Introduction
Breast cancer (BC) is the most common malignant
tumor in women patients (1,2). BC is responsible for the majority of
skeletal metastases, and the incidence of bone metastases is 65–75%
in advanced diseases (3). Bone
metastases are a major cause for morbidity, the median survival of
BC patients with bone metastases is 19–25 months (4). Bone metastases are characterized by
severe pain, impaired mobility, pathologic fractures, spinal cord
compression, bone marrow aplasia and hypercalcemia (5,6).
Metastatic cancer cells could secrete a series of factors into the
bone-tumor microenvironment and form an osteolytic lesion, and bone
matrix released growth factors could reversely stimulate BC cell
proliferation. The interactions of BC cells and bone formed a
‘vicious osteolytic cycle’ (7,8). With the
advantages of modern science, treatment strategies for primary
breast tumors are well developed, while clinical interventions for
bone lesions are still unsatisfying (9,10).
Therefore, finding targets which could prevent or cure bone
metastases is a priority for cancer treatment.
Platelet-activating factor (PAF,
1-O-alkyl-2-acetyl-snglycero-3-phosphocholine), a potent
phospholipid mediator, has been shown to play a role in a number of
biological pathways including inflammatory diseases, cardiovascular
homeostasis as well as in cancer, by binding to the G-protein
coupled receptor (GPCR) PAF receptor (PTAFR) (11). PAF play important roles in platelet
aggregation, stimulation of neutrophils and macrophages,
inflammation and allergic responses (12,13). It
also play vital roles in tumor neo-angiogenesis by activation of
nuclear factor-κB (NF-κB) (14,15). PAF
has been reported highly expressed in BC cells but not in normal
MECs. The Kaplan-Meier analysis showed that high levels of PAF
expression were strongly associated with poor prognosis in BC
(16) Bussolati et al reported
that BC cell lines MDA-MB-231 and MCF-7 could secret PAF and
increase the motility of cancer cells by an autocrine manner
(17). The upregulation of PTAFR has
also been observed in several BC cell lines. Recently, Anandi et
al reported that PAF could promote motility in BC cells and
distupts non-transformed breast acinar structures (18). All these findings highlighted the
potential role of PAF/PTAFR signaling pathway in BC
progression.
The bone microenvironment is a highly dynamic system
balanced between osteoclastic breakdown and osteoblastic rebuilding
under tightly control in a local, coordinated, and sequential
manner (19). Interruption of the
homeostasis of bone microenvironment by tumor cells is one of the
most important mechanisms of BC bone metastases. In vitro
studies has demonstrated that PAF could directly enhance osteoclast
motility and resorptive activity (20,21).
Clinical studies also indicates that higher plasma PAF levels are
associated with increased risk of vertebral fracture and lower bone
mineral density in postmenopausal women (22). Hence, blocking PAF induced
osteoclastogenesis might be an effective strategy in treatment of
osteoclast related diseases, including osteolytic BC bone
metastases.
Kadsurenone is a natural product isolated from stems
of Piper kadsura. This herb is used in traditional Chinese
medicine for the relief of diseases as asthma and rheumatoid
arthritis (RA) (23). Kadsurenone
have been demonstrated as a natural PAF inhibitor which could stops
or diminishes all unwanted reactions induced by PAF (24). In the current study, we revealed that
the upregulation of PTAFR is associated with increased incidence of
bone metastases. We also demonstrated that Kadsurenone could
effectively inhibit PAF induced BC cell migration. Furthermore,
Kadsurenone could also attenuate BC induced osteolytic bone
metastases by blocking the PAF/PTAFR signaling pathway.
Materials and methods
Bioinformatics analysis
The online Oncomine database (www.oncomine.org) were used to reveal PTAFR expression
patterns between normal breast tissues and invasive breast
carcinomas. Involved expression data were deposited in the National
Center for Biotechnology Information/Gene Expression Omnibus
database entries GSE9014. Data for survival analysis of bone
metastasis and PTAFR expression patterns between osteoclatogenic
(OG) and non-OG cell lines were obtained from GSE2603 and GSE43811,
respectively. Survival analyses were based on Kaplan-Meier method,
cutoff value was determined by ROC curves.
Cells and culture conditions
MDA-MB-231, RAW 264.7 and 293T cells were all
obtained from the Type Culture Collection of the Chinese Academy of
Sciences (Shanghai, China). MDA-MB-231 and 293T cells were cultured
in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), supplied with 10% fetal bovine
serum (FBS; HyClone; GE Healthcare Life Sciences, Logan, UT, USA)
and 100 U/ml penicillin-streptomycin (P/S; Gibco; Thermo Fisher
Scientific, Inc.). RAW264.7 cells and mouse bone marrow monocytes
(BMMs) were cultured in α-minimum essential medium (α-MEM; Gibco;
Thermo Fisher Scientific, Inc.), supplied with 10% FBS and 100 U/ml
P/S. Cells were cultured in a humidified incubator (Thermo Fisher
Scientific, Inc.) with 5% CO2 at 37°C.
Cell viability assay
The viability and cytotoxicity effect of Kadsurenone
was determined by the MTS method following the manual of CellTiter
96 Aqueous One Solution Cell Proliferation assay (Promega
Corporation, Madison, WI, USA) with VERSA max microplate reader
(Molecular Devices, LLC, Sunnyvale, CA, USA) as described
previously.
Transwell asssy
The transwell assay were performed with Boyden
chambers (Corning Incorporated, Corning, NY, USA). MDA-MB-231 cells
were collected and resuspend in blank DMEM after 12-h serum starve.
6×105 cells were plated in the top chambers with or
without 200 nM PAF and different concentrations (0, 0.5, 1, 2.5 and
5 µM) of Kadsurenone. The bottom chambers were filled with 600 µl
mediums supplemented with 2% FBS. After 8 h incubation, migrated
cells were fixed with 4% paraformaldehyde (PFA) and stained with 1%
crystal violet. Images were taken using an Olympus inverted
microscope (Olympus Corporation, Tokyo, Japan) and migrated cells
were counted using Image-Pro Plus 6.0 (Media Cybernetics, Inc.,
Rockville, MD, USA).
