Furthermore, miRs may function
as tumor suppressors or oncogenes in tumorigenesis, and have
demonstrated prognostic significance for several tumor types
(12,13). miR-650 is a previously reported miR,
which has been revealed to target ING4 in order to promote gastric
cancer tumorigenicity (14). Further
investigation indicates that miR-650 targets the promoter region of
the NDRG2 gene and represses its transcription (15). The upregulation of miR-650 has been
associated with ING4 downregulation and the progression of
hepatocellular carcinoma (16). In an
additional study, miR-650 was reported to be upregulated in several
types of human colorectal cancer (17). However, the underlying molecular
mechanism of miR-650 in CRC progression remains unclear.
Mitogen-activated protein kinases (MAPKs) belong to
a family of serine/threonine-specific protein kinases, including
three major MAPKs: Extracellular signal-regulated kinases (ERK1/2),
c-Jun N-terminal kinases and p38 MAPKs (18,19). MAPKs
are involved in directing cellular responses to a diverse array of
stimuli, including mitogens, osmotic stress, heat shock proteins
and proinflammatory cytokines (20).
MAPKs also function as essential modulators in signal transduction
pathways, regulating gene transcription in response to external
stimuli (18,21,22).
Furthermore, MAPKs have been identified to serve important
functions in mediating cell proliferation, differentiation,
transformation and apoptosis, and are involved in the development
and progression of tumors (23).
In the present study, it was demonstrated that
ING4 was a target gene of miR-650 in CRC. Elevated levels of
miR-650 following transfection with miR-650 mimics contributed to
increased cell vitality, elevated cell invasion and
epithelial-to-mesenchymal transition (EMT). Overexpression of
miR-650 induced the activation of Ras homolog gene family member A
(RhoA)/Ras-related C3 botulinum toxin (Rac1) GTPase and MAPK
signaling. These results indicate that miR-650 serves an important
function in promoting CRC progression, and suggest that miR-650 and
ING4 may be promising biomarkers for CRC diagnosis and therapy.
Materials and methods
Cell lines
Cell lines derived from human CRC (SW480, SW620,
RKO, 320DM, 320HSR, NCI-H716, H508) and the normal liver cell line
CCD841CoN used in this study were purchased from the cell bank of
the American Type Culture Collection (Manassas, VA, USA).
Reagents
MTT was purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). Four synthetic, chemically modified short
double-stranded RNA oligonucleotides (miR-650 mimics, mimics
negative control, miR-650 inhibitor and inhibitor negative control)
were obtained from Guangzhou RiboBio Co., Ltd. (Guangzhou, China).
Primary antibodies against p38 (#9212), phosphorylated (p)-p38
(#4511), ERK1/2 (#4695), p-ERK1/2 (#4370) and RhoA (#2117) were
obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA);
active Rac1 (#26903) and active RhoA (#26904) from NewEast
Biosciences (King of Prussia, PA, USA); E-cadherin (ab1416),
β-catenin (ab32572), Rac1 (ab33186) and FITC-conjugated anti-rat
(ab6717)/anti-mouse IgG (ab6785) secondary antibodies from Abcam
(Cambridge, UK); α-smooth muscle actin (α-SMA, 55135–1-AP) from
ProteinTech Group, Inc. (Chicago, IL, USA); vimentin (BM0135) from
Wuhan Boster Biological Technology, Ltd. (Wuhan, China); and
β-actin (sc-58673) and HRP conjugated goat anti-rabbit/goat
anti-mouse secondary antibodies (sc-2004/sc-2005) from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). The primary antibody ING4
(#40-7700) and the fluorescent dye Alexa Fluor 546 conjugated
phalloidin (A22283, 1:5,000) were purchased from Invitrogen (Thermo
Fisher Scientific, Inc., Waltham, MA, USA).
Cell culture
Cells were cultured in L15 medium (Biological
Industries, Kibbutz Beit Haemek, Israel) supplemented with 10%
fetal bovine serum (Biological Industries USA, Cromwell, CT, USA),
100 U/ml penicillin and 100 µg/ml streptomycin, at 37°C in a
humidified incubator containing 5% CO2.
Transfection
For transfection, miR-650 mimics (100 nM), miR-650
inhibitors and their aforementioned negative controls were
transfected into the SW620 and SW480 cells using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. ING4
3′UTR and the mutant 3′UTR were inserted into the plasmid with a
luciferase reporter gene and then ING4 3′UTR and the mutant 3′UTR
were transfected into the HEK293T cells. The sequences of the
primers are listed as follows: ING4-3′F-UTR-P1:
5′-GCGCGCAAGCTTCAACACAGTTTCTTCCACATCCCC-3′ m-ING4-3′R-UTR-P1:
5′-GCGCTCTAGACTCTACAAAACATTCTTCCATTGTATAGCTTTTATTTAC-3′. ING4-3′
F-UTR-P4: 5′-GCGCATTCTAGACTCTACAATAAACACAGCAGGC-3′
m-ING4-3′R-UTR-P4:
5′-GCGCGCTCTAGACTCTACAATAAACATTCTTTCCCATCTTGTATAGCTTTTATTTACCTACCC-3′.
For ING4 knockdown, the specific shING4, sense:
5′-GATCCGAGGCTGATCTCAAGGAGAAATTCAAGAGATTTCTCCTTGAGATCAGCCTCAGA-3′;
negative control short hairpin RNA (shRNA), sense:
5′-AAGCTGAAGTACAACCTTCTTCAAGAGAGAAGGTTGTACTTCAGCTTAG-3′ was
transfected into SW480 and SW620 cells using Lipofectamine 2000
(Thermo Fisher Scientific, Inc.) with standard transfection
procedures. At 48 h post transfection, 600 µg/ml G418
(Sigma-Adrich; Merck KGaA) was added to select stable
transfectants, and individual clones were isolated and maintained
in a medium containing G418 (200 µg/ml). For ING4 overexpression,
ING4 was inserted into the pcDNA3.1 vector (Invitrogen;
Thermo Fisher Scientific, Inc.), which was then transfected into
the cells. Transfection was performed using Lipofectamine 2000
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. At 48 h post transfection, 600 µg/ml G418 (Sigma-Aldrich;
Thermo Fisher Scientific, Inc.) was added to select stable
transfectants, and individual clones were isolated and maintained
in a medium containing G418 (200 µg/ml).
Cell viability assay
An MTT assay was used to determine cell viability.
Individual wells of a 96-well plate were inoculated with 100 µl of
L15 medium containing 5×104 cells. Cells were
transfected with miR-650 mimics, miR-650 inhibitors and their
negative controls. A total of 20 µl MTT solution (0.5 mg/ml) was
added to each well and incubated at 37°C for 4 h. The culture
medium was removed and 200 µl dimethyl sulfoxide was added to each
well to dissolve the purple formazan for 10 min at room
temperature. The absorbance values were read at 570 nm using an
Infinite m200pro microplate spectrophotometer (Tecan Group, Ltd.,
Männedorf, Switzerland).
