Introduction
Nasopharyngeal cancer (NPC) is a rare cancer
globally, but is prevalent in Southeast Asia (1). Due to its radiosensitivity, radiotherapy
is the primary treatment of NPC. In locally advanced stages,
combined radiotherapy and chemotherapy have been considered to be
an effective treatment in order to improve survival, preferred to
radiotherapy alone (2). Local control
rate of NPC has improved markedly in the past decade (3). However, local recurrence and metastasis
remain the primary causes of mortality from this cancer,
particularly in advanced stages (4),
and management of local treatment failure remains a challenge in
NPC treatment.
Emerging evidence supports the notion that cancer
stem cells (CSCs) contribute to NPCs resistance to chemoradiation,
which results in a poor prognosis for numerous different types of
human cancer (5). CSCs possess the
ability to recreate the complete phenotypic heterogeneity of the
parental tumor cells. These cells possess distinct surface markers
allowing for self-renewal (6).
Multiple stem cell markers, including nanog homeobox (Nanog),
SRY-box 2 (Sox-2) and aldehyde dehydrogenase (ALDH) have been used
successfully to identify CSCs in normal and tumor tissue (7,8).
Furthermore, side population (SP) cells exhibit CSC characteristics
in NPC (9).
The anchorage-independent serum-free culture of stem
cells was instrumental in the research of CSCs (10,11).
Sphere formation may be specifically used to enrich the potential
CSC subpopulation as a functional method (12,13).
Therefore, the suspension culture system may maintain CSCs in their
undifferentiated condition, facilitating their enrichment. However,
few reports exist at present regarding tumorspheres in NPC.
Therefore, the present study evaluated NPC cell subsets with CSC
properties.
Enhanced chemoradioresistance to therapy is another
characteristic of CSCs and has been identified in numerous
different types of cancer cells (14,15). The
NPC tumorsphere may be a valuable model for the further research of
CSCs and chemoradioresistance. In the present study, it was
therefore evaluated as to whether NPC tumorsphere cells acquired
the chemoradiation-resistant characteristics of CSCs.
Although NPC CSCs may be experimentally identified,
drugs or compounds that selectively target NPC CSCs have not yet
emerged. Salinomycin is a carboxylic polyether ionophore extracted
from Streptomyces albus (16).
Salinomycin has been identified as a selective inhibitor of breast
and lung CSCs (17,18), however its function in the inhibition
of NPC CSCs remains to be revealed. In the present study, a
tumorsphere was successfully used to enrich NPC CSCs, and the
results demonstrated that salinomycin was able to kill NPC
CSCs.
Materials and methods
Cells and culture conditions
SUNE-1 and 5-8F human nasopharyngeal cancer cell
lines were purchased from the Type Culture Collection of the
Chinese Academy of Sciences (Shanghai, China). SUNE-1 and 5-8F
cells were cultured in DMEM medium (Hyclone; GE Healthcare Life
Sciences, Logan, UT, USA) supplemented with 10% fetal bovine serum
(FBS; Hyclone; GE Healthcare Life Sciences) and 100 U/ml
penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Cells were cultured in a humidified air with 5%
CO2 at 37°C.
Tumorsphere culture and selection
SUNE-1 and 5-8F cells (1,000 cells/ml) were
cultivated in serum-free Ham's F-12 medium (Gibco; Thermo Fisher
Scientific, Inc.), supplemented with B27 (1:50; Gibco; Thermo
Fisher Scientific, Inc.), 20 ng/ml epidermal growth factor
(Invitrogen; Thermo Fisher Scientific, Inc.) and 20 ng/ml
fibroblast growth factor (Invitrogen; Thermo Fisher Scientific,
Inc.) at 37°C. To expand spheres in vitro, spheres were
harvested by centrifugation at 125 × g for 5 min at 24°C, separated
to single cells and then cultured for 72 h at 37°C to produce
tumorspheres of the next generation. The tumorspheres were filtered
using a cell strainer (BD Biosciences, San Jose, CA, USA).
Spheroids with a diameter >40 µm were selected to perform the
experiment.
Western blot analysis
The tumorspheres and parental cells were washed
three times with 5 ml phosphate-buffered saline. Total protein was
extracted from cells using a cell lysis buffer (20 mmol/l Tris-HCl,
150 mmol/l NaCl, 1% NP40, 5 mmol/l EDTA, 1 mmol/l Na3VO4; pH 7.5)
supplemented with a protease inhibitor cocktail and a phosphatase
inhibitor (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), and was
incubated on ice for 30 min. Protein concentration was determined
using a bicinchoninic acid protein assay kit (Pierce; Thermo Fisher
Scientific, Inc.). Protein (50 µg/lane) were loaded and separated
on a 10% gel using SDS-PAGE and then transferred to polyvinylidene
membranes. Following blocking in 50 g/l non-fat milk in
tris-buffered saline with Tween 20 (20 mmol/l Tris-HCl, 137 mmol/l
NaCl and 1 g/l Tween 20; pH 7.6) for 2 h at room temperature, the
membranes were incubated at 4°C overnight with the following
primary antibodies: Mouse anti-Nanog (dilution, 1:1,000; cat. no.