Luciferase reporter gene assay
Dual luciferase assays were conducted in a 24-well
plate format. Vectors were transfected into 70% confluent HEK293
cells, PAF or receptor activator for NF-κB ligand (RANKL)
inducement and different concentrations of Kadsurenone were added.
After 48-h transfection, frefly and renilla luciferase were
quantified sequentially using the Dual Luciferase Assay kit
(Promega Corporation) following the manufacturer's
recommendations.
BC cells induced osteoclast
differentiation assay
2×103 MDA-MB-231 cells and
5X103 RAW264.7 cells were pooled together and seeded
into 24-well plates. Cells were cultured in α-MEM added with 10%
FBS and different concentrations of Kadsurenone. After 5–7 days,
cells were fixed with 4% PFA and permeabilized with 0.1% Triton-X
100 inPBS for 5 min, and subjected to tartrate-resistant acid
phosphatase (Trap) staining with Leukocyte acid phosphatase kit
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). Trap positive
osteoclasts were photographed and counted thereafter.
Mouse BMMs isolation and osteoclast
differentiation assay
Mouse BMMs were flushed and isolated from C57/BL6
mice as previously described. 8×103 BMMs were seeded
into 96-well plates and incubated with 10 ng/ml M-CSF, 50 ng/ml
RANKL and different concentrations of Kadsurenone. After 5–7 days,
cells were fixed and subjected to Trap staining and photographed.
All experimental protocols were approved by the Review Committee
for the Use of Human or Animal Subjects of East China Normal
University and Chang Zheng Hospital (Shanghai, China).
Acting ring formation assay
Mouse BMMs were isolation and induced for osteoclast
differentiation for 5–7 days. Cells were then permeabilized with
Triton X-100 and incubated with rhodamine conjugated phalloidin
(Molecular Probes; Thermo Fisher Scientific, Inc.) to visualize
F-actin.
RNA extraction and gene expression
analysis
Mouse BMMs were isolated and induced for osteoclast
differentiation with or without indicated concentrations of
Kadsurenone. Cells were collected and total RNA were extracted with
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Then,
500 ng total RNA were reverse transcribed with PrimeScript™ RT
Master Mix (Takara Biotechnology Co., Ltd., Dalian, China),
according to the manufacturer's instructions. The complementary DNA
was used for reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) and a final volume of 20 µl was adopted as
follows: 10 µl 2X SYBR Premix Ex Taq (Takara Biotechnology Co.,
Ltd.), 1 µl forward and reverse primers, 2.5 µM, 2 µl cDNA, 7 µl
ddH2O. PCR and data collection were performed on Mx3000P
QPCR system (Stratagene; Agilent Technologies, Inc., Santa Clara,
CA, USA) and data analysis was operated with the 2−ΔΔCq
method normalized to the endogenous control β-actin. Primers used
in RT-qPCR are listed in Table I.
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Direction | Sequence |
|---|
| β-actin | Forward |
5′-GTACGCCAACACAGTGCTG-3′ |
|
| Reverse |
5′-CGTCATACTCCTGCTTGCTG-3′ |
| Ctsk | Forward |
5′-CTTCCAATACGTGCAGCAGA-3′ |
|
| Reverse |
5′-TCGGTTTCTTCTCCTCTGGA-3′ |
| Trap | Forward |
5′-GCTGGAAACCATGATCACCT-3′ |
|
| Reverse |
5′-GAGTTGCCACACAGCATCAC-3′ |
| Nfatc1 | Forward |
5′-TGGAGAAGCAGAGCACAGAC-3′ |
|
| Reverse |
5′-GCGGAAAGGTGGTATCTCAA-3′ |
Statistical analysis
Experiments were performed with 3 or more
replicates. The results were reported as the mean ± standard error
of the mean. The differences between control and experimental
groups were determined using an unpaired Student's t-test or
one-way analysis of variance followed by Tukey's post hoc test.
Survival curves were analyzed by log-rank test. P<0.05 was
considered to indicate a statistically significant difference.
Results
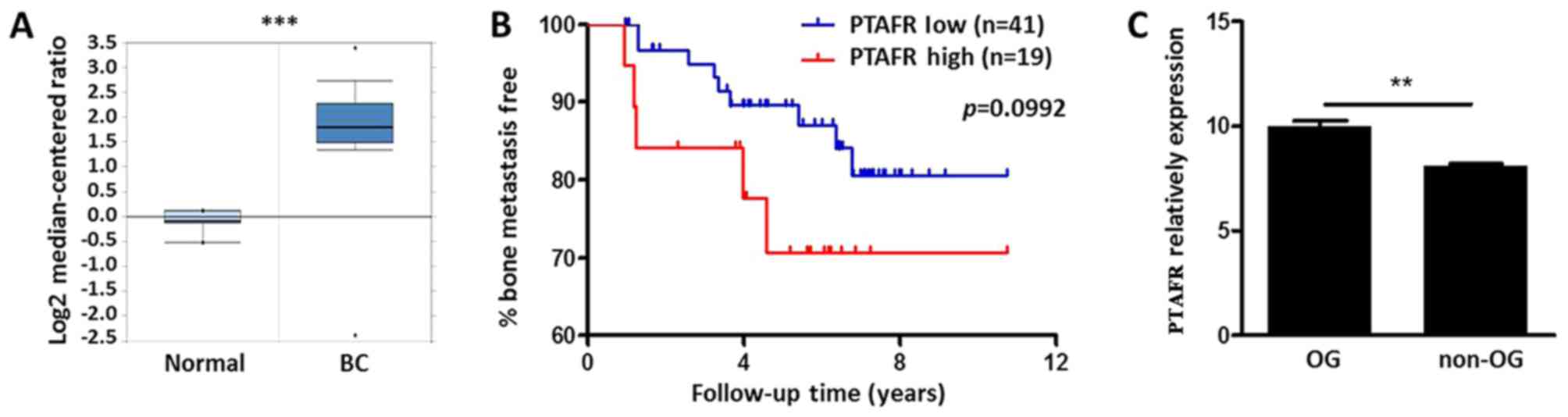
Bioinformatics analysis of PTAFR
expression in BC tissues and osteoclastogenic (OG) cell lines
Using the Oncomine database (www.oncomine.org), we compared the expression patterns
of PTAFR between normal breast tissues and invasive breast
carcinomas PTAFR was significantly upregulated in cancer patients
with a fold change of 3.88 (P<0.001; Fig. 1A). We further examined prognostic
value of PTAFR expression level in BC bone metastasis by Minn's
dataset (25). Although no
significance was drown (P=0.0992, P<0.1), BC with higher
expression levels of PTAFR (n=19) still indicated a tendency for
bone metastases (Fig. 1B). We also
revealed the expression patterns of PTAFR between OG and non-OG
cell lines in GSE43811. PTAFR was significantly upregulated after
RANKL stimulation in OG Raw264.7 cells (Fig. 1C).
