Introduction
Breast cancer is the most common malignancy
affecting women's health, with increasing incidence worldwide.
About 1 in 8 women will be diagnosed with breast cancer in their
lifetimes; moreover, 1 in 5 cases of breast cancer is the
triple-negative subtype, that is, negative for estrogen receptor,
progesterone receptor, and human epidermal growth factor receptor 2
(HER2) (1). Triple-negative breast
cancer (TNBC) tends to be particularly aggressive, with a higher
propensity for metastasis and locoregional recurrence compared with
other subtypes (2). Although several
prognostic criteria and markers have already been introduced to
assist management after curative surgery for TNBC (3), identifying novel molecular markers in
order to discriminate individual variability and predict survival
and thus provide individualized treatment is necessary.
Aquaporins (AQPs), a family of small (30
kDa/monomer) water channel proteins that are integral membrane
proteins, play a crucial role in water homeostasis by regulating
cellular water transport (4). Thus
far, 13 AQPs (AQP0-AQP12) have been identified, and their
expression is widely distributed in various tissues throughout the
body. Among them, AQP0, AQP1, AQP2, AQP4, AQP5, AQP6, and AQP8 are
primarily involved in water transport, whereas AQP3, AQP7, AQP9,
and AQP10 are also involved in the transport of other small
solutes, such as glycerol and urea (5). AQP11 and AQP12, also named
‘superaquaporins’ or ‘subcellular aquaporins,’ are located
intracellularly with no clearly established selectivity (6). Accumulating studies have revealed that
AQPs are involved in many physiological functions, such as urine
concentration, exocrine gland secretion, brain swelling, neural
signal transduction, skin moisturization, and fat metabolism
(7–10).
Recently, some studies have demonstrated certain AQP
subtypes as novel targets for antitumor therapy because of their
involvement in carcinogenesis, tumor progression, and invasion
(11,12). AQP3 and AQP5 are highly expressed in
stomach, tongue, liver, pancreatic, colorectal, lung, and cervical
cancers (13–16). In previous studies, prominent AQP3 and
AQP5 expression was also observed in breast cancer tissues
(17). However, to date, there has
been no investigation of the clinicopathological relevance of AQP3
and AQP5 expression in TNBC. Accordingly, the aim of this study was
to investigate the expression patterns of AQP3 and AQP5 and to
evaluate their relationships with clinicopathological
characteristics and prognosis in TNBC patients.
Materials and methods
Patient and tissue samples
The present study was approved by the Research
Ethics Committees of Jiangsu Taizhou People's Hospital and Soochow
University, Suzhou, China. Written informed consent was obtained
from all patients. All specimens were handled anonymously according
to the local ethical and legal standards.
A total of ninety-six tumor samples and matched
adjacent normal samples were obtained from 96 women who underwent
surgery for TNBC at Jiangsu Taizhou People's Hospital, China
between 2007 and 2012. Patients with ductal carcinoma in
situ, lobular carcinoma, or those who underwent any type of
neoadjuvant treatment prior to surgery were not enrolled in the
present study. The average patient age was 48.86±10.32 years. All
patients were classified according to the pathological
tumor/node/metastasis (TNM) system based on the seventh edition of
America Joint Committee on Cancer. Clinicopathological
characteristics, including age, menopausal status, operation
method, histological grade, tumor size, nodal status, and local
relapse/distant metastasis, are summarized in Table I.
 | Table I.Association of AQP3 and AQP5
expression with established clinicopathological parameters in 96
patients with TNBC. |
Table I.
Association of AQP3 and AQP5
expression with established clinicopathological parameters in 96
patients with TNBC.