sc-374001; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), Sox-2
(dilution, 1:1,000; cat. no. sc-365823, Santa Cruz Biotechnology)
and GAPDH (dilution, 1:1,000; cat. no. sc-51907; Santa Cruz
Biotechnology). The membranes were then incubated for 1 h at 24°C
with horseradish peroxidase-conjugated anti-mouse immunoglobulin
secondary antibodies (dilution, 1:1,000; cat. no. A32729,
Invitrogen; Thermo Fisher Scientific, Inc.). Finally, the membranes
were visualized using the Image lab 3.0.1 software (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) following analysis using an
enhanced chemiluminescence-Plus detection system (Bio-Rad
Laboratories, Inc.).
ALDEFLUOR assay by
fluorescence-activated cell sorting (FACS)
The identification of ALDH activity using the
ALDEFLUOR assay (Stem Cell Technologies, Inc., Vancouver, BC,
Canada) was followed by FACS analysis. Cells were suspended in
ALDEFLUOR assay buffer, which contains ALDH substrate, and were
incubated for 40 min at 37°C. As a negative control, for each
sample of cells an aliquot was treated with 50 mM
diethylaminobenzaldehyde (DEAB), a specific ALDH inhibitor. FACS
analysis was performed using a FACSAria flow cytometer (BD
Biosciences). The results were analyzed using FlowJo 7.6.3 software
(FlowJo LLC, Ashland, OR, USA).
Hoechst staining and SP cell
assay
The parental or spheroid cells were suspended in
DMEM/2% FBS at a density of 1×106 cells/ml. The cells
were then dispersed into single cells and incubated with Hoechst
33342 dye (5 µg/ml; Sigma-Aldrich; Merck KGaA) either alone or in
combination with verapamil (50 mmol/ml; Sigma-Aldrich; Merck KGaA)
for 90 min at 37°C. Following incubation, cells were washed with
PBS and stained with propidium iodide (1 µg/ml; Sigma-Aldrich;
Merck KGaA) for 30 min at 4°C. Finally, the cells were maintained
at 4°C for the flow cytometric analysis and for the sorting of the
SP fraction using a FACSAria flow cytometer. The results were
analyzed using the FlowJo 7.6.3 software.
Drug sensitivity assay
Parental or spheroid cells were collected in 96-well
microplates at a density of 3,000 cells per well. The cells were
then treated with increasing concentrations of cisplatin
(10−7, 10−6, 10−5 and
10−4 M; Sigma-Aldrich, St. Louis, MO, USA). Subsequent
to incubation for 72 h at 37°C, an MTT assay was used to evaluate
the cell viability. The number of living cells was calculated
according to the absorbance at 490 nm. Each experiment was repeated
three times.
Clone formation assay
Parental or spheroid cells were irradiated at
indicated doses (0, 2, 4, 6, 8 and 10 Gy). Irradiation of cells was
performed using 250 kV orthovoltage X-rays by a linear accelerator
(Elekta Instrument AB, Stockholm, Sweden). Following irradiation,
the cells were collected and subsequently replated in a 30-mm
culture dish at a density of 200-5,000 cells per dish. Subsequent
to culturing for 14 days at 37°C, cells were fixed with 10%
formalin and stained with 0.1% crystal violet for 15 min at 24°C;
clones consisting of >50 cells were selected. The survival
fraction was calculated by dividing the number of colonies formed
by the number of cells plated. The data were entered into single
hit multi-target formula, as follows:
S=1-(1-e-D/D°)N (where D, quasi-threshold
dose; D°, mean lethal dose; N, extrapolation number; and S,
survival fraction). Graphpad Prism 5.0 (Graphpad Software, Inc., La
Jolla, CA, USA) was used to draw the survival fraction curve.
Experiments were repeated three times.
Sphere formation efficiency assay
Parental or spheroid cells were collected in 96-well
microplates at a density of 3,000 cells per well. The cells were
pretreated with dimethyl sulfoxide (DMSO; Sigma-Aldrich; Merck
KGaA), 2 µM cisplatin (Sigma-Aldrich; Merck KGaA) and 2 µM
salinomycin (Sigma-Aldrich; Merck KGaA) for 72 h at 37°C.
Subsequently, the cells were transferred into serum-free Ham's F-12
medium in 24-well microplates at a density of 100 cells/well. After
48 h, the tumorspheres were counted under a light microscope
(magnification, ×200; Nikon Corporation, Tokyo, Japan). Each
experiments was repeated for three times.