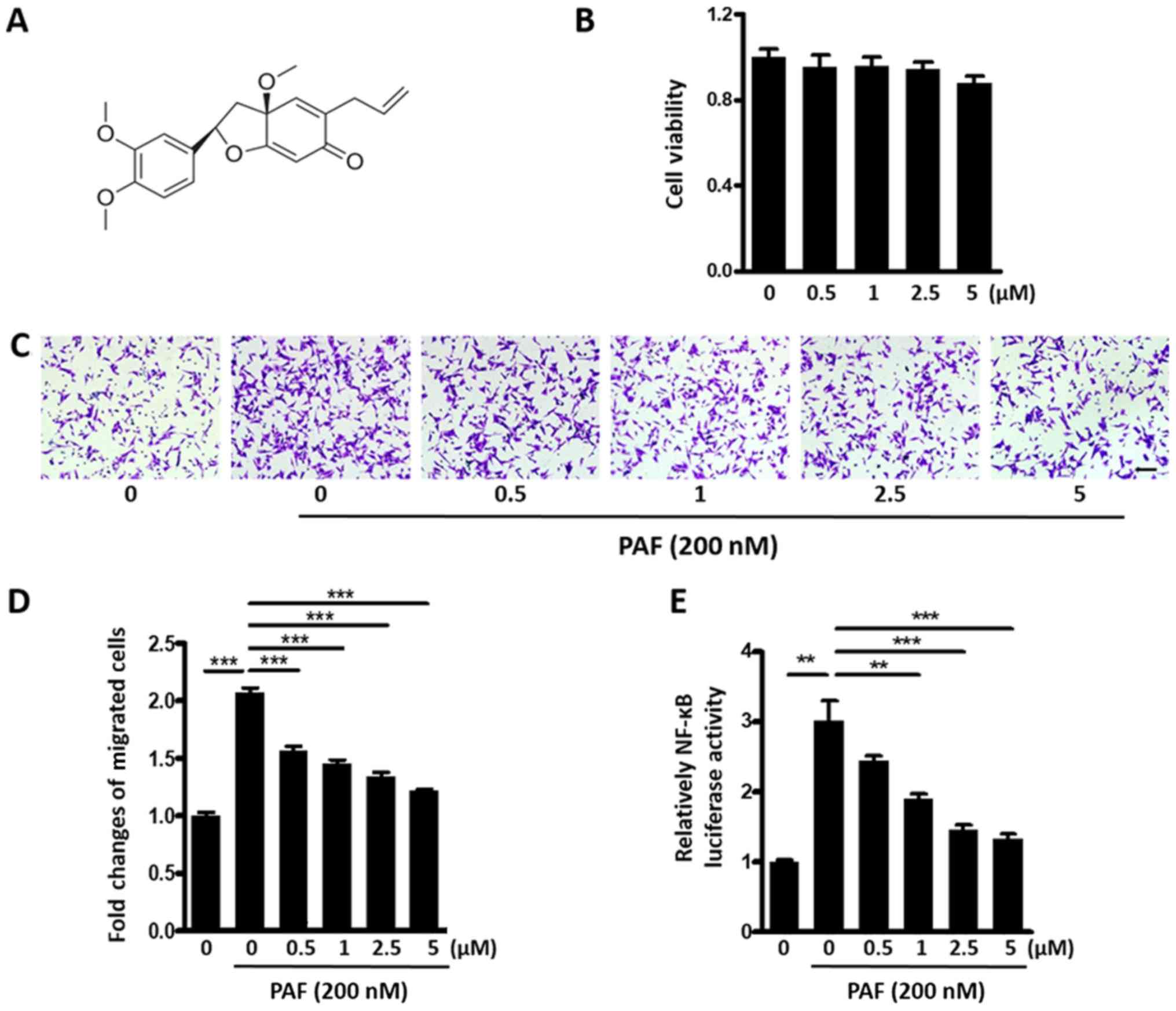
Kadsurenone inhibits PAF induced BC
cell migration
Kadsurenone is a natural product isolated from stems
of Piper kadsura. Chemical structure of Kadsurenone was
shown in Fig. 2A. We first tested
cytotoxicity of Kadsurenone on BC cell line MDA-MB-231. No
significant disturb of cell viability was observed with a highest
concentration of 5 µM (Fig. 2B).
We then examined effects of Kadsurenone on PAF
induced MDA-MB-231 cells migration. Transwell assay shown that
Kadsurenone could dose dependently inhibit PAF induced BC cells
migration (Fig. 2C and D). Luciferase
assay indicated that the NF-κB activity of MDA-MB-231 cells were
significantly inhibited by Kadsurenone dose dependently (Fig. 2E). Our data proved that Kadsurenone
did not disturb the cell viability significantly but suppressed
cell migration in MDA-MB-231 cells, which indicated that PAF/PTAFR
played key role in cell migration but not cell viability.