|
|
| AQP3 expression n
(%) |
| AQP5 expression n
(%) |
|
|---|
|
|
|
|
|
|
|
|---|
| Factor | No. | Low | High | P-value | Low | High | P-value |
|---|
| Age |
|
|
|
|
|
|
|
| ≤50
years | 51 | 21 (41.2) | 30 (58.8) | 0.734 | 24 (47.1) | 27 (52.9) | 0.172 |
| >50
years | 45 | 17 (37.8) | 28 (62.2) |
| 15 (33.3) | 30 (66.7) |
|
| Menopausal
status |
|
|
|
|
|
|
|
|
Pre-menopause | 58 | 23 (39.7) | 35 (60.3) | 0.986 | 26 (44.8) | 32 (55.2) | 0.300 |
|
Post-menopause | 38 | 15 (39.5) | 23 (60.5) |
| 13 (34.2) | 25 (65.8) |
|
| Surgery |
|
|
|
|
|
|
|
|
Mastectomy | 67 | 25 (37.3) | 42 (62.7) | 0.489 | 27 (40.3) | 40 (59.7) | 0.921 |
| Breast
conserving | 29 | 13 (44.8) | 16 (55.2) |
| 12 (41.4) | 17 (58.6) |
|
| Histological
grade |
|
|
|
|
|
|
|
| I | 16 | 5 (31.3) | 11 (68.8) | 0.321 | 7 (43.8) | 9 (56.3) | 0.287 |
| II | 44 | 21 (47.7) | 23 (52.3) |
| 21 (47.7) | 23 (52.3) |
|
|
III | 36 | 12 (33.3) | 24 (66.7) |
| 11 (30.6) | 25 (69.4) |
|
| Tumor size |
|
|
|
|
|
|
|
| T1 | 36 | 20 (55.6) | 16 (44.4) | 0.035 | 22 (61.1) | 14 (38.9) | 0.001 |
| T2 | 46 | 15 (32.6) | 31 (67.4) |
| 16 (34.8) | 30 (65.2) |
|
|
T3~T4 | 14 | 3 (21.4) | 11 (78.6) |
| 1 (7.1) | 13 (92.9) |
|
| Nodal status |
|
|
|
|
|
|
|
|
Negative | 59 | 28 (47.5) | 31 (52.5) | 0.046 | 30 (50.8) | 29 (49.2) | 0.010 |
|
Positive | 37 | 10 (27.0) | 27 (73.0) |
| 9 (24.3) | 28 (75.7) |
|
| Distant
metastasis |
|
|
|
|
|
|
|
| M0 | 79 | 36 (45.6) | 43 (54.4) | 0.021 | 38 (48.1) | 41 (51.9) | 0.003 |
| M1 | 17 | 2 (11.8) | 15 (88.2) |
| 1 (5.9) | 16 (94.1) |
|
| Ki-67 level |
|
|
|
|
|
|
|
| Low
expression | 18 | 8 (44.4) | 10 (55.6) | 0.640 | 12 (66.7) | 6 (33.3) | 0.013 |
| High
expression | 78 | 30 (38.5) | 48 (61.5) |
| 27 (34.6) | 51 (65.4) |
|
The postoperative evaluation of all TNBC patients
was followed up every 3 months for the first 2 years, then at least
every 6 months thereafter. Follow-up was completed on June 30,
2017. The median follow-up time was 39 months (range 5–60 months).
We excluded any patient with concurrent non-breast malignant tumors
or non-tumor related death. Hence, any death reported in the cases
enrolled in this study is attributed to the original TNBC tumor,
its recurrence or distant metastasis. At each follow-up visit, a
complete medical history was taken and clinical examinations were
performed. Tests for tumor markers such as carcinoembryonic antigen
(CEA), cancer antigen 125 (CA125), and cancer antigen 153 (CA153),
mammogram, and ultrasound were performed every 3–12 months. CT,
MRI, or FDG-PET/CT was performed selectively in case of any
abnormalities during the examination. Relapse or metastasis was
confirmed by biopsy where possible. Disease-free survival (DFS) and
overall survival (OS) were defined as the time from the date of
surgery to the date of recurrence and the date of the last
follow-up or death, respectively.
