Introduction
Breast cancer is the most fatal type of malignancy
and the second leading cause of cancer-associated mortalities among
women worldwide (1). Approximately
90% of breast cancer-associated mortalities are due to metastasis
to distant sites and not by the primary tumor itself. Therefore,
screening for more effective compounds to control breast cancer
metastasis has become the most important factor in improving breast
cancer treatment.
During cancer progression, epithelial-mesenchymal
transition (EMT) process is one of the primary mechanisms involved
in breast cancer metastasis (2). The
process of EMT, during which cells lose epithelial characteristics
and obtain mesenchymal properties, promotes cancer cell metastasis
from primary tumors (3). In addition
to EMT, angiogenesis is another important factor that promotes
tumor growth metastasis (4).
Accumulating evidence suggests that vascular endothelium growth
factor (VEGF) is an essential factor in the development of tumor
angiogenesis (5). Hypoxia inducible
factor 1α (HIF-1α), upstream of the VEGF, is associated with poor
prognosis, and an increased risk of metastasis and mortality
(6). Hypoxia, a common characteristic
of solid tumors, has been reported to reactivate EMT through HIF-1α
in several tumor models (7). HIF-1α
directly or indirectly regulates EMT regulators, including Snail,
Twist1 and Slug, and other transcription factors, and these
transcription factors then trans activate EMT-associated genes,
including E-cadherin, Vimentin and N-cadherin, to mediate EMT
(8). Accordingly, EMT and
angiogenesis have become therapeutic targets for cancer metastasis
treatment.
Oridonin, an active diterpenoid compound isolated
from Rabdosia rubescens, is currently one of the most
important active Chinese medicinal components. Notably, several
studies reported that oridonin also demonstrates significant
anti-metastasis effect in a variety of cancer types (9). For instance, Dong et al (10) confirmed that oridonin inhibits tumor
growth and metastasis through anti-angiogenesis by blocking the
Notch signaling. Liu et al (11) indicated that the oridonin anti-tumor
effect was primarily based on its anti-proliferation and
anti-angiogenesis properties. Although accumulating evidence have
indicated the anti-metastatic potential of oridonin, the effects
and underlying mechanisms of oridonin on human breast cancer cells
have not been fully elucidated. The present study investigated the
effects of oridonin on EMT and angiogenesis in breast cancer, and
its underlying mechanisms.
Materials and methods
Animals
A total of 24 female BALB/c mice (5–6 weeks old,
14–16 g) were purchased from the Laboratory Animal Center of the
Academy of Military Medical Sciences (Beijing, China) and housed in
a temperature-controlled vivarium on a 12/12 h light/dark cycle.
Standard rodent diet and water were provided ad libitum
except where noted. All animal experiments were approved by the
Animal Ethics Committee of Tianjin Medical University (Tianjin,
China) and complied with its regulations.
Reagents and antibodies
Oridonin (CAS no. 28957-04-2) was acquired from
Aladdin Shanghai Biochemical Technology Co., Ltd. (Shanghai,
China). MTT, dimethyl sulfoxide (DMSO) and albumin fraction V (BSA)
were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS)
and penicillin/streptomycin were purchased from Gibco (Thermo
Fisher Scientific Inc., Waltham, MA, USA). Antibodies against
E-cadherin (mouse; cat. no. 14472), N-cadherin (rabbit; cat. no.
13116), Vimentin (mouse; cat. no. 3390) and Snail (mouse; cat. no.
3895) were purchased from Cell Signaling Technology, Inc. (Danvers,
MA, USA). Antibodies against HIF-1α (mouse; cat. no. ab113642),
VEGF-A (rabbit; cat. no. ab46154), VEGFR-2 (rabbit; cat. no.
ab2349), β-actin (rabbit; cat. no. ab8227) and CD31 (rabbit; cat.
no. ab32457) were purchased from Abcam (Cambridge, MA, USA). Goat
anti-mouse IgG-horseradish peroxidase (HRP) (cat. no. sc-2005) and
goat anti-rabbit IgG-HRP (cat. no. sc-2004) were purchased from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Cell culture
Human breast cancer cells MDA-MB-231,
non-tumorigenic human breast cells MCF-10A and human umbilical vein
epithelial cells (HUVECs; cat. no. PCS-100-013) were obtained from
the American Type Culture Collection (Manassas, VA, USA). Mouse
breast cancer cells 4T1 were purchased from the Type Culture
Collection of the Chinese Academy of Sciences (Shanghai, China).
The cells were cultured in DMEM supplemented with 10% FBS, 100 U/ml
penicillin and 100 µg/ml streptomycin. Hypoxia was induced by
exposing the cells to 150 µM CoCl2 (Sigma-Aldrich; Merck
KGaA) in DMEM with 0.5% FBS for 24 h (12). All cell cultures were maintained at
37°C in an atmosphere containing 5% CO2.
MTT assay
Cell viability was measured using an MTT assay. The
MDA-MB-231, MCF-10A and 4T1 cells in the logarithm phase were
seeded in 96-well culture plates at the density of 5×103
cells/well. When the cells were adherent to the walls, the cells
were treated with oridonin (2, 4, 8, 16, 32 and 64 µM) for 48 h
followed by the addition of 100 µl MTT (1 mg/ml) and incubation for
4 h. Then, the medium was removed and 150 µl of DMSO was added to
each well. Absorbance of each well was detected at 490 nm using
amicroplate reader. The experiment was repeated three times. The
inhibition rate (% of control) was calculated as follows:
[1-(absorbance of test sample/absorbance of control)] ×100%.
Wound healing migration assay
Cell migration was detected using a wound-healing
assay. MDA-MB-231 and 4T1 cells were cultured in 60-mm dishes at
the density of 8×105 cells/dish to 100% confluence.
Following wounding with a pipette tip, the cells were washed with
PBS and serum-free medium with 4, 8 or 16 µM oridonin. Then, the
cells were allowed to migrate for 24 h at 37°C in 5%
CO2. At predetermined time points (0, 3, 6, 9, 12 and 24
h), the widths of wound were measured using a ruler, and images of
the cells were captured at 0 and 24 h under a light microscope with
×100 magnification (IX71; Olympus Corporation, Tokyo, Japan).
Transwell invasion assay
Cell invasion was detected using a Transwell assay.
Following pre-treatment with 4, 8 or 16 µM oridonin for 24 h,
MDA-MB-231 and 4T1 cells were harvested and seeded in the upper
chamber of the Transwell chamber coated with Matrigel (BD
Biosciences, San Jose, CA, USA) at a density of 4×104
cells/well in serum-free medium. The lower chambers were filled
with standard medium. The cells were allowed to invade for 24 h
incubated at 37°C in 5% CO2. The invading cells were
fixed with 4% methanol for 20 min at room temperature. Cell numbers
were counted in five separate fields using a computer-based
microcopy imaging DP2-BSW system (version 1.3; Olympus
Corporation).
