Introduction
The tumor-node-metastasis (TNM) classification is a
widely accepted system for estimating the prognosis in various
types of cancer (1,2). The TNM staging system in head and neck
squamous cell carcinoma (SCC), including oral SCC, is reported to
be unable to predict the survival outcomes of patients at the same
stage (2–4). Numerous studies have researched the
prognostic parameters of oral SCC using various approaches,
including clinical and pathological procedures (5–8). The
pathological tumor volume (PTV) of the primary tumor is calculated
after surgical resection by measuring its diameters in three
dimensions (9–13). Recently, the PTV in tongue SCC, which
is part of the oral cavity, was reported to be significantly
correlated with the survival outcome (9). However, to the best of our knowledge,
the association between the PTV and overall survival in patients
with SCC of the entire oral cavity has not been investigated.
In the present study, we investigated the possible
association between the PTV and overall survival in patients with
both oral SCC and clinical lymph node metastasis.
Materials and methods
Patients and methods
From January 2008 to December 2013, 56 patients, who
were newly diagnosed with oral cancer with clinical lymph node
metastasis, underwent surgery without preoperative treatment at the
Department of Head and Neck Surgery, Aichi Cancer Center Hospital.
We excluded six patients with histological types other than SCC,
one patient with carcinoma in situ of the primary site, one
patient with synchronous colon cancer, and one patient in whom it
was not possible to define a theoretically reconstructed normal
mucosal line in the pathological examination. Thus, a total of 47
patients were enrolled in this study. The sites of the primary
tumor were as follows: Tongue, n=29; lower gum, n=6; upper gum,
n=5; floor of mouth, n=3; buccal mucosa, n=2; and hard palate, n=2.
This study was approved by the institutional review board, and all
patients provided their informed consent for all of the
examinations and treatments. The clinical stage was determined by
physical examinations and enhanced cervical computed tomography,
18F-fluorodeoxyglucose-positron emission tomography with
computed tomography or magnetic resonance imaging. The TNM
classification system of the International Union Against Cancer
(seventh edition) was used for the staging of the tumors (14). Surgically resected tissues were fixed
with formalin and embedded in paraffin. Representative sections of
the paraffin-embedded tissues were cut and stained with hematoxylin
and eosin. The pathological examinations were performed by two
experienced pathologists.
The measurement of the pathological
parameters
The tumor size was defined as the greatest dimension
of the primary tumor, as measured in the pathological examination.
The tumor thickness, depth of invasion, and PTV were measured as
described previously (7,10). Both the tumor thickness and the depth
of invasion were measured by microscopic examinations using an
ocular micrometer with an accuracy of 0.1 mm. The tumor thickness
was assessed as the distance from the surface of the tumor to the
site that showed the deepest invasion. The depth of invasion was
measured as the distance from the theoretically reconstructed