Immunohistochemistry analysis
Immunohistochemistry was performed on
formalin-fixed, paraffin-embedded, 4-µm tissue sections using the
EnVision complex method. Briefly, the Ethylene Diamine Tetraacetic
Acid (EDTA) heat repair method was performed for 20 min for antigen
retrieval, and sections were placed in an endogenous peroxidase
blocker for 15 min at room temperature to quench endogenous
peroxidase. The slides were washed thrice (1 min each) with
phosphate-buffered saline (PBS) and incubated with the primary
antibodies against AQP3 (rabbit polyclonal antibody, cat no.:
ab-125219; Abcam, Cambridge, MA, USA) and AQP5 (rabbit monoclonal
antibody, cat no.: ab-92320; Abcam) at a 1:500 dilution overnight
at 4°C. After several washes, the sections were incubated in the
EnVision™+/HRP rabbit working solution (Dako; Agilent
Technologies, Inc., Santa Clara, CA, USA) for 30 min and then
stained with diaminobenzidine. The slides were washed with
distilled water and counterstained with hematoxylin. After thorough
washes, each chip was dehydrated and then sealed with a neutral
gum. In each immunohistochemistry run, PBS was used instead of the
primary antibody as a negative control, and an internal control was
used for each slide.
Immunolabeled slides were scored by two independent
expert pathologists blinded to the clinicopathological data and
clinical prognosis. Owing to the homogeneousness of the
immunostaining of the target proteins, tumor specimens were scored
in a semiquantitative manner on the basis of a well-established
immunoreactivity scoring system (IRS) (18). The staining intensity score (IS) was
stratified as follows: 0 (no staining), 1 (faint), 2 (moderate),
and 3 (strong). The percentage of positive cells (PC) was scored as
follows: 0 (0%), 1 (1–10%), 2 (11–50%), 3 (51–80%), and 4
(>80%). The final IRS was obtained for each case by multiplying
the IS and PC. Expression levels of target protein were further
analyzed by stratifying IRS values as low (≤ the average value) and
high (> the average value).
Statistical analysis
SPSS v21.0 software (SPSS, Inc., Chicago, IL, USA)
was used for statistical analysis. Results are expressed as mean
and standard deviation. Student's t-test was used to compare the
expression levels of AQP3 and AQP5 between carcinoma tissues and
adjacent normal tissues. Categorical variables were compared using
the chi-square test. The correlation between the expression levels
of AQP3, AQP5, and Ki-67 was analyzed using the Spearman's rank
correlation coefficient. The survival curves were constructed based
on the Kaplan-Meier method. Cox proportional hazards regression
models were performed for the univariate and multivariate analysis.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression and localization of AQP3
and AQP5 in TNBC and adjacent normal tissue
AQP3 and AQP5 were expressed mainly in the membrane
and cytoplasm of tumor cells in TNBC tissues. AQP3 (IRS: 6.31±1.24
vs. 2.87±0.58, P<0.001) and AQP5 (IRS: 5.95±1.36 vs. 2.96±0.43,
P<0.001) expression was remarkably stronger in the carcinoma
tissues than in the adjacent normal tissues (Fig. 1). Moreover, in some of the TNBC
tissues, AQP5 was more prominent on the invasive front, and AQP5
staining was decreased in areas adjacent to necrosis (Fig. 1C). In adjacent normal tissues,
however, there was weak immunostaining of AQP3 and AQP5 in the
periductal or intralobular stroma, and little in the endothelial
cells of the capillary, small veins, and peripheral nerve fibers
(Fig. 1B and D).