Cell adhesion assay
Following pre-treatment with 4, 8 or 16 µM oridonin
for 24 h, MDA-MB-231 and 4T1 cells were harvested, re-suspended in
serum free medium at the density of 2×105 cells/well and
seeded to the 24-well plates coated with fibronectin (10 ng/ml).
Following further incubations for 5, 15 and 30 min, non-adherent
cells were removed by three PBS washes. The adherent cells were
fixed with 4% methanol for 20 min at room temperature and counted
in five separate fields under a light microscope with ×200
magnification (Olympus Corporation).
Tube formation assay
AHUVEC capillary-like formation assay was performed
on Matrigel (BD Biosciences) (13).
Matrigel was added into 96-well plate and cultured in 37°C for 2 h
to solidify the Matrigel. Following pre-treatment with 4, 8 or 16
µM oridonin for 24 h, HUVECs were seeded in plates at the density
of 8×103 cells/well. According to previous methods
(14,15), morphological changes (formation of
capillary-like structures) of the cells and tube formation were
observed under a phase-contrast microscope and imaged at ×200
magnification after a 6 h incubation.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Following pre-treatment with 4, 8 or 16 µM oridonin
for 24 h, the total RNA of MDA-MB-231 or 4T1 cells was extracted
using TRIzol reagent (Life Technologies; Thermo Fisher Scientific,
Inc.). Reverse transcription was performed with the fast quant RT
kit (Tiangen Biotech Co., Ltd., Beijing, China) according to the
manufacturer's protocol. The PCR mixture volume was 25 µl,
including 12.5 µl SYBR green mix (Tiangen Biotech Co., Ltd.), 0.2
µl cDNA, 1.5 µl primer/mix (10 µM of each primer) and 10.8 µl
RNAse-free H2O. The experiment was then set up using the
following PCR program on a ABI 7500 system (Applied Biosystems;
Thermo Fisher Scientific, Inc.): 95°C for 15 min, 1 cycle; followed
by 40 cycles of 95°C for 10 sec, 60°C for 20 sec and 72°C for 30
sec. Specific primers were designed using Gene Runner software
(version 6.5.48) produced by Dr. Spruyt Michael (Mahwah, NJ, USA)
and Dr. Buquicchio Frank (Old Tappan, NJ, USA). Specific primers
were synthesized by Beijing Century Aoke Biotechnology Co., Ltd.
(Beijing, China). The specific primers are presented in Table I. The Cq value was automatically
calculated by 7500 software (version 2.0.5, Applied Biosystems;
Thermo Fisher Scientific, Inc.), the Ct values were all normalized
against the quantity of the β-actin control RNA, and the relative
quantification of gene expression was calculated by the
2−∆∆Cq method according to the formula: ∆Cq (target
gene)=Cq (target gene)-Cq (control gene) (16). All assays were performed in triplicate
and independently repeated three times.
 | Table I.Primers sequences used in RT-qPCR. |
Table I.
Primers sequences used in RT-qPCR.
| Gene name | Primer sequence
(5′-3′) |
|---|
| E-cadherin |
|
| F |
GGATTGCAAATTCCTGCCATTC |
| R |
AACGTTGTCCCGGGTGTCA |
| Vimentin |
|
| F |
GCAGGAGGCAGAAGAATGGTA |
| R |
GGGACTCATTGGTTCCTTTAAGG |
| Snail |
|
| F |
TCTAGGCCCTGGCTGCTACAA |
| R |
CATCTGAGTGGGTCTGGAGGTG |
| N-cadherin |
|
| F |
GAGAGGAAGACCAGGACTATGA |
| R |
CAGTCATCACCACCACCATAC |
| β-actin |
|
| F |
CCACGAAACTACCTTCAACTCCA |
| R |
GTGATCTCCTTCTGCATCCTGTC |
Western blotting assay
Western blotting assay was used for the detection of
E-cadherin, N-cadherin, Vimentin, Snail, HIF-1α, VEGF-A and VEGFR-2
expression levels in MDA-MB-231 and 4T1 cells. Following
pre-treatment with 4, 8 or 16 µM oridonin for 24 h, the 10 µg
protein lysates from cultured cells were separated by 10% SDS-PAGE
systems and transferred to polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA). Following blocking with 5% skim
milk in TBS containing 0.1% Tween 20 (TBST) for 2 h at room
temperature, the membranes were incubated with the aforementioned
primary antibodies at 1:500 to 1:1,000 dilutions with 5% BSA in
TBST overnight at 4°C. The antibodies and dilution factors were as
follows: E-cadherin (1:1,000); N-cadherin (1:1,000); Vimentin
(1:1,000); Snail (1:1,000); HIF-1α (1:500); VEGF-A (1:800); and
VEGFR-2 (1:800). The blots were washed with PBS and incubated with
HRP-conjugated secondary antibodies for 1 h at room temperature.
Membranes were visualized using enhanced chemiluminescencekit (EMD
Millipore) and were imaged using G-BOX (Gene Company, Ltd., Hong
Kong, China). The bands analyzed using Image Pro Plus 6.0 software
(Media Cybernetics Inc., Rockville, MD, USA).
Animal experiments
The 4T1 (3×106) cells were subcutaneously
inoculated into the mammary fat pad of mice. Once each animal
developed distinct tumors, animals were randomly segregated into 1
vehicle group and 3 oridonin group (n=6/group) and respectively
treated with 0.5% DMSO (the vehicle) or oridonin at doses of 2.5, 5
or 10 mg/kg. All administrations were carried out via intratumoral
injections every 3 days four times. Bodyweight and tumor volumes
were measured twice a week. The tumor volume was estimated using
the following formula: (ab)2/2, where a and b are tumor
length and width, respectively (17).
The animals were sacrificed on day 10, and the tumors of the
oridonin-treated and vehicle-treated groups were excised.
Immunohistochemical analysis
Immunohistochemical analysis was performed to
evaluate the tumor vessels density. Tumor tissues were fixed in 8%
neutral formaldehyde at room temperature, embedded in paraffin and
cut into sections of 5-mm thickness. Following antigen retrieval,
tissue sections were blocked with PBS containing blocking serum
(Gibco; Thermo Fisher Scientific, Inc.) for 2 h at room temperature
and were incubated overnight at 4°C with primary antibodies against
CD31 at 1:500 dilutions with 5% BSA in TBST (cat. no. AR0195;
Boster, Wuhan, China). Subsequently, sections were incubated with
universal HRP-conjugated secondary antibodies (OriGene
Technologies, Inc., Beijing, China) for 30 min. The chromogenic
reaction was performed using DAB peroxidase substrate (OriGene
Technologies, Inc.) by incubating sections for 2 min at room
temperature. Images were captured with an upright fluorescent
microscope (BX51; Olympus Corporation). The number of CD31-positive
vessels (brown staining) in five randomly selected fields
(magnification, ×200) was counted and vessels were calculated as
the sum of CD31-positive vessels in the five fields (18).