normal mucosal line to the site that showed the deepest invasion.
The PTV was calculated using the following formula: PTV=π/6
*(Xpathx Ypathx Zpath). Both
Xpath and Ypath were obtained from the
pathological report, and Zpath was assessed by the tumor
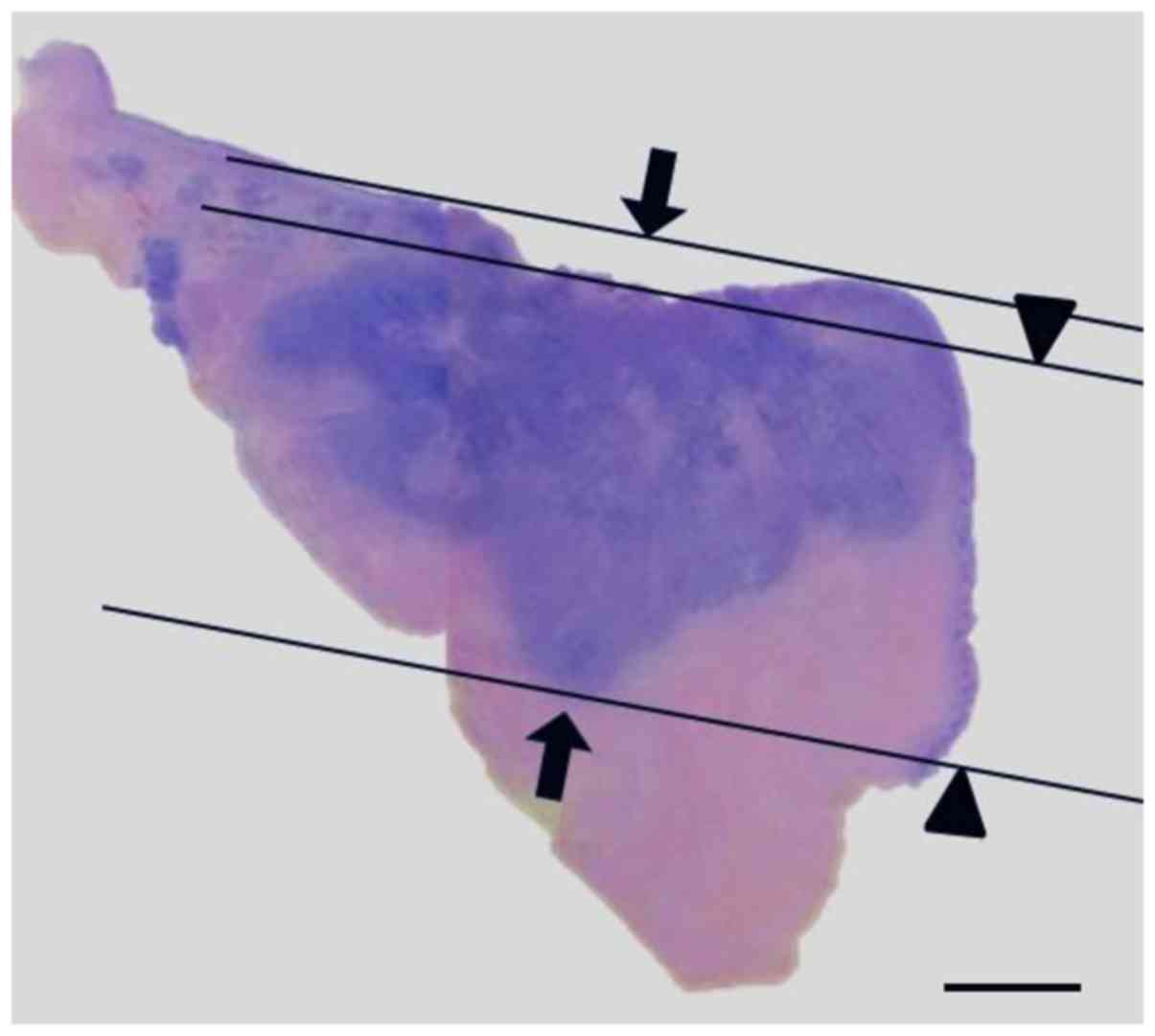
thickness. A representative image from the pathological examination
is shown in Fig. 1.
Statistical analysis
All of the statistical analyses were performed using
the JMP software program (version 11; SAS; Cary, NC, USA). The
relationships between the PTV and the clinicopathological
parameters (gender, age, tumor site, pathological T classification,
pathological N classification, pathological stage, positive
surgical margin, extracapsular extension, postoperative radiation,
and positive surgical margin and/or extracapsular extension) were
analyzed using a t-test, Mann-Whitney U test, or Kruskal-Wallis
test, as appropriate. The relationships between the PTV and the
pathological parameters (size, tumor thickness, and depth of
invasion) were assessed by a simple regression analysis. The
Kaplan-Meier method was used to estimate the survival time. We
defined the survival time as the period from surgery to a target
event or last contact. The target events included death (for
overall survival), local recurrence (for local recurrence-free
survival), regional recurrence (for regional recurrence-free
survival), and distant metastasis (for distant metastasis-free
survival) (15). Applying a
modification of a previously described method (15,16),
various PTV cut-off values were tested using a Cox proportional
hazards model of univariate overall survival. Since a PTV of 18
cm3 was found to significantly differentiate the shorter
survival group from the longer survival group in the univariate
analysis of overall survival, all of the patients were separated
into two groups based on the PTV (PTV <18 cm3 or PTV
≥18 cm3). The relationship between both the
clinicopathological parameters and the pathological parameters in
these two groups was compared using the chi-squared test and the
Mann-Whitney U test. The two groups (PTV <18 cm3 and
PTV ≥18 cm3) were compared by univariate analyses of
local recurrence-free survival, regional recurrence-free survival,
and distant metastasis-free survival. We performed multivariate
analyses (overall survival and local recurrence-free survival) with
adjustments for the primary site (tongue/others), pathological
stage (stage I–II/stage III–IV), and positive surgical margin
and/or extracapsular extension (absent/present) using a Cox
proportional hazards model. P-values of <0.05 were considered to
indicate statistical significance.
Results
The PTV and the clinicopathological
parameters
The mean ± standard deviation PTV of the whole study
population was 10.30±12.03 cm3. The associations between
the PTV and the clinicopathological parameters are shown in
Table I. The PTV was significantly
correlated with the pathological T classification (P<0.01),
pathological stage (P<0.02), and a positive surgical margin
(P<0.03).
 | Table I.Association between pathological tumor
volume and clinicopathological parameters (n=47). |
Table I.
Association between pathological tumor
volume and clinicopathological parameters (n=47).