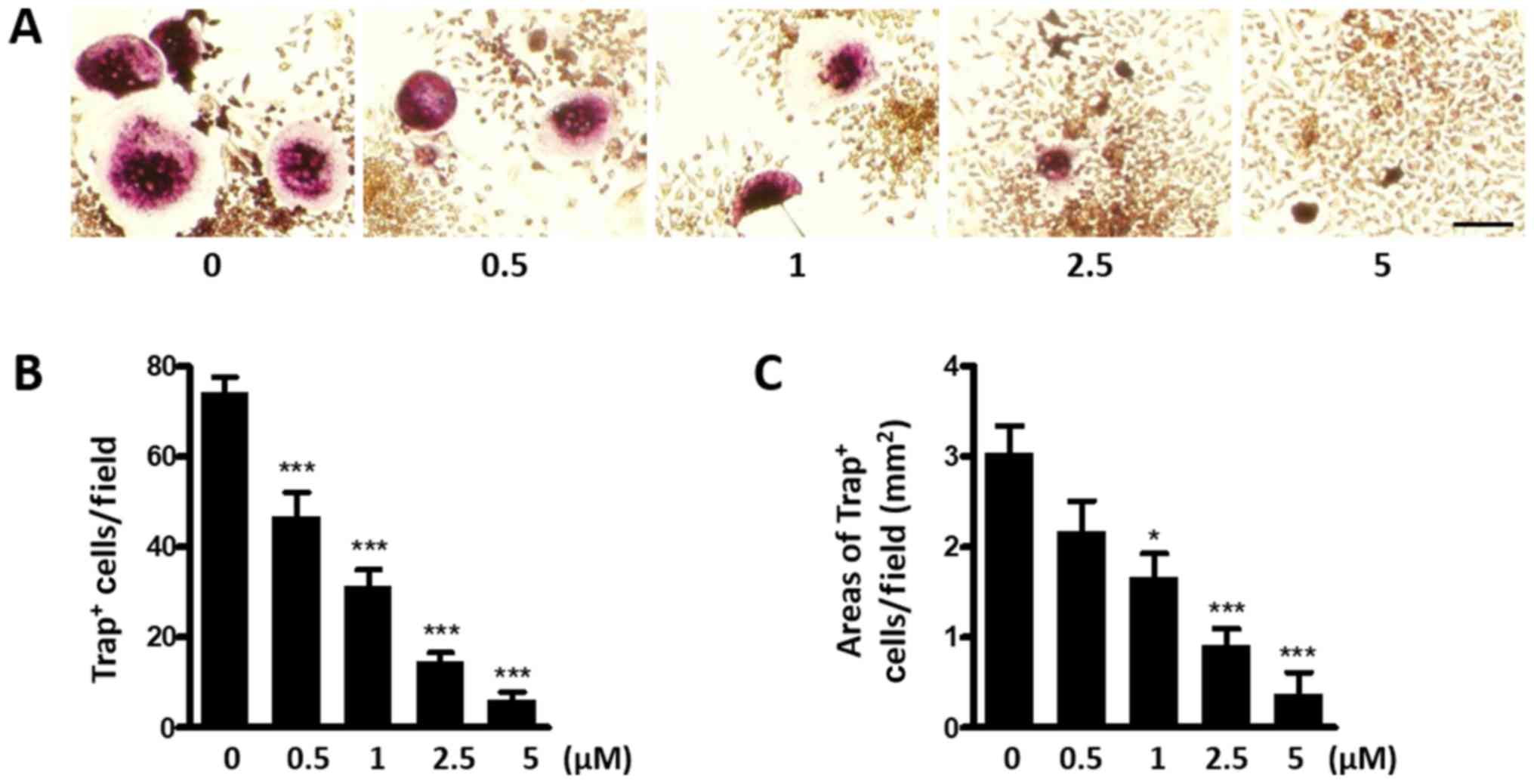
Kadsurenone inhibits BC cells induced
osteoclastogenesis
In the bone microenvironment, BC cells could
stimulate osteoclast differentiation directly through excretion of
cytokines such as interleukin (IL)-1β, tumor necrosis factor-α
(TNFα). BC cells could also indirectly promote osteoclast
differentiation via upregulation of RANKL by osteoblast. Therefore,
we intended to study whether Kadsurenone could directly inhibit BCs
induced osteoclastogenesis. MDA-MB-231 cells, which could secret
PAF, were co-cultured with the OG cell line RAW264.7 cells. We
proposed to use this co-culture system mimic the BC bone metastases
microenvironment. BC cells could induce RAW264.7 cells
differentiate into Trap positive osteoclasts. However, this process
could be attenuated by Kadsurenone dose dependently (Fig. 3).