Wound healing assay
Cells were seeded in a 24-well plate and cultured to
100% confluency, following which the cell monolayer was scratched
in a straight line using a pipette tip (200 µl). In order to remove
the debris and smooth the edge of the scratch, culture medium was
removed and the wells were washed three times with 1 ml growth
medium. After 24 h of culture, the scratch was viewed using a
microscope (24).
Total RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA from the cultured cell lines was isolated
using TRIzol® reagent (Life Sciences; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
relative expression of miR-650 and ING4 were determined using the
SYBR Green I method on a CFX96 Real-Time C1000 Touch Thermocycler
system (Bio-Rad Laboratories, Inc., Hercules, CA, USA) with
U6 and GAPDH as the internal controls. Each reaction
was carried out in triplicate.
Immunofluorescence staining
Cells cultured in 6-well plates were fixed with 4%
paraformaldehyde in PBS (pH=7.35) for 15 min at room temperature.
Nonspecific binding was blocked for 2 h with goat serum
(Sigma-Aldrich; Merck KGaA,), and the cells were subsequently
incubated with the aforementioned primary antibodies against
E-cadherin, β-catenin, α-SMA and vimentin (all diluted to 1:50) at
4°C overnight. The cells were washed and incubated with the
FITC-conjugated anti-rat (dilution, 1:2,000) or anti-mouse IgG
(dilution, 1:2,000) secondary antibodies for 1 h. The cells were
visualized using a confocal microscope (magnification −100).
Protein extraction and western
blotting
Cell samples were lysed in radioimmunoprecipitation
assay buffer (Thermo Fisher Scientific, Inc.) with a protease
inhibitor and phosphatase inhibitor cocktail (cat no. ab201119;
Abcam), prior to protein extraction. Proteins were quantified using
a Bio-Rad protein assay kit (cat no. 5000002, Bio-Rad Laboratories,
Inc., Hercules, CA, USA), and equal quantities (10 µg) were
fractionated using SDS-PAGE (4%) and transferred to a
polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA,
USA). The membranes were blocked with 5% bovine serum albumin
(Sigma-Aldrich, Merck KGaA) for 2 h at room temperature with
agitation, and then incubated with the aforementioned primary
antibodies, including those against ING4, E-cadherin, β-catenin,
α-SMA, vimentin, RhoA, active RhoA, Rac1, active Rac1, ERK1/2,
p-ERK1/2, p38 and p-p38, each at a dilution of 1:1,000, at 4°C
overnight. Following three washes with Tris-buffered saline
containing 0.5% Tween 20, the membranes were incubated with
horseradish peroxidase-conjugated secondary antibodies (dilution,
1:5,000) at room temperature for 1 h. Bands were observed using an
Odyssey infrared imaging system (LI-COR Biosciences, Lincoln, NE,
USA). β-actin was used as the internal control.
Statistical analysis
All data are presented as the mean ± standard error
of the mean. Multiple comparisons were analyzed statistically using
Mann-Whitney U test or one-way analysis of variance followed by a
Student-Newman-Keuls post-hoc test. All analysis was performed
using GraphPad Prism software version 5.0 (GraphPad Software, Inc.,
La Jolla, CA, USA). A two-tailed value of P<0.05 was considered
to indicate a statistically significant difference.
Results
The expression of ING4 in various CRC
cell lines
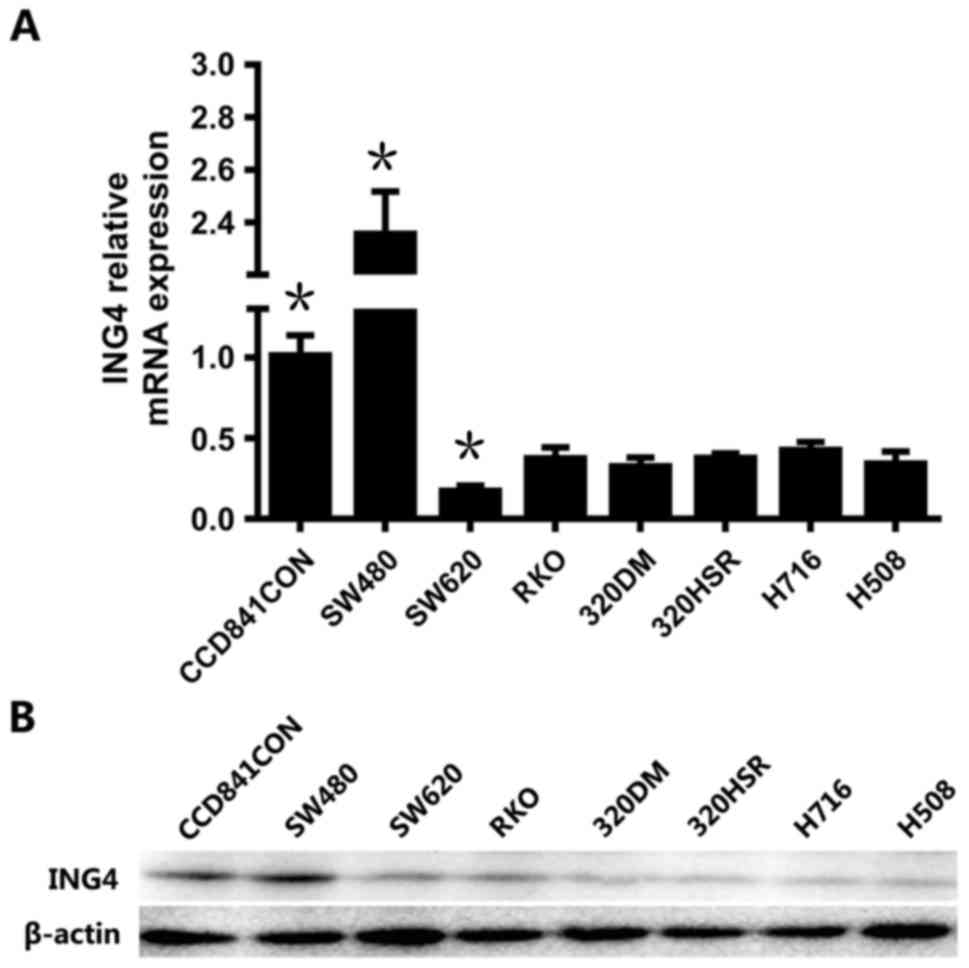
In order to investigate ING4 expression in CRC
cells, including the cell lines SW480, SW620, RKO, 320DM, 320HSR,
NCI-H716 and H508, RT-qPCR and western blotting were performed. The
normal colonic epithelial cell line CCD841CoN was used as the
control. As presented in Fig. 1A, the
mRNA expression of ING4 was decreased compared with the
control in all the CRC cell lines except SW480 (P<0.05),
suggesting the decreasing trend of ING4 in CRC, which is consistent
with the expression in tissues of patients (9). The expression level of ING4 in
SW480 was markedly increased compared with the control (P<0.05).