| Parameter | Number | PTV (Mean ± standard
deviation cm3) | P-value |
|---|
| Age |
|
| 0.61ª |
|
<64 | 23 |
9.37±12.30 |
|
| ≥64 | 24 | 11.20±11.96 |
|
| Sex |
|
| 0.57b |
| Male | 25 | 12.53±14.28 |
|
|
Female | 22 | 7.77±8.44 |
|
| Site |
|
| 0.68ª |
|
Tongue | 29 |
9.73±11.34 |
|
|
Others | 18 | 11.24±13.35 |
|
| Pathological T
classification |
|
| <0.01c |
| T1 | 5 | 0.71±0.69 |
|
| T2 | 22 | 5.33±3.71 |
|
| T3 | 9 | 16.96±9.65 |
|
| T4 | 11 | 19.16±18.31 |
|
| Pathological N
classification |
|
| 0.58c |
| N0 | 14 |
8.35±10.09 |
|
| N1 | 5 | 5.43±3.86 |
|
| N2 | 28 | 12.15±13.61 |
|
| Pathological
stage |
|
| <0.02c |
| I | 3 | 0.37±0.16 |
|
| II | 6 | 4.14±2.61 |
|
| III | 7 | 8.91±5.96 |
|
| IV | 31 | 12.77±13.76 |
|
| Radiation
therapy |
|
| 0.52b |
|
Absent | 31 | 7.90±8.00 |
|
|
Present | 16 | 14.97±16.75 |
|
| Extracapsular
extension |
|
| 0.51a |
|
Absent | 33 | 9.50±11.28 |
|
|
Present | 14 | 12.11±13.92 |
|
| Positive surgical
margin |
|
|
<0.03b |
|
Absent | 38 | 7.71±8.73 |
|
|
Present | 9 | 21.27±17.68 |
|
| Positive surgical
margin and/or extracapsular extension |
|
| 0.38b |
|
Absent | 29 | 8.01±8.97 |
|
|
Present | 18 | 14.00±15.35 |
|
The PTV and the pathological
parameters
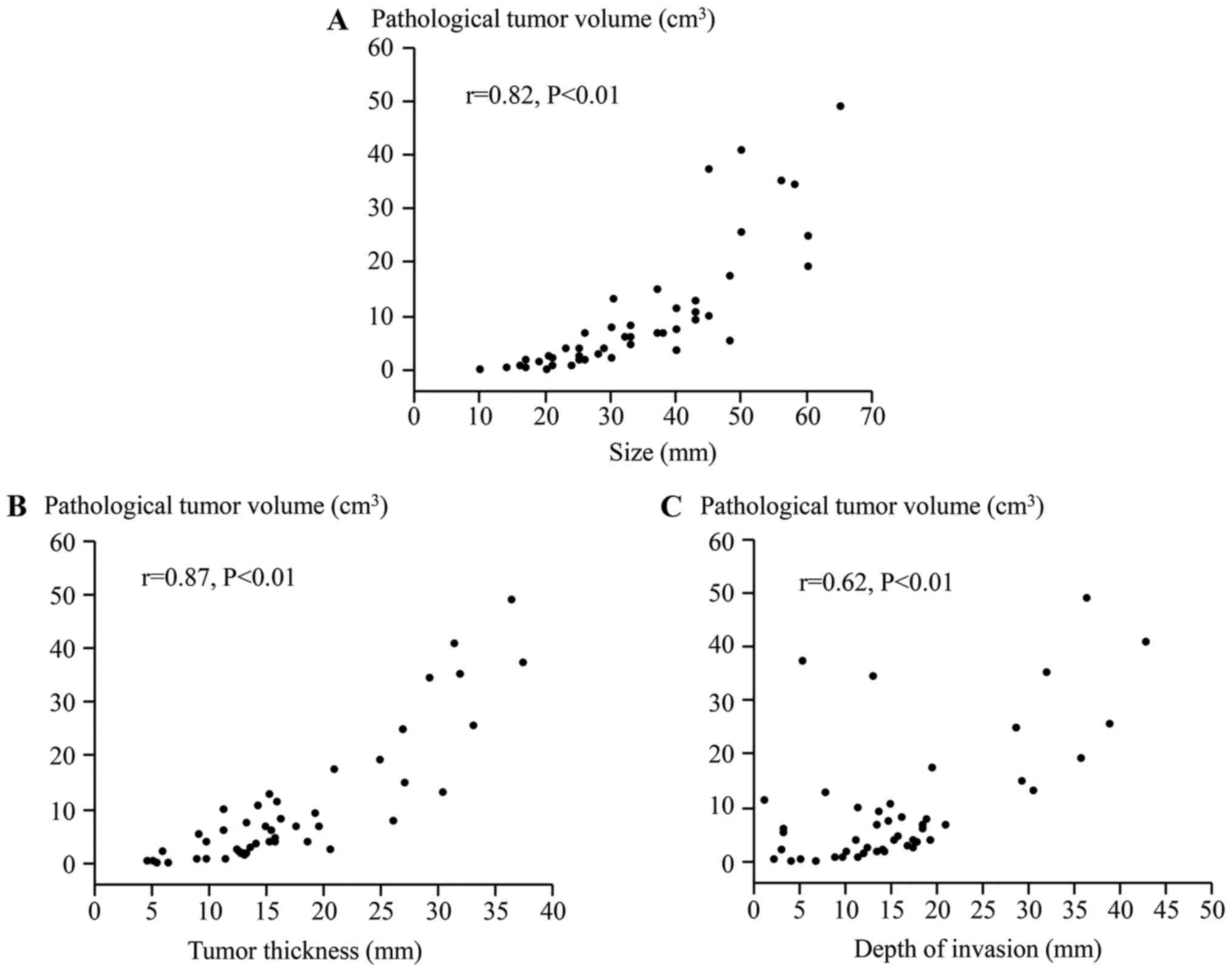
A simple regression analysis was performed to
analyze the associations between the PTV and the pathological
parameters (Fig. 2). The PTV was
significantly correlated with the size (r=0.82, P<0.01), tumor
thickness (r=0.87, P<0.01), and depth of invasion (r=0.62,
P<0.01).