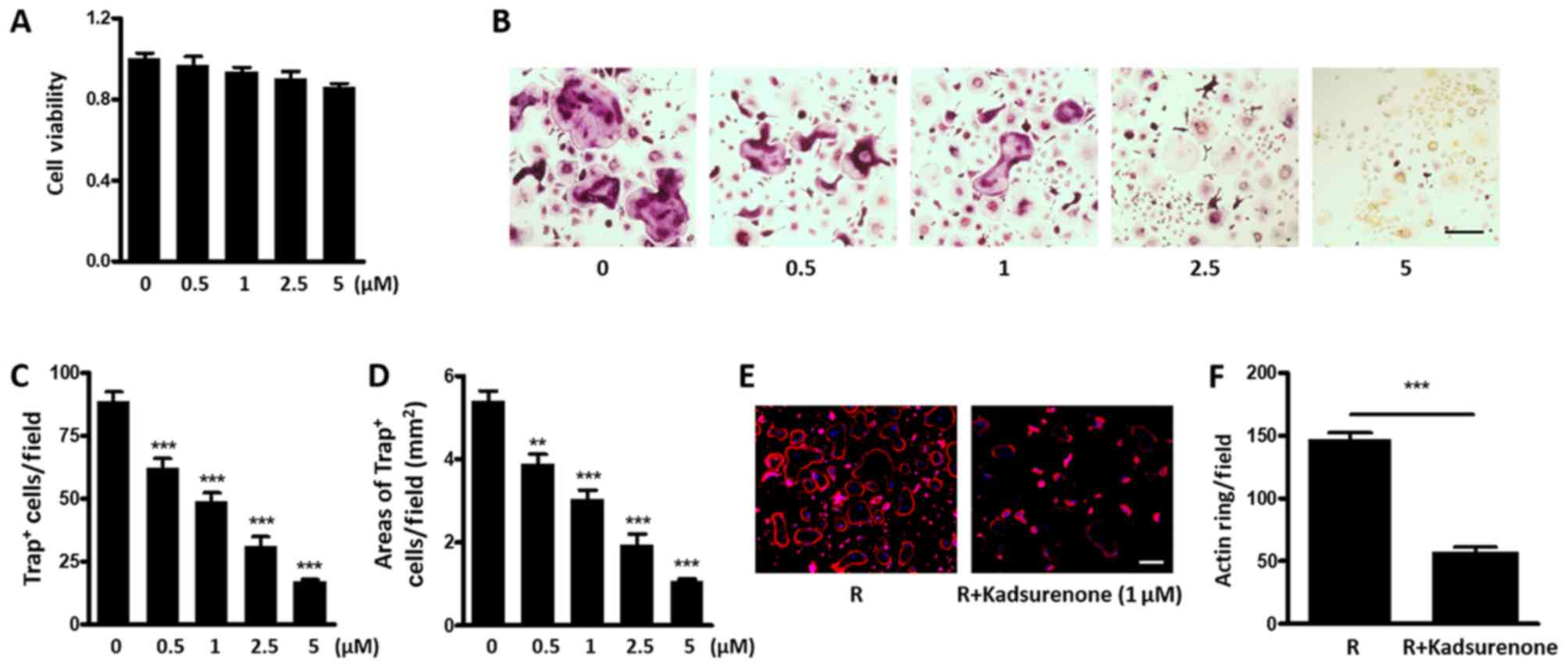
Kadsurenone directly inhibits RANKL
induced osteoclastogenesis
It is reported that BMMs could secret PAF as an
autocrine factor and promote the OG process (26). Therefore, we intend to investigate
whether Kadsurenone could directly affect osteoclastogenesis. We
firstly confirmed that no significant cytotoxicity of Kadsurenone
on mouse BMMs under a certain concentration (Fig. 4A). However, the process of RANKL
induced Trap positive osteoclasts differentiated by BMMs were
significantly attenuated by Kadsurenone dose dependently (Fig. 4B-D). Meanwhile, acting ring formation
were also restrained (Fig. 4E and
F).
Kadsurenone inhibits RANKL induced OC
marker genes expression by inhibiting the NF-κB pathway
To investigate potential mechanisms of the
inhibitory function of Kadsurenone on BMMs differentiation. RT-PCR
(Fig. 5A) and RT-qPCT (Fig. 5B) assay were performed. Results
indicated that the expression of osteoclast marker genes Ctsk,
Trap, and nuclear factor of activated T cells 1 (Nfatc1) were all
inhibited by Kadsurenone. Luciferase assay indicated that the
transcription factors NF-κB and Nfatc1 activities were all
attenuated (Fig. 5C).
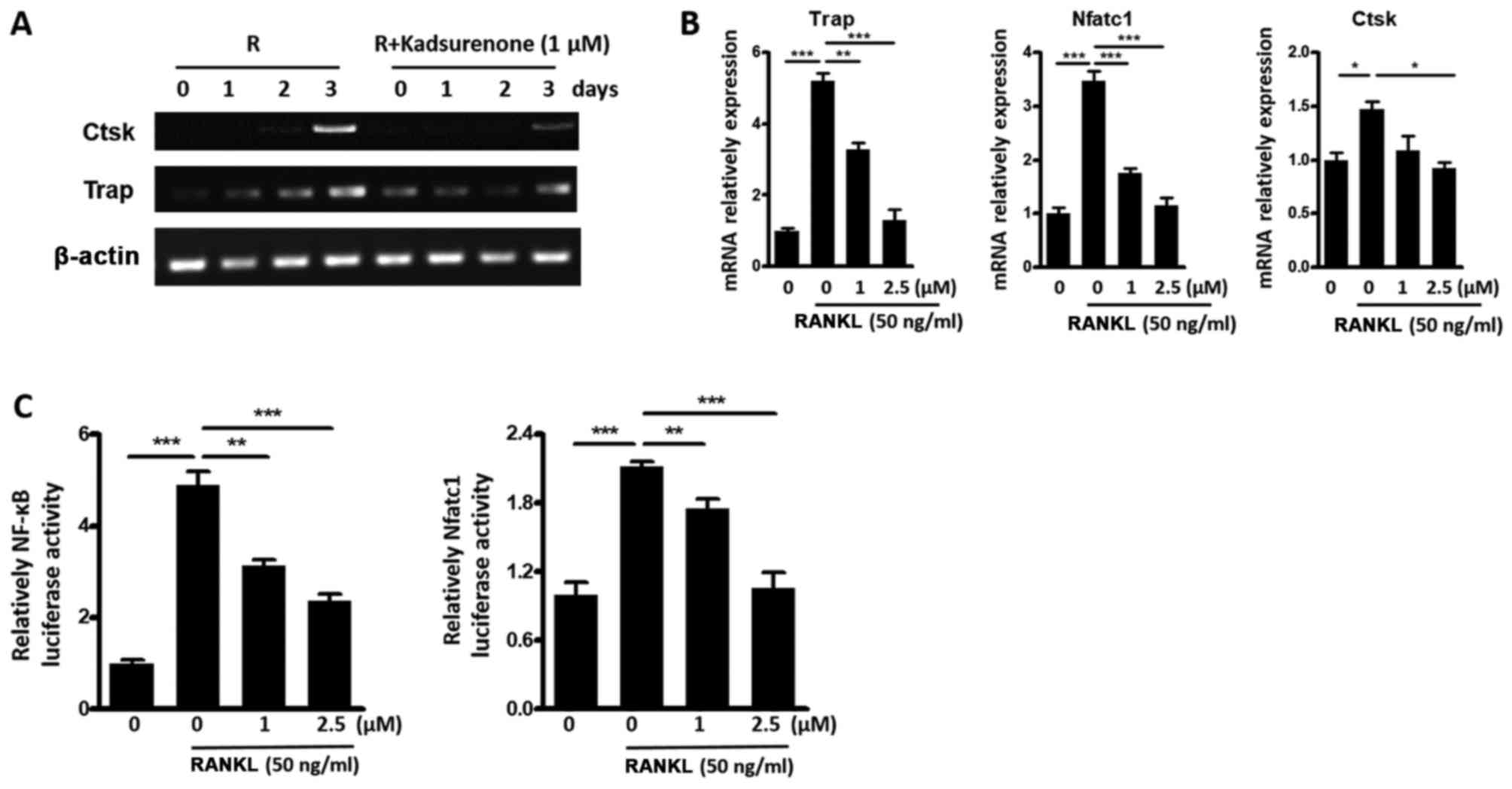 | Figure 5.Kadsurenone inhibits RANKL induce OC
marker gene expression by inhibiting the NF-κB signaling pathway.
