Introduction
Air pollution, also known as smog, has become much
more severe in China in recent years. Smog is the result of
interactions between specific climatic conditions and human
activities. The economic and social activities of high-density
populations result in the emission of large amounts of fine
particulate matter; one such common pollutant is known as
particulate matter with diameter ≤2.5 µm (PM2.5) due to the
aerodynamic diameter is ≤2.5 µm. When the discharge of PM2.5
exceeds the circulation and carrying capacity of the atmosphere,
these fine particles accumulate in the air, leading to smog.
Numerous studies have demonstrated that air pollution damages the
respiratory system, cardiovascular system, and organs, including
the heart and lungs (1–8) Additionally, PM2.5 increases the
incidence of infectious diseases and decreases male fertility
(9–13).
Leukemia, a malignant clonal disease originating
from hematopoietic stem cells, is a major public health threat, and
the sixth and eighth leading cause of cancer-associated mortality
in men and women in China, respectively. Notably, leukemia is the
leading cause of cancer-associated mortality in children and
patients <35 years old. In recent years, several studies have
demonstrated the association between PM2.5 and the risk of
leukemia. Studies by Brosselin and Steffen have revealed that
living near gas stations or garages may increase the risk of
developing acute lymphoblastic leukemia and acute myeloid leukemia
(AML) (14,15). Another study performed in Denmark
indicated that there is an association between traffic-associated
air pollution and the risk of developing AML (16).
Despite extensive studies, the mechanisms mediating
the effects of PM2.5 on the occurrence and development of leukemia
remain unclear. However, changes in the bone marrow
microenvironment are considered to be involved in the progress of
leukemia (17,18). A study performed in Taiyuan, China
demonstrated that PM2.5 affects cell proliferation, but does not
cause cell injury in exposed leukemia cells and that low doses of
PM2.5 accelerates leukemia development through a reactive oxygen
species-mediated pathway (19).
In the present study, the effects of various
concentrations of PM2.5 on cell proliferation were investigated in
three AML cell lines (U937, HL-60 and KG-1a). The expression levels
of several cytokines, including interleukin-2 (IL-2),
IL-10, IL-17A and tumor necrosis factor (TNF) α, after PM2.5
exposure in AML cell lines and rats were also evaluated, with the
aim of elucidating the mechanisms through which PM2.5 affects the
occurrence and development of leukemia.
Materials and methods
Reagents and cell lines
PM2.5 particles purchased from the National
Institute of Standards and Technology (Gaithersburg, MD, USA) were
diluted in sterilized PBS solution to 5 mg/ml and preserved at
−20°C in aliquots. The samples were then diluted to a working
concentration in RPMI-1640 (cat. no. SH30809.01; Hyclone; GE
Healthcare Life Sciences, Logan, UT, USA) prior to use.
Three human AML cell lines (U937, HL-60 and KG-1a)
were purchased from the National Infrastructure of Cell Line
Resource (Beijing, China). U937 and HL-60 cells were cultured in
RPMI-1640 supplemented with 10% fetal bovine serum (cat. no.
10099141; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
whereas KG-1a cells were cultured in RPMI-1640 supplemented with
20% fetal bovine serum. All cell lines were cultured at 37°C in a
5% CO2 humidified atmosphere without antibiotics.
Treatment of U937, HL-60 and KG-1a
cell lines
U937, HL-60 and KG-1a cells were treated with PM2.5
solution at concentrations of 0–20 mg/ml for 24, 48 and 72 h after
reaching a steady-state of exponential growth in normal medium.
Measurement of cell proliferation
Cell proliferation rates were measured using a Cell
Counting Kit-8 (Dojindo Molecular Technologies, Inc., Kumamoto,
Japan) according to the manufacturer's protocol. The relative cell
proliferation ratio (%) following treatment was compared with
controls (cultured cells without PM2.5 treatment or blank controls
with RPMI-1640 with 10% FBS), and calculated as follows:
[(Acontrol-Ablank of
control)-(Asample-Ablank of
sample)]/(Acontrol-Ablank of control)
×100%.
Measurement of cytokine mRNA
expression levels in AML cell lines
The mRNA expression levels of IL-2, IL-10,
IL-17A, and TNFα were detected by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) in
AML cells following treatment with 0.1 mg/ml PM2.5 solution for 0,
24, 48 and 72 h. The housekeeper gene actin β was used as a
control. The RNA of cells was extracted using Trizol reagent (cat.
no. 15596018; Invitrogen; Thermo Fisher Scientific, Inc.). cDNA
templates from AML cell lines were prepared using a TIANScript RT
kit (Tiangen Biotech, Co., Ltd., Beijing, China) according to the
manufacture's protocol.
RT-qPCR cycling was performed in 96-well plates on a
LightCycler 480 Real-Time PCR system (Roche Applied Science,
Penzberg, Germany). The reaction was performed in a 20-µl total
volume containing 10 µl SYBR Select Master mix (cat. no. 4472908;
Thermo Fisher Scientific, Inc.), 1 µl of each primer (10 µM), and 2
µl template cDNA. The primer sequences used for PCR are presented
in Table I. The amplification
protocol consisted of an initial denaturation step at 95°C for 5
min, followed by two-step PCR for 40 cycles at 95°C for 30 sec and
60°C for 30 sec. The mRNA expression levels of each target were
determined based on the cycle threshold (Cq) value for the
reference and each target and calculated as 2−ΔCq
(20). Three independent experiments
were performed.
 | Table I.Primer sequences and lengths of
detected genes. |
Table I.
Primer sequences and lengths of
detected genes.
| Primer name | Sequence
(5′-3′) | Product length
(bp) |
|---|
| IL-2 | F:
TACAAGAACCCGAAACTGACTCG | 29 |
|
| R:
ACATGAAGGTAGTCTCACTGCC | 28 |
| IL-10 | F:
TCAAGGCGCATGTGAACTCC | 26 |
|
| R:
GATGTCAAACTCACTCATGGCT | 28 |
| IL-17A | F:
AGATTACTACAACCGATCCACCT | 29 |
|
| R:
GGGGACAGAGTTCATGTGGTA | 27 |
| TNFα | F:
CCTCTCTCTAATCAGCCCTCTG | 28 |
|
| R:
GAGGACCTGGGAGTAGATGAG | 27 |
| ACTB | F:
CATGTACGTTGCTATCCAGGC | 27 |
|
| R:
CTCCTTAATGTCACGCACGAT | 27 |
Measurement of protein expression
levels in rats
All 26 Sprague Dawley (SD) rats were 6-week-old
males (173–190 g), purchased from Charles River Laboratories
(Beijing, China), and housed at a temperature of 25°C,
1.013×105 pa atmosphere, 12/12 h dark/light cycle and
ad libitum access to food and water. The present study was
approved by Ethics Committee of Beijing Luhe Hospital Affiliated to
Capital Medical University (Beijing, China). Exposed chambers and
clean chambers were constructed to raise rats. Air in the exposed
chambers was the same as the true air outside, whereas air in the
clean chambers was filtered to remove PM2.5 particles. Fourteen
rats were kept in exposed chambers (n=8) or clean chambers (n=6)
for 6 weeks (between August 9, 2016 and September 19, 2016), and 12
rats were kept in exposed chambers (n=8) or clean chambers (n=4)
for 12 weeks (between August 9, 2016 and October 10, 2016). The
PM2.5 concentration in the atmosphere during the study is presented
in Table II.
 | Table II.PM2.5 concentration of the atmosphere
during the study. |
Table II.