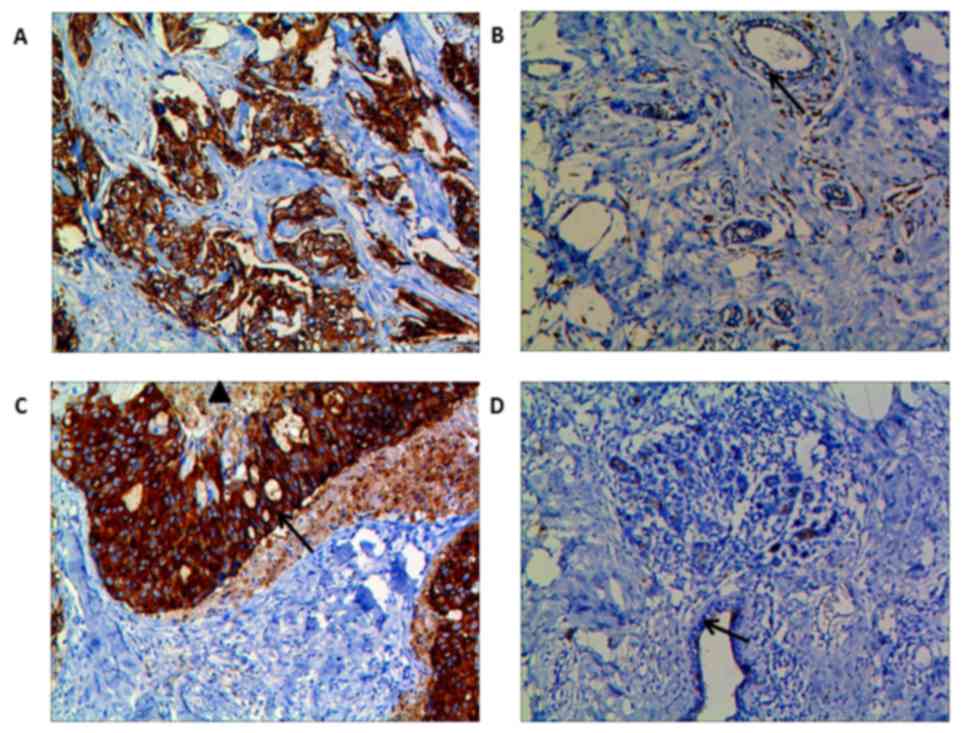 | Figure 1.Immunohistochemical staining for AQP3
and AQP5 proteins in TNBC tissues and adjacent normal tissues. AQP3
(A) and AQP5 (C) were expressed mainly in the membrane and
cytoplasm of tumor cells in TNBC tissues, and their expression was
stronger in the carcinoma tissues than in the adjacent normal
tissues. Furthermore, immunostaining of AQP5 was more prominent on
the invasive front of the tumor (C, arrow ↑), and decreased near
necrotic areas (C, triangle ▲). (B and D) In adjacent normal
tissues, however, there was weak immunostaining of AQP3 and AQP5 in
the periductal or intralobular stroma, and little in the
endothelial cells of the capillary, small veins, and peripheral
nerve fibers. Magnification, ×200. AQP, aquaporin; TNBC,
triple-negative breast cancer. |
Relationship between
clinicopathological features and AQP3/AQP5 expression in TNBC
patients
To evaluate whether AQP3 and AQP5 expression was
associated with clinicopathological features of TNBC patients, we
investigated the association of AQP3 and AQP5 expression with age,
menopausal status, surgical method, histological grade, tumor size,
nodal status, local relapse/distant metastasis and Ki-67 expression
(Table I). The average IRS values of
AQP3 and AQP5 expression in TNBC tissues were 6.31 and 5.95,
respectively. TNBC patients with AQP3 or AQP5 expression less than
or equal to the average value were assigned to the AQP3-low or
AQP5-low expression group, and those with expression above the
average level were assigned to the AQP3-high or AQP5-high
expression group. As shown in Table
I, combined AQP3 and AQP5 overexpression was significantly
associated with tumor size (P=0.035 and 0.001, respectively), nodal
status (P=0.046 and 0.01, respectively), and local relapse/distant
metastasis (P=0.021 and 0.003, respectively). There was no
significant difference in age, menopausal status, surgical method,
or histological grade between the groups. In addition, aberrant
overexpression of AQP5 was observed more frequently in TNBC tissues
with high Ki-67 expression than in those with low Ki-67 expression
(P=0.013). As analyzed by the Spearman's rank correlation analysis,
AQP5 expression was closely associated with ki-67 expression (r =
0.255, P=0.012; Table II). A similar
correlation was not found for AQP3 (r=0.048, P=0.064; Table II).
 | Table II.Correlation of AQP3, AQP5 and Ki-67
in 96 patients with TNBC. |
Table II.