(A) RT-qPCR analysis of the OC marker gene Ctsk and Trap
expression. Mouse BMMs were isolated and induced for
osteoclastogenesis with Rank l (50 ng/ml). Cells were treated with
or without 1 µM Kadsurenone. mRNAs were harvested on the indicated
days. (B) RT-qPCR analysis of the OC marker genes Trap, Nfatc1 and
Ctsk expression. Mouse BMMs were isolated and induced for
osteoclastogenesis with RANKL (50 ng/ml). Cells were treated with
the indicated concentrations of Kadsurenone. mRNAs were harvested
on day 3. (C) Relative NF-κB (left) and Nfatc1 (right) luciferase
activity of mouse BMMs induced by RANKL (50 ng/ml) with the
presence or absence of the indicated concentrations of Kadsurenone.
Data are presented as the mean ± standard error of the mean (n=3).
P-values were calculated using one-way analysis of variance
followed by Tukey's post hoc test. *P<0.05, **P<0.01 and
***P<0.001 vs. the control. BMMs, bone marrow monocytess;
RT-qPCR, reverse transcription-quantitative polymerase chain
reaction; OC, osteoclast; Ctsk, cathepsin K; Nfatc1, nuclear factor
of activated T cells 1; Trap, tartrate-resistant acid phosphatase;
RANKL, receptor activator for NF-κB ligand; NF, nuclear factor. |
Discussion
Bone metastasis is a deleterious and debilitating
aspect of BC patients. Up to 70% of BC patients who succumb to
disease present with bone metastases on autopsy (27). Bone metastases lesions are primarily
osteolytic since BC cells could regulate the bone-tumor
microenvironment (28–30). Osteoclasts were over activated by BC
cells while BC cells were reversely promoted by cytokines released
from bone matrix degradation. Current treatment strategies for BC
bone metastases mainly focused on breaking such ‘vicious osteolytic
cycle’ (31–33). Bioinformatics analysis provided clues
that the PAF/PTAFR signaling pathway may play important roles in
regulating BC bone metastasis and osteoclastogenesis. In the
current study, we demonstrated that PAF could promote BC cell
migration and induce BMMs differentiation. Besides, it has been
reported that absence of PAFR protects mice from osteoporosis
following ovariectomy, and PAF could enhance osteoclast
differentiation directly. Kadsurenone could attenuate both
processes, and could be considered as a potential therapeutic
reagent for BC bone metastases.
PAF can be synthesized within variety of cells like
platelets, macrophages, eosinophils, basophils and endothelial
cells by de novo or remodeling pathways and cause different
physiological reactions (34,35). The expression of PAF and its receptor
PTAFR in BC cell lines have also been demonstrated (17,18). In
the tumor microenvironment, PAF is secreted and accumulated by
infiltrated inflammation cells and tumor cells per se, which
forms an autocrine loop. PTAFR is a GPCR. PAF/PTAFR activation
resulted complex cell responses including increase of cell
motility, upregulation of IL-6 and matrix metalloproteinase (MMP)
expression, and activation of NF-κB signaling (34). The increased migration ability is an
important characteristic for cancer cells forming metastatic
lesions, including bone metastases (36). Both of published literatures and our
results revealed increased cell migration ability of BC cells after
PAF treatment. To avoid the harmful effects of PAF, mostly PAF
inhibitors are used to abolish or attenuate the PAF/PTAFR signaling
activation (37,38). Comparing to synthetic PAF inhibitors,
natural inhibitors are preferred due to the safety reason (34). In the current study, we used the
natural herbal extraction Kadsurenone effectively abolished PAF
induced BC migration, and little cytotoxicity manifested.
The great majority of BC produces osteolytic bone
metastases (9,39,40). This
process is characterized by over activated osteoclastogenesis and
subsequent bone destruction. BC cells could form a complex and
multigenic programed ‘vicious osteolytic cycle’ to remold the bone
microenvironment and promote cancer progression after, or even
before, bone colonization (28,36,41).
Cytokines secreted by BC cells like IL-6, parathyroid hormone like
hormone (PTHRP) and TNFα could stimulate osteoblasts and upregulate
the RANKL/osteoprotegerin (OPG) ratio, which further stimulates the
development of osteoclasts from myeloid precursors. At the same
time, other cytokines such as IL-11 and osteopontin (OPN) could
directly promote ostoclastogenesis. Activated osteoclasts degrade
the bone matrix, and release transforming growth factor β (TGFβ),
bone morphogenetic protein (BMPs) and insulin-like growth factor
(IGFs), which are mainly stored in bone matrix, reversely stimulate
BC progression.