PM2.5 concentration of the atmosphere
during the study.
| A, PM2.5
concentration between August 09 2016 and September 19 2016 |
|---|
|
|---|
| Date | PM2.5 (µg/m3)
within chambers | PM2.5 (µg/m3) in
Beijing outside | Date | PM2.5 (µg/m3) | Beijing |
|---|
| Aug-09-2016 | 30 | 44 | Aug-30-2016 | 25 | 28 |
| Aug-10-2016 | 41 | 82 | Aug-31-2016 | 15 | 16 |
| Aug-11-2016 | 36 | 85 | Sep-01-2016 | 6 | 7 |
| Aug-12-2016 | 23 | 59 | Sep-02-2016 | 8 | 11 |
| Aug-13-2016 | 10 | 21 | Sep-03-2016 | 13 | 18 |
| Aug-14-2016 | 9 | 18 | Sep-04-2016 | 56 | 80 |
| Aug-15-2016 | 15 | 20 | Sep-05-2016 | 25 | 27 |
| Aug-16-2016 | 18 | 22 | Sep-06-2016 | 22 | 17 |
| Aug-17-2016 | 35 | 59 | Sep-07-2016 | 28 | 41 |
| Aug-18-2016 | 38 | 74 | Sep-08-2016 | 17 | 20 |
| Aug-19-2016 | 11 | 17 | Sep-09-2016 | 19 | 17 |
| Aug-20-2016 | 31 | 52 | Sep-10-2016 | 18 | 15 |
| Aug-21-2016 | 40 | 51 | Sep-11-2016 | 28 | 32 |
| Aug-22-2016 | 37 | 51 | Sep-12-2016 | 33 | 31 |
| Aug-23-2016 | 35 | 69 | Sep-13-2016 | 62 | 86 |
| Aug-24-2016 | 41 | 79 | Sep-14-2016 | 66 | 101 |
| Aug-25-2016 | 10 | 19 | Sep-15-2016 | 72 | 69 |
| Aug-26-2016 | 9 | 9 | Sep-16-2016 | 82 | 133 |
| Aug-27-2016 | 12 | 14 | Sep-17-2016 | 28 | 41 |
| Aug-28-2016 | 9 | 10 | Sep-18-2016 | 17 | 22 |
| Aug-29-2016 | 14 | 17 | Sep-19-2016 | 12 | 10 |
| Average value | 44 | 61 | Average value | 44 | 61 |
|
| B, PM2.5
concentration between September 19 2016 and October 31
2016 |
|
| Date | PM2.5 (µg/m3)
within chambers | PM2.5 (µg/m3) in
Beijing outside | Date | PM2.5
(µg/m3) | Beijing |
|
| Sep-20-2016 | 32 | 18 | Oct-11-2016 | 75 | 116 |
| Sep-21-2016 | 56 | 63 | Oct-12-2016 | 77 | 77 |
| Sep-22-2016 | 73 | 99 | Oct-13-2016 | 148 | 150 |
| Sep-23-2016 | 77 | 121 | Oct-14-2016 | 133 | 241 |
| Sep-24-2016 | 85 | 111 | Oct-15-2016 | 96 | 190 |
| Sep-25-2016 | 80 | 162 | Oct-16-2016 | 83 | 119 |
| Sep-26-2016 | 44 | 58 | Oct-17-2016 | 52 | 59 |
| Sep-27-2016 | 10 | 24 | Oct-18-2016 | 80 | 117 |
| Sep-28-2016 | 20 | 13 | Oct-19-2016 | 130 | 225 |
| Sep-29-2016 | 56 | 51 | Oct-20-2016 | 42 | 94 |
| Sep-30-2016 | 76 | 97 | Oct-21-2016 | 49 | 42 |
| Oct-01-2016 | 106 | 165 | Oct-22-2016 | 23 | 14 |
| Oct-02-2016 | 96 | 183 | Oct-23-2016 | 43 | 26 |
| Oct-03-2016 | 62 | 120 | Oct-24-2016 | 79 | 60 |
| Oct-04-2016 | 37 | 33 | Oct-25-2016 | 87 | 96 |
| Oct-05-2016 | 66 | 55 | Oct-26-2016 | 24 | 43 |
| Oct-06-2016 | 48 | 72 | Oct-27-2016 | 36 | 30 |
| Oct-07-2016 | 35 | 39 | Oct-28-2016 | 17 | 10 |
| Oct-08-2016 | 26 | 11 | Oct-29-2016 | 38 | 23 |
| Oct-09-2016 | 52 | 31 | Oct-30-2016 | 34 | 43 |
| Oct-10-2016 | 88 | 87 | Oct-31-2016 | 10 | 8 |
| Average value | 44 | 61 | Average value | 44 | 61 |
The peripheral blood of rats was collected from
hearts after feeding for 6 or 12 weeks. Blood samples were
centrifuged at 1,000 × g for 20 min at room temperature to obtain
serum. Milliplex Map Rat Cytokine/Chemokine Magnetic Bead
Panel-Immunology Multiplex assays (cat. no. RECYTMAG-65K; EMD
Millipore, Billerica, MA, USA) were used to detect the expression
of cytokines in serum according to the manufacturer's protocol.
Statistical analysis
Statistical analysis was performed using Microsoft
Excel 2010 (Microsoft Corporation, Redmond, WA, USA) and Graphpad
Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA). All data were
derived from at least three independent experiments and are
presented as means ± standard deviations. The results were
evaluated using two-tailed unpaired Student's t-test and one-way
analysis of variance (ANOVA) with Dunnett's multiple comparison
post-hoc test. P<0.05 was considered to indicate a statistically
significant difference. In RT-qPCR analysis, statistical
significance was assumed if the 2−Δct (target mean
value-reference mean value) value was >150% or
<75%.
