Introduction
Prostate cancer is one of the most common cancers
among men, which affects one in seven men in the US (1). Theoretically, prostate cancer cells may
spread anywhere in the body. If prostate cancer spreads to other
parts of the body, bone is the preferred location for prostate
cancer to metastasize to, which results in complications including
bone pain, pathological fractures, spinal compression and
hypercalcemia (2). Osteogenesis
serves an important function in osteolytic bone lesions.
Connective tissue growth factor (CTGF) is a
matricellular protein that belongs to the extracellular
matrix-associated heparin-binding protein family (3,4), which has
notable functions in numerous different biological processes,
including cell migration and proliferation (5). Previous experimental studies demonstrate
that CTGF expression levels are high in mouse models and patients
with primary prostate cancer that have metastasized to the bone
(6). CTGF possesses multiple
functions in different types of cancer cell (7).
Previous studies have demonstrated that CTGF
possesses an inhibitory function in the development of brain
glioma, melanoma and prostate cancer cells (8). However, in contrast, a previous study
has identified that CTGF has a facilitating effect on the
metastasis of melanoma in addition to being able to promote the
proliferation, migration and metastasis of Ewing sarcoma cells, and
increasing expression levels result in an undesirable prognosis
(9). To the best of our knowledge,
the functional mechanisms of CTGF in association with prostate
cancer metastasizing within the bone remain unclear.
The present study investigated the effect of CTGF on
prostate cancer bone metastasis using RM1 murine prostate cancer
cell line and analyzed the underlying molecular mechanisms
regarding to mouse osteoblast differentiation dysregulation.
Materials and methods
Cell culture
All experiments of the present study were approved
by the ethics committee of The First Hospital of Qiqihar
(Heilongjiang, China; no. 2015017). Mouse prostate cancer cells
RM1, PNEC30, P25.48, MyC-CaP and VCaP cell line of human prostate
cancer exhibited different metastatic capacities (10), and were purchased from the American
Association for Cancer Research (Philadelphia, PA, USA). Cells were
cultured in Dulbecco's modified Eagle's medium containing Ham's F12
(Corning Incorporated, Corning, NY, USA), and placed in a 5%
CO2 incubator at 37°C and passaged once cells reached
confluency. Following the incubation period, cells were resuspended
in 0.25% trypsin/0.02% EDTA solution for 3 min at room
temperature.
Total RNA extraction and quantitative
polymerase chain reaction (qPCR)
RNA was isolated using TRIzol™ Reagent
(15596026, Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) according to the manufacturer's protocol. cDNA was prepared
from primary cultured astrocytes using Fast Lane Cell cDNA Kit
(Qiagen, CA, USA) by using 500 ng RNA, and levels of mRNA were
assessed using SYBR supermix (1708880, Bio-Rad, CA, USA). The
thermocycling conditions were as follows: 95°C for 3 min, 39 cycles
of 95°C for 30 sec, 60°C for 30 sec and 72°C for 30 sec. The
following forward (F) and reverse (R) primers were used to amplify
CTGF and actin: CTGF (F) 5-GATGTACGGAGACATGGCGT-3, CTGF (R)
5-AGTAGGCACACTGCTGCTTT-3 (primer blast, NCBI, USA); actin (F)
5-GAGATTACTGCCCTGGCTCCTA-3, actin (R) 5-TCATCGTACTCCTGCTTGCTGAT-3
(11) (Invitrogen, Thermo Fisher
Scientific, Inc.). Gene level was calculated using the
2−ΔΔCq method and presented as relative fold change
(12).
Western blotting
Total protein was extracted from RM1, PNEC30 and
P25.48 cell lines using RIPA buffer (Beyotime, Beijing, China). The
protein concentration was determined using a bicinchoninic acid
protein assay kit. Protein samples (10 µg) were separated using
denaturing SDS-PAGE (10% gel) and transferred onto a polyvinylidene
fluoride membrane. The membrane was blocked in PBS/5% skimmed milk
at room temperature for 1 h, followed by an incubation period with
primary antibody (anti-CTGF: 1:1,000; cat. no. ab6992; Abcam,
Cambridge, UK) and α-tubulin (T9026, 1:5,000, Sigma-Aldrich, Merck
KGaA, Darmstadt, Germany) overnight at 4°C. Secondary antibody
(anti-rabbit horseradish peroxidase conjugated antibody, 1:5,000;
cat. no. ab99848; Abcam and anti-mouse HRP antibody, 1:5,000;
Bio-Rad Laboratories, Inc., Hercules, CA, USA) was added prior to
incubation at room temperature for 1 h. Protein was visualized
using enhanced chemiluminscence reagent (Thermo Fisher Scientific,
Inc., Waltham, MA, USA). Expression levels of protein were analyzed
using ImageJ analysis software (V.2.1.4.7, National Institutes of
Health, Bethesda, MD, USA).