Statistical analyses
P<0.05 was considered to indicate a statistically
significant difference. Data were analyzed using the SPSS 19.0
statistical software package (IBM Corp., Armonk, NY, USA) and were
presented as the mean ± the standard deviation. Differences between
the groups were determined using a one-way analysis of variance and
least significant difference method for multiple comparisons.
Results
NPC tumorspheres contain cells with
cancer stem-like properties
It has been reported that breast CSC populations may
be generated in vitro as mammospheres under serum-free
culture conditions (19). In the
present study, the NPC CSC population was enriched from the SUNE-1
cell line. Parental cells were cultivated in serum-free culture.
After culturing for 72 h, floating tumorspheres were formed
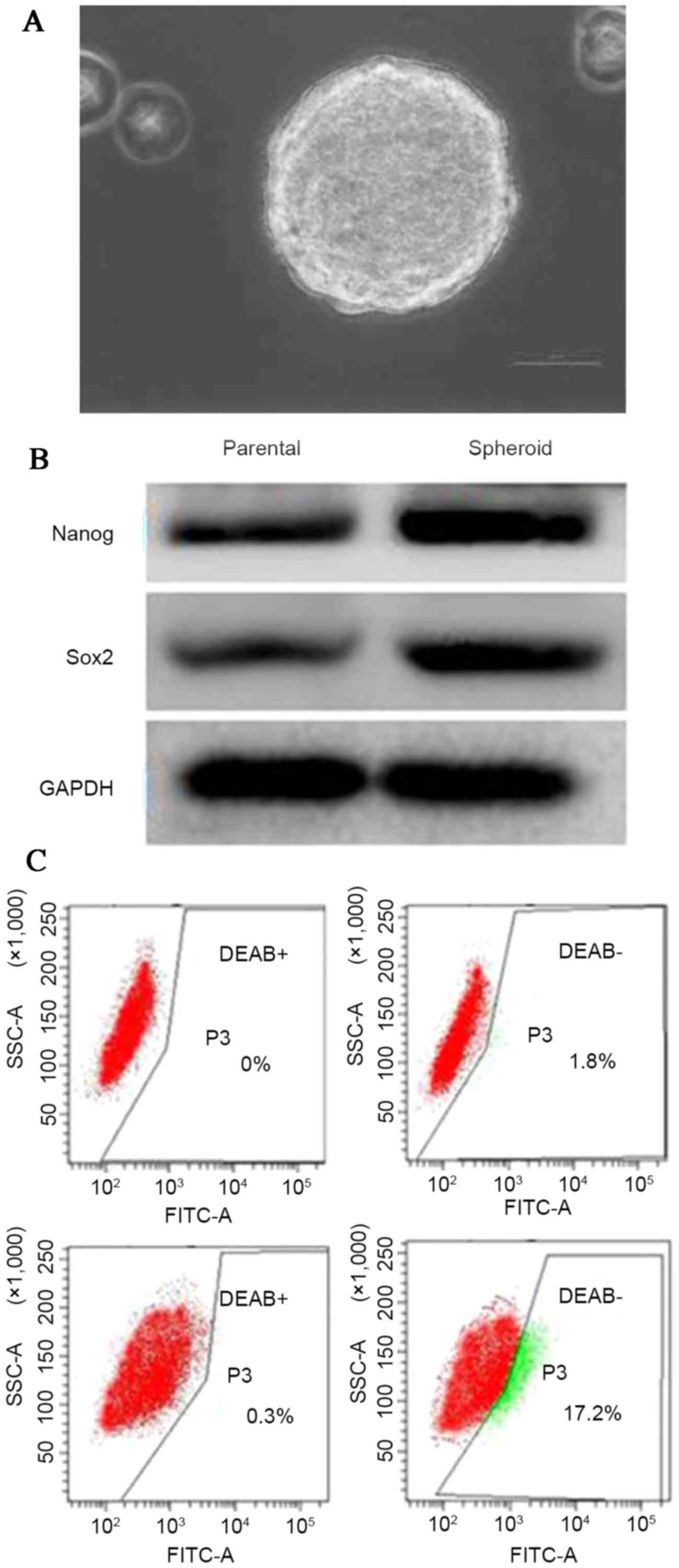
(Fig. 1A). SUNE-1 spheroids with a
diameter of >40 µm, which were filtered out using a cell
strainer, were selected. Two typical CSC markers, Nanog and Sox2,
were detected using immunoblotting. As revealed in Fig. 1B, a marked increase in the expression
of Nanog and Sox2 were observed in the SUNE-1 spheroids, compared
with the parental cells. ALDH has been identified as a potential
marker for NPC CSCs (20). ALDH is a
detoxifying enzyme responsible for the oxidation of intracellular
aldehydes. To further confirm this finding, an ALDEFLUOR assay was
used to assess ALDH enzymatic activity in the SUNE-1 spheroids.