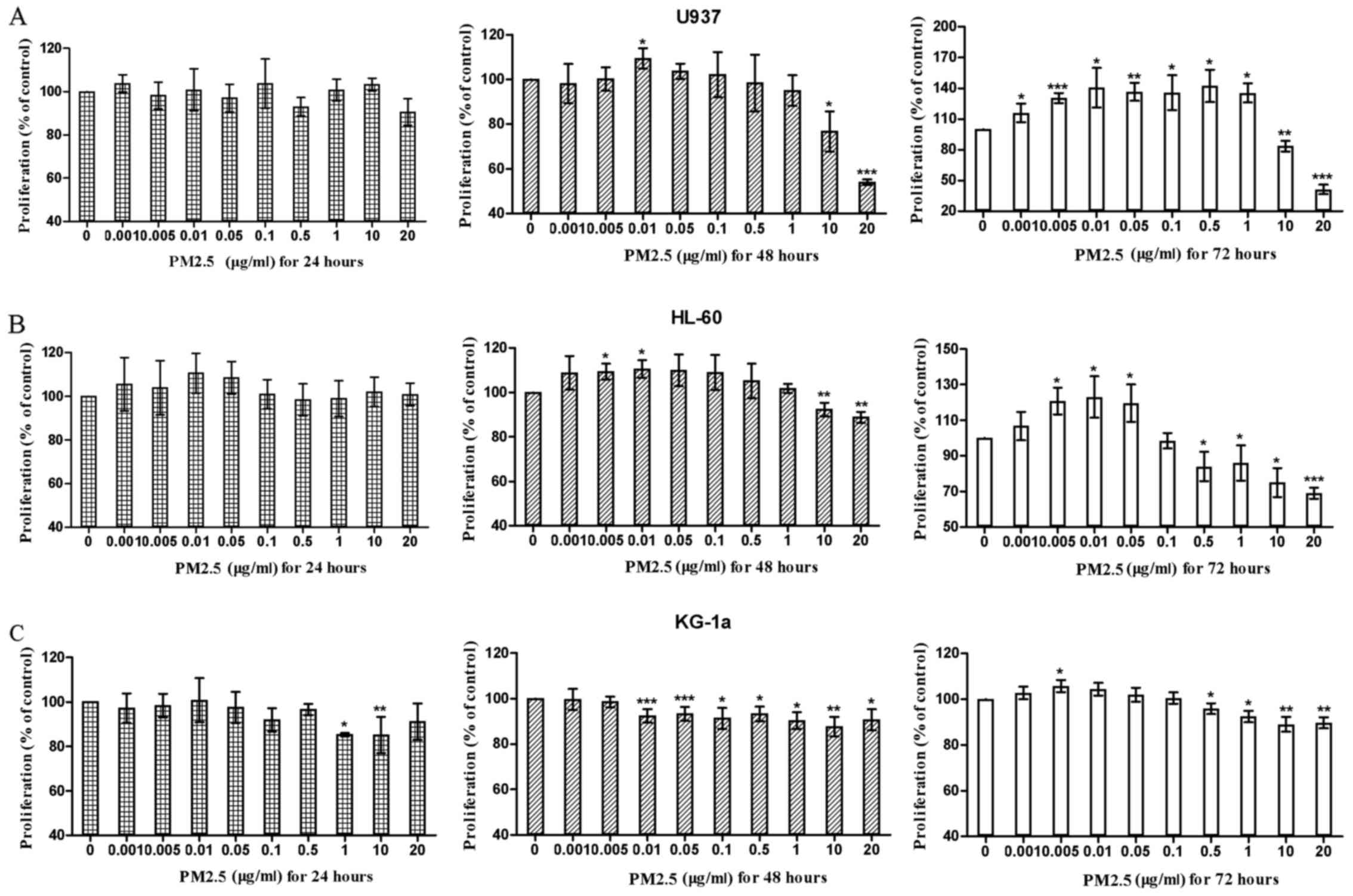
Results
Low concentrations of PM2.5 promote
proliferation, whereas high concentrations of PM2.5 inhibit
proliferation in AML cells
First, the effects of PM2.5 on the proliferation of
AML cell lines (U937, HL-60, and KG-1a) were evaluated. The results
demonstrated that 24 h of treatment with PM2.5 did not induce or
inhibit the proliferation of U937 cells at any concentration.
However, after 48 or 72 h of treatment, PM2.5 first promoted and
then significantly inhibited the proliferation of U937 cells as the
concentration of PM2.5 increased. These effects were more evident
at 72 h compared with at 48 h. A similar situation was observed in
HL-60 and KG-1a cells. However, the stimulatory and inhibitory
effects of PM2.5 on KG-1a cells were less evident compared with
those on U937 and HL-60 cells (Fig.
1).
PM2.5 significantly alters the
expression of cytokines in AML cells
Next, the expression levels of IL-2, IL-10,
IL-17A and TNFα were analyzed in U937, HL-60 and KG-1a
cells following treatment with PM2.5. The results revealed that the
mRNA expression levels of IL-2 in U937 cells, IL-17A
in HL-60 cells, and IL-2 and IL-17A in KG-1a cells
were below the limit of detection (data not shown). In contrast,
the expression of IL-10, IL-17A and TNFα in U937
cells; IL-10 and TNFα in HL-60 cells; and
IL-10 in KG-1a cells significantly increased in a time- and
concentration-dependent manner (Figs.
2 and 3). Notably, IL-2
expression in HL-60 cells and TNFα expression in KG-1a also
increased after treatment with 0.1 µg/ml PM2.5 for 24 h or ≥0.01
µg/ml for 72 h; however, these effects did not persist over time
(Fig. 2) and did not increase as the
concentration was increased (Fig.
3).