Correlation of AQP3, AQP5 and Ki-67
in 96 patients with TNBC.
|
|
| Ki-67 |
|
|
|---|
|
|
|
|
|
|
|---|
|
| Expression | Low (n) | High (n) | r-value | P-value |
|---|
| AQP3 | Low | 8 | 30 | 0.048 | 0.644 |
|
| High | 10 | 48 |
|
|
| AQP5 | Low | 12 | 27 | 0.255 | 0.012 |
|
| High | 6 | 51 |
|
|
Univariate analyses were performed to evaluate the
effect of co-expression of AQP3 and AQP5 and other
clinicopathological parameters on TNBC prognosis. Five-year DFS and
OS of TNBC patients in our study were significantly associated with
increased expression of both AQP3 and AQP5 (P=0.015 and P=0.028,
respectively), tumor size (P=0.036 and 0.041, respectively), nodal
status (P=0.026 and 0.018, respectively), local relapse/distant
metastasis (P=0.018 and 0.021, respectively), and Ki-67 expression
(P=0.032 and 0.043, respectively) (Table III). The significant parameters in
univariate analysis were further evaluated in multivariate
analysis. We found that nodal status (P=0.026 and 0.038,
respectively), local relapse/distant metastasis (P=0.032 and 0.039,
respectively), and increased expression of both AQP3 and AQP5
(P=0.014 and 0.025, respectively) were independent poor prognostic
factors for OS and DFS in TNBC patients (Table IV).
 | Table III.Univariate analysis of different
prognostic factors in 96 patients with TNBC. |
Table III.
Univariate analysis of different
prognostic factors in 96 patients with TNBC.
|
| Overall
survival | Disease-free
survival |
|---|
|
|
|
|
|---|
| Factor | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age | 1.025
(0.948–1.108) | 0.534 | 1.049
(0.857–1.284) | 0.643 |
| Menopausal | 1.125
(0.820–1.543) | 0.465 | 1.624
(0.541–4.872) | 0.387 |
| Surgery | 1.434
(0.811–2.535) | 0.215 | 1.826
(1.566–5.896) | 0.314 |
| Histological
grade | 2.231
(0.390–12.755) | 0.367 | 1.526
(0.557–4.180) | 0.411 |
| Tumor size | 1.845
(1.041–3.271) | 0.036 | 1.485
(1.016–2.170) | 0.041 |
| Nodal status | 2.044
(1.089–3.836) | 0.026 | 2.851
(1.197–6.792) | 0.018 |
| Distant
metastasis | 4.426
(1.291–15179) | 0.018 | 3.895
(1.228–12.359) | 0.021 |
| Ki-67 | 1.174
(1.014–1.359) | 0.032 | 1.252
(1.007–1.556) | 0.043 |
| AQP3/AQP5
expression | 4.356
(1.331–14.258) | 0.015 | 3.892
(1.158–13.080) | 0.028 |
 | Table IV.Multivariate analysis of different
prognostic factors in 96 patients with TNBC. |
Table IV.
Multivariate analysis of different
prognostic factors in 96 patients with TNBC.
|
| Overall
survival | Disease-free
survival |
|---|
|
|
|
|
|---|
| Factors | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Tumor size | 1.151
(0.979–1.354) | 0.089 | 1.088
(0.977–1.212) | 0.126 |
| Nodal status | 1.847
(1.076–3.170) | 0.026 | 1.775
(1.032–3.052) | 0.038 |
| Distant
metastasis | 3.245
(1.107–9.516) | 0.032 | 4.122
(1.074–15.818) | 0.039 |
| Ki-67 | 1.266
(0.952–1.684) | 0.105 | 1.528
(0.812–2.877) | 0.189 |
| AQP3/AQP5
expression | 5.324
(1.403–20.207) | 0.014 | 4.248
(1.199–15.049) | 0.025 |
Discussion
TNBC is associated with a younger age, a higher
mitotic index and more advanced stage at diagnosis. Because neither
endocrine nor targeted therapies are effective in the treatment of
TNBC, its prognosis is poor compared with other breast cancer
subtypes (19). Therefore,
determining markers that can both serve as prognosticators and
potential therapeutic targets are urgently required to advance
effective treatment approaches. In the current study, we
investigated the expression patterns of AQP3 and AQP5 in 96 TNBC
patients through immunohistochemistry. Overexpression of AQP3 and
AQP5 was observed in 60.41 and 59.37% of TNBC tissues,
respectively. Both AQP3 and AQP5 proteins were expressed mainly in
the membrane and cytoplasm of tumor cells. Furthermore,
immunostaining of AQP5 was more prominent on the invasive front of
the tumor, and decreased near necrotic areas. Besides, combined
AQP3 and AQP5 overexpression was significantly associated with
tumor size, nodal status, and local relapse/distant metastasis in
TNBC patients. Aberrant overexpression of AQP5 protein was observed
more frequently in patients with higher Ki-67 than in those with
lower Ki-67. Moreover, pairwise comparisons showed that the
patients with AQP3-high/AQP5-high expression had the poorest DFS
and OS. In multivariate analysis, high expression of AQP3 and AQP5
was found to be an independent prognostic factor of relapse and
decreased survival for TNBC. To the best of our knowledge, there
are no reports on the co-expression of AQP3 and AQP5 on TNBC.