ALDEFLUOR-positive cells were increased 9–10-fold in tumorsphere
cells, compared with the parental cells (1.8 vs. 16.9%; Fig. 1C). The results indicated that NPC
tumorspheres possessed increased stem-like cancer cells.
NPC tumorspheres exhibit increased
chemoresistance
Tumor cells resistant to chemotherapy occur partly
due to the overexpression of the ATP-binding cassette sub-family
(21). This characteristic is
associated with the ability to expel dyes, identified by flow
cytometry to be a SP (22). SP cells
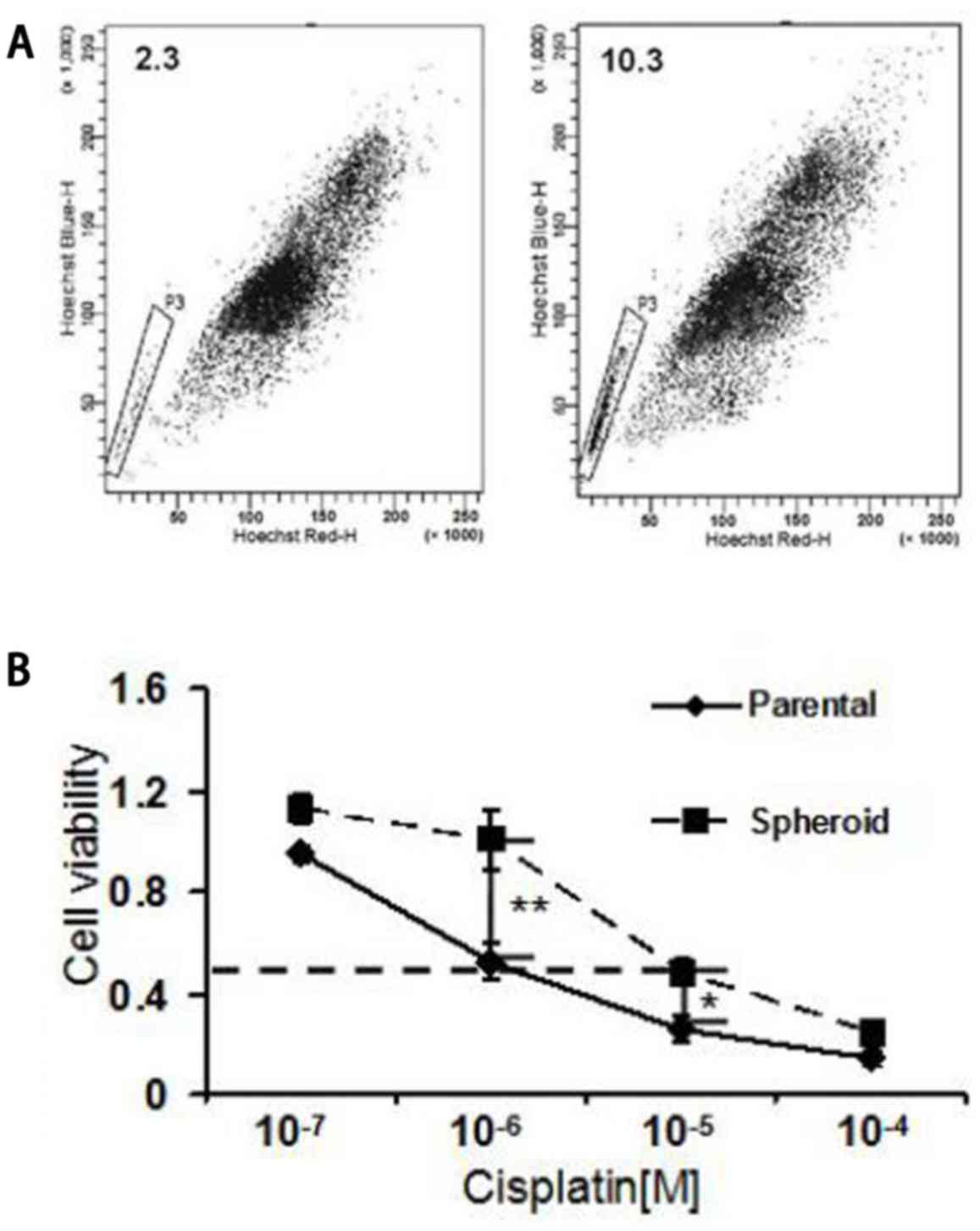
have been reported to possess NPC CSC properties (23). In the present study, NPC tumorsphere
cells cultured in serum-free cultures were detected to possess a
4.5-fold increase in the proportion of SP cells compared with the
parental cells (10.3 vs. 2.3%; Fig.
2A). Furthermore, the sensitivity of NPC tumorsphere cells and
parental cells to cisplatin, which is usually used for chemotherapy
against NPC, was examined. The tumorsphere cells from the spheroids
demonstrated an increased half maximal inhibitory concentration
value of >10-fold with cisplatin compared with the control
parental cells (Fig. 2B). These
results indicate that NPC tumorspheres possess increased
chemoresistant properties of CSCs.