The clinical course
The median follow-up period was 23 months (range
3–77 months). Eighteen of the overall patients (38.3%) died before
the end of the study. Nine (19.1%, vs. all), 10 (21.3%, vs. all),
and 10 (21.3%, vs. all) patients exhibited local recurrence,
regional recurrence, and distant metastasis, respectively. At the
end of the study, the rates of overall survival, local
recurrence-free survival, regional recurrence-free survival, and
distant metastasis-free survival in the whole study population were
58.5, 78.2, 37.8, and 74.4%, respectively.
The univariate survival analysis
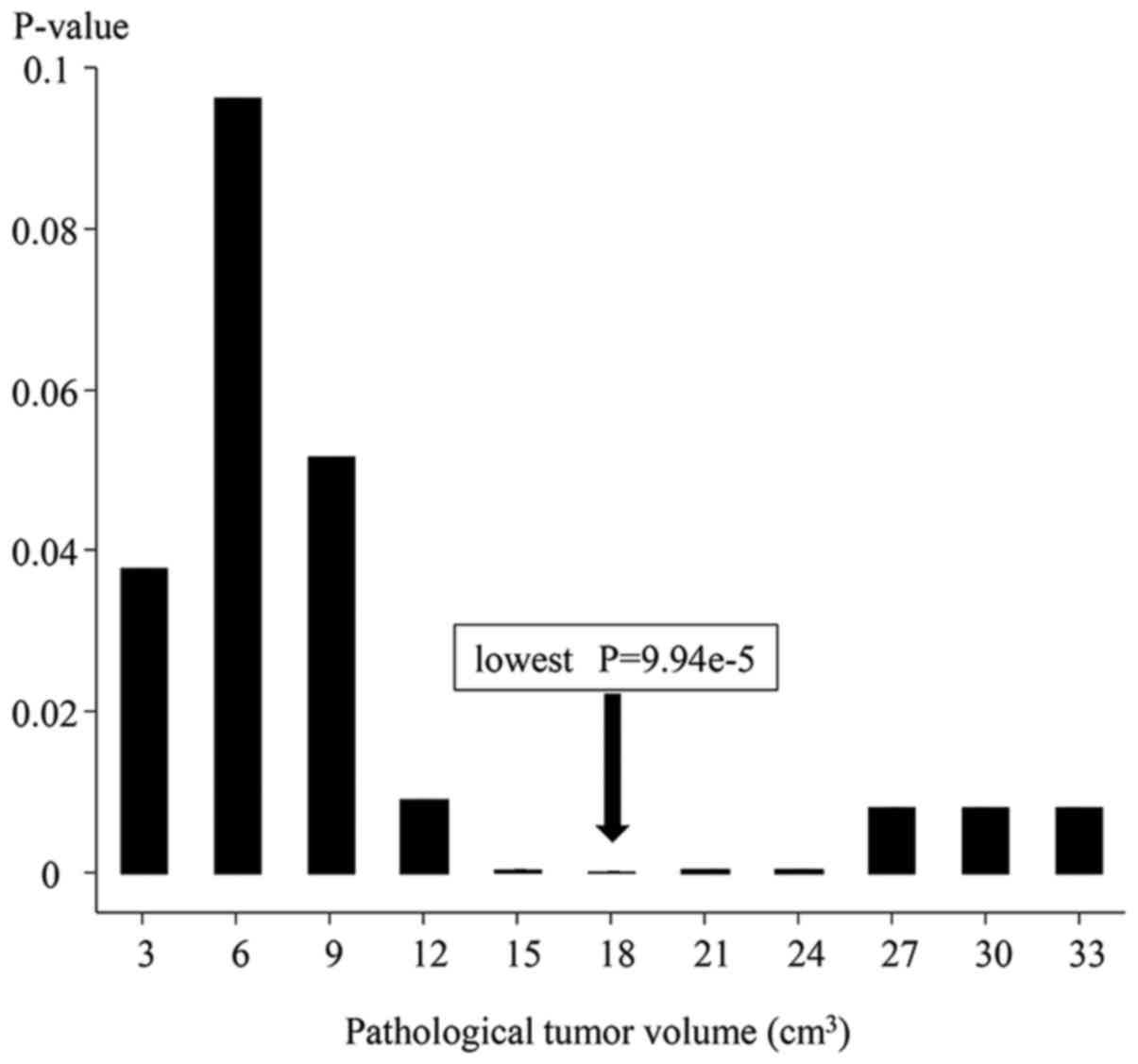
Various cut-off PTV values were tested using a Cox
proportional hazard model in the univariate analysis of overall
survival, and a PTV of 18 cm3 was found to have the
lowest P-value (Fig. 3). The
univariate analysis of overall survival revealed that a PTV of 18
cm3 significantly differentiated the shorter overall
survival group (PTV ≥18 cm3) from the longer overall
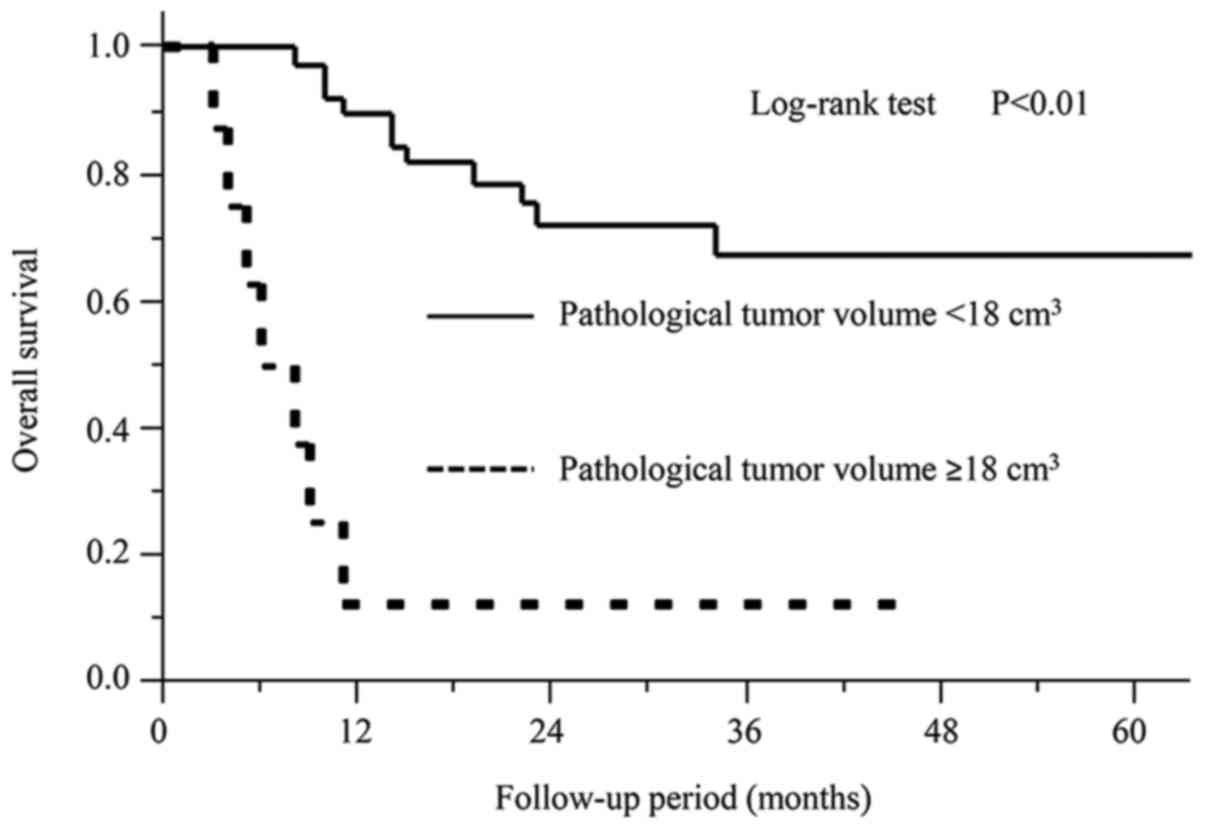
survival group (PTV <18 cm3). The Kaplan-Meier curves
from the univariate overall survival analysis are shown in Fig. 4. The correlation of both the
clinicopathological parameters and the pathological parameters
between the two groups (PTV ≥18 cm3; PTV <18
cm3) is shown in Table
II. A PTV of ≥18 cm3 was more frequently found in