Immunohistochemical analysis
Paraffin specimens confirmed by pathology were
collected from a patient with prostate cancer which had
metastasized to the bone. Patient was male, 55 years old, and the
sample was collected at the Department of Orthopedic Surgery at The
First Hospital of Qiqihaer City (Quiqihaer, China) in December
2015. Paraffin specimens (fixed in 4% paraformaldehyde at 4°C for
at least 48 h and then were cut into 5 µm thick slices) were
processed using anti-CTGF immunohistochemical staining. Subsequent
to being blocked using 2% bovine serum albumin at room temperature
for 1 h (BSA; Amresco, LLC, Solon, OH, USA), the primary antibody,
anti-CTGF antibody (1,400; cat. no. ab6992; Abcam), was applied to
the specimens overnight at 4°C. Following incubation, the secondary
antibody, biotin-labeled sheep anti-mouse antibody (1:1,000; cat
no. A0277s; Beyotime Institute of Biotechnology, Haimen, China),
was added. All photographs were captured using a Zeiss Axioplan 2
microscope (Carl Zeiss AG, Oberkochen, Germany). Immunostained
slides were analyzed using the Image-Pro Plus system (Media
Cybernetics, Inc., Rockville, MD, USA).
Cell proliferation assay
An automated cell counter (Cellometer; Nexcelom
Bioscience LLC, Lawrence, MA, USA) was used to analyze cell
proliferation. RM1 cells (8×103 cells/well) were
transfected with empty vector or stable overexpression of CTGF in
12-well plates and incubated for 48 h. Following incubation, a
number of cells (5×106) were removed daily for a total
of 4 days, for further experiments.
Tumor metastasis assay
A C57BL/6 mouse model carrying a form of bone
metastasis, developed according to Kubota and Takigawa (5), was used to investigate prostate cancer
and orthotopic implantation. The model was established by the
subcapsular implantation of histologically intact prostate tumor
tissues into the prostate gland of C57BL6 syngeneic mice (male;
Vital River, Beijing, China; 20–25 g; 2–3 months old). The numbers
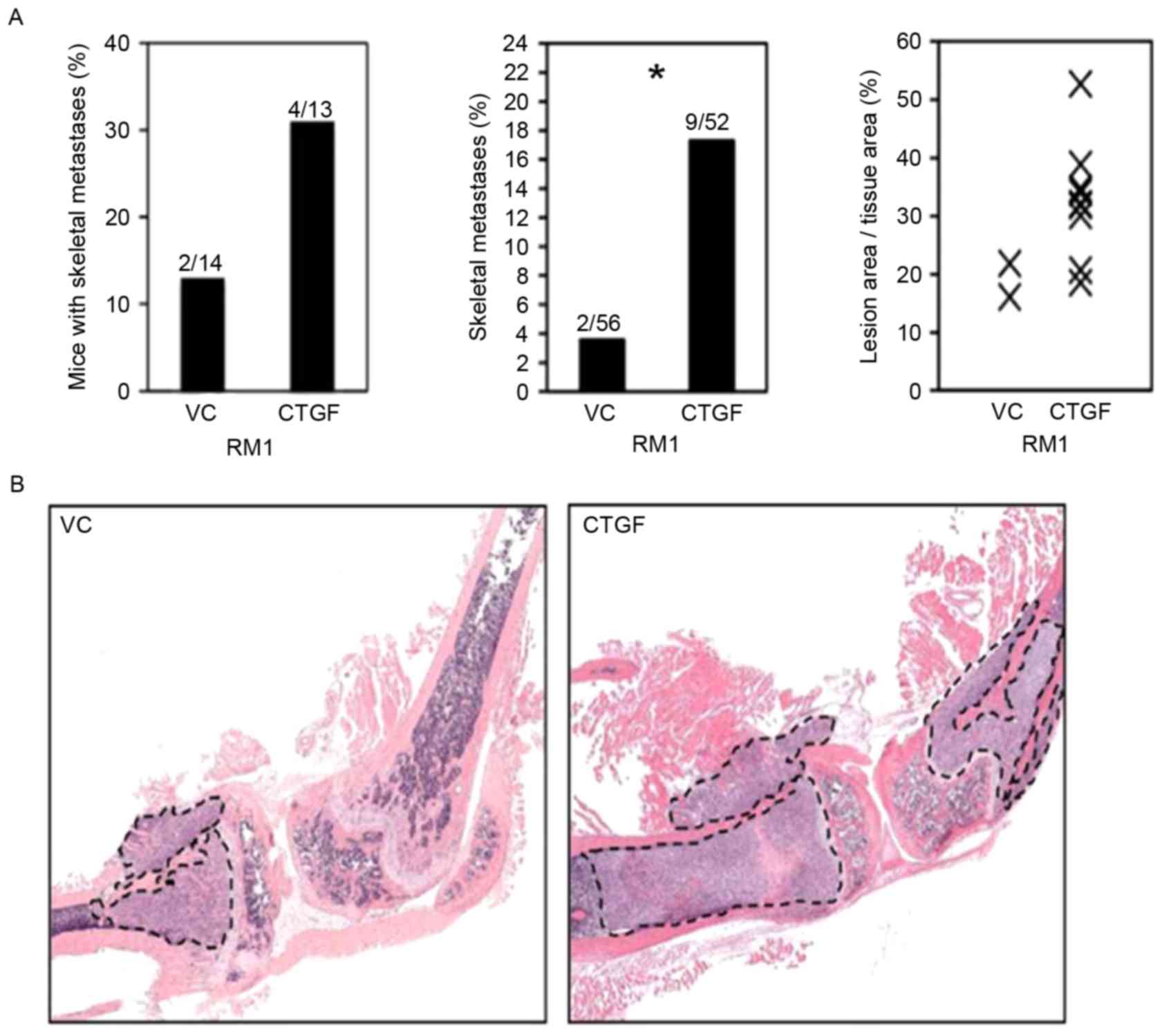
of mice were 13, 14, 52, 56 respectively (Fig. 4A), the diameter of tumor pieces were
<3 mm, which were harvested from a subcutaneous tumor by RM1
cells. All animals were maintained on a 12-h light/dark cycle at
20–25°C and had free access to food and water. The subcutaneous
implantation model was used as a control. Alterations in carcinoma
tumor volume(s) (volume calculation formula: πLW2/6, where L is
length and W is width) in situ and the survival rate time(s)
were recorded. X-radiography (XPERT80-L; Kubtec, Stratford, CT,
USA) was used to record radiographs on the effects of implantation
after 7 days. Bone samples from the model were collected and fixed
in 4% paraformaldehyde overnight at room temperature. Samples were
stored in 70% ethanol, embedded in paraffin, sagittally sectioned
at 7 µm, and stained with hematoxylin and eosin, according to
standard methods (13). Samples were
imaged using a Zeiss Axioplan 2 microscope (Carl Zeiss, Inc.,
Thornwood, NY, USA).