NPC tumorsphere cells demonstrate
enhanced resistance to radiation
Radiotherapy is the primary treatment of NPC due to
its radiosensitivity (24). To assess
whether self-renewing cells from spheroids possessed a radiotherapy
resistant phenotype, the radiosensitivity of parental and
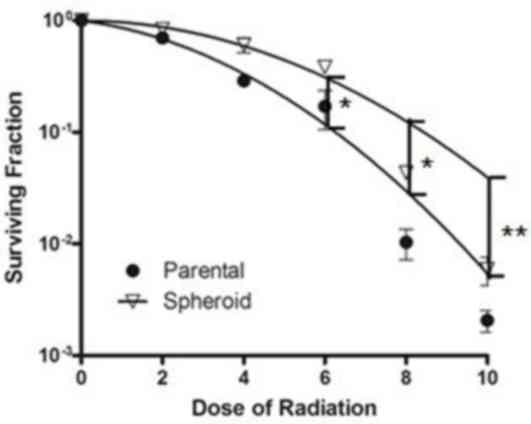
tumorsphere cells was analyzed using a clone formation assay.
Following radiotherapeutic treatment, the survival fraction (SF) of
the cells cultured as spheroids was significantly decreased
compared with that of the parental cells [spheroid cells, mean SF
at 6 Gy (SF6 Gy)=0.383±0.064 vs. parental cells SF6 Gy=0.171±0.113;
P<0.05; Fig. 3]. These results
supported radioresistance characteristics of NPC CSC-like
cells.
Salinomycin selectively kills NPC
CSCs
Salinomycin has been reported to possess potent
anti-CSC activity (25). As a
functional measure of CSC frequency, the ability of SUNE-1 and 5-8F
cells to form tumorspheres following treatment for 72 h with
salinomycin, cisplatin or a DMSO control when cultured in
suspension cultures, was tested as an in vitro measure of
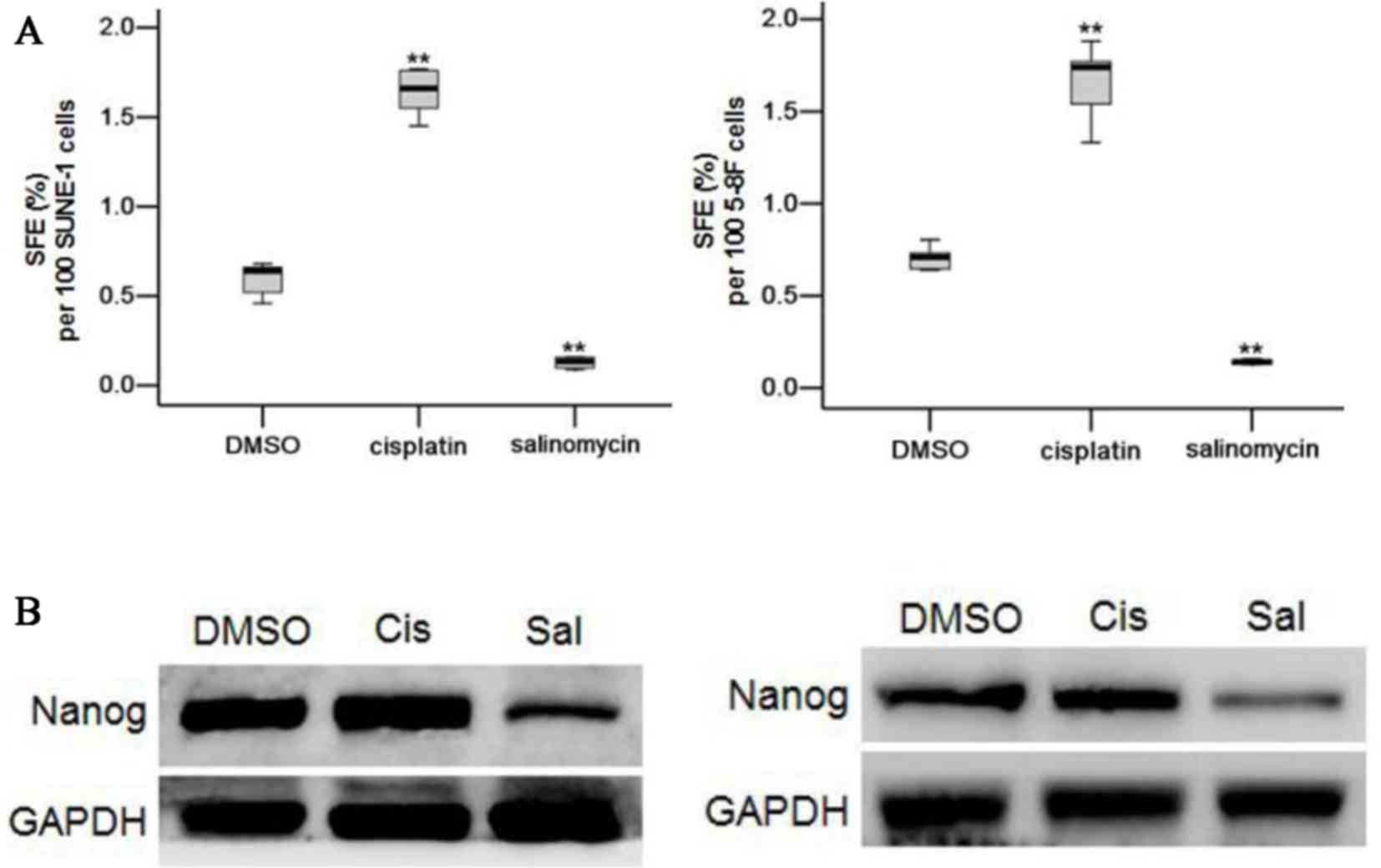
CSC activity. Parental SUNE-1 and 5-8F cells were treated by DMSO,
cisplatin (2 µM) or salinomycin (2 µM) for 72 h. Following
treatment, tumor cells were cultivated in suspension cultures.