ING4 expression in SW620 was lower than all other CRC cell lines
(P<0.05). Therefore, SW480 and SW620 cell lines were selected
for subsequent experiments. The protein levels of ING4 in various
cell lines revealed similar trends with the mRNA levels (Fig. 1B).
MiR-650 targets the 3′UTR of ING4 in
CRC cells
It has been reported that miR-650 targets
ING4 to promote gastric cancer tumorigenicity (14). Therefore, the present study
investigated whether miR-650 targets ING4 in CRC. Following
the transfection of miR-650 mimics, miR-650 mimics negative control
(NC), miR-650 inhibitors and miR-650 inhibitors NC, the level of
miR-650, and the mRNA and protein levels of ING4 were analyzed
using RT-qPCR and western blotting, respectively. As presented in
Fig. 2A, the levels of miR-650 in
SW480 and SW620 were significantly increased compared with those in
the miR-650 mimics NC, miR-650 inhibitors and miR-650 inhibitors NC
groups (P<0.05). As predicted, the mRNA level of ING4 was
significantly attenuated following miR-650 transfection, relative
to the controls (P<0.05). The changes in ING4 protein levels
were consistent with the mRNA levels (Fig. 2B).
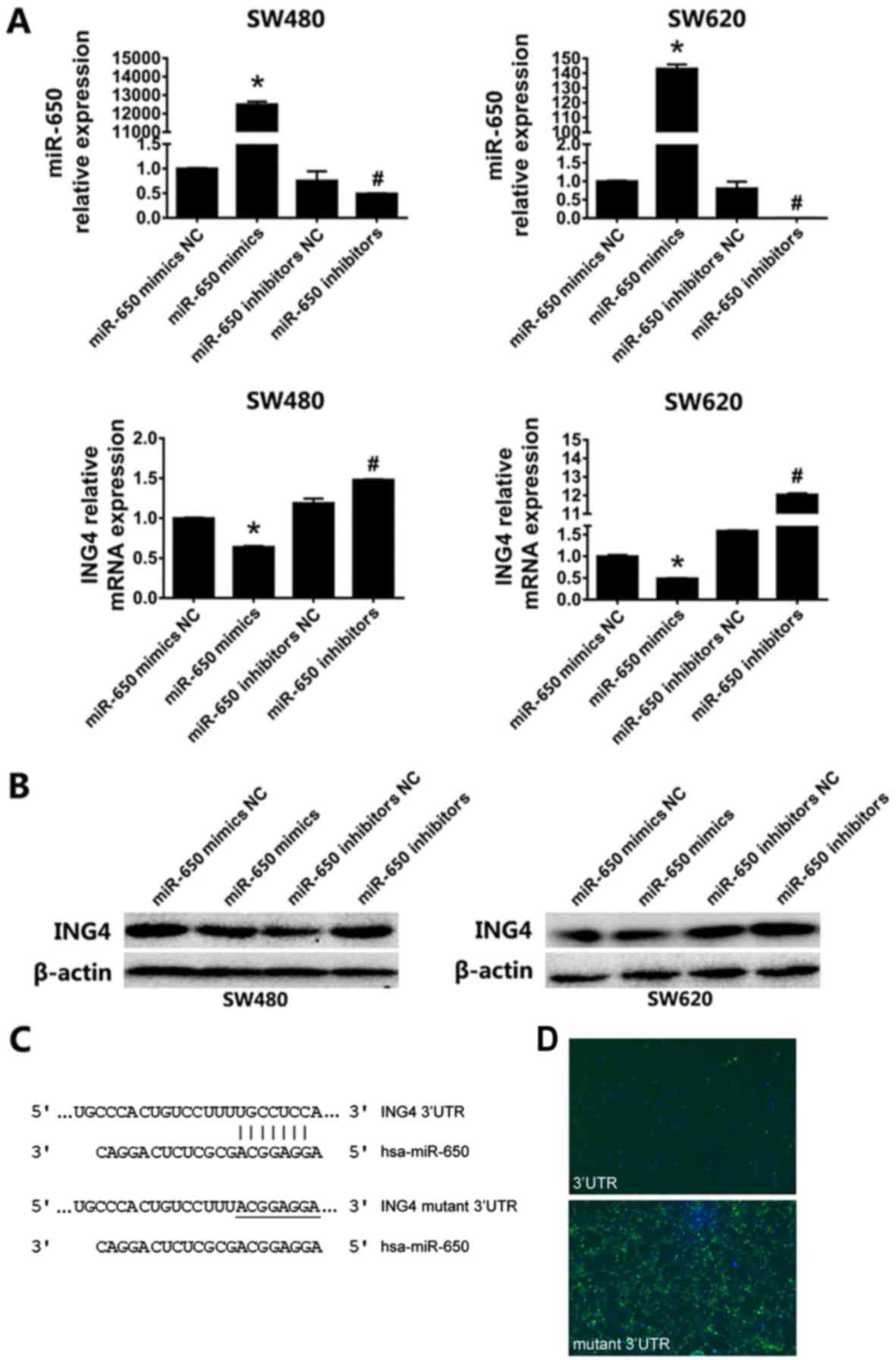 | Figure 2.MiR-650 targets the 3′UTR of ING4 in
CRC cells. (A) Reverse transcription-quantitative polymerase chain
reaction analysis of the expression of miR-650 and ING4 in
SW480 and SW620 cells following transfection with miR-650 mimics,
miR-650 mimics NC, miR-650 inhibitors and miR-650 inhibitors NC.
The relative expression of miR-650 and ING4 was normalized
to that of U6 and β-actin, respectively. (B) Western
blot analysis of the protein levels of ING4 following miR-650
mimics transfection. (C) The core nucleotide sequence of
ING4 3′UTR containing the predicted binding site matched by
miR-650. The matched nucleotides are indicated with vertical lines,
and the mutant residues in ING4 3′UTR are underlined. (D)
Immunofluorescence verification the targeting of miR-650 to the
3′UTR of ING4. The ING4 3′UTR and its mutant were
constructed into the plasmid with luciferase reporter and
transfected with miR-650 mimics into HEK293T cells, respectively.
Slides were analyzed under a light microscope (BX53; Olympus
Corporation, Tokyo, Japan) using a BX3-URA fluorescence system
(Olympus Corporation) (magnification −100). *P<0.05 vs. control
group.miR-650 mimics NC, miR-650 inhibitors and miR-650 inhibitors
NC groups. #P<0.05 vs.control group.miR-650 mimics
NC, miR-650 mimics and miR-650 inhibitors NC groups. NC, negative
control; miR, micro RNA; ING4, inhibitor of growth protein 4. |
According to the miRDB and TargetScan databases,
miR-650 was predicted to bind to the 3′UTR of ING4, and the
core sequence containing the binding site is presented in Fig. 2C. The ING4 3′UTR and the mutant
3′UTR were inserted into the plasmid with a luciferase reporter
gene. Following the co-transfection of miR-650 mimics with the
plasmid containing ING4 3′UTR, almost no fluorescence was
observed. However, when co-transfected with the mutant 3′UTR,
marked fluorescence was observed (Fig.