Comparing to the ‘vicious osteolytic cycle’, PAF
could promote BMMs differentiate into osteoclasts by two parallel
manners. On one hand, PAF could stimulate BC cells upregulate
downstream signaling such as IL-6, IL-11, MMPs and NF-κB, which
further activate osteoclastogenesis (34). On the other hand, PAF could also
directly activate BMMs differentiate into osteoclasts (20,21). In
the current study, we demonstrated that Kadsurenone could inhibit
both the BC induced or PAF directly stimulated osteoclastogenesis.
Expression of osteoclast differentiation markers are all
downregulated after Kadsurenone treatment. It is known that NFATc1
is the master transcription factor for osteoclast differentiation.
NF-κB regulates RANKL induced osteoclast differentiation mainly
through promoting NFATc1 transcription activity. NF-κB is a
critical signal for OC differentiation downstream of RANKL, Mice
lacking the subunit p50 and p52 of NF-κB signal pathway have been
shown to be osteopetrotic p50-/- or p52-/- precursors fail to form
OCs. The results in our study indicated that PAF might partially
inhibited NF-κB activation and osteoclast differentiation via
blocking NFATc1 transcription activity.
In conclusion, Kadsurenone maybe an effectively
strategy to attenuate BC formed osteolytic bone metastases by
blocking the PAF/PTAFR signaling. Nonetheless, many questions
pertaining to be answered. Further studies are requested to reveal
detailed mechanisms of Kadsurenone function and in vivo
studies are needed.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HT performed the experiments and drafted the
manuscript. LY, LS and ZC performed the data analyses and wrote the
manuscript. JY, WD, TL, ZM, XW, QM and WZ performed the
experiments. ZJ and WH helped perform the data analysis. LZ
contributed to the conception of the study. XJ contributed to the
conception of the study and revised the manuscript critically for
important intellectual content.
Ethics approval and consent to
participate
All experimental protocols were approved by the
Review Committee for the Use of Human or Animal Subjects of East
China Normal University and Chang Zheng Hospital.
Consent for publication
Not applicable.
Competing interests
The authors confirm that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BC
|
breast cancer
|
|
PAF
|
platelet-activating factor
|
|
PTAFR
|
platelet-activating factor
receptor
|
|
OG
|
osteoclatogenic
|
|
NF-κB
|
nuclear factor-κB
|
|
P/S
|
penicillin-streptomycin
|
|
BMMs
|
bone marrow monocytes
|
|
α-MEM
|
α-minimum essential medium
|
|
Trap
|
tartrate-resistant acid
phosphatase
|
|
RANKL
|
receptor activator for NF-κB
ligand
|
|
GPCR
|
G-protein coupled receptor
|
|
IL
|
interleukin
|
|
MMP
|
matrix metalloproteinase
|
|
PTHRP
|
parathyroid hormone like hormone
|
|
TNFα
|
tumor necrosis factor-α
|
|
OPG
|
osteoprotegerin
|
|
OPN
|
osteopontin
|
|
TGFβ
|
transforming growth factor-β
|
|
BMP
|
bone morphogenetic protein
|
|
IGF
|
insulin-like growth factor
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coleman RE: Metastatic bone disease:
Clinical features, pathophysiology and treatment strategies. Cancer
Treat Rev. 27:165–176. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Selvaggi G and Scagliotti GV: Management
of bone metastases in cancer: A review. Crit Rev Oncol Hematol.
56:365–378. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sutcliffe P, Connock M, Shyangdan D, Court
R, Kandala NB and Clarke A: A systematic review of evidence on
malignant spinal metastases: natural history and technologies for
identifying patients at high risk of vertebral fracture and spinal
cord compression. Health Technol Assess. 17:1–274. 2013. View Article : Google Scholar
|
|
6
|
Kelly ML, Kshettry VR, Rosenbaum BP,
Seicean A and Weil RJ: Effect of a randomized controlled trial on
the surgical treatment of spinal metastasis, 2000 through 2010: A
population-based cohort study. Cancer. 120:901–908. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nguyen DX, Bos PD and Massagué J:
Metastasis: From dissemination to organ-specific colonization. Nat
Rev Cancer. 9:274–284. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sutherland A, Forsyth A, Cong Y, Grant L,
Juan TH, Lee JK, Klimowicz A, Petrillo SK, Hu J, Chan A, et al: The
role of prolactin in bone metastasis and breast cancer
cell-mediated osteoclast differentiation. J Natl Cancer Inst.
108:2015.PubMed/NCBI
|
|
9
|
von Moos R, Sternberg C, Body JJ and
Bokemeyer C: Reducing the burden of bone metastases: Current
concepts and treatment options. Support Care Cancer. 21:1773–1783.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li BT, Wong MH and Pavlakis N: Treatment
and prevention of bone metastases from breast cancer: A
comprehensive review of evidence for clinical practice. J Clin Med.
3:1–24. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Damiani E and Ullrich SE: Understanding
the connection between platelet-activating factor, a UV-induced
lipid mediator of inflammation, immune suppression and skin cancer.
Prog Lipid Res. 63:14–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pałgan K and Bartuzi Z: Platelet
activating factor in allergies. Int J Immunopathol Pharmacol.
28:584–589. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Y, Shields LBE, Gao Z, Wang Y, Zhang
YP, Chu T, Zhu Q, Shields CB and Cai J: Current understanding of
platelet-activating factor signaling in central nervous system
diseases. Mol Neurobiol. 54:5563–5572. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McHowat J, Gullickson G, Hoover RG, Sharma
J, Turk J and Kornbluth J: Platelet-activating factor and
metastasis: Calcium-independent phospholipase A2β deficiency
protects against breast cancer metastasis to the lung. Am J Physiol
Cell Physiol. 300:C825–C832. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu JT and Kral JG: The NF-kappaB/IkappaB
signaling system: A molecular target in breast cancer therapy. J
Surg Res. 123:158–169. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang X, Jung YS, Jun S, Lee S, Wang W,
Schneider A, Oh Sun Y, Lin SH, Park BJ, Chen J, et al: PAF-Wnt
signaling-induced cell plasticity is required for maintenance of
breast cancer cell stemness. Nat Commun. 7:106332016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bussolati B, Biancone L, Cassoni P, Russo
S, Rola-Pleszczynski M, Montrucchio G and Camussi G: PAF produced
by human breast cancer cells promotes migration and proliferation
of tumor cells and neo-angiogenesis. Am J Pathol. 157:1713–1725.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Anandi VL, Ashiq KA, Nitheesh K and Lahiri
M: Platelet-activating factor promotes motility in breast cancer
cells and disrupts non-transformed breast acinar structures. Oncol
Rep. 35:179–188. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Langdahl B, Ferrari S and Dempster DW:
Bone modeling and remodeling: Potential as therapeutic targets for
the treatment of osteoporosis. Ther Adv Musculoskelet Dis.