patients with a pathological T classification of 3–4 (P<0.01),
who had received postoperative radiation (P<0.01), who showed a
positive surgical margin (P<0.01), and who showed a positive
surgical margin and/or extracapsular extension (P<0.02). A PTV
of ≥18 cm3 was significantly correlated with larger size
(P<0.01), tumor thickness (P<0.01), and the depth of invasion
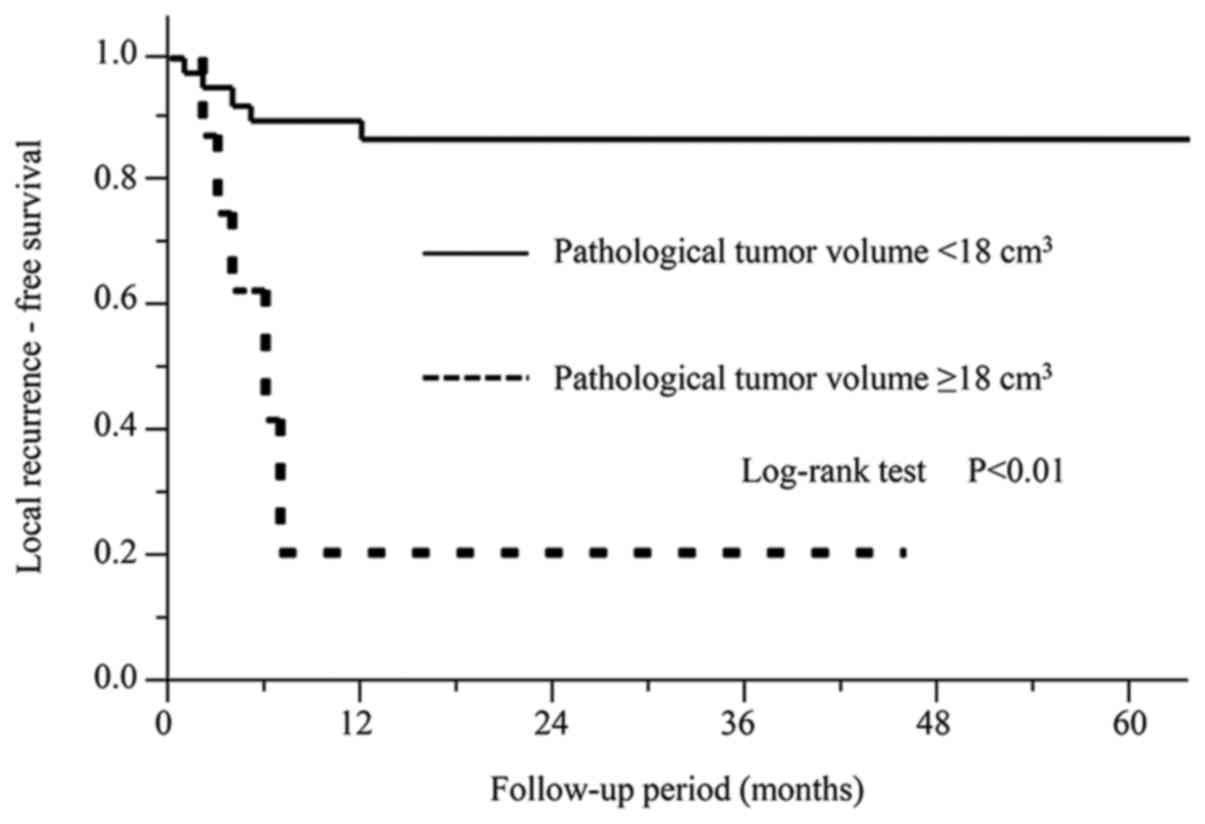
(P<0.01). The patients with a PTV of ≥18 cm3 showed
significantly shorter local recurrence-free survival than those
with a PTV of <18 cm3 (P<0.01). However, the
patients with PTV of ≥18 cm3 did not show shorter
regional recurrence-free survival (P=0.58), or distant
metastasis-free survival (P=0.24). The Kaplan-Meier curves from the
univariate analysis of the local recurrence-free survival are shown
in Fig. 5.
 | Table II.Association of clinicopathological
parameters and continuative pathological parameters with PTV
(<18 cm3/≥18 cm3). |
Table II.
Association of clinicopathological
parameters and continuative pathological parameters with PTV
(<18 cm3/≥18 cm3).
| Parameter | PTV <18
cm3 (n=39) | PTV ≥18
cm3 (n=8) | P-value |
|---|
| Age |
|
| 0.48a |
|
<64 | 20 | 3 |
|
|
≥64 | 19 | 5 |
|
| Sex |
|
| 0.17a |
|
Male | 19 | 6 |
|
|
Female | 20 | 2 |
|
| Site |
|
| 0.45a |
|
Tongue | 25 | 4 |
|
|
Others | 14 | 4 |
|
| Pathological T
classification |
|
|