2D). These results indicate that miR-650 negatively regulates
the expression of ING4 by targeting the 3′UTR of ING4
transcripts in CRC cells.
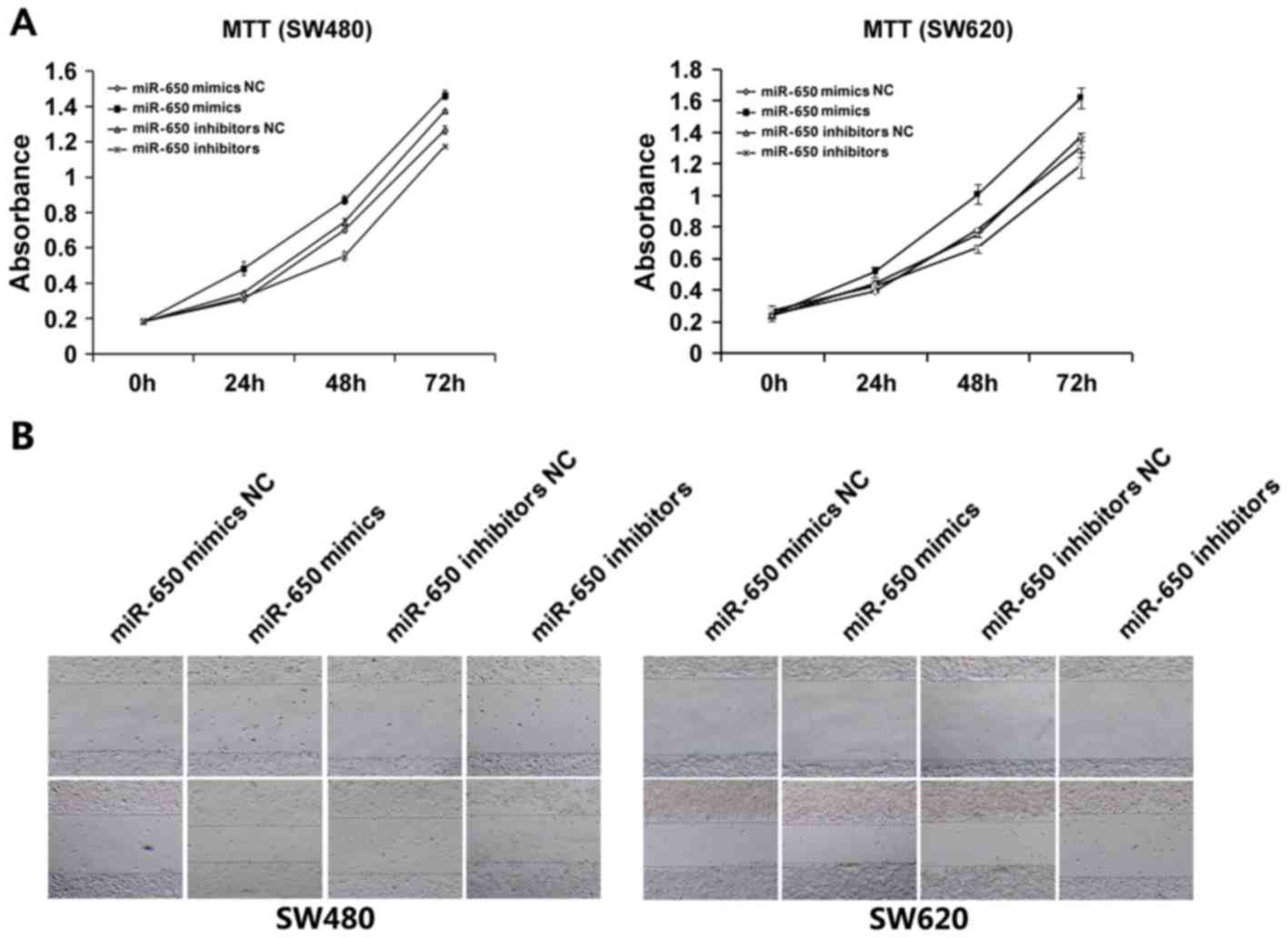
MiR-650 promotes the proliferation and
migration of CRC cells
To further resolve the function of miR-650 during
tumorigenesis, SW480 and SW620 cells were transfected with miR-650
mimics, miR-650 mimics NC, miR-650 inhibitors and miR-650
inhibitors NC. An MTT assay was performed to examine cell
viability. The results revealed that cell proliferation in the
group transfected with miR-650 mimics was increased compared with
in the group transfected with miR-650 mimics NC; proliferation in
the group transfected with the miR-650 inhibitor was decreased
compared with in the group transfected with the miR-650 inhibitor
NC (Fig. 3A).
The effect of miR-650 on the cell migration was
evaluated using a wound healing assay. The results indicated that
transfection of miR-650 mimics increased the migration of SW480
cell lines compared with the respective control groups (Fig. 3B), which suggest that miR-650
upregulation may be associated with tumor growth and migration in
CRC.