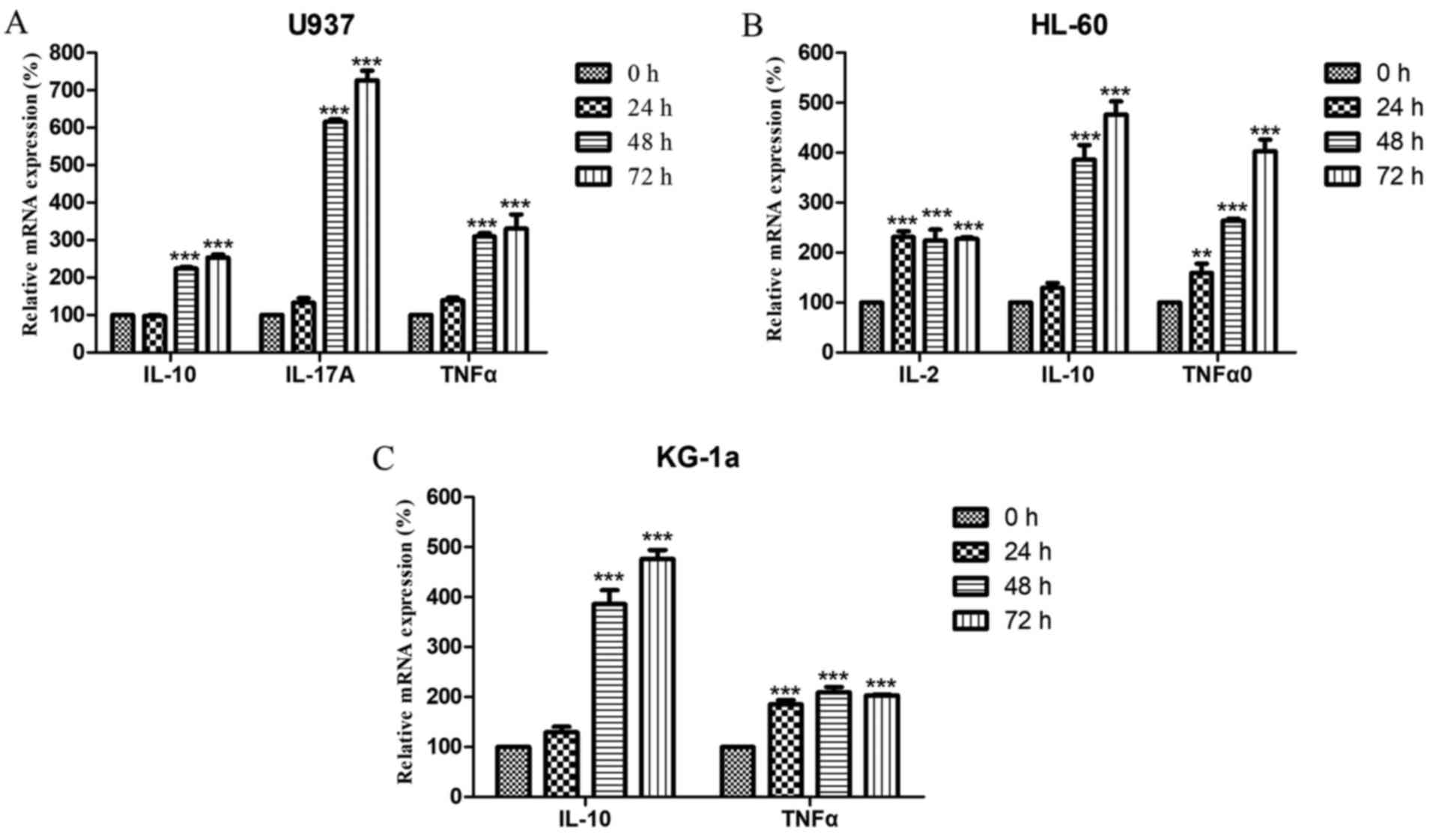 | Figure 2.Relative mRNA expression levels of
IL-10, IL-17A, and TNFα in (A) U937 cells; (B) IL-2, IL-10, and
TNFα in HL-60 cells; and (C) IL-10 and TNFα in KG-1a cells treated
with 0.1 µg/ml PM2.5 for 24, 48, or 72 h. mRNA expression levels
are relative to actin β. Data are presented as the means ± standard
deviations of three identical experiments with four replicates
each. **P<0.005, ***P<0.0005 vs. 0h. IL, interleukin; TNF,
tumor necrosis factor; PM2.5, particulate matter with diameter ≤2.5
µm. |
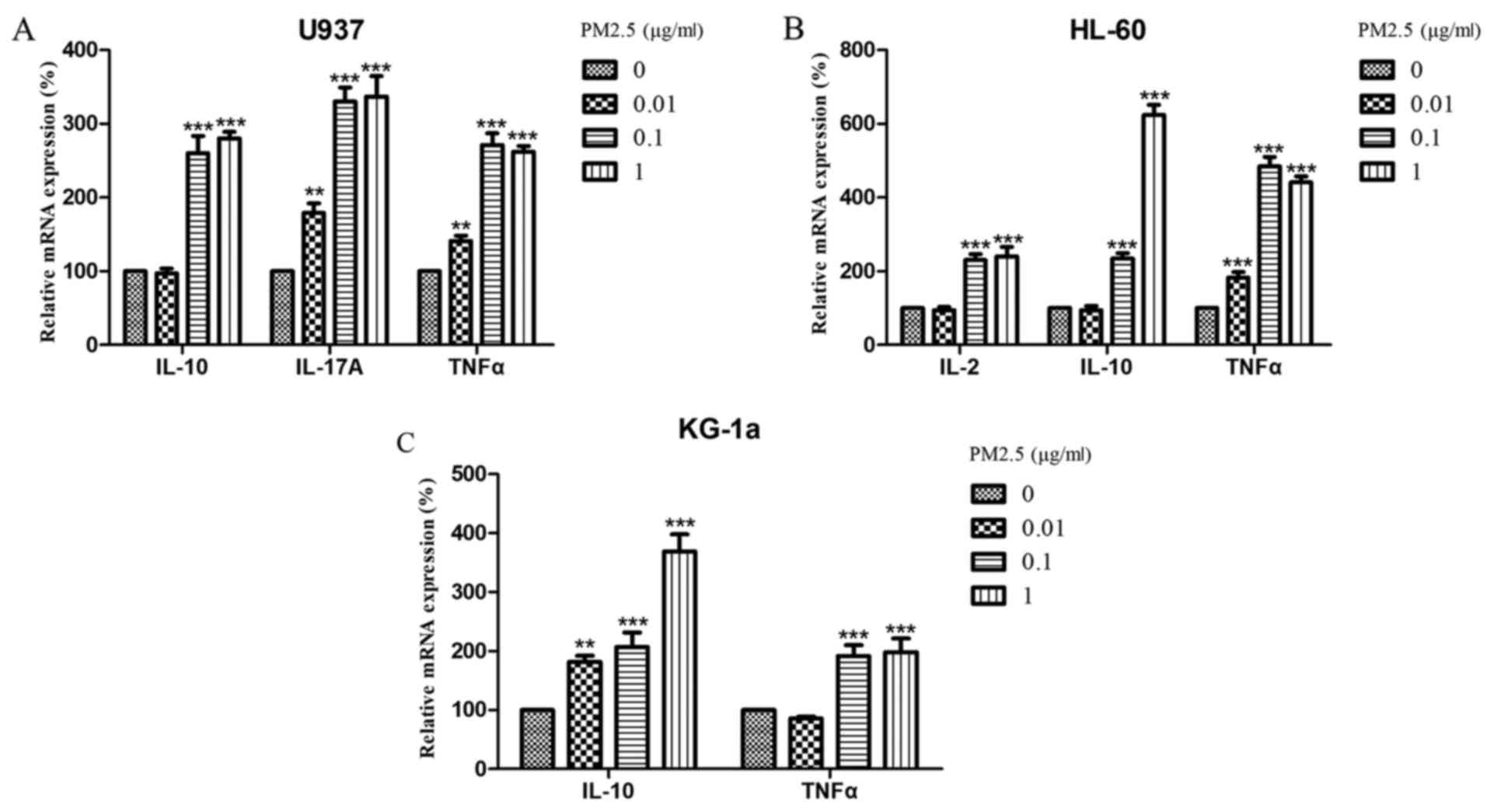 | Figure 3.Relative mRNA expression levels of
IL-10, IL-17A, and TNFα in (A) U937 cells; (B) IL-2, IL-10, and
TNFα in HL-60 cells; and (C) IL-10 and TNFα in KG-1a cells treated
with 0, 0.01, 0.1, or 1 µg/ml PM2.5 for 72 h. mRNA expression
levels are relative to actin β. Data are presented as the means ±
standard deviations of three identical experiments with four
replicates each. **P<0.005, ***P<0.0005 vs. 0 µg/ml. IL,
interleukin; TNF, tumor necrosis factor; PM2.5, particulate matter
with diameter ≤2.5 µm. |
PM2.5 significantly increases serum
IL-2 and IL-6 in SD rats following treatment for 12 weeks
Four cytokines, IL-2, IL-10, IL-17A and
TNFα, were detected in SD rat serum using enzyme-linked
immunosorbent assays following treatment with PM2.5 for 6 or 12
weeks. The results demonstrated that exposure to PM2.5 for 6 weeks
did not significantly alter serum levels of IL-2, IL-10,
IL-17A and TNFα in SD rats (Table III). However, when the exposure time
was increased to 12 weeks, serum IL-2 and IL-10
levels in rats were significantly higher compared with those of
untreated rats (Table IV).