Statistical analysis
Statistical analysis was performed using SPSS 19.0
(IBM Corp., Armonk, NY, USA). All data are presented as the mean ±
standard deviation. One-way analysis of variance was used to
analyze the difference between the groups. The least significant
difference method was used to analyze post-hoc multiple
comparisons. P-values were two-tailed; P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of oridonin on the viability
of MDA-MB-231, 4T1 and MCF-10A cells
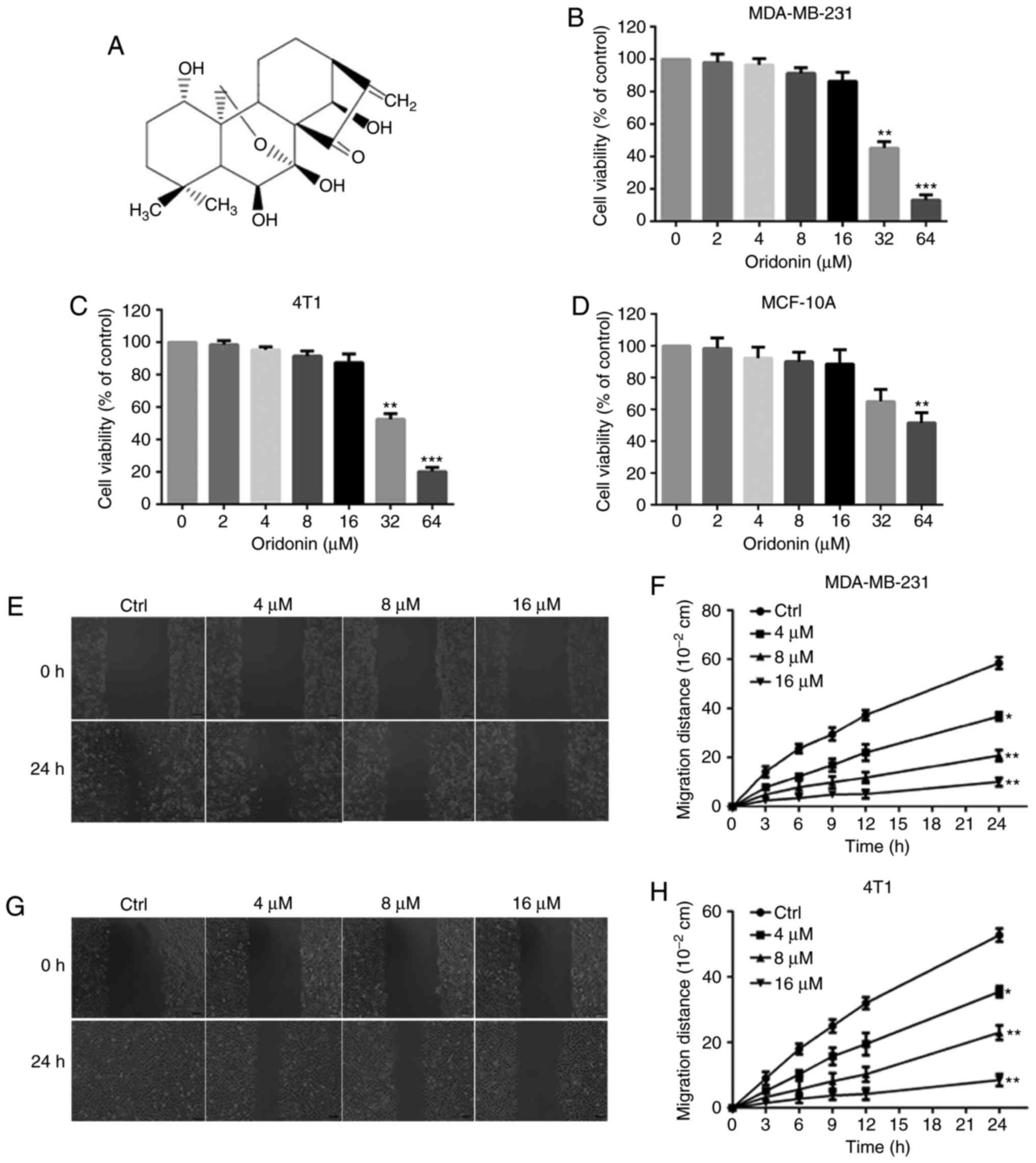
Oridonin has a molecular weight of 364.44 g/mol and
its molecular structure is presented in Fig. 1A. Firstly, the inhibitory effects of
oridonin on the proliferation of MDA-MB-231 and 4T1 cells were
investigated using an MTT assay. The results demonstrated that
oridonin treatment caused significant cell proliferation inhibition
in a dose-dependent manner in MDA-MB-231 and 4T1 cells (Fig. 1B and C). The IC50 value of
oridonin after 48 h of incubation was 29.33 and 33.78 µM for
MDA-MB-231 and 4T1 cells, respectively. No significant differences
were observed in the viability of MDA-MB-231 and 4T1 cells
following exposureto oridonin (4, 8 and 16 µM) for 48 h. Oridonin
at 4, 8 and 16 µM alone exhibited no significant anti-proliferative
and cytotoxic effects in non-tumorigenic human breast cells MCF-10A
(IC50 value, 67.94 µM) (Fig.
1D). Thus, in the subsequent experiments, the concentrations 4,
8 and 16 µM oridonin were chosen for further investigation. For all
in vitro experiments, an untreated group was used as the
control.
Oridonin inhibits the migration and
invasion of MDA-MB-231 and 4T1 cells
In the absence of oridonin (control group),
MDA-MB-231 and 4T1 cells exhibited high migratory and invasive
capabilities as indicated through the complete healing of the wound
scratch and penetration of cells through the Matrigel-coated
filters. The migration (Fig. 1E-H)
and invasion (Fig. 2A-C) of cancer
cells was significantly suppressed by oridonin in a
concentration-dependent manner.