AQPs are a family of water-transporting integral
membrane proteins. However, increasing evidence shows that AQPs
play important roles in tumorigenesis, tumor progression, invasion,
and metastasis (20). AQP3 and AQP5,
as subtypes of the AQP family, are overexpressed in a variety of
tumor types, suggesting an important role in tumorigenesis. We have
reported on the high expression of AQP3 and AQP5 in gastric cancer
and their important role in the migration and proliferation of
gastric cancer cells, suggesting that AQP3 and AQP5 may be
potentially important determinants of tumor growth and metastasis
(21,22). In lung cancer, overexpression of AQP3
is associated with tumor pathological grade and clinical stage
(23). In a mouse model, AQP3
knockdown inhibits tumor growth and decreases angiogenesis in human
non-small cell lung cancer xenografts (24). In human breast cancer, Shi et
al found that AQP1 and AQP3-5 exhibited differential expression
between breast cancer and normal breast tissues, suggesting that
some subtypes of the AQP family play a key role in human breast
carcinogenesis (25). Kang et
al (26), demonstrated that, in
patients with early HER2-positive breast cancer, AQP3 expression
was significantly related to survival and was an independent
prognostic marker of DFS. Furthermore, the common chemotherapy drug
cisplatin induces ovarian tumor cell death by inhibiting AQP5
expression and the NF-κB pathway, supporting AQP5 as a potential
target for therapy in ovarian cancer (27). Similarly, expression of both AQP3 and
AQP5 are increased in squamous cell carcinoma. Treatment with the
AQP inhibitor, CuSO4, or AQP5-specific siRNA shows
inhibition of cell growth in squamous cell carcinoma cell lines via
the inhibition of integrins and the mitogen-activated protein
kinase pathway (28).
Little is known about the expression and role of
AQP3 and AQP5 in TNBC. The current study indicates that AQP3 and
AQP5 overexpression were positively correlated with Ki-67
expression, tumor size, lymph node metastasis, and local
relapse/distant metastasis. The biologic marker Ki-67 has been
identified as an important prognostic and predictive marker in
breast cancer (29). Moreover, Guo
et al (14) demonstrated that
the overexpression of AQP3 in combination with upregulation of AQP5
was an unfavorable prognostic factor in hepatocellular carcinoma.
Therefore, combined expression of AQP3 and AQP5 may be a potential
promising marker in TNBC. Excitingly, AQPs have been targeted in
the clinical treatment of some diseases, such as nephrogenic
diabetes insipidus (AQP2), and neuromyelitis optica, an autoimmune
demyelinating disease (AQP4) (30,31),
identifying AQP3 and AQP5 as novel targets for anti-cancer
treatment still need further investigation.
Though our study generated some important findings,
a limitation due to the relatively short follow-up and small sample
size should be acknowledged. Only immunohistochemistry was
performed in present study, and mRNA and protein expression of AQP3
and AQP5 were not assessed in cancer tissues. Additionally, the
molecular mechanisms for this aberrant expression, as well as the
roles of AQP3 and AQP5 in TNBC patients, have not been fully
elucidated. Further studies are needed to more fully understand
their molecular function in TNBC.
In summary, the present study demonstrates for the
first time that increased co-expression of AQP3 and AQP5 may be
associated with tumor clinicopathological characteristics and could
serve as an independent prognostic factor in TNBC patients.