Osteoblast differentiation
Bone marrow cells (2–4×105) were obtained
from mouse tibia and femur. Following overnight culture, 50 ng/ml
ascorbic acid (AA; Merck KGaA) which was able to induce the
differentiation of osteoblast cells, was added, and 600 ng/ml BSA
(Amresco, LLC) and 600 ng/ml CTGF were added to the control group
and experimental group respectively every 2 days for a 9-day
period. Cells were fixed with 4% paraformaldehyde for 10 min at
room temperature, washed with PBS and then alkaline phosphatase
(ALP) staining was performed using Fast Red (F4381; Merck KGaA),
according to the manufacturer's protocol. Image analysis software
BIOQUANT Imaging Extensions (Bioquant Image Analysis Corporation,
Nashville, TN, USA) was used to perform statistical analysis and
calculate the mean covering rate.
Statistical analysis
Statistical analysis was performed using SPSS
(version 16.0; SPSS, Inc., Chicago, IL, USA). All measurement data
are presented as the mean ± standard deviation. A paired Student's
t-test was used for all measurement data analyzed using VassarStats
software (http://vassarstats.net/). Enumeration
data were analyzed using the χ2 goodness-of-fit test.
All experiments were performed at least three times. P<0.05
indicated a statistically significant difference.
Results