 | Table III.Content of rat serum cell factors
after raising in exposed and clean chambers for 6 weeks. |
Table III.
Content of rat serum cell factors
after raising in exposed and clean chambers for 6 weeks.
|
|
| Content (pg/ml) in
each rat |
|
|---|
|
|
|
|
|
|---|
| Cell factors | Chamber | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | P-value |
|---|
| IL-2 | Exposed
chamber | 35.38 | 16.18 | 33.35 | 9.6 | 25.42 | 13.61 | 9.6 | 8.71 | 0.33 |
|
| Clean chamber | 17.95 | 23.5 | 41.6 | 16.18 | 17.95 | 31.33 |
|
|
|
| IL-10 | Exposed
chamber | 25.53 | 89.42 | 222.9 | 56.52 | 23.3 | 23.3 | 155.46 | 41.05 | 0.79 |
|
| Clean chamber | 28.97 | 72.71 | 115.15 | 9.45 | 129.69 | 67.25 |
|
|
|
| IL-17A | Exposed
chamber | 3.49 | 5.23 | 5.23 | 8.31 | 19.99 | 10.63 | 3.49 | 6.2 | 0.42 |
|
| Clean chamber | 5.23 | 13.13 | 5.23 | 8.31 | 11.86 | 17.15 |
|
|
|
| TNFα | Exposed
chamber | 1.35 | 2.45 | 2.17 | 3.02 | 3.02 | 2.17 | 1.35 | 1.9 | 0.25 |
|
| Clean chamber | 2.73 | 2.45 | 1.9 | 1.9 | 3.02 | 3.62 |
|
|
|
 | Table IV.Content of rat serum cell factors
following raising in exposed and clean chambers for 12 weeks. |
Table IV.
Content of rat serum cell factors
following raising in exposed and clean chambers for 12 weeks.
|
|
| Content (pg/ml) in
each rat |
|
|---|
|
|
|
|
|
|---|
| Cell factors | Chamber | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | P-value |
|---|
| IL-2 | Exposed
chamber | 9.6 | 8.71 | 6.78 | 14.45 | 12.78 | 9.6 | 6.78 | 5.92 | 0.01 |
|
| Clean chamber | 21.61 | 16.18 | 14.45 | 11.16 |
|
|
|
|
|
| IL-10 | Exposed
chamber | 14.95 | 82.4 | 59.17 | 22.2 | 10.31 | 17.96 | 28.97 | 39.81 | 0.04 |
|
| Clean chamber | 351.6 | 192.02 | 66.11 | 14.95 |
|
|
|
|
|
| IL-17A | Exposed
chamber | 4.33 | 5.23 | 2.7 | 5.23 | 8.31 | 5.23 | 2.7 | 4.33 | 0.08 |
|
| Clean chamber | 5.23 | 11.86 | 6.2 | 6.2 |
|
|
|
|
|
| TNFα | Exposed
chamber | 1.9 | 1.68 | 1.68 | 1.51 | 2.17 | 1.9 | 1.68 | 1.9 | 0.68 |
|
| Clean chamber | 1.9 | 1.68 | 1.68 | 2.17 |
|
|
|
|
|
Discussion
Several epidemiological studies have demonstrated
positive associations between PM2.5 exposure and increased leukemia
risk (14–16,21–23).
However, the mechanism explaining this association has not yet been
elucidated. The present study aimed to explain the effects of PM2.5
on the occurrence and development of leukemia through its influence
on cytokines in vitro and in vivo.
In the current study, low doses of PM2.5 promoted
the proliferation of AML cells (U937, HL-60, and KG-1a), whereas
high doses of PM2.5 resulted in cytotoxicity, thereby inhibiting
cell proliferation. Furthermore, PM2.5 exposure resulted in
significantly increased levels of IL-2, IL-10, IL-17A and
TNFα in AML cells. Thus, these results suggested that PM2.5
was capable of inducing an inflammatory response in human AML
cells.
CD4+ T cells, also known as helper T
cells, are divided into three classes: Th1, Th2, and Th17,
according to their different cytokine secretion patterns. The
differentiation of Th1 and Th2 cells is influenced by local
environmental cytokines, and these two groups complement or
antagonize each other to regulate immune functions.
IL-2 is a Th1 cytokine that is indispensable
in immune system regulation. IL-2 is secreted by activated T
or natural killer (NK) cells by autocrine or paracrine secretion,
and serves an important role in the activation and maintenance of
the immune response and in the promotion of lymphocyte development.
Numerous studies have confirmed that IL-2 induces NK cells
and enhances their antitumor activity (24,25). The
application of IL-2 as an antitumor drug in the clinical
treatment of patients with AML began in the 1980s (26). In the present study, it was revealed
that IL-2 levels increased in a time- and
concentration-dependent manner in HL-60 cells treated with PM2.5,
but was not detected in U937 or KG-1a cells. In rats, IL-2
expression was significantly enhanced after 12 weeks of exposure to
PM2.5. These results suggested that PM2.5 may affect different
subtypes of AML cells in different ways; thus, there may be
multiple complex cytokine networks in vivo.