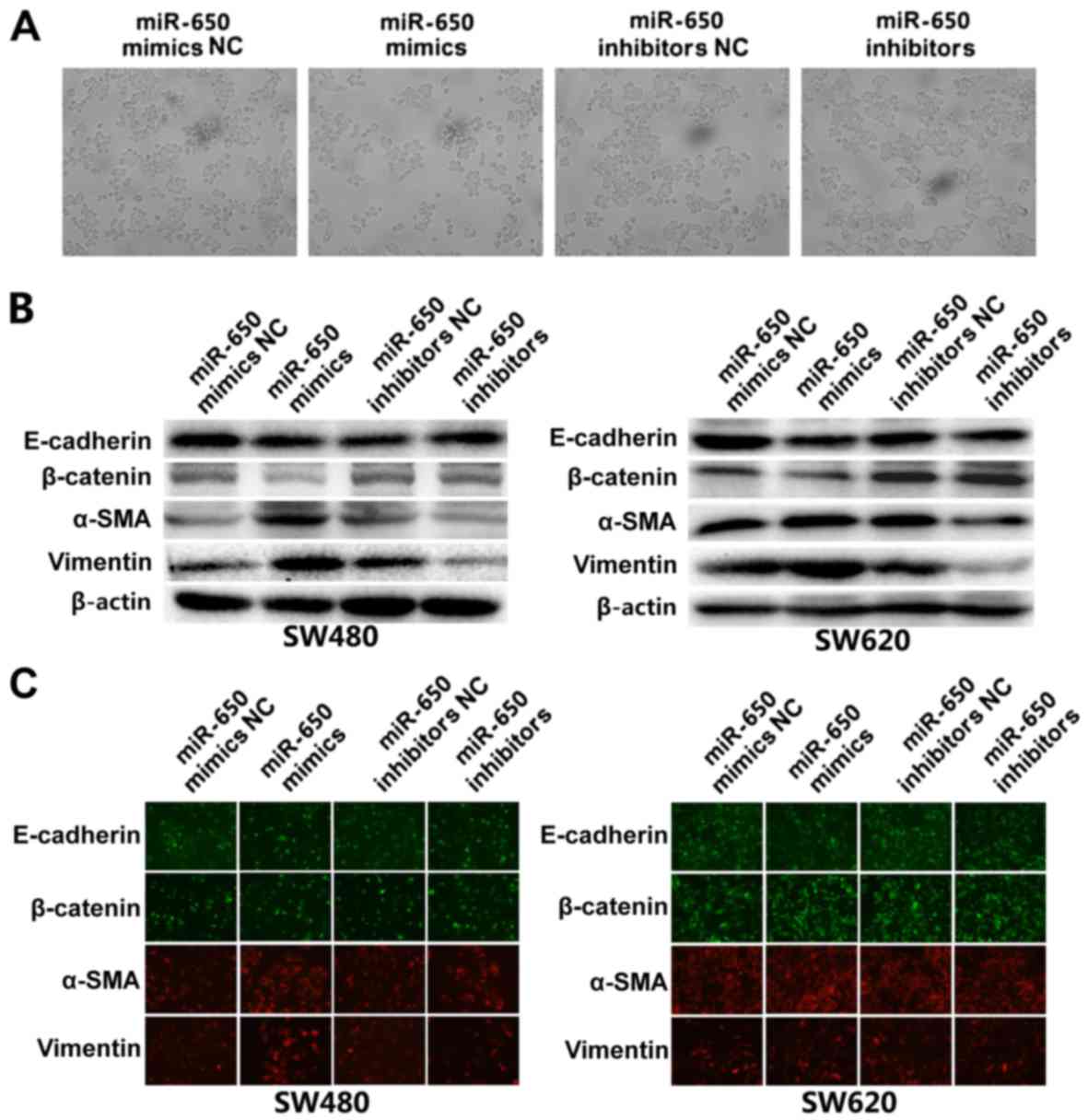
MiR-650 induces EMT in SW620 and SW480
cell lines
Following transfection with miR-650 mimics, various
morphological changes, including spindle-shaped and fibroblastic
cell morphology, as well as scattering and decreased cell-cell
contact were observed in SW480 and SW620 cells (Fig. 4A), suggesting that these cells may
undergo EMT progression. EMT is an essential event in tumor
invasion and metastasis, which is characterized by the decreased
presence of certain epithelial markers (E-cadherin and β-catenin)
and upregulated mesenchymal markers (α-SMA and vimentin) (25). In the present study, western blotting
was used to further verify this EMT progression in the cell lines.
As presented in Fig. 4B, in miR-650
mimics-transfected cells, the expression of E-cadherin and
β-catenin was markedly decreased. On the contrary, mesenchymal
markers, including vimentin and α-SMA, were markedly elevated in
SW480 and SW620 cells (Fig. 4B).
Immunofluorescence indicated decreased expression of E-cadherin,
β-catenin and increased expression of SMA in miR-650
mimics-transfected cells compared with control (Fig. 4C). These data reinforce that the
overexpression of miR-650 induces EMT in SW480 and SW620 cell
lines.
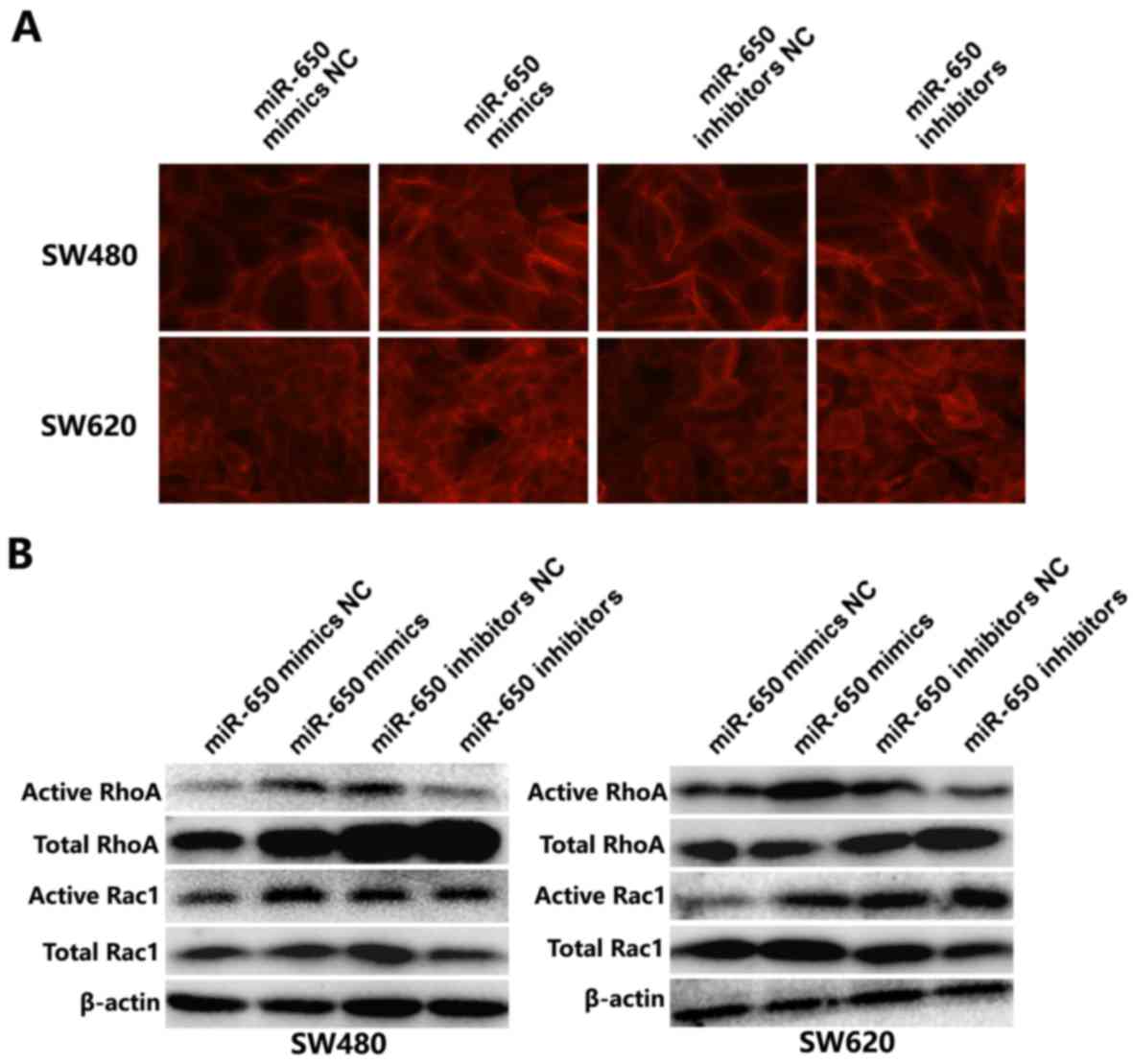
MiR-650 induces the activation of
Rho/Rac GTPases in SW620 and SW480 cell lines
Since miR-650 transfected cells exhibited
morphological alterations, the effects of miR-650 on modulating
cytoskeleton rearrangement were further investigated. F-actin
staining by phalloidin revealed that stress fibers and lamellipodia
were observed in miR-650 transfected cells, but fewer were present
in control cells. By contrast, miR-650 inhibitor group exhibited
reduced stress fibers in the SW620 and SW480 cells (Fig. 5A). During tumor progression,
activation of Rho-GTPase contributes to actin cytoskeleton
reorganization, which in turn causes disruption of adherent
junctions (26,27). Therefore, considering the
morphological alterations, it was further determined whether
Rho-GTPases were activated following miR-650 overexpression.