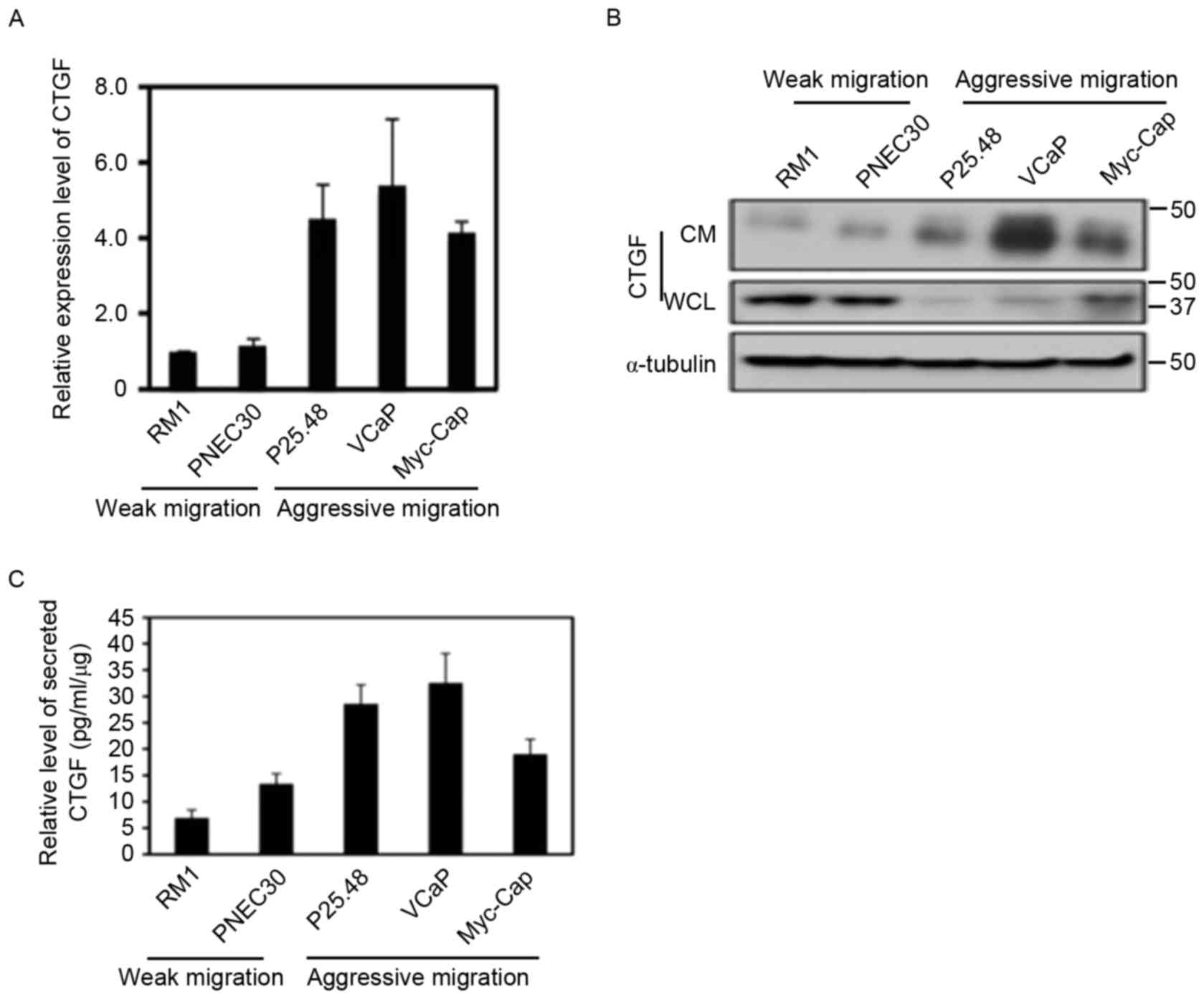
Increased expression of CTGF in highly
metastatic prostate cancer cells
Reverse transcription-qPCR analysis demonstrated
that CTGF mRNA expression was significantly increased in aggressive
bone metastatic prostate cancer cells (P=0.032; Fig. 1A). Western blotting results
demonstrated that CTGF protein expression in whole cell lysate and
culture supernatant was significantly decreased in high bone
metastatic prostate cancer cells (P=0.025); however, secretory CTGF
protein levels in cell culture supernatant was markedly increased
compared with prostate cancer cells with a weaker metastatic
potential (P=0.0011; Fig. 1B). In the
ELISA, CTGF secreted by aggressively metastatic prostate cancer
cells (MyC-CaP, P25.48 and VCaP cell lines) was increased
1.9-fold compared with weakly metastatic prostate cancer cells (RM1
and PNEC30 cell lines; P=0.002; Fig.
1C).
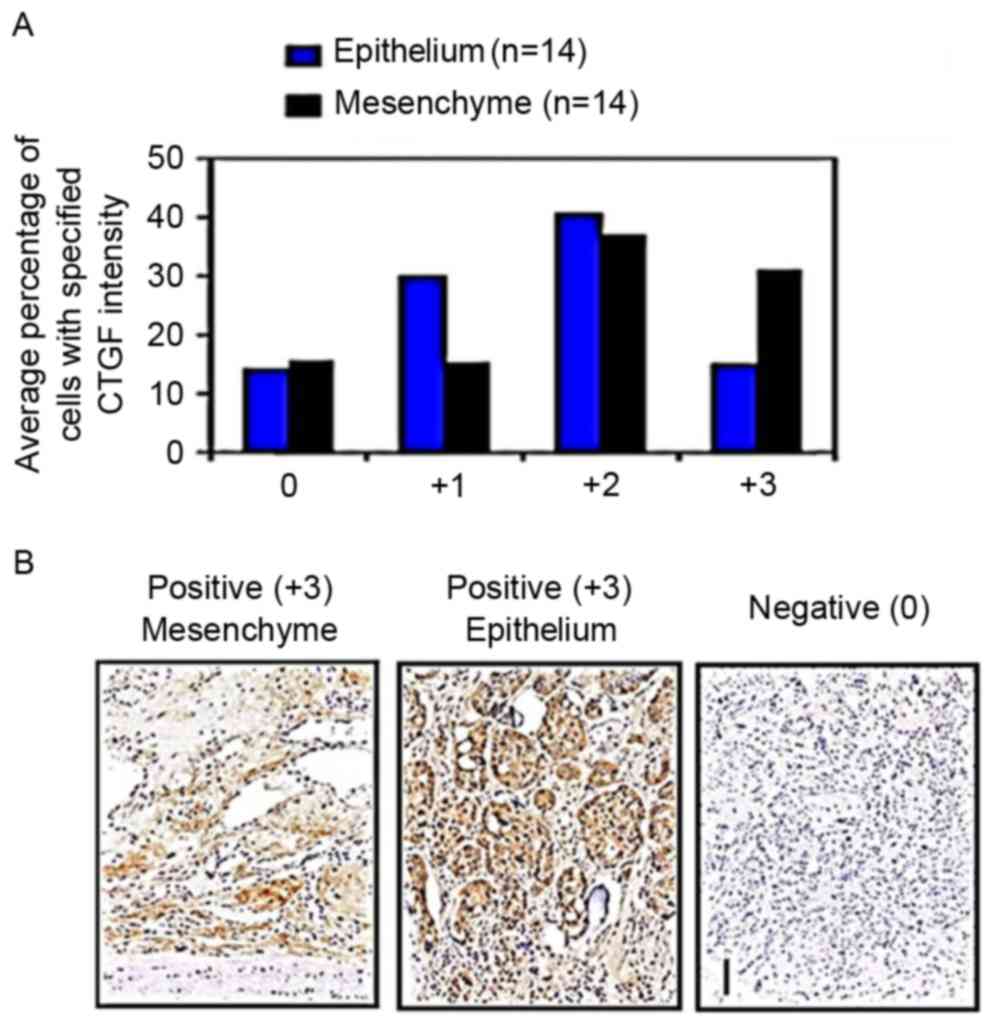
Increased CTGF expression levels in
patients with prostate cancer bone metastasis
Immunohistochemical staining detecting CTGF
expression levels in clinical samples demonstrated mid to deep
staining (scoring 2–4) in bone metastasis samples. Expression of
CTGF in mesenchymal cells was markedly increased compared with
epithelial cells (P=0.013; Fig. 2A).