Sphere formation efficiency (SFE) of SUNE-1 and 5-8F cells with the
salinomycin treatment demonstrated a significant 4.7-fold and
5.0-fold decrease relative to DMSO treatment (0.592 spheres vs.
0.126 spheres per 100 SUNE-1 cells, P<0.01; 0.706 spheres vs.
0.142 spheres per 100 5-8F cells, P<0.05; Fig. 4A). In contrast, cisplatin treatment
demonstrated a significant increase in the SFE of SUNE-1 and 5-8F
cells compared with DMSO treatment (P<0.01; Fig. 4A). Nanog, a CSC marker, of SUNE-1 and
5-8F tumorsphere cells treated for 72 h with DMSO, cisplatin (2 µM)
and salinomycin (2 µM) was also directly assayed. SUNE-1 and 5-8F
tumorsphere cells treated with salinomycin presented a decrease in
Nanog expression, compared with the DMSO control. The expression of
Nanog did not decrease in the cisplatin-treated SUNE-1 and 5-8F
cells (Fig. 4B). These results
suggested that salinomycin may inhibit NPC CSC properties.
Discussion
Radiotherapy is the initial treatment mode of NPC
and using radiotherapy in combination with chemotherapy is
recommended for the treatment of locally advanced tumors (26). Tumor recurrence and metastasis often
result in the failure of treatment due to chemoradioresistance
(27).
A previous study reported the application of
serum-free culture to enrich and isolate potential CSC
subpopulations in multiple different types of tumor (28). In general, as with all stem cells, the
tumorsphere forming cells are capable of proliferation,
self-renewal and exhibit increased tumorigenicity (29). In the present study, a comprehensive
investigation of tumorsphere cells that are derived from the SUNE-1
cell line was provided. It was revealed that SUNE-1 tumorsphere
cells acquire the characteristics of CSCs, with the increased
expression of stem cell markers (Nanog and Sox-2), compared with
the parental cells. It has been demonstrated that in SUNE-1
spheroids, a comparatively large subpopulation of cells had
elevated the enzymatic activity of ALDH. These results suggest that
NPC tumorsphere cells are associated with cancer stem-like
populations.
Enhanced chemoresistance to therapy is another
characteristic of CSCs that has been identified in numerous
different types of cancer cells (30). In the present study, the tumorsphere
cells demonstrated increased resistance, compared with that in the
parental cells, to cisplatin treatment. NPC tumorspheres also
exhibited an increased prevalence of SP cells. Therefore, it was
suggested that the non-adherent tumorspheres cultured in serum-free
conditions possessed NPC CSC properties. Suspension culture may be
used to enrich drug-resistant NPC cells.
Radioresistance has been implied to be associated
with CSCs in multiple types of cancer (31,32).
Radiotherapy is the most important method in the treatment of NPC.
NPC cells are more sensitive to radiation than other cancer cells
(33). In the present study,
tumorsphere cells displayed enhanced resistance to radiation
compared with that displayed by its radiosensitive SUNE-1 parental
cells. Therefore, eradicating radiotherapy-resistant cells is
critical for successful anti-NPC therapy.
Salinomycin is a polyether anticoccidial drug
produced by an S. albus strain. Previously, salinomycin had
been reported to possess potent anti-CSC activity (34). The present study revealed that a
decrease of SFE was observed following salinomycin treatment in
vitro, implying that salinomycin may kill NPC CSCs selectively.