IL-10 is a Th2 cytokine that has multiple
pleiotropic effects on immunoregulation and inflammation, and is
capable of inhibiting the synthesis of pro-inflammatory cytokines,
including interferon-γ, IL-2, IL-3, TNFα and
granulocyte-macrophage colony-stimulating factor, produced by
macrophages and Th1 T cells (27–29).
Despite the inhibitory effects of IL-10 on Th1 cytokines,
certain Th1 cells are also able to produce IL-10, and a
negative feedback pathway is formed when IL-10 regulates Th1
cell activation (27). Previous
studies have demonstrated that IL-10 directly inhibits the
proliferation and migration of effector T cells, and blocks the
production of associated cytokines, serving an important role in
inducing tumor immune escape. When the IL-10 content is
increased, the killing effect of T cells on tumor cells is markedly
inhibited (30–32). Blocking IL-10 expression in
animal models improves the killing ability of the immune system on
tumor cells (33,34). In addition, IL-10 has been
shown to inhibit T cells, forming an immunosuppressive environment
and inducing tumor immune escape by inhibiting antigen-presenting
cells activation (35). In the
present study, the mRNA expression levels of IL-10 were
significantly increased in a time- and concentration-dependent
manner following treatment of AML cell lines with PM2.5 solution.
Similar results were also obtained in an in vivo experiment;
that is, serum IL-10 contents were significantly increased
in rats exposed to PM2.5 compared with those of unexposed rats
after 12 weeks. These results demonstrate the potentially
carcinogenetic effects of PM2.5 in AML.
IL-17A is a pro-inflammatory cytokine
produced by activated Th17 cells, which function to amplify
inflammation, and activate neutrophils to engulf and digest
extracellular bacteria and molds by releasing pro-inflammatory
cytokines (36). IL-17 and Th17 cells
mediate carcinogenesis in rat tumor models and patients with
cancer. The mechanisms through which IL-17 and Th17 cells mediate
carcinogenesis involve angiogenesis and the induction of cytokines
in the tumor microenvironment, which promotes tumor growth
(37–40). Several studies have demonstrated that
IL-17 induces IL-6 production, and IL-6 activates the signal
transducer and activator of transcription 3 pathway, which then
upregulates the expression of prosurvival and angiogenic genes to
modulate tumor angiogenesis (41–45). The
tumor promoting effects of IL-17 and Th1 exist in various types of
common tumors (40,46–48). Wu
et al (49) demonstrated that
Th17 cells were significantly increased in the peripheral blood of
patients with AML compared with that in normal healthy individuals.
In addition, IL-17 content increased as the number of Th17 cells
increased, resulting in promotion of bone marrow cell proliferation
and inhibition of immune function. Additionally, the slow growth of
TNFα in the tumor microenvironment enhanced the recruitment
of IL-17-dependent bone marrow cells and promoted tumor
development. In the present study, the mRNA expression of
IL-17A was significantly increased in U937 AML cells a time-
and concentration-dependent manner following treatment with PM2.5
solution; however, the IL-17A content in HL-60 and KG-1a
cells was below the detection range. Thus, these findings indicated
that different mechanisms mediated the effects of PM2.5 on AML
cells. The in vivo experiment revealed no significant
increases in exposed rats compared with unexposed rats after 6 or
12 weeks, indicating that the mechanisms through which PM2.5
affected AML differed in vitro and in vivo. However,
further studies with increased numbers of animals are required to
confirm these findings.
TNFα, which is also a Th1 cytokine, is
produced ectopically by malignant and immune cells in the
tumor-associated microenvironment, creating a tumor-supportive
inflammatory niche that modulates the development and progression
of malignant disease (50).
TNFα is produced by various types of leukemia cells
(51–55). In several clinical studies, a positive
association between the expression levels of TNFα and
adverse clinical parameters in leukemia was observed (53–57) In
AML, high levels of TNFα expression are associated with
greater fatigue and poorer quality of life (58). Previous studies have revealed that
TNFα activates nuclear factor κB and c-Jun N-terminal
kinase/activator protein-1 to exert anti-apoptotic and
proproliferative effects in leukemia cells, thereby facilitating
leukemia cell survival and progression (32–34,
59–61). In the current study, TNFα
levels were significantly increased in U937 and HL-60 cells in a
time- and concentration-dependent manner following PM2.5 treatment.
In KG-1a cells, TNFα levels were also increased, although
the time- and concentration-dependent effects were not as evident.
This in vitro experiment reflects the potential ability of
PM2.5 to promote the occurrence and development of leukemia by
regulating intracellular TNFα. In vivo, significant
changes in TNFα expression were not observed after 6 or 12
weeks of treatment with PM2.5, further supporting that there may be
different mechanisms mediating the effects of PM2.5 on AML in
vitro and in vivo. However, again, further studies with
greater numbers of animals are required.
Acknowledgements
The authors would like to thank Dr Xiaokun Geng and
Mr Longfei Guan (China-America Institute of Neuroscience, Beijing
Luhe Hospital, Capital Medical University, Beijing, China) for
their valuable technical assistance with the animal
experiments.
Funding
The present study was supported by the Air Pollution
Subject of Science Committee of Tongzhou District (grant no.
CK2016KJ035).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TC performed the cell and animal experiments of the
present study and analyzed the obtained data. JZ and HuZ
interpreted the data. YuZ, YoZ and XZ helped TC to perform the
animal experiments. DZ and YF helped with the animal experiments
and participated in writing the manuscript. HeZ was a major
contributor to the idea of the study and participated in writing
the manuscript.
Ethics approval and consent to
participate
The present study was approved by Ethics Committee
of Beijing Luhe Hospital Affiliated to Capital Medical University
(Beijing, China).
Consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
References
|
1
|
Henriquez G and Urrea C: Association
between air pollution and emergency consultations for respiratory
diseases. Rev Med Chil. 145:1371–1377. 2017.(In Spanish).