Combined expression of the two proteins may be a potential
promising marker in patients with TNBC and could be a novel target
for anti-cancer treatment.
Acknowledgements
The authors would like to thank Professor Lizong
Shen (Division of Gastrointestinal Surgery, Department of General
Surgery, First Affiliated Hospital, Nanjing Medical University) for
his helpful suggestions.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81600434).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Author's contributions
ZCZ, LHJ and LT performed the surgery and drafted
the manuscript. ZCZ and HGW participated in the design of the study
and performed the histological examination. WW performed the
statistical analysis. HXQ made substantial contributions to the
manuscript including conception and design, acquisition of data,
analysis and interpretation of data and contributed to the
supervision and revision of the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All study participants provided informed consent and
the study design was approved by the Research Ethics Committees of
Jiangsu Taizhou People's Hospital and Soochow University (Suzhou,
China).
Consent for publication
Written informed consent for publication of their
clinical details and images was obtained from all participants in
the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen VE, Gillespie EF, Zakeri K, Murphy
JD, Yashar CM, Lu S and Einck JP: Pathologic response after
neoadjuvant chemotherapy predicts locoregional control in patients
with triple negative breast cancer. Adv Radiat Oncol. 2:105–109.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Santana-Davila R and Perez EA: Treatment
options for patients with triple-negative breast cancer. J Hematol
Oncol. 3:422010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Verkman AS, Anderson MO and Papadopoulos
MC: Aquaporins: Important but elusive drug targets. Nat Rev Drug
Discov. 13:259–277. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee SJ, Chae YS, Kim JG, Kim WW, Jung JH,
Park HY, Jeong JY, Park JY, Jung HJ and Kwon TH: AQP5 expression
predicts survival in patients with early breast cancer. Ann Surg
Oncol. 21:375–383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Verkman AS: Aquaporins in clinical
medicine. Annu Rev Med. 63:303–316. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ishibashi K, Tanaka Y and Morishita Y: The
role of mammalian superaquaporins inside the cell. Biochim Biophys
Acta. 1840:1507–1512. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Y, Wang W, Jiang T and Yang B:
Aquaporins in urinary system. Adv Exp Med Biol. 969:131–148. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu S, Zhang S, Jiang H, Yang Y and Jiang
Y: Co-expression of AQP3 and AQP5 in esophageal squamous cell
carcinoma correlates with aggressive tumor progression and poor
prognosis. Med Oncol. 30:6362013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Filippidis AS, Carozza RB and Rekate HL:
Aquaporins in brain edema and neuropathological conditions. Int J
Mol Sci. 18:pii: E55. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ikarashi N, Kon R, Kaneko M, Mizukami N,
Kusunoki Y and Sugiyama K: Relationship between aging-related skin
dryness and aquaporins. Int J Mol Sci. 18:pii: E1559. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Esteva-Font C, Jin BJ and Verkman AS:
Aquaporin-1 gene deletion reduces breast tumor growth and lung
metastasis in tumor-producing MMTV-PyVT mice. FASEB J.
28:1446–1453. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kang BW, Kim JG, Lee SJ, Chae YS and Jeong
JY, Yoon GS, Park SY, Kim HJ, Park JS, Choi GS and Jeong JY:
Expression of aquaporin-1, aquaporin-3, and aquaporin-5 correlates
with nodal metastasis in colon cancer. Oncology. 88:369–376. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen J, Wang T, Zhou YC, Gao F, Zhang ZH,
Xu H, Wang SL and Shen LZ: Aquaporin 3 promotes
epithelial-mesenchymal transition in gastric cancer. J Exp Clin
Cancer Res. 33:382014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo X, Sun T, Yang M, Li Z, Li Z and Gao
Y: Prognostic value of combined aquaporin 3 and aquaporin 5
overexpression in hepatocellular carcinoma. Biomed Res Int.
2013:2065252013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Direito I, Paulino J, Vigia E, Brito MA
and Soveral G: Differential expression of aquaporin-3 and
aquaporin-5 in pancreatic ductal adenocarcinoma. J Surg Oncol.