Results indicate that high CTGF expression levels in mesenchymal
and epithelial cells was a common characteristic of prostate cancer
with bone metastasis.
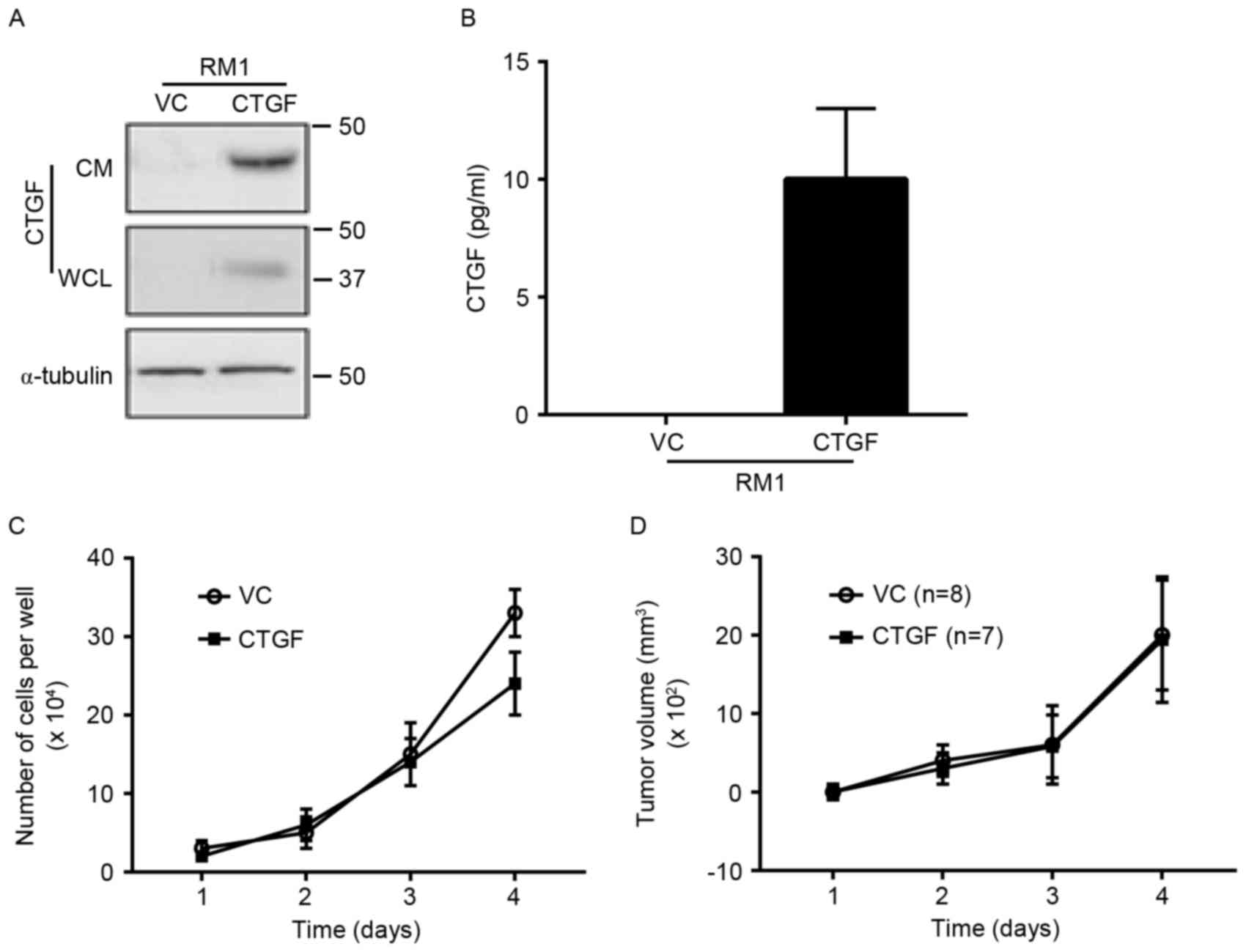
Increased CTGF expression levels do
not promote tumor cell proliferation and xenotransplantation
RM1 cells were transfected with an empty plasmid and
a CTGF plasmid to produce a stable RM1 cell line. CTGF expression
in whole cell lysate and culture supernatant was significantly
increased in RM1 cells (Fig. 3A and
B). The in vitro amplification curve demonstrated that
high expression levels of CTGF had no significant effect on the
proliferation of tumor cells (P>0.05). RM1 cell lines that were
implanted into mice demonstrated that increased CTGF expression
levels would not cause an alteration in tumor volume (P>0.05;
Fig. 3C and D).