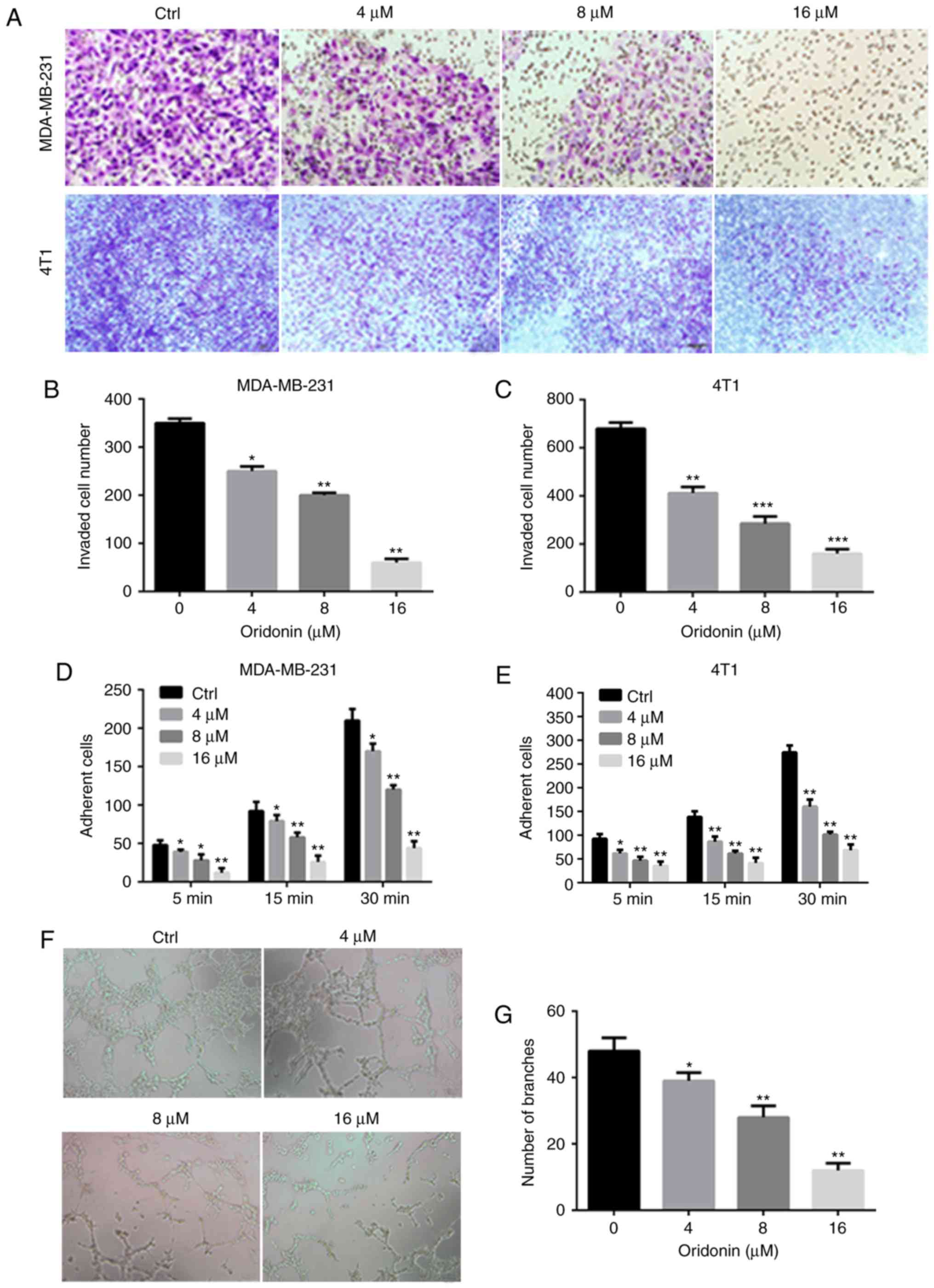
Oridonin inhibits the adhesion of
MDA-MB-231 and 4T1 cells
Fibronectin was used as the basement membrane to
mimic the adhesion of MDA-MB-231 and 4T1 cells (19). Following pre-treatment with 4, 8 and
16 µM oridonin, the number of cells adhering to the fibronectin
significantly decreased compared with the control group (Fig. 2D and E). The results demonstrated that
oridonin inhibited the adhesion of MDA-MB-231 and 4T1 cells to
fibronectin.
Oridonin inhibits tube formation of
HUVECs cells in vitro
To investigate the effect of oridonin on
angiogenesis in vitro, a capillary tube formation assayusing
HUVECs on Matrigel was performed. As presented in Fig. 2F and G, the capillary tube formation
of HUVECs was significantly inhibited after 24 h exposure to
oridonin when compared with the control group.
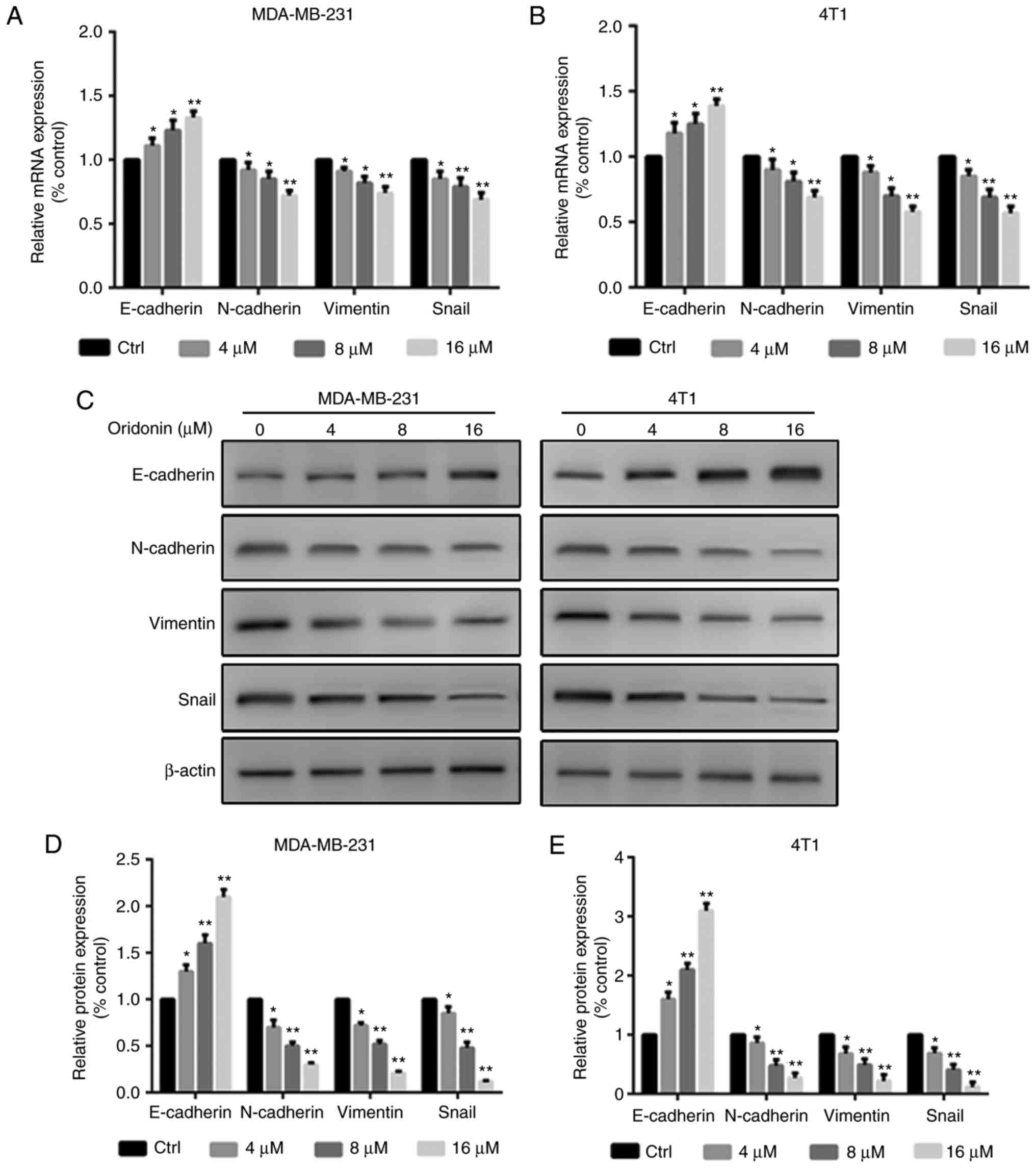
Oridonin regulates the expression of
EMT markers in MDA-MB-231 and 4T1 cells
The results demonstrated that the effects of
oridonin were dose-dependent, with significantly increasing
expression levels of E-cadherin, and significantly decreasing
expression levels of N-cadherin, Vimentin and Snail in MDA-MB-231
and 4T1 cells compared with the control group (Fig. 3).