PubMed/NCBI
|
|
2
|
Rabiei K, Hosseini SM, Sadeghi E,
Jafari-Koshki T, Rahimi M, Shishehforoush M, Lahijanzadeh A,
Sadeghian B, Moazam E, Mohebi MB, et al: Air pollution and
cardiovascular and respiratory disease: Rationale and methodology
of CAPACITY study. ARYA Atheroscler. 13:264–273. 2017.PubMed/NCBI
|
|
3
|
Trnjar K, Pintarić S, Mornar Jelavić M,
Nesek V, Ostojić J, Pleština S, Šikić A and Pintarić H: Correlation
between occurrence and deterioration of respiratory diseases and
air pollution within the legally permissible limits. Acta Clin
Croat. 56:210–217. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Faridi S, Shamsipour M, Krzyzanowski M,
Künzli N, Amini H, Azimi F, Malkawi M, Momeniha F, Gholampour A,
Hassanvand MS and Naddafi K: Long-term trends and health impact of
PM2.5 and O3 in Tehran, Iran, 2006–2015. Environ Int. 114:37–49.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kolpakova AF, Sharipov RN and Kolpakov FA:
Air pollution by particulate matter as the risk factor for the
cardiovascular diseases. Gig Sanit. 96:133–137. 2017.PubMed/NCBI
|
|
6
|
Stachyra K, Kiepura A and Olszanecki R:
Air pollution and atherosclerosis-a brief review of mechanistic
links between atherogenesis and biological actions of inorganic
part of particulate matter. Folia Med Cracov. 57:37–46.
2017.PubMed/NCBI
|
|
7
|
Hüls A, Vierkötter A, Sugiri D, Abramson
MJ, Ranft U, Krämer U and Schikowski T: The role of air pollution
and lung function in cognitive impairment. Eur Respir J.
51:17019632018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim H, Kim J, Kim S, Kang SH, Kim HJ, Kim
H, Heo J, Yi SM, Kim K, Youn TJ and Chae IH: Cardiovascular effects
of long-term exposure to air pollution: A population-based study
with 900 845 person-years of follow-up. J Am Heart Assoc.
6:e0071702017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brunekreef B and Holgate ST: Air pollution
and health. Lancet. 360:1233–1242. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li R, Kou X, Geng H, Xie J, Tian J, Cai Z
and Dong C: Mitochondrial damage: An important mechanism of ambient
PM2.5 exposure-induced acute heart injury in rats. J Hazard Mater.
287:392–401. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang C, Tu Y, Yu Z and Lu R: PM2.5 and
cardiovascular diseases in the elderly: An overview. Int J Environ
Res Public Health. 12:8187–8197. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dabass A, Talbott EO, Venkat A, Rager J,
Marsh GM, Sharma RK and Holguin F: Association of exposure to
particulate matter (PM2.5) air pollution and biomarkers of
cardiovascular disease risk in adult NHANES participants
(2001–2008). Int J Hyg Environ Health. 219:301–310. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang A, Janssen NA, Brunekreef B, Cassee
FR, Hoek G and Gehring U: Children's respiratory health and
oxidative potential of PM2.5: The PIAMA birth cohort study. Occup
Environ Med. 73:154–160. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brosselin P, Rudant J, Orsi L, Leverger G,
Baruchel A, Bertrand Y, Nelken B, Robert A, Michel G, Margueritte
G, et al: Acute childhood leukaemia and residence next to petrol
stations and automotive repair garages: The ESCALE study (SFCE).
Occup Environ Med. 66:598–606. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Steffen C, Auclerc MF, Auvrignon A,
Baruchel A, Kebaili K, Lambilliotte A, Leverger G, Sommelet D,
Vilmer E, Hémon D and Clavel J: Acute childhood leukaemia and
environmental exposure to potential sources of benzene and other
hydrocarbons; a case-control study. Occup Environ Med. 61:773–778.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Raaschou-Nielsen O, Ketzel M, Poulsen
Harbo A and Sørensen M: Traffic-related air pollution and risk for
leukaemia of an adult population. Int J Cancer. 138:1111–1117.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chiarini F, Lonetti A, Evangelisti C,
Buontempo F, Orsini E, Evangelisti C, Cappellini A, Neri LM,
McCubrey JA and Martelli AM: Advances in understanding the acute
lymphoblastic leukemia bone marrow microenvironment: From biology
to therapeutic targeting. Biochim Biophys Acta. 1863:449–463. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kumar B, Garcia M, Murakami JL and Chen
CC: Exosome-mediated microenvironment dysregulation in leukemia.
Biochim Biophys Acta. 1863:464–470. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jin XT, Chen ML, Li RJ, An Q, Song L, Zhao
Y, Xiao H, Cheng L and Li ZY: Progression and inflammation of human
myeloid leukemia induced by ambient PM2.5 exposure. Arch Toxicol.
90:1929–1938. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-delta delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Castro-Jimenez MÁ and Orozco-Vargas LC:
Parental exposure to carcinogens and risk for childhood acute
lymphoblastic leukemia, Colombia, 2000–2005. Prev Chronic Dis.
8:A1062011.PubMed/NCBI
|
|
22
|
McHale CM, Zhang L and Smith MT: Current
understanding of the mechanism of benzene-induced leukemia in
humans: Implications for risk assessment. Carcinogenesis.
33:240–252. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Filippini T, Heck JE, Malagoli C, Del
Giovane C and Vinceti M: A review and meta-analysis of outdoor air
pollution and risk of childhood leukemia. J Environ Sci Health C
Environ Carcinog Ecotoxicol Rev. 33:36–66. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sharma A, Rajappa M, Satyam A and Sharma
M: Cytokines (TH1 and TH2) in patients with advanced cervical
cancer undergoing neoadjuvant chemoradiation: Correlation with
treatment response. Int J Gynecol Cancer. 19:1269–1275. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Becker Y: Molecular immunological
approaches to biotherapy of human cancers-a review, hypothesis and
implications. Anticancer Res. 26:1113–1134. 2006.PubMed/NCBI
|
|
26
|
Min G: Interleukin-2 and its application
in the treatment of patients with acute myelogenous leukemia. J
Leukemia Lymphoma. 17:152–155. 2008.(In Chinese).
|
|
27
|
Shouval DS, Ouahed J, Biswas A, Goettel
JA, Horwitz BH, Klein C, Muise AM and Snapper SB: Interleukin 10
receptor signaling: Master regulator of intestinal mucosal
homeostasis in mice and humans. Adv Immunol. 122:177–210. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qing Yang ZL: Interleukin family cytokines
and stem cell mobilization. Chin J Comp Med. 21:62–65. 2011.(In
Chinese).
|
|
29
|
Lobo-Silva D, Carriche GM, Castro AG,
Roque S and Saraiva M: Balancing the immune response in the brain:
IL-10 and its regulation. J Neuroinflammation. 13:2972016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
de Waal Malefyt R, Haanen J, Spits H,
Roncarolo MG, te Velde A, Figdor C, Johnson K, Kastelein R, Yssel H
and de Vries JE: Interleukin-10 (IL-10) and viral IL-10 strongly
reduce antigen-specific human T cell proliferation by diminishing
the antigen-presenting capacity of monocytes via downregulation of
class II major histocompatibility complex expression. J Exp Med.