Increased expression of CTGF promotes
bone metastasis in prostate cancer
The incidence of bone metastasis in mice injected
with control plasmid was 14% (2/14), whereas mice injected CTGF
plasmids had an incidence of 31% (4/13; Fig. 4A). Bone lesions caused by RM1 cells
transfected with control plasmids and CTGF plasmids were
significantly different (P=0.025). The area of tumors with RM1
cells transfected with CTGF was 1.7-fold larger compared with that
with RM1 cells transfected with a control plasmid (P=0.018;
Fig. 4B). Results demonstrate that
CTGF may significantly improve the ability of bone metastasis in
primary prostate cancer.
CTGF causes dysregulation in
osteoblast differentiation
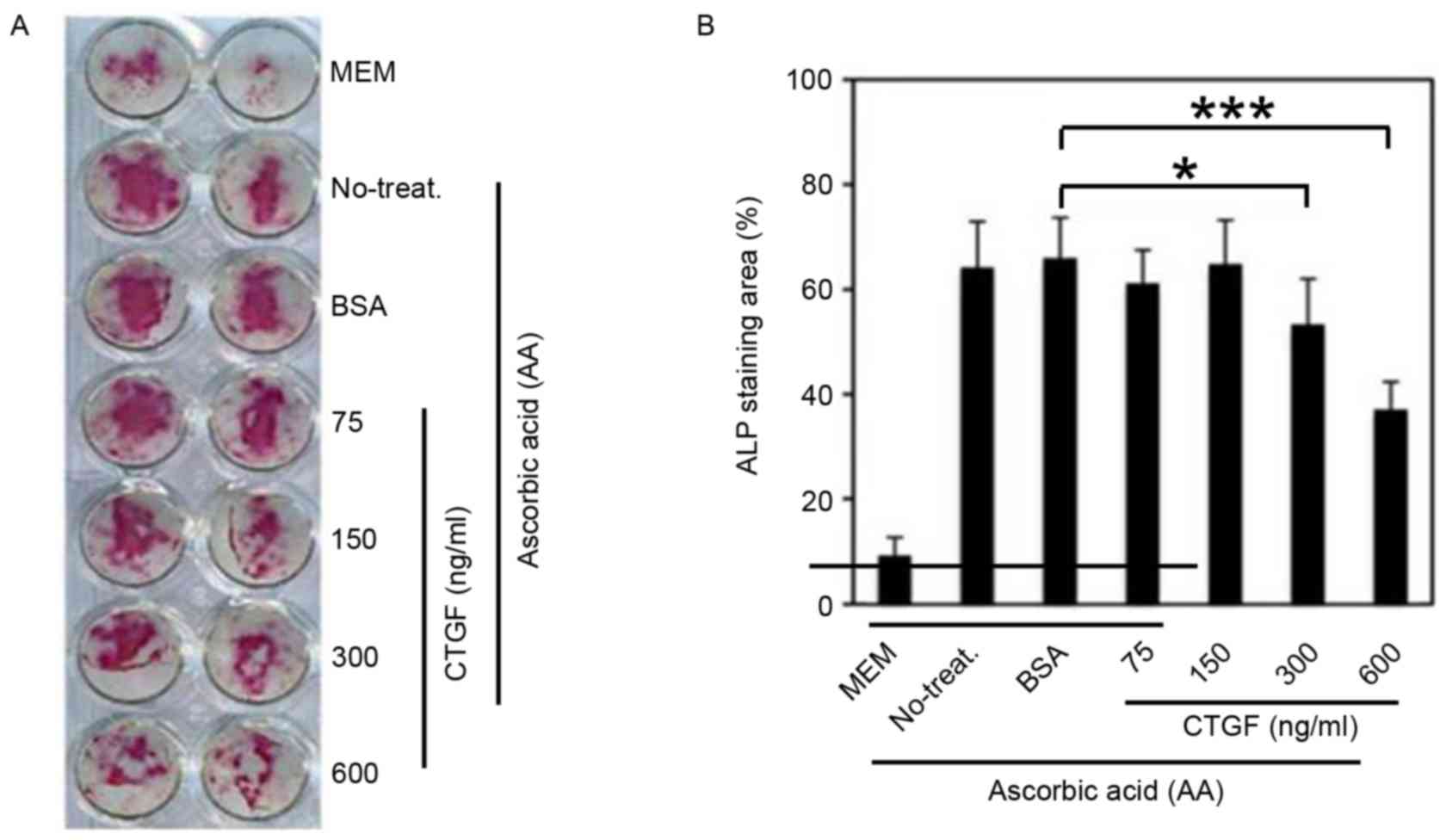
ALP staining was used to calculate the mean coverage
of osteoblast differentiation. The ability of osteoblast
differentiation significantly increased following the addition of
AA (P=0.006; Fig. 5A and B). However,
when adding AA and CTGF simultaneously, the staining area was
significantly decreased as the concentration of CTGF increased
(P=0.014). Results demonstrated that CTGF promoted prostate cancer
bone metastasis by causing dysregulation in osteoblast
differentiation.
Discussion
The association between CTGF expression levels and
prostate cancer with bone metastasis has been investigated
previously (14). Previous reports
demonstrated that CTGF increased in cancer tissue (15), which is consistent with the results of
the present study, and it had different regulation abilities and
effects on different types of tumor cell (16–19).