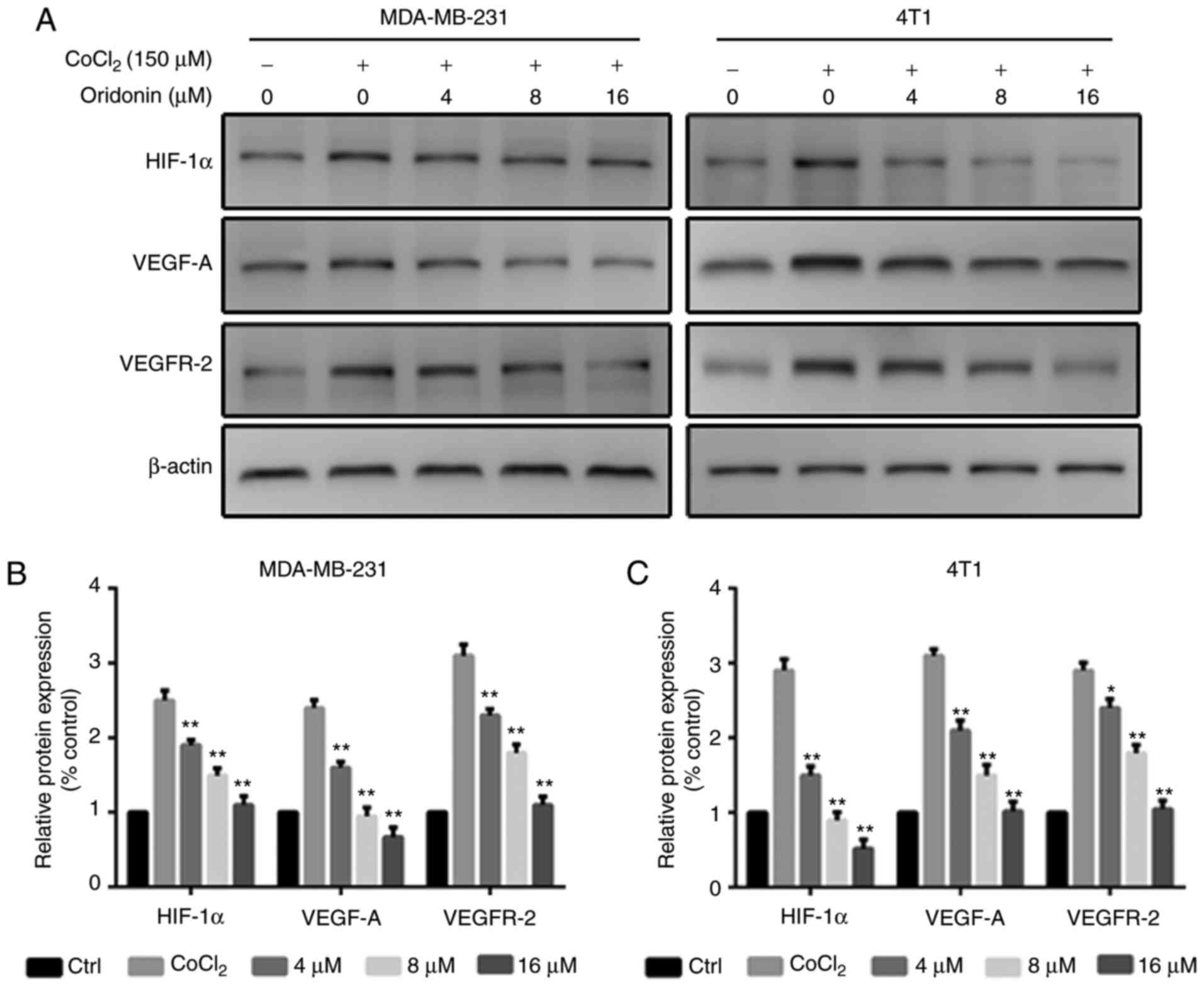
Oridonin suppresses the HIF-1α/VEGF
signaling pathway in MDA-MB-231 and 4T1 cells
The expression levels of angiogenic molecules,
including HIF-1α, VEGF-A and VEGFR-2 were measured in MDA-MB-231
and 4T1 cells using western blotting. The data revealed that the
levels of HIF-1α, VEGF-A and VEGFR-2 were significantly reduced
following oridonin treatment (Fig.
4A-C).