Western blotting results revealed that the active form of RhoA was
increased in SW480 and SW620 cells when miR-650 was overexpressed,
and decreased following transfection with the miR-650 inhibitor.
The expression of active Rac1 was also elevated by miR-650
overexpression (Fig. 5B). There was
no notable reduction in the expression of active Rac1 in miR-650
inhibitors-transfected cells compared with the NC-transfected
group. These results indicate that miR-650 activates small GTPase
Rac1 and RhoA, disrupting adherent junctions between cells and
promoting actin cytoskeleton reorganization.
MiR-650 exerts its function through
MAPK signaling
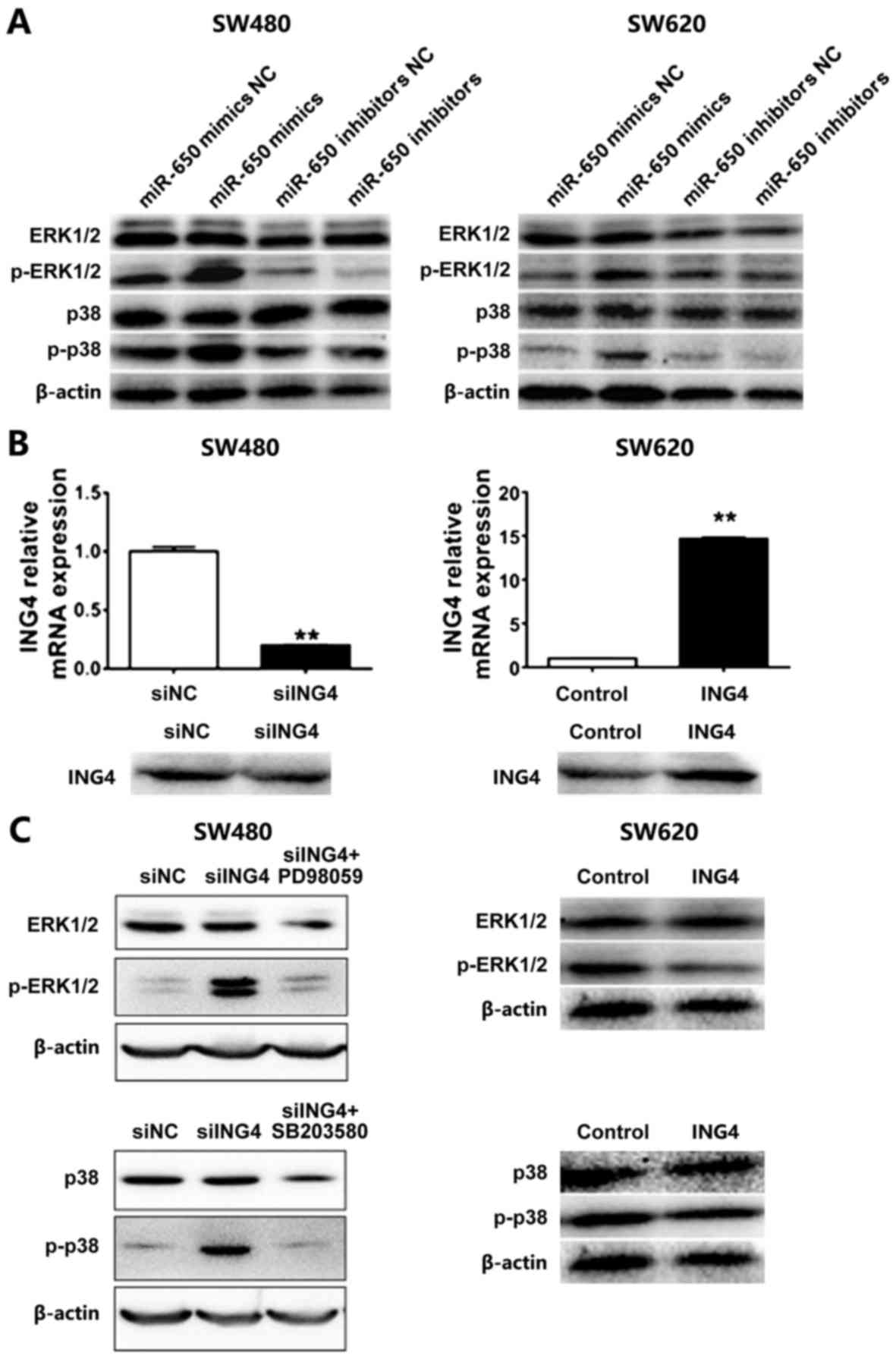
MAPK signaling serves functions in mediating cell
proliferation, differentiation, transformation and apoptosis, and
is involved in the development and progression of tumors (19). Promotion of tumor progression by
miR-650 via the two major MAPKs, including ERK1/2 and p38 MAPK was
investigated. Western blotting revealed that transfection of
miR-650 elevates the levels of p-ERK1/2 and p-p38 MAPK, while the
inhibition of miR-650 attenuates their expression in SW480 and
SW620 cells (Fig. 6A). These results
suggest that ERK and p38 MAPK may be involved in the modulation of
tumor development and progression in CRC. To determine the
association between ING4 and these MAPKs, ING4 was overexpressed in
SW480 and SW620, respectively (Fig.
6B). Western blotting revealed that the levels of
phosphorylated ERK and p38 MAPK were increased when ING4 was
knocked down but decreased when ING4 was overexpressed (Fig. 6C). The application of the inhibitors
of ERK and p38 MAPK could reduce their phosphorylation levels.
Taken together, these results suggest that miR-650 targets ING4 and
then activates ERK and p38 MAPK to promote CRC progression.