NPC CSCs are more sensitive to salinomycin compared with the
parental cells. In contrast, an increase in SFE was observed
following cisplatin treatment in vitro; it was theorized
that the increased SFE was due to the already increased proportion
of CSCs present in the NPC cells treated with cisplatin. This may
be due to the fact that cisplatin may only kill common tumor cells
rather than CSCs (35).
To conclude, the present study demonstrated that
chemoresistant NPC tumorsphere cells are rich in ‘stem-cell-like’
tumor cells and may be inhibited by salinomycin selectively. An
effective method for the enrichment of CSCs was provided, which is
beneficial for the research of the characteristics of NPC stem-like
cells in terms of their biology and their unique cell surface
markers. Finding novel therapies to overcome chemoresistance in NPC
CSCs is key to improving long-term survival rates for patients with
NPC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81402458) and the
Basic Research Project of Shanxi Province (grant no.
2014021037-4).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
GZ performed the FACS analysis, and SZ performed the
irradiation. JR performed the tumorsphere culture and selection. CY
and ZZ conducted the western blot analysis. XQ and XZ conducted the
clone formation assay. SW was responsible for the drug sensitivity
assay. GZ and LL performed the statistical analysis. GZ designed
the study and wrote the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lee AW, Ma BB, Ng WT and Chan AT:
Management of nasopharyngeal carcinoma: Current practice and future
perspective. J Clin Oncol. 33:3356–3364. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tan WL, Tan EH, Lim DW, Ng QS, Tan DS,
Jain A and Ang MK: Advances in systemic treatment for
nasopharyngeal carcinoma. Chin Clin Oncol. 5:212016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sze H, Blanchard P, Ng WT, Pignon JP and
Lee AW: Chemotherapy for nasopharyngeal carcinoma-current
recommendation and controversies. Hematol Oncol Clin North Am.
29:1107–1122. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li JG, Venigalla P, Leeman JE, LaPlant Q,
Setton J, Sherman E, Tsai J, McBride S, Riaz N and Lee N: Patterns
of nodal failure after intensity modulated radiotherapy for
nasopharyngeal carcinoma. Laryngoscope. 127:377–382. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Greve B, Kelsch R, Spaniol K, Eich HT and
Götte M: Flow cytometry in cancer stem cell analysis and
separation. Cytometry A. 81:284–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dalerba P, Cho RW and Clarke MF: Cancer
stem cells: Models and concepts. Annu Rev Med. 58:267–284. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mathieu J, Zhang Z, Zhou W, Wang AJ,
Heddleston JM, Pinna CM, Hubaud A, Stadler B, Choi M, Bar M, et al:
HIF induces human embryonic stem cell markers in cancer cells.
Cancer Res. 71:4640–4652. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu A, Luo W, Zhang Q, Yang Z, Zhang G, Li
S and Yao K: Aldehyde dehydrogenase 1, a functional marker for
identifying cancer stem cells in human nasopharyngeal carcinoma.
Cancer Lett. 330:181–189. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guo D, Xu BL, Zhang XH and Dong MM: Cancer
stem-like side population cells in the human nasopharyngeal
carcinoma cell line cne-2 possess epithelial mesenchymal transition
properties in association with metastasis. Oncol Rep. 28:241–247.
2012.PubMed/NCBI
|
|
10
|
Bez A, Corsini E, Curti D, Biggiogera M,
Colombo A, Nicosia RF, Pagano SF and Parati EA: Neurosphere and
neurosphere-forming cells: Morphological and ultrastructural
characterization. Brain Res. 993:18–29. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi X, Gipp J and Bushman W:
Anchorage-independent culture maintains prostate stem cells. Dev
Biol. 312:396–406. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee CH, Yu CC, Wang BY and Chang WW:
Tumorsphere as an effective in vitro platform for screening
anti-cancer stem cell drugs. Oncotarget. 7:1215–1226.
2016.PubMed/NCBI
|
|
13
|
Dontu G, Abdallah WM, Foley JM, Jackson
KW, Clarke MF, Kawamura MJ and Wicha MS: In vitro propagation and
transcriptional profiling of human mammary stem/progenitor cells.
Genes Dev. 17:1253–1270. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lan X, Wu YZ, Wang Y, Wu FR, Zang CB, Tang
C, Cao S and Li SL: CD133 silencing inhibits stemness properties
and enhances chemoradiosensitivity in CD133-positive liver cancer
stem cells. Int J Mol Med. 31:315–324. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang CF, Peng LX, Huang TJ, Yang GD, Chu
QQ, Liang YY, Cao X, Xie P, Zheng LS, Huang HB, et al: Cancer
stem-like cell characteristics induced by EB virus-encoded LMP1
contribute to radioresistance in nasopharyngeal carcinoma by
suppressing the p53-mediated apoptosis pathway. Cancer Lett.