<0.01a |
|
T1-2 | 27 | 0 |
|
|
T3-4 | 12 | 8 |
|
| Pathological N
classification |
|
| 0.24a |
| N0 | 13 | 1 |
|
|
N1-2 | 26 | 7 |
|
| Pathological
stage |
|
| 0.13a |
|
I–II | 9 | 0 |
|
|
III–IV | 30 | 8 |
|
| Radiation
therapy |
|
|
<0.01a |
|
Absent | 29 | 2 |
|
|
Present | 10 | 6 |
|
| Extracapsular
extension |
|
| 0.17a |
|
Absent | 29 | 4 |
|
|
Present | 10 | 4 |
|
| Positive surgical
margin |
|
|
<0.01a |
|
Absent | 35 | 3 |
|
|
Present | 4 | 5 |
|
| Positive surgical
margin and/or extracapsular extension |
|
|
<0.02a |
|
Absent | 27 | 2 |
|
|
Present | 12 | 6 |
|
| Size (mm) |
|
|
<0.01b |
| Mean ±
standard deviation | 29.56±9.97 | 55.50±6.63 |
|
| Tumor thickness
(mm) |
|
|
<0.01b |
| Mean ±
standard deviation | 14.15±5.77 | 31.32±4.32 |
|
| Depth of invasion
(mm) |
|
|
<0.01b |
| Mean ±
standard deviation | 13.07±6.70 | 28.95±13.16 |
|
The multivariate survival
analyses
We performed multivariate analyses of overall
survival and local recurrence-free survival with adjustments for
the primary site, pathological stage, and positive surgical margin
and/or extracapsular extension (Table
III). A PTV of ≥18 cm3 was significantly associated
with shorter overall survival (P<0.01) and local recurrence-free
survival (P<0.01) in the multivariate analysis.
 | Table III.Multivariate analysis of overall
survival and local recurrence-free survival. |
Table III.