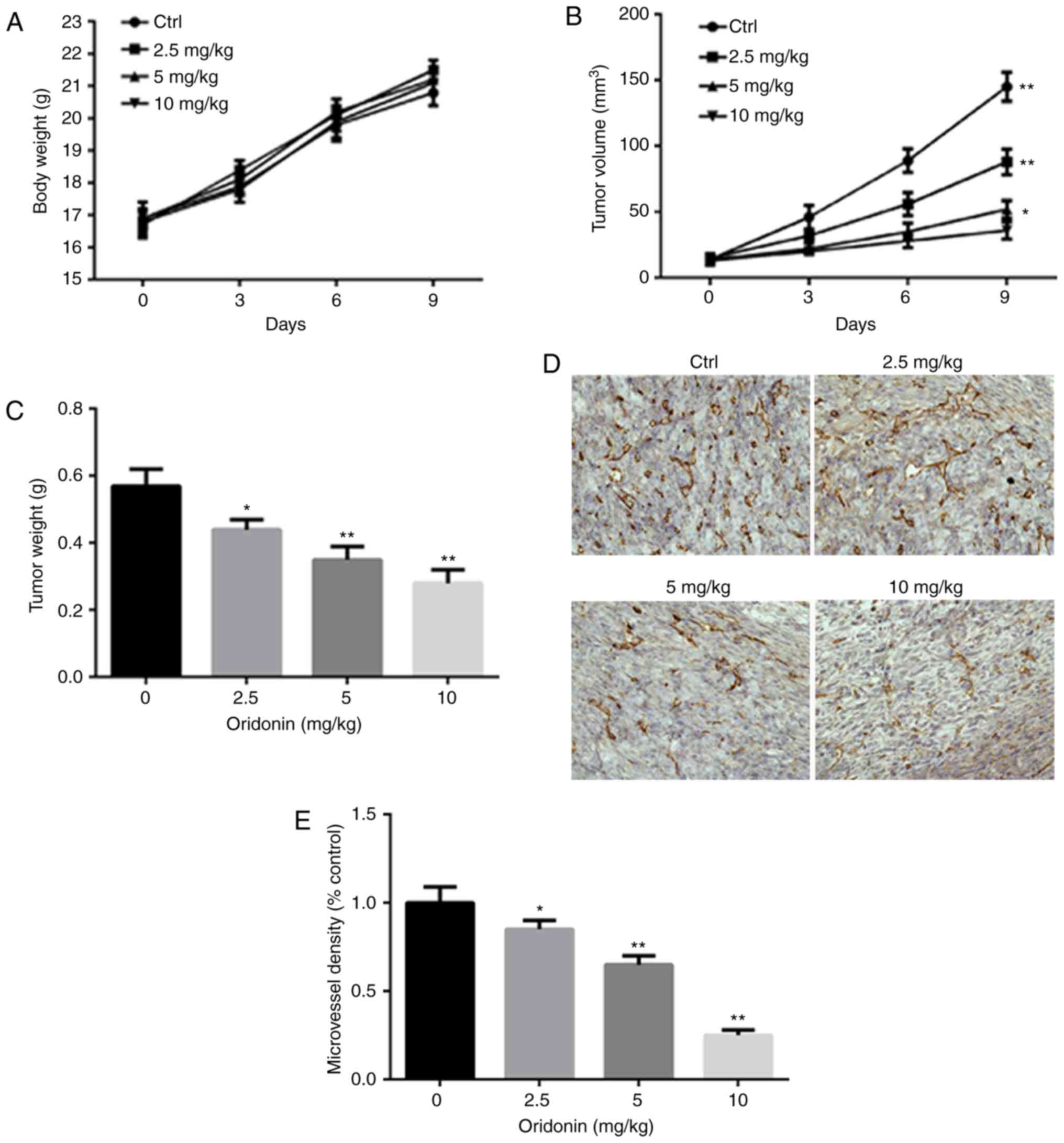
Oridonin inhibits the tumor growth and
angiogenesis in vivo
To assess the effect of oridonin on tumor growth and
angiogenesis in vivo, BALB/c mice were implanted with 4T1
cells. Following the injection of 4T1 cells, the tumors were
allowed to grow, subsequently mice were treated with different
concentrations of oridonin. During the experiment, no mice
presented multiple tumors. The longest diameter exhibited by a
single subcutaneous tumor was ~6 mm. No significant changes in the
body weight of mice were noted followed oridonin treatment
(Fig. 5A). The mean tumor volume
after 9 days of treatment was significantly decreased in the group
treated with indicated concentrations (2.5, 5 and 10 mg/kg) of
oridonin (Fig. 5B). After 9 days of
treatment, the mice were sacrificed; and the tumors were excised
and weighted. The mean tumor weight after 9 days treatment was
significantly decreased in all three oridonin treatment groups
compared with the vehicle control (Fig.
5C). Additionally, the data demonstrated that oridonin
significantly downregulated CD31 expression when compared with the
control group (Fig. 5D and E).
However, a limitation of the present study was absence of the
determination of CD31 expression in the tissues by western
blotting.
Discussion
Recently, oridonin has attracted increasing
attention due to its excellent antitumor activity against various
cancer types, including breast cancer (20). The present study was designed to
investigate the anti-metastatic effect of oridonin on breast
cancer. The results of the present study demonstrated that oridonin
significantly suppressed the migratory and invasive potential of
MDA-MB-231 and 4T1 cells in vitro possibly by inhibition of
EMT and angiogenesis via downregulation of the HIF-1α/VEGF
signaling pathway.
As numerous literatures have reported, oridonin
effectively inhibited the proliferation of a variety of cancer
cells, including those from prostate (LNCaP, DU145, PC3), breast
(MCF-7, MDA-MB231) and non-small cell lung (NCI-H520, NCI-H460,
NCI-H1299) cancer, and acute promyelocytic leukemia (NB4) and
glioblastoma multiforme (U118, U138) with the ED50 range
between 1.8 and 7.5 mg/ml (21). The
majority of reported researches have investigated the cytotoxicity
of oridonin. However, in the current study, the anti-metastatic
ability of breast cancer cells below the cytotoxicity dosage was
focused upon. Firstly, an MTT assay was performed to evaluate the
inhibitory effects of oridonin on the proliferation of MDA-MB-231
and 4T1 cells. Oridonin significantly inhibited the proliferation
of MDA-MB-231 and 4T1 cells in a dose-dependent manner. In order to
eliminate the influence of cytotoxic effects of oridonin on the
migratory and invasive capacities of cells, non-cytotoxic
concentrations of 4, 8 and 16 µM oridonin were chosen for further
investigation. Cell invasion, migration and adhesion assays were
performed to evaluate the anti-migratory ability of oridonin. Based
on our previous study (22), the time
of cell exposure to oridonin in the aforementioned experiments were
as follows: Transwell invasion assay 24 h; wound healing migration
assay 24 h; and cell adhesion assay 24 h. Hence, an exposure time
of 48 h was chosen for the MTT assay, to demonstrate that the
cytotoxicity of oridonin did not affect the migratory ability of
tumor cells. The non-cytotoxic effects of 4, 8 and 16 µM doses of
oridonin were confirmed in non-tumorigenic human breast cells
MCF-10A. No evident cytotoxic effects following exposure to
oridonin for 48 h were observed in the MCF-10A cells. One
limitation of the MTT assay was the absence of a time-dependent
experiment on the effects of oridonin on cell viability, this may
be investigated in further studies.