Bennewith et al (20)
demonstrated that CTGF protects cells from hypoxia-mediated
apoptosis, providing an in vivo selection for tumor cells
that express high levels of CTGF. Meanwhile, Kwon et al
(21) revealed that CTGF is induced
by TGF-β in diverse cell types, and CTGF was impaired in pancreatic
cancers and cell lines. In different types of cancer cell,
particularly breast and prostate, there are increased levels of
CTGF expression, which are associated with a poor clinical outcome
for patients (22,23). Furthermore, previous research
demonstrated that CTGF had an association with vasculogenesis,
infiltration and metastasis of malignancy tumor (24).
Results from the present study demonstrated that the
transcription and expression levels of CTGF were increased in
prostate cancer cells that were highly metastatic, and the level of
CTGF secreted from these cells was increased 1.7-fold compared with
prostate cancer cells that had a decreased metastatic ability (low
metastasis). This indicated that CTGF may be able to promote
metastasis and invasion of prostate cancer. Increased CTGF
expression levels in liver cancer, brain glioma and infiltrative
gastric carcinoma may exacerbate medical conditions and decrease
overall survival rates compared with diseases that have low levels
of CTGF (25). Results of the present
study suggested that the same biological functions of CTGF are
present in prostate cancer cells and that high levels of expression
are present in highly metastatic cells. Increased expression levels
of CTGF was the primary characteristic of prostate cancer bone
metastasis, with no significant difference between mesenchymal and
epithelial cells.
CTGF may promote the occurrence, differentiation,
proliferation, pathological fracture healing and bone mass
maintenance of cartilage and osteogenesis. Overexpression of CTGF
may promote the expression of type X collagen in HCS-2/8
chondrosarcoma cells and the secretion of chondroproteoglycan
(26). CTGF also promotes the
adhesion of osteoblasts by inhibiting the combination of fibrinogen
and integrin receptors and inhibit the synthesis of osteocalcin in
rat osteoblast-like cells (27).
Collectively, these previous studies indicate that CTGF serves a
key function in regulating bone transformation and metastasis.
The present study demonstrated that increased
expression levels of CTGF were not able to promote the
proliferation of cancer cells, and also had no influence on
alterations in tumor volume by xenotransplantation of RM1 cells.
However, increased levels of CTGF expression may be able to promote
bone metastasis in primary prostate cancer. Furthermore, ALP
staining experiments demonstrated that bone metastasis in prostate
cancer was caused by the dysregulation of osteoblast
differentiation. However, further research is required to clarify
the underlying molecular mechanism(s) of CTGF in promoting bone
formation.
In summary, the results of the present study
identified that CTGF promotes the bone metastatic process of
prostate cancer by dysregulating the differentiation of
osteoblasts, and forms tumor microenvironment characteristics for
osteolytic metastatic carcinoma. These identified characteristics
provide an evidence base for the development of effective
prevention strategies and therapeutic options for patients with
prostate cancer, as well as having important implications in the
prevention of bone metastasis.
Acknowledgements
The present study was supported by a Harbin First
Hospital talent introduction funded project (grant no.
2013SYYRCYJ01-1).
Glossary
Abbreviations
Abbreviations:
|
CTGF
|
connective tissue growth factor
|
|
ALP
|
alkaline phosphatase
|
|
AA
|
ascorbic acid
|
References
|
1
|
Vickers AJ, Sjoberg DD, Ulmert D,
Vertosick E, Roobol MJ, Thompson I, Heijnsdijk EA, De Koning H,
Atoria-Swartz C, Scardino PT and Lilja H: Empirical estimates of
prostate cancer overdiagnosis by age and prostate-specific antigen.
BMC Med. 12:262014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cives M, Rizzo F, Simone V, Bisceglia F,
Stucci S, Seeber A, Spizzo G, Montrone T, Resta L and Silvestris F:
Reviewing the osteotropism in neuroendocrine tumors: The role of
epithelial-mesenchymal transition. Neuroendocrinology. 103:321–334.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen CC and Lau LF: Functions and
mechanisms of action of CCN matricellular proteins. Int J Biochem
Cell Biol. 41:771–783. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Holbourn KP, Acharya KR and Perbal B: The
CCN family of proteins: Structure-function relationships. Trends
Biochem Sci. 33:461–473. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kubota S and Takigawa M: The role of CCN2
in cartilage and bone development. J Cell Commun Signal. 5:209–217.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hou R, Wang YW, Liang HF, Zhang ZG, Liu
ZM, Zhang BH, Zhang BX and Chen XP: Animal and cellular models of
hepatocellular carcinoma bone metastasis: Establishment and
characterisation. J Cancer Res Clin Oncol. 141:1931–1943. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jun JI and Lau LF: Taking aim at the
extracellular matrix: CCN proteins as emerging therapeutic targets.
Nat Rev Drug Discov. 10:945–963. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tai HC, Chang AC, Yu HJ, Huang CY, Tsai
YC, Lai YW, Sun HL, Tang CH and Wang SW: Osteoblast-derived
WNT-induced secreted protein 1 increases VCAM-1 expression and
enhances prostate cancer metastasis by down-regulating miR-126.