115:980–996. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ribatti D, Ranieri G, Annese T and Nico B:
Aquaporins in cancer. Biochimica et Biophysica Acta.
1840:1550–1553. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang J, Feng L, Zhu Z, Zheng M, Wang D,
Chen Z and Sun H: Aquaporins as diagnostic and therapeutic targets
in cancer: How far we are? J Transl Med. 13:962015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gatto F, Feelders RA, van der Pas R, Kros
JM, Waaijers M, Sprij-Mooij D, Neggers SJ, van der Lelij AJ, Minuto
F, Lamberts SW, et al: Immunoreactivity score using an anti-sst2A
receptor monoclonal antibody strongly predicts the biochemical
response to adjuvant treatment with somatostatin analogs in
acromegaly. J Clin Endocrinol Metab. 98:E66–E71. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rampurwala M, Wisinski KB and Regan RO:
Role of the androgen receptor in triple-negative breast cancer.
Clin Adv Hematol Oncol. 14:186–193. 2016.PubMed/NCBI
|
|
20
|
Satooka H and Hara-Chikuma M: Aquaporin-3
controls breast cancer cell migration by regulating hydrogen
peroxide transport and its downstream cell signaling. Mol Cell
Biol. 36:1206–1218. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shen L, Zhu Z, Huang Y, Shu Y, Sun M, Xu
H, Zhang G, Guo R, Wei W and Wu W: Expression profile of multiple
aquaporins in human gastric carcinoma and its clinical
significance. Biomed Pharmacother. 64:313–318. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang Y, Zhu Z, Sun M, Wang J, Guo R, Shen
L and Wu W: Critical role of aquaporin-3 in the human epidermal
growth factor-induced migration and proliferation in the human
gastric adenocarcinoma cells. Cancer Biol Ther. 9:1000–1007. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Papadopoulos MC and Saadoun S: Key roles
of aquaporins in tumor biology. Biochim Biophys Acta.
1848:2576–2583. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xia H, Ma YF, Yu CH, Li YJ, Tang J, Li JB,
Zhao YN and Liu Y: Aquaporin 3 knockdown suppresses tumour growth
and angiogenesis in experimental non-small cell lung cancer. Exp
Physiol. 99:974–984. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shi Z, Zhang T, Luo L, Zhao H, Cheng J,
Xiang J and Zhao C: Aquaporins in human breast cancer:
Identification and involvement in carcinogenesis of breast cancer.
J Surg Oncol. 106:267–272. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kang S, Chae YS, Lee SJ, Kang BW, Kim JG,
Kim WW, Jung JH, Park HY, Jeong JH, Jeong JY and Park JY: Aquaporin
3 expression predicts survival in patients with HER2-positive early
breast cancer. Anticancer Res. 35:2775–2782. 2015.PubMed/NCBI
|
|
27
|
Yang J, Yan C, Zheng W and Chen X:
Proliferation inhibition of cisplatin and aquaporin 5 expression in
human ovarian cancer cell CAOV3. Arch Gynecol Obstet. 285:239–245.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ishimoto S, Wada K, Usami Y, Tanaka N,
Aikawa T, Okura M, Nakajima A, Kogo M and Kamisaki Y: Differential
expression of aquaporin 5 and aquaporin 3 in squamous cell
carcinoma and adenoid cystic carcinoma. Int J Oncol. 41:67–75.
2012.PubMed/NCBI
|
|
29
|
Schlotter CM, Tietze L, Vogt U, Heinsen CV
and Hahn A: Ki67 and lymphocytes in the pretherapeutic core biopsy
of primary invasive breast cancer: Positive markers of therapy
response prediction and superior survival. Horm Mol Biol Clin
Investig. 32:pii:/j/hmbci. 2017.PubMed/NCBI
|
|
30
|
Kowarik MC, Soltys J and Bennett JL: The
treatment of neuromyelitis optica. J Neuroophthalmol. 34:70–82.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Soveral G and Casini A: Aquaporin
modulators: A patent review (2010–2015). Expert Opin Ther Pat.
27:49–62. 2017. View Article : Google Scholar : PubMed/NCBI
|