Discussion
Colorectal cancer (CRC) has become the third most
common type of malignant disease worldwide, and is one of the
leading causes of cancer-associated mortality. However, the
pathogenesis of CRC is not completely understood. Therefore, it is
important to resolve the underlying molecular mechanism of CRC
progression and carry out effective treatments to prevent and cure
the disease. Emerging studies have demonstrated that miRs serve
crucial functions in the progression of CRC (28).
At present, limited reports have demonstrated that
miR-650 is involved in cancer. Mraz et al (29) reported that miR-650 expression is
associated with the prognosis of chronic lymphocytic leukemia and
has influences on B-cell proliferation. A high level of miR-650 was
identified to be a prognostic indicator for lymph node involvement
and more aggressive clinical outcomes of lung adenocarcinoma
(30). In CRC, little information is
known about the function of miR-650, except for its involvement in
regulating expression of the NDRG gene (15). In the present study, it was
demonstrated that in CRC, miR-650 targets ING4 and has effects on
promoting proliferation, migration and activation of ERK and p38
MAPK which provides further evidence of the involvement of miR-650
in cancer progression.
ING4 was initially identified to be involved in the
regulation of glioma growth and angiogenesis and interacting with
nuclear factor NF-κB (31). ING4 has
been demonstrated to induce cell growth inhibition in human lung
adenocarcinoma cells and gastric carcinoma cells (32,33),
inhibit melanoma cell invasion (34)
and attenuate cellular transformation (35). Similarly, in the present study,
inhibition of ING4 through the overexpression of miR-650 promoted
CRC cell proliferation and migration (Fig. 3B).
In the present study, a series of assays were
carried out to characterize the function of miR-650 in regulating
CRC cell growth, invasiveness, EMT and actin cytoskeleton
reorganization. The expression of ING4 in patient CRC tissues,
compared with in the adjacent non-tumor tissues, was previously
evaluated, and the results revealed that ING4 sharply decreased in
cancer (36). To confirm the
decreased level of ING4 in CRC, the expression of ING4 was
evaluated in 8 CRC cell lines, which is consistent with the
previous results. Two cell lines (SW480 and SW620) were selected
for further experiments. miR-650 was overexpressed by transfecting
miR-650 mimics into SW480 and SW620 cells, and inhibited by
transfecting with an miR-650-inhibitor. The results demonstrated
that overexpression of miR-650 significantly inhibited ING4. As
ING4 is a tumor suppressor gene, the function of miR-650 in tumor
progression was assessed, including cell growth, cell invasion, EMT
and cell actin cytoskeleton reorganization. The underlying
molecular mechanisms of miR-650 in promoting cancer progression
were also evaluated. Western blotting results indicate that
overexpression of miR-650 enhanced the activation of ERK and p38
MAPK. In addition, knockdown of ING4 results in elevated levels of
p-ERK and p-p38 MAPK. These results suggest that miR-650 targets
ING4 which functions upstream of ERK and p38 MAPK to promote CRC
progression. Whether ING4 interacts with ERK and p38 MAPK or other
cancer-associated proteins to activate ERK and p38 MAPK must still
be further explored.
To the best of our knowledge, this is the first
study to identify the critical functions of miR-650 in CRC. miR-650
may target ING4 to promote CRC progression through the MAPK
signaling pathway. The present study not only evaluated the
functions of miR-650 in CRC progression, but also provided a solid
basis to explore the pathogenesis of and develop therapeutic
strategies for CRC.
Acknowledgements
The authors would like to thank Professor Dawei Yuan
(Geneis Beijing Co. Ltd., Beijing, China) for his technical
assistance with cell culture support.
Funding
This work was supported by the National Nature
Science Foundation of China (grant nos. 81600539, 81400443,
81372178), the Natural Science Foundation of Heilongjiang Province
of China (grant nos. QC2012C041, LC2016038), the Foundation of
Heilongjiang Administration of Traditional Chinese Medicine
(Huining Li, grant no. ZHY16-032), the Chinese Postdoctoral Science
Foundation (grant no. 2015M581472), the Special Financial Grant
from the China Postdoctoral Science Foundation (grant no.
2016T90310), the Postdoctoral Science Foundation of Heilongjiang
Province of China (grant nos. LBH-Z16101, LBH-TZ0616), the
Heilongjiang Human Resources and Social Security Bureau (Hongxue
Meng), the Harbin Special Fund Project for Science and Technology
Innovation (grant no. 2016RAQXJ203) and the Youth Elite Training
Foundation of Harbin Medical University Cancer Hospital (grant no.
JY2016-06).
Availability of data and materials
All data that were generated or analyzed in this
study are included in this manuscript.
Author's contributions
QY, HL and YL conceived and designed the study. YX
and SM conducted the experiments. GY and YX performed the
statistical analysis. JG, XJ and HM interpreted the statistical
analysis, reviewed and final approved the version to be published.
All authors read and approved the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chia VM, Newcomb PA, Bigler J, Morimoto
LM, Thibodeau SN and Potter JD: Risk of microsatellite-unstable
colorectal cancer is associated jointly with smoking and
nonsteroidal anti-inflammatory drug use. Cancer Res. 66:6877–6883.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou Y, Feng X, Liu YL, Ye SC, Wang H, Tan
WK, Tian T, Qiu YM and Luo HS: Down-regulation of miR-126 is
associated with colorectal cancer cells proliferation, migration
and invasion by targeting IRS-1 via the AKT and ERK1/2 signaling
pathways. PLoS One. 8:e812032013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen Z, He X, Xia W, Huang Q, Zhang Z, Ye
J, Ni C, Wu P, Wu D, Xu J, et al: Prognostic value and
clinicopathological differences of HIFs in colorectal cancer:
Evidence from meta-analysis. PLoS One. 8:e803372013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guo Y, Meng X, Wang Q, Wang Y and Shang H:
The ING4 binding with p53 and induced p53 acetylation were
attenuated by human papillomavirus 16 E6. PLoS One. 8:e714532013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li J and Li G: Cell cycle regulator ING4
is a suppressor of melanoma angiogenesis that is regulated by the
metastasis suppressor BRMS1. Cancer Res. 70:10445–10453. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Byron SA, Min E, Thal TS, Hostetter G,
Watanabe AT, Azorsa DO, Little TH, Tapia C and Kim S: Negative
regulation of NF-κB by the ING4 tumor suppressor in breast cancer.
PLoS One. 7:e468232012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Culurgioni S, Muñoz IG, Moreno A, Palacios
A, Villate M, Palmero I, Montoya G and Blanco FJ: Crystal structure
of inhibitor of growth 4 (ING4) dimerization domain reveals
functional organization of ING family of chromatin-binding
proteins. J Biol Chem. 287:10876–10884. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Palacios A, Moreno A, Oliveira BL, Rivera
T, Prieto J, Garcia P, Fernández-Fernández MR, Bernadó P, Palmero I
and Blanco FJ: The dimeric structure and the bivalent recognition
of H3K4me3 by the tumor suppressor ING4 suggests a mechanism for
enhanced targeting of the HBO1 complex to chromatin. J Mol Biol.