Multivariate analysis of overall
survival and local recurrence-free survival.
|
| Overall
survival | Local
recurrence-free survival |
|---|
|
|
|
|
|---|
| Parameter | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Primary site |
|
| 0.07 |
|
| 0.35 |
|
Tongue | 1 |
|
| 1 |
|
|
|
Others | 0.38 | 0.12–1.08 |
| 1.87 | 0.50–7.83 |
|
| Pathological
stage |
|
| 0.12 |
|
| 0.94 |
|
I–II | 1 |
|
| 1 |
|
|
|
III–IV | 3.98 | 0.73–74.07 |
| 1.09 | 0.15–22.03 |
|
| Positive surgical
margin and/or extracapsular extension |
|
| 0.44 |
|
| 0.59 |
|
Absent | 1 |
|
| 1 |
|
|
|
Present | 1.57 | 0.49–4.92 |
| 0.66 | 0.14–3.03 |
|
| PTV |
|
| <0.01 |
|
| <0.01 |
| <18
cm3 | 1 |
|
| 1 |
|
|
| ≥18
cm3 | 8.30 | 2.46–29.45 |
| 8.54 | 1.81–44.72 |
|
Discussion
The results of the using univariate and multivariate
analyses in the present study showed-for the first time-that a PTV
of ≥18 cm3 was significantly associated with shorter
overall survival and local recurrence-free survival in patients
with oral SCC.
Both the depth of invasion and the tumor thickness
were considered representative prognostic parameters for oral SCC
(6,7).
In a review of 55 clinical studies, both the depth of invasion and
the tumor thickness were significantly correlated with overall
survival in oral SCC (6). Indeed, the
significant correlation between overall survival and these
pathological parameters (tumor thickness and depth of invasion)
that was found in our previous and present studies was in good
agreement with previous findings (6,7).
The PTV, which is calculated by three-dimensional
measurements, has been reported as a pathological parameter in
various sites of cancer (9–13). Mucke et al (9) showed a significant correlation between a
larger PTV and shorter overall survival in 437 patients with tongue
SCC. We have also reported on the PTV in hypopharyngeal SCC
(10). We hypothesized that a larger
PTV would be related to shorter overall survival in patients with
SCC of the entire oral cavity. The significant correlation between
the PTV and overall survival in the present study supported this
hypothesis.
In a previous study about the PTV in tongue SCC
(9), the rate of local
recurrence-free survival was not estimated. For the first time, we
demonstrated that a PTV of ≥18 cm3 is significantly
associated with shorter local recurrence-free survival. The result
suggests that postoperative therapy, such as chemoradiotherapy has
the potential to improve local recurrence-free survival in patients
with a PTV of ≥18 cm3. Furthermore, we demonstrated-for
the first time-that the PTV in oral SCC is significantly correlated
with the pathological parameters (size, tumor thickness, and depth
of invasion).
The extracapsular extension is considered a
significant prognostic factor in head and neck cancer, including
oral cancer (8). In the present
study, the PTV was not correlated with the extracapsular extension
in Tables I and II, and the patients with a PTV of ≥18
cm3 did not show a shorter regional recurrence-free
survival than those with a PTV of <18 cm3 (P=0.58).
We believe that this fundamental difference in the prognostic
utility of PTV and extracapsular extension may have been because
PTV was a prognostic factor obtained from the primary tumor, while
extracapsular extension was a prognostic factor obtained from the
cervical lymph node.
We showed the results of multivariate analyses which
were adjusted by the Cox proportional hazards model in the present
study. We considered PTV not to be an independent prognostic
factor, although it was a prognostic factor in multivariate
analyses after adjustments were made for the primary site,
pathological stage, and positive surgical margin and/or
extracapsular extension. To exclude the influence of the
confounding factors, we underwent the multivariate analyses in the
present study. Since PTV was significantly associated with
pathological T classification, pathological stage, positive
surgical margin, size, tumor thickness, and depth of invasion in
the present study, we considered that PTV is not an independent
prognostic factor.
The present study is associated with some
limitations, including the relatively small study population and
its retrospective nature. A future prospective analysis of a larger
study population will yield more accurate results and will
hopefully provide insight into the potential application of the PTV
as a prognostic tool.
In conclusion, we demonstrated, for the first time,
that a PTV of ≥18 cm3 was significantly correlated with
shorter overall survival and local recurrence-free survival in
patients with SCC of the entire oral cavity. Thus, these results
suggest that the PTV is a prognostic parameter in oral SCC.
Acknowledgements
Not applicable.
Funding
This study was supported by JSPS KAKENHI (grant no.
16K11253).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NM contributed to the data analysis and drafting of
the manuscript. HS acquired the data, contributed to the study
design and revised the manuscript critically for intellectual
content. NH and YH also acquired the data and revised the
manuscript critically for intellectual content. MS contributed to
the study design and revised the manuscript critically for
intellectual content. All authors approved the final version of the
manuscript.