174:915–924. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mumm JB, Emmerich J, Zhang X, Chan I, Wu
L, Mauze S, Blaisdell S, Basham B, Dai J, Grein J, et al: IL-10
elicits IFNγ-dependent tumor immune surveillance. Cancer Cell.
20:781–796. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Y, Ma Y, Fang Y, Wu S, Liu L, Fu D
and Shen X: Regulatory T cell: A protection for tumour cells. J
Cell Mol Med. 16:425–436. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tanikawa T, Wilke CM, Kryczek I, Chen GY,
Kao J, Núñez G and Zou W: Interleukin-10 ablation promotes tumor
development, growth, and metastasis. Cancer Res. 72:420–429. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mocellin S, Marincola F, Rossi CR, Nitti D
and Lise M: The multifaceted relationship between IL-10 and
adaptive immunity: Putting together the pieces of a puzzle.
Cytokine Growth Factor Rev. 15:61–76. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mittal SK and Roche PA: Suppression of
antigen presentation by IL-10. Curr Opin Immunol. 34:22–27. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Han L, Yang J, Wang X, Li D, Lv L and Li
B: Th17 cells in autoimmune diseases. Front Med. 9:10–19. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Housseau F, Wu S, Wick EC, Fan H, Wu X,
Llosa NJ, Smith KN, Tam A, Ganguly S, Wanyiri JW, et al: Redundant
innate and adaptive sources of IL17 production drive colon
tumorigenesis. Cancer Res. 76:2115–2124. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Patil RS, Shah SU, Shrikhande SV, Goel M,
Dikshit RP and Chiplunkar SV: IL17 producing γδ T cells induce
angiogenesis and are associated with poor survival in gallbladder
cancer patients. Int J Cancer. 139:869–881. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Benevides L, da Fonseca DM, Donate PB,
Tiezzi DG, De Carvalho DD, de Andrade JM, Martins GA and Silva JS:
IL17 promotes mammary tumor progression by changing the behavior of
tumor cells and eliciting tumorigenic neutrophils recruitment.
Cancer Res. 75:3788–3799. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Numasaki M, Fukushi J, Ono M, Narula SK,
Zavodny PJ, Kudo T, Robbins PD, Tahara H and Lotze MT:
Interleukin-17 promotes angiogenesis and tumor growth. Blood.
101:2620–2627. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee EJ, Park HJ, Lee IJ, Kim WW, Ha SJ,
Suh YG and Seong J: Inhibition of IL-17A suppresses enhanced-tumor
growth in low dose pre-irradiated tumor beds. PLoS One.
9:e1064232014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ju X, Ijaz T, Sun H, Ray S, Lejeune W, Lee
C, Recinos A III, Guo DC, Milewicz DM, Tilton RG and Brasier AR:
Interleukin-6-signal transducer and activator of transcription-3
signaling mediates aortic dissections induced by angiotensin II via
the T-helper lymphocyte 17-interleukin 17 axis in C57BL/6 mice.
Arterioscler Thromb Vasc Biol. 33:1612–1621. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kumar P: Natarajan K and Shanmugam N: High
glucose driven expression of pro-inflammatory cytokine and
chemokine genes in lymphocytes: Molecular mechanisms of IL-17
family gene expression. Cell Signal. 26:528–539. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen XW and Zhou SF: Inflammation,
cytokines, the IL-17/IL-6/STAT3/NF-κB axis, and tumorigenesis. Drug
Des Devel Ther. 9:2941–2946. 2015.PubMed/NCBI
|
|
45
|
Hu Z, Luo D, Wang D, Ma L, Zhao Y and Li
L: IL-17 activates the IL-6/STAT3 signal pathway in the
proliferation of hepatitis B virus-related hepatocellular
carcinoma. Cell Physiol Biochem. 43:2379–2390. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang JP, Yan J, Xu J, Pang XH, Chen MS,
Li L, Wu C, Li SP and Zheng L: Increased intratumoral
IL-17-producing cells correlate with poor survival in
hepatocellular carcinoma patients. J Hepatol. 50:980–989. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Du JW, Xu KY, Fang LY and Qi XL:
Interleukin-17, produced by lymphocytes, promotes tumor growth and
angiogenesis in a mouse model of breast cancer. Mol Med Rep.
6:1099–1102. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mucida D, Park Y, Kim G, Turovskaya O,
Scott I, Kronenberg M and Cheroutre H: Reciprocal TH17 and
regulatory T cell differentiation mediated by retinoic acid.
Science. 317:256–260. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wu C, Wang S, Wang F, Chen Q, Peng S,
Zhang Y, Qian J, Jin J and Xu H: Increased frequencies of T helper
type 17 cells in the peripheral blood of patients with acute
myeloid leukaemia. Clin Exp Immunol. 158:199–204. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Waters JP, Pober JS and Bradley JR: Tumour
necrosis factor and cancer. J Pathol. 230:241–248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Gallipoli P, Pellicano F, Morrison H,
Laidlaw K, Allan EK, Bhatia R, Copland M, Jørgensen HG and Holyoake
TL: Autocrine TNF-α production supports CML stem and progenitor
cell survival and enhances their proliferation. Blood.
122:3335–3339. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sanchez-Correa B, Bergua JM, Campos C,
Gayoso I, Arcos MJ, Bañas H, Morgado S, Casado JG, Solana R and
Tarazona R: Cytokine profiles in acute myeloid leukemia patients at
diagnosis: Survival is inversely correlated with IL-6 and directly
correlated with IL-10 levels. Cytokine. 61:885–891. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Potapnev MP, Petyovka NV, Belevtsev MV,
Savitskiy VP and Migal NV: Plasma level of tumor necrosis
factor-alpha (TNF-alpha) correlates with leukocytosis and
biological features of leukemic cells, but not treatment response
of children with acute lymphoblastic leukemia. Leuk Lymphoma.
44:1077–1079. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Foa R, Massaia M, Cardona S, Tos AG,
Bianchi A, Attisano C, Guarini A, di Celle PF and Fierro MT:
Production of tumor necrosis factor-alpha by B-cell chronic
lymphocytic leukemia cells: A possible regulatory role of TNF in
the progression of the disease. Blood. 76:393–400. 1990.PubMed/NCBI
|
|
55
|
Lech-Maranda E, Grzybowska-Izydorczyk O,
Wyka K, Mlynarski W, Borowiec M, Antosik K, Cebula-Obrzut B,
Makuch-Lasica H, Nowak G, Klimkiewicz-Wojciechowska G, et al: Serum
tumor necrosis factor-alpha and interleukin-10 levels as markers to
predict outcome of patients with chronic lymphocytic leukemia in
different risk groups defined by the IGHV mutation status. Arch
Immunol Ther Exp (Warsz). 60:477–486. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ferrajoli A, Keating MJ, Manshouri T,