To the best of our knowledge, the results of the
present study are the first to investigate the anti-migratory and
invasive ability of oridonin on breast cancer cells below the
cytotoxicity dosage. The metastasis potential of cancer cells is
primarily determined by the migratory, invasive and adhesive
capacities, which is commonly determined with wounding healing,
Transwell chamber with Matrigel-coated filter and adhesion assays,
all three of which were used in the present study (23). The data demonstrated that oridonin
effectively attenuated the migratory, invasive and adhesive
abilities of MDA-MB-231 and 4T1 cells. In addition, to investigate
the effect of oridonin on angiogenesis in vitro, a capillary
tube formation assay was performed on HUVECs using Matrigel. It was
observed that oridonin significantly inhibited capillary tube
formation of HUVECs.
EMT is an essential step that occurs during cancer
development. The initiation of EMT arises from a loss of cell
polarity and cell-cell adhesion, which enhances the cancer cells
migratory and invasive abilities. In breast cancer, EMT may be
induced by multiple extracellular signaling factors, including
tumor growth factor-β, Notch, phosphatidylinositol 3-kinase/protein
kinase B, Wnt and receptor tyrosine kinases (24,25). The
snail transcriptional repressor complex promotes EMT by repressing
the junction component E-cadherin (26). Decreased E-cadherin has been
associated with cancer cell migration, invasion and the advanced
cancer stages, as well as metastasis, leading to poor prognosis in
breast cancer (27). The results of
the present study demonstrated that the expression of E-cadherin
was significantly increased, and the expression of Snail,
N-cadherin and Vimentin was significantly decreased following
exposure to oridonin, indicating that the inhibition of EMT is
involved in the anti-migratory and anti-invasive effects of
oridonin.
Breast cancer shares the characteristics of tissue
hypoxia, particularly when the tumor grows too quickly and
angiogenesis fails to catch up with the speed of tumor growth.
HIF-1α is a key regulatory molecule that responds to hypoxia in
cell migration, invasion, adhesion and angiogenesis (28). In addition, VEGF is associated with
the induction of neovascularization in human breast cancer, which
is mediated by multiple interacting genetic and environmental
signals (29). A hypoxic
microenvironment maybe a leading cause of angiogenesis, which is an
important determinant of malignant tumor development, by activation
of the expression of VEGF via HIF-1α (30). In the in vitro study, it was
revealed that oridonin exhibited an anti-angiogenesis effect. It is
well known that angiogenesis is important for tumor metastasis.
Thus, we hypothesized that the mechanism underlying the
anti-migratory and anti-invasive effects of oridonin is associated
with its anti-angiogenesis effect. Thus, an in vivo study
was performed to confirm this hypothesis. As previously reported
(31), the subcutaneously xenograft
model is a better model to evaluate the effect of adrug or compound
on angiogenesis using CD31 staining. Thus, this model was chosen in
the present study. A metastatic mouse model will be established to
evaluate the anti-metastatic effect of oridonin in future studies.
The current study demonstrated that oridonin effectively inhibited
tumor growth and angiogenesis in vivo. Further
investigations revealed that the expression of HIF-1α, VEGF-A and
VEGFR-2 was significantly decreased, indicating that the inhibition
of angiogenesis was involved in the antitumor effect of oridonin.
Loss-of-function and gain-of-function experiments will be performed
to confirm the mechanism underlying the antitumor effects of
oridonin in further studies.
In conclusion, the findings of the present study
suggest that oridonin significantly inhibited the migration,
invasion, adhesion and angiogenesis through inhibition of EMT and
downregulation of the HIF-1α/VEGF signal pathway in vitro
and in vivo. Thus, oridonin may be a potentially useful
anti-metastatic agent in breast cancer treatment.
Acknowledgements
The authors would like to thank Mr David Adam Jin
from the International Medical School, Tianjin Medical School,
China, for revising the manuscript.
Funding
The present study was supported by the Natural
Science Foundation of Tianjin Medical University (grant no.
2015KYZQ13), Postdoctoral Science Foundation of China (grant no.
2016M591398) and Basic Scientific Research Fund of Tianjin
Municipal Education Commission (grant no. 2016YD07).
Availability of data and materials
The datasets used or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CL and QW conceived and designed the experiments.
CL, QW, SS and XW performed the experiments. CL and GL analyzed the
data. CL and QW contributed reagents/materials/analysis tools and
wrote the manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the Animal
Ethics Committee of Tianjin Medical University and complied with
its regulations.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ramasamy T, Sundaramoorthy P, Ruttala HB,
Choi Y, Shin WH, Jeong JH, Ku SK, Choi HG, Kim HM, Yong CS and Kim
JO: Polyunsaturated fatty acid-based targeted nanotherapeutics to
enhance the therapeutic efficacy of docetaxel. Drug Deliv.
24:1262–1272. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Neelakantan D, Zhou H, Oliphant MUJ, Zhang
X, Simon LM, Henke DM, Shaw CA, Wu MF, Hilsenbeck SG, White LD, et
al: EMT cells increase breast cancer metastasis via paracrine GLI
activation in neighbouring tumour cells. Nat Commun. 8:157732017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou T, Zhang A, Kuang G, Gong X, Jiang R,
Lin D, Li J and Li H, Zhang X, Wan J and Li H: Baicalin inhibits
the metastasis of highly aggressive breast cancer cells by
reversing epithelial-to-mesenchymal transition by targeting
β-catenin signaling. Oncol Rep. 38:3599–3607. 2017.PubMed/NCBI
|
|
4
|
Sohn EJ, Jung DB, Lee H, Han I, Lee J, Lee
H and Kim SH: CNOT2 promotes proliferation and angiogenesis via
VEGF signaling in MDA-MB-231 breast cancer cells. Cancer Lett.
412:88–98. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang Y, Liu J, Lin J, Zhou L, Song Y, Wei
B, Luo X, Chen Z, Chen Y, Xiong J, et al: The transcription factor
GATA1 and the histone methyltransferase SET7 interact to promote
VEGF-mediated angiogenesis and tumor growth and predict clinical
outcome of breast cancer. Oncotarget. 7:9859–9875. 2016.PubMed/NCBI
|
|
6
|
Yehia L, Boulos F, Jabbour M, Mahfoud Z,
Fakhruddin N and El-Sabban M: Expression of HIF-1α and markers of
angiogenesis are not significantly different in triple negative
breast cancer compared to other breast cancer molecular subtypes:
Implications for future therapy. PLoS One. 10:e01293562015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pakravan K, Babashah S, Sadeghizadeh M,
Mowla SJ, Mossahebi-Mohammadi M, Ataei F, Dana N and Javan M:
MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes
suppresses in vitro angiogenesis through modulating the
mTOR/HIF-1α/VEGF signaling axis in breast cancer cells. Cell Oncol.