Oncotarget. 5:7589–7598. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ren L, Wang X, Dong Z, Liu J and Zhang S:
Bone metastasis from breast cancer involves elevated IL-11
expression and the gp130/STAT3 pathway. Med Oncol. 30:6342013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gao YB, Xiang ZL, Zhou LY, Wu ZF, Fan J,
Zeng HY and Zeng ZC: Enhanced production of CTGF and IL-11 from
highly metastatic hepatoma cells under hypoxic conditions: An
implication of hepatocellular carcinoma metastasis to bone. J
Cancer Res Clin Oncol. 139:669–679. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang X, Sha L, Sun N, Shen Y and Xu Q:
Deletion of mTOR in reactive astrocytes suppresses chronic seizures
in a mouse model of temporal lobe epilepsy. Mol Neurobiol.
54:175–187. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fischer AH, Jacobson KA, Rose J and Zeller
R: Hematoxylin and eosin staining of tissue and cell sections. CSH
Protoc. 2008:pdb.prot4986. 2008.
|
|
14
|
Juárez P, Mohammad KS, Yin JJ, Fournier
PG, McKenna RC, Davis HW, Peng XH, Niewolna M, Javelaud D, Chirgwin
JM, et al: Halofuginone inhibits the establishment and progression
of melanoma bone metastases. Cancer Res. 72:6247–6256. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chien W, O'Kelly J, Lu D, Leiter A, Sohn
J, Yin D, Karlan B, Vadgama J, Lyons KM and Koeffler HP: Expression
of connective tissue growth factor (CTGF/CCN2) in breast cancer
cells is associated with increased migration and angiogenesis. Int
J Oncol. 38:1741–1747. 2011.PubMed/NCBI
|
|
16
|
Cawthorn TR, Amir E, Broom R, Freedman O,
Gianfelice D, Barth D, Wang D, Holen I, Done SJ and Clemons M:
Mechanisms and pathways of bone metastasis: Challenges and pitfalls
of performing molecular research on patient samples. Clin Exp
Metastasis. 26:935–943. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fong YC, Lin CY, Su YC, Chen WC, Tsai FJ,
Tsai CH, Huang CY and Tang CH: CCN6 enhances ICAM-1 expression and
cell motility in human chondrosarcoma cells. J Cell Physiol.
227:223–232. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mohammad KS, Javelaud D, Fournier PG,
Niewolna M, McKenna CR, Peng XH, Duong V, Dunn LK, Mauviel A and
Guise TA: TGF-beta-RI kinase inhibitor SD-208 reduces the
development and progression of melanoma bone metastases. Cancer
Res. 71:175–184. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gorlov IP, Sircar K, Zhao H, Maity SN,
Navone NM, Gorlova OY, Troncoso P, Pettaway CA, Byun JY and
Logothetis CJ: Prioritizing genes associated with prostate cancer
development. BMC Cancer. 10:5992010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bennewith KL, Huang X, Ham CM, Graves EE,
Erler JT, Kambham N, Feazell J, Yang GP, Koong A and Giaccia AJ:
The role of tumor cell-derived connective tissue growth factor
(CTGF/CCN2) in pancreatic tumor growth. Cancer Res. 69:775–784.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kwon S, Munroe X, Crawley SC, Lee HY,
Spong S, Bradham D, Gum JR Jr, Sleisenger MH and Kim YS: Expression
of connective tissue growth factor in pancreatic cancer cell lines.
Int J Oncol. 31:693–703. 2007.PubMed/NCBI
|
|
22
|
Tan TW, Lai CH, Huang CY, Yang WH, Chen
HT, Hsu HC, Fong YC and Tang CH: CTGF enhances migration and MMP-13
up-regulation via alphavbeta3 integrin, FAK, ERK, and
NF-kappaB-dependent pathway in human chondrosarcoma cells. J Cell
Biochem. 107:345–356. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shimo T, Kubota S, Yoshioka N, Ibaragi S,
Isowa S, Eguchi T, Sasaki A and Takigawa M: Pathogenic role of
connective tissue growth factor (CTGF/CCN2) in osteolytic
metastasis of breast cancer. J Bone Miner Res. 21:1045–1059. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bartholin L, Wessner LL, Chirgwin JM and
Guise TA: The human Cyr61 gene is a transcriptional target of
transforming growth factor beta in cancer cells. Cancer Lett.
246:230–236. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kang Y, Siegel PM, Shu W, Drobnjak M,
Kakonen SM, Cordón-Cardo C, Guise TA and Massagué J: A multigenic
program mediating breast cancer metastasis to bone. Cancer Cell.
3:537–549. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Manara MC, Perbal B, Benini S, Strammiello
R, Cerisano V, Perdichizzi S, Serra M, Astolfi A, Bertoni F, Alami
J, et al: The expression of ccn3(nov) gene in musculoskeletal
tumors. Am J Pathol. 160:849–859. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Inoue M, Otsuka K and Shibata H:
Circulating tumor cell count as a biomarker of a specific gastric
cancer subgroup characterized by bone metastasis and/or
disseminated intravascular coagulation-an early indicator of
chemotherapeutic response. Oncol Lett. 11:1294–1298. 2016.
View Article : Google Scholar : PubMed/NCBI
|