344:260–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou S, Wang F, Wong ET, Fonkem E, Hsieh
TC, Wu JM and Wu E: Salinomycin: A novel anti-cancer agent with
known anti-coccidial activities. Curr Med Chem. 20:4095–4101. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fu YZ, Yan YY, He M, Xiao QH, Yao WF, Zhao
L, Wu HZ, Yu ZJ, Zhou MY, Lv MT, et al: Salinomycin induces
selective cytotoxicity to MCF-7 mammosphere cells through targeting
the Hedgehog signaling pathway. Oncol Rep. 35:912–922. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang Y: Effects of salinomycin on cancer
stem cell in human lung adenocarcinoma A549 cells. Med Chem.
7:106–111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ponti D, Costa A, Zaffaroni N, Pratesi G,
Petrangolini G, Coradini D, Pilotti S, Pierotti MA and Daidone M:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yu F, Sim AC, Li C, Li Y, Zhao X, Wang DY
and Loh KS: Identification of a subpopulation of nasopharyngeal
carcinoma cells with cancer stem-like cell properties by high
aldehyde dehydrogenase activity. Laryngoscope. 123:1903–1911. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McIntosh K, Balch C and Tiwari AK:
Tackling multidrug resistance mediated by efflux transporters in
tumor-initiating cells. Expert Opin Drug Metab Toxicol. 12:633–644.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Boesch M, Zeimet AG, Fiegl H, Wolf B,
Huber J, Klocker H, Gastl G, Sopper S and Wolf D: High prevalence
of side population in human cancer cell lines. Oncoscience.
3:85–87. 2016.PubMed/NCBI
|
|
23
|
Yu S, Zhang R, Liu F, Wang H, Wu J and
Wang Y: Notch inhibition suppresses nasopharyngeal carcinoma by
depleting cancer stem-like side population cells. Oncol Rep.
28:561–566. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pan JJ, Ng WT, Zong JF, Lee SW, Choi HC,
Chan LL, Lin SJ, Guo QJ, Sze HC, Chen YB, et al: Prognostic
nomogram for refining the prognostication of the proposed 8th
edition of the AJCC/UICC staging system for nasopharyngeal cancer
in the era of intensity-modulated radiotherapy. Cancer.
122:3307–3315. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang C, Tian Y, Song F, Fu C, Han B and
Wang Y: Salinomycin inhibits the growth of colorectal carcinoma by
targeting tumor stem cells. Oncol Rep. 34:2469–2476. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang B, Hu Y, Xiong RH, Pan YF, Xu QL,
Kong XY, Cai R, Chen QQ, Tang HY and Jiang W: Matched analysis of
induction chemotherapy plus chemoradiotherapy versus induction
chemotherapy plus radiotherapy alone in locoregionally advanced
nasopharyngeal carcinoma: A multicenter study. Oncotarget.
8:14078–14088. 2017.PubMed/NCBI
|
|
27
|
Xu C, Chen YP and Ma J: Clinical trials in
nasopharyngeal carcinoma-past, present and future. Chin Clin Oncol.
5:202016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hirschhaeuser F, Menne H, Dittfeld C, West
J, Mueller-Klieser W and Kunz-Schughart LA: Multicellular tumor
spheroids: An underestimated tool is catching up again. J
Biotechnol. 148:3–15. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gou S, Liu T, Wang C, Yin T, Li K, Yang M
and Zhou J: Establishment of clonal colony-forming assay for
propagation of pancreatic cancer cells with stem cell properties.
Pancreas. 34:429–435. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu W, Gao Q, Chen K, Xue X, Li M, Chen Q,
Zhu G and Gao Y: Hiwi facilitates chemoresistance as a cancer stem
cell marker in cervical cancer. Oncol Rep. 32:1853–1860. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pranatharthi A, Ross C and Srivastava S:
Cancer stem cells and radioresistance: Rho/ROCK pathway plea
attention. Stem Cells Int. 2016:57857862016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
De Bacco F, D'Ambrosio A, Casanova E,
Orzan F, Neggia R, Albano R, Verginelli F, Cominelli M, Poliani PL,
Luraghi P, et al: MET inhibition overcomes radiation resistance of
glioblastoma stem-like cells. EMBO Mol Med. 8:550–568. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee AW, Ng WT, Chan YH, Sze H, Chan C and
Lam TH: The battle against nasopharyngeal cancer. Radiother Oncol.
104:272–278. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gupta PB, Onder TT, Jiang G, Tao K,
Kuperwasser C, Weinberg RA and Lander ES: Identification of
selective inhibitors of cancer stem cells by high-throughput
screening. Cell. 138:645–659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang J, Zhang K, Wu J, Shi J, Xue J, Li J,
Chen J, Zhu Y, Wei J, He J and Liu X: Wnt5a increases properties of
lung cancer stem cells and resistance to cisplatin through
activation of Wnt5a/PKC signaling pathway. Stem Cells Int.
2016:16908962016. View Article : Google Scholar : PubMed/NCBI
|