8:225–235. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng ZG, Wood DA, Sims SM and Dixon SJ:
Platelet-activating factor stimulates resorption by rabbit
osteoclasts in vitro. Am J Physiol. 264:E74–E81. 1993.PubMed/NCBI
|
|
21
|
Wood DA, Hapak LK, Sims SM and Dixon SJ:
Direct effects of platelet-activating factor on isolated rat
osteoclasts. Rapid elevation of intracellular free calcium and
transient retraction of pseudopods. J Biol Chem. 266:15369–15376.
1991.PubMed/NCBI
|
|
22
|
Kim H, Kim BJ, Ahn SH, Lee SH and Koh JM:
Higher plasma platelet-activating factor levels are associated with
increased risk of vertebral fracture and lower bone mineral density
in postmenopausal women. J Bone Miner Metab. 33:701–707. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang SP, Lin LC, Wu YT and Tsai TH:
Pharmacokinetics of kadsurenone and its interaction with
cyclosporin A in rats using a combined HPLC and microdialysis
system. J Chromatogr B Analyt Technol Biomed Life Sci. 877:247–252.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang N, Li R, Yu H, Shi D, Dong N, Zhang
S and Wang H: Development of an LC-MS/MS method for quantification
of kadsurenone in rat plasma and its application to a
pharmacokinetic study. Biomed Chromatogr. 27:1754–1758. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu
W, Giri DD, Viale A, Olshen AB, Gerald WL and Massagué J: Genes
that mediate breast cancer metastasis to lung. Nature. 436:518–524.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hikiji H, Ishii S, Shindou H, Takato T and
Shimizu T: Absence of platelet-activating factor receptor protects
mice from osteoporosis following ovariectomy. J Clin Invest.
114:85–93. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Johnson RW, Finger EC, Olcina MM, Vilalta
M, Aguilera T, Miao Y, Merkel AR, Johnson JR, Sterling JA, Wu JY
and Giaccia AJ: Induction of LIFR confers a dormancy phenotype in
breast cancer cells disseminated to the bone marrow. Nat Cell Biol.
18:1078–1089. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kang Y, Siegel PM, Shu W, Drobnjak M,
Kakonen SM, Cordón-Cardo C, Guise TA and Massagué J: A multigenic
program mediating breast cancer metastasis to bone. Cancer Cell.
3:537–549. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Roodman GD: Mechanisms of bone metastasis.
N Engl J Med. 350:1655–1664. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Macedo F, Ladeira K, Pinho F, Saraiva N,
Bonito N, Pinto L and Goncalves F: Bone metastases: An overview.
Oncol Rev. 11:3212017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Trémollieres FA, Ceausu I, Depypere H,
Lambrinoudaki I, Mueck A, Pérez-López FR, van der Schouw YT,
Senturk LM, Simoncini T, Stevenson JC, et al: Osteoporosis
management in patients with breast cancer: EMAS position statement.
Maturitas. 95:65–71. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Van Acker HH, Anguille S, Willemen Y,
Smits EL and Van Tendeloo VF: Bisphosphonates for cancer treatment:
Mechanisms of action and lessons from clinical trials. Pharmacol
Ther. 158:24–40. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Santini D, Stumbo L, Spoto C, D'Onofrio L,
Pantano F, Iuliani M, Fioramonti M, Zoccoli A, Ribelli G, Virzì V,
et al: Bisphosphonates as anticancer agents in early breast cancer:
preclinical and clinical evidence. Breast Cancer Res. 17:1212015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Singh P, Singh IN, Mondal SC, Singh L and
Garg VK: Platelet-activating factor (PAF)-antagonists of natural
origin. Fitoterapia. 84:180–201. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tsoupras AB, Fragopoulou E, Nomikos T,
Iatrou C, Antonopoulou S and Demopoulos CA: Characterization of the
de novo biosynthetic enzyme of platelet activating factor,
DDT-insensitive cholinephosphotransferase, of human mesangial
cells. Mediators Inflamm. 2007:276832007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Weilbaecher KN, Guise TA and McCauley LK:
Cancer to bone: A fatal attraction. Nat Rev Cancer. 11:411–425.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kasperska-Zajac A, Brzoza Z and Rogala B:
Platelet-activating factor (PAF): A review of its role in asthma
and clinical efficacy of PAF antagonists in the disease therapy.
Recent Pat Inflamm Allergy Drug Discov. 2:72–76. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Imrie CW and McKay CJ: The possible role
of platelet-activating factor antagonist therapy in the management
of severe acute pancreatitis. Baillieres Best Pract Res Clin
Gastroenterol. 13:357–364. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Waning DL and Guise TA: Molecular
mechanisms of bone metastasis and associated muscle weakness. Clin
Cancer Res. 20:3071–3077. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cox TR, Gartland A and Erler JT: Lysyl
oxidase, a targetable secreted molecule involved in cancer
metastasis. Cancer Res. 76:188–192. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cox TR, Rumney RM, Schoof EM, Perryman L,
Høye AM, Agrawal A, Bird D, Latif NA, Forrest H, Evans HR, et al:
The hypoxic cancer secretome induces pre-metastatic bone lesions
through lysyl oxidase. Nature. 522:106–110. 2015. View Article : Google Scholar : PubMed/NCBI
|