40:457–470. 2017. View Article : Google Scholar
|
|
8
|
Shao C, Zhang J, Fu J and Ling F: The
potential role of Brachyury in inducing epithelial-to-mesenchymal
transition (EMT) and HIF-1α expression in breast cancer cells.
Biochem Biophys Res Commun. 467:1083–1089. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He Z, Xiao X, Li S, Guo Y, Huang Q, Shi X,
Wang X and Liu Y: Oridonin induces apoptosis and reverses drug
resistance in cisplatin resistant human gastric cancer cells. Oncol
Lett. 14:2499–2504. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dong Y, Zhang T, Li J, Deng H, Song Y,
Zhai D, Peng Y, Lu X, Liu M, Zhao Y and Yi Z: Oridonin inhibits
tumor growth and metastasis through anti-angiogenesis by blocking
the Notch signaling. PLoS One. 9:e1138302014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu H, Qian C and Shen Z: Anti-tumor
activity of oridonin on SNU-5 subcutaneous xenograft model via
regulation of c-Met pathway. Tumour Biol. 35:9139–9146. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cui XY, Tinholt M, Stavik B, Dahm AE,
Kanse S, Jin Y, Seidl S, Sahlberg KK, Iversen N, Skretting G and
Sandset PM: Effect of hypoxia on tissue factor pathway inhibitor
expression in breast cancer. J Thromb Haemost. 14:387–396. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lopes FC, Ferreira R, Albuquerque DM,
Silveira AA, Costa R, Soares R, Costa FF and Conran N: In vitro and
in vivo anti-angiogenic effects of hydroxyurea. Microvasc Res.
94:106–113. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen H, Zhang L, Long X, Li P, Chen S,
Kuang W and Guo J: Sargassum fusiforme polysaccharides inhibit
VEGF-A-related angiogenesis and proliferation of lung cancer in
vitro and in vivo. Biomed Pharmacother. 85:22–27. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kang Z, Jiang W, Luan H, Zhao F and Zhang
S: Cornin induces angiogenesis through PI3K-Akt-eNOS-VEGF signaling
pathway. Food Chem Toxicol. 58:340–346. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitiative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
An J, Wang L, Zhao Y, Hao Q, Zhang Y,
Zhang J, Yang C, Liu L, Wang W, Fang D, et al: Effects of FSTL1 on
cell proliferation in breast cancer cell line MDA-MB-231 and its
brain metastatic variant MDA-MB-231-BR. Oncol Rep. 38:3001–3010.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu H, Huang G, Wang H, Li X, Wang X, Feng
Y, Tan B and Chen T: Inhibition effect of triptolide on human
epithelial ovarian cancer via adjusting cellular immunity and
angiogenesis. Oncol Rep. 39:1191–1196. 2017.PubMed/NCBI
|
|
19
|
Ko P, Kim D, You E, Jung J, Oh S, Kim J,
Lee KH and Rhee S: Extracellular matrix rigidity-dependent
sphingosine-1-phosphate secretion regulates metastatic cancer cell
invasion and adhesion. Sci Rep. 6:215642016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xia S, Zhang X, Li C and Guan H: Oridonin
inhibits breast cancer growth and metastasis through blocking the
Notch signaling. Saudi Pharm J. 25:638–643. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ikezoe T, Chen SS, Tong XJ, Heber D,
Taguchi H and Koeffler HP: Oridonin induces growth inhibition and
apoptosis of a variety of human cancer cells. Int J Oncol.
23:1187–1193. 2003.PubMed/NCBI
|
|
22
|
Li CY, Wang Q, Shen S, Wei XL and Li GX:
Oridonin inhibits migration, invasion, adhesion and TGF-β1-induced
epithelial-mesenchymal transition of melanoma cells by inhibiting
the activity of PI3K/Akt/GSK-3β signaling pathway. Oncol Lett.
15:1362–1372. 2018.PubMed/NCBI
|
|
23
|
Li Y and Galileo DS: Soluble L1CAM
promotes breast cancer cell adhesion and migration in vitro, but
not invasion. Cancer Cell Int. 10:342010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matysiak M, Kapka-Skrzypczak L,
Jodłowska-Jędrych B and Kruszewski M: EMT promoting transcription
factors as prognostic markers in human breast cancer. Arch Gynecol
Obstet. 295:817–825. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yan LX, Liu YH, Xiang JW, Wu QN, Xu LB,
Luo XL, Zhu XL, Liu C, Xu FP, Luo DL, et al: PIK3R1 targeting by
miR-21 suppresses tumor cell migration and invasion by reducing
PI3K/AKT signaling and reversing EMT, and predicts clinical outcome
of breast cancer. Int J Oncol. 48:471–484. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang T, Chen Z and Fang L: Curcumin
inhibits LPS-induced EMT through downregulation of NF-κB-Snail
signaling in breast cancer cells. Oncol Rep. 29:117–124. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu JM, Sun W, Hua F, Xie J, Lin H, Zhou DD
and Hu ZW: BCL6 induces EMT by promoting the ZEB1-mediated
transcription repression of E-cadherin in breast cancer cells.
Cancer Lett. 365:190–200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim DH, Sung B, Kim JA, Kang YJ, Hwang SY,
Hwang NL, Suh H, Choi YH, Im E, Chung HY and Kim ND: HS-1793, a
resveratrol analogue, downregulates the expression of
hypoxia-induced HIF-1 and VEGF and inhibits tumor growth of human
breast cancer cells in a nude mouse xenograft model. Int J Oncol.
51:715–723. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang E, Shi H, Yang L, Wu X and Wang Z:
Ginsenoside Rd regulates the Akt/mTOR/p70S6K signaling cascade and
suppresses angiogenesis and breast tumor growth. Oncol Rep.
38:359–367. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liang S, Chen Z, Jiang G, Zhou Y, Liu Q,
Su Q, Wei W, Du J and Wang H: Activation of GPER suppresses
migration and angiogenesis of triple negative breast cancer via
inhibition of NF-κB/IL-6 signals. Cancer Lett. 386:12–23. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cook MT, Liang Y, Besch-Williford C,
Goyette S, Mafuvadze B and Hyder SM: Luteolin inhibits
progestin-dependent angiogenesis, stem cell-like characteristics,
and growth of human breast cancer xenografts. Springerplus.
4:4442015. View Article : Google Scholar : PubMed/NCBI
|