Ethics approval and consent to
participate
Ethics approval was obtained from the institutional
review board of Aichi Cancer Center Hospital. All patients provided
their informed consent for all of the examinations and
treatments.
Consent for publication
All patients provided their informed consent.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PTV
|
pathological tumor volume
|
|
SCC
|
squamous cell carcinoma
|
|
TNM
|
tumor-node-metastasis
|
References
|
1
|
Ko B, Parvathaneni U, Hudgins PA and Anzai
Y: Do radiologists report the TNM staging in radiology reports for
head and neck cancers? A national survey study. AJNR Am J
Neuroradiol. 37:1504–1509. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Joo YH, Hwang SH, Sun DI, Cho KJ, Park JO
and Kim MS: Relationships between tumor volume and lymphatic
metastasis and prognosis in early oral tongue cancer. Clin Exp
Otorhinolaryngol. 6:243–248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schwartz DL, Rajendran J, Yueh B, Coltrera
MD, Leblanc M, Eary J and Krohn K: FDG-PET prediction of head and
neck squamous cell cancer outcomes. Arch Otolaryngol Head Neck
Surg. 130:1361–1367. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu H, Cheng KL, Xu XQ, Wu FY, Tyan YS,
Tsai CH and Shen CY: Predicting the prognosis of oral tongue
carcinoma using a simple quantitative measurement based on
preoperative MR imaging: Tumor thickness versus tumor volume. AJNR
Am J Neuroradiol. 36:1338–1342. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Benhamou Y, Picco V and Pages G: The
telomere proteins in tumorigenesis and clinical outcomes of oral
squamous cell carcinoma. Oral Oncol. 57:46–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pentenero M, Gandolfo S and Carrozzo M:
Importance of tumor thickness and depth of invasion in nodal
involvement and prognosis of oral squamous cell carcinoma: A review
of the literature. Head Neck. 27:1080–1091. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Suzuki H, Fukuyama R, Hasegawa Y, Tamaki
T, Nishio M, Nakashima T and Tatematsu M: Tumor thickness, depth of
invasion and Bcl-2 expression are correlated with FDG-uptake in
oral squamous cell carcinomas. Oral Oncol. 45:891–897. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fan S, Tang QL, Lin YJ, Chen WL, Li JS,
Huang ZQ, Yang ZH, Wang YY, Zhang DM, Wang HJ, et al: A review of
clinical and histological parameters associated with contralateral
neck metastases in oral squamous cell carcinoma. Int J Oral Sci.
3:180–191. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mucke T, Mitchell DA, Ritschl LM,
Tannapfel A, Wolff KD, Kesting MR, Loeffelbein DJ and Kanatas A:
Influence of tumor volume on survival in patients with oral
squamous cell carcinoma. J Cancer Res Clin Oncol. 141:1007–1011.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Suzuki H, Nishio M, Nakanishi H, Hanai N,
Hirakawa H, Kodaira T, Tamaki T and Hasegawa Y: Impact of total
lesion glycolysis measured by 18F-FDG-PET/CT on overall survival
and distant metastasis in hypopharyngeal cancer. Oncol Lett.
12:1493–1500. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Murphy JD, Chisholm KM, Daly ME, Wiegner
EA, Truong D, Iagaru A, Maxim PG, Loo BW Jr, Graves EE, Kaplan MJ,
et al: Correlation between metabolic tumor volume and pathologic
tumor volume in squamous cell carcinoma of the oral cavity.
Radiother Oncol. 101:356–361. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Knoedler JJ, Karnes RJ, Thompson RH,
Rangel LJ, Bergstralh EJ and Boorjian SA: The association of tumor
volume with mortality following radical prostatectomy. Prostate
Cancer Prostatic Dis. 17:144–148. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sridhar P, Mercier G, Tan J, Truong MT,
Daly B and Subramaniam RM: FDG PET metabolic tumor volume
segmentation and pathologic volume of primary human solid tumors.
AJR Am J Roentgenol. 202:1114–1119. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sobin LH, Gospodarowicz MK and Wittekind
C: International Union against CancerTNM classification of
malignant tumours. 7th edition. Wiley-Blackwell (UK); pp. 22–62.
2010
|
|
15
|
Suzuki H, Hanai N, Nishikawa D, Fukuda Y,
Koide Y, Kodaira T, Tachibana H, Tomita N, Makita C and Hasegawa Y:
The Charlson comorbidity index is a prognostic factor in sinonasal
tract squamous cell carcinoma. Jpn J Clin Oncol. 46:646–651. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Strongin A, Yovino S, Taylor R, Wolf J,
Cullen K, Zimrin A, Strome S, Regine W and Suntharalingam M:
Primary tumor volume is an important predictor of clinical outcomes
among patients with locally advanced squamous cell cancer of the
head and neck treated with definitive chemoradiotherapy. Int J
Radiat Oncol Biol Phys. 82:1823–1830. 2012. View Article : Google Scholar : PubMed/NCBI
|