396:1117–1127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
You Q, Wang XS, Fu SB and Jin XM:
Downregulated expression of inhibitor of growth 4 (ING4) in
advanced colorectal cancers: A non-randomized experimental study.
Pathol Oncol Res. 17:473–477. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang WC, Chan SH, Jang TH, Chang JW, Ko
YC, Yen TC, Chiang SL, Chiang WF, Shieh TY, Liao CT, et al:
miRNA-491-5p and GIT1 serve as modulators and biomarkers for oral
squamous cell carcinoma invasion and metastasis. Cancer Res.
74:751–764. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Esquela-Kerscher A and Slack FJ:
Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer.
6:259–269. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen D, Chen Z, Jin Y, Dragas D, Zhang L,
Adjei BS, Wang A, Dai Y and Zhou X: MicroRNA-99 family members
suppress Homeobox A1 expression in epithelial cells. PLoS One.
8:e806252013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sugihara H, Ishimoto T, Watanabe M,
Sawayama H, Iwatsuki M, Baba Y, Komohara Y, Takeya M and Baba H:
Identification of miR-30e* regulation of Bmi1 expression mediated
by tumor-associated macrophages in gastrointestinal cancer. PLoS
One. 8:e818392013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang X, Zhu W, Zhang J, Huo S, Zhou L, Gu
Z and Zhang M: MicroRNA-650 targets ING4 to promote gastric cancer
tumorigenicity. Biochem Bioph Res Commun. 395:275–280. 2010.
View Article : Google Scholar
|
|
15
|
Feng L, Xie Y, Zhang H and Wu Y:
Down-regulation of NDRG2 gene expression in human colorectal cancer
involves promoter methylation and microRNA-650. Biochem Bioph Res
Commun. 406:534–538. 2011. View Article : Google Scholar
|
|
16
|
Zeng ZL, Li FJ, Gao F, Sun DS and Yao L:
Upregulation of miR-650 is correlated with down-regulation of ING4
and progression of hepatocellular carcinoma. J Surg Oncol.
107:105–110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bartley AN, Yao H, Barkoh BA, Ivan C,
Mishra BM, Rashid A, Calin GA, Luthra R and Hamilton SR: Complex
patterns of altered MicroRNA expression during the
adenoma-adenocarcinoma sequence for microsatellite-stable
colorectal cancer. Clin Cancer Res. 17:7283–7293. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Peres TV, Pedro DZ, de Cordova FM, Lopes
MW, Goncalves FM, Mendes-de-Aguiar CB, Walz R, Farina M, Aschner M
and Leal RB: In vitro manganese exposure disrupts MAPK signaling
pathways in striatal and hippocampal slices from immature rats.
Biomed Res Int. 2013:7692952013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thomas GM and Huganir RL: MAPK cascade
signalling and synaptic plasticity. Nat Rev Neurosci. 5:173–183.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cargnello M and Roux PP: Activation and
function of the MAPKs and their substrates, the MAPK-activated
protein kinases. Microbiol Mol Biol Rev. 75:50–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guo G, Yao W, Zhang Q and Bo Y: Oleanolic
acid suppresses migration and invasion of malignant glioma cells by
inactivating MAPK/ERK signaling pathway. PLoS One. 8:e720792013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Su N, Peng L, Xia B, Zhao Y, Xu A, Wang J,
Wang X and Jiang B: Lyn is involved in CD24-induced ERK1/2
activation in colorectal cancer. Mol Cancer. 11:432012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gout S, Morin C, Houle F and Huot J: Death
receptor-3, a new E-Selectin counter-receptor that confers
migration and survival advantages to colon carcinoma cells by
triggering p38 and ERK MAPK activation. Cancer Res. 66:9117–9124.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen L, Li M, Li Q, Wang CJ and Xie SQ:
DKK1 promotes hepatocellular carcinoma cell migration and invasion
through β-catenin/MMP7 signaling pathway. Mol Cancer. 12:1572013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Scanlon CS, van Tubergen EA, Inglehart RC
and D'Silva NJ: Biomarkers of epithelial-mesenchymal transition in
squamous cell carcinoma. J Dent Res. 92:114–121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Parri M and Chiarugi P: Rac and Rho
GTPases in cancer cell motility control. Cell Commun Signal.
8:232010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yilmaz M and Christofori G: EMT, the
cytoskeleton, and cancer cell invasion. Cancer Metast Rev.
28:15–33. 2009. View Article : Google Scholar
|
|
28
|
Slaby O, Svoboda M, Michalek J and Vyzula
R: MicroRNAs in colorectal cancer: Translation of molecular biology
into clinical application. Mol Cancer. 8:1022009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mraz M, Dolezalova D, Plevova K, Stano
Kozubik K, Mayerova V, Cerna K, Musilova K, Tichy B, Pavlova S,
Borsky M, et al: MicroRNA-650 expression is influenced by
immunoglobulin gene rearrangement and affects the biology of
chronic lymphocytic leukemia. Blood. 119:2110–2113. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zuo ZH, Yu YP, Ding Y, Liu S, Martin A,
Tseng G and Luo JH: Oncogenic activity of miR-650 in prostate
cancer is mediated by suppression of CSR1 expression. Am J Pathol.
185:1991–1999. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Garkavtsev I, Kozin SV, Chernova O, Xu L,
Winkler F, Brown E, Barnett GH and Jain RK: The candidate tumour
suppressor protein ING4 regulates brain tumour growth and
angiogenesis. Nature. 428:328–332. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li X, Cai L, Liang M, Wang Y, Yang J and
Zhao Y: ING4 induces cell growth inhibition in human lung
adenocarcinoma A549 cells by means of Wnt-1/beta-catenin signaling
pathway. Anat Rec (Hoboken). 291:593–600. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li S, Fan T, Liu H, Chen J, Qin C and Ren
X: Tumor suppressor ING4 overexpression contributes to
proliferation and invasion inhibition in gastric carcinoma by
suppressing the NF-kappaB signaling pathway. Mol Biol Rep.
40:5723–5732. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li J, Martinka M and Li G: Role of ING4 in
human melanoma cell migration, invasion and patient survival.
Carcinogenesis. 29:1373–1379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hung T, Binda O, Champagne KS, Kuo AJ,
Johnson K, Chang HY, Simon MD, Kutateladze TG and Gozani O: ING4
mediates crosstalk between histone H3 K4 trimethylation and H3
acetylation to attenuate cellular transformation. Mol Cell.
33:248–256. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lou C, Jiang S, Guo X and Dong XS: ING4 is
negatively correlated with microvessel density in colon cancer.
Tumour Biol. 33:2357–2364. 2012. View Article : Google Scholar : PubMed/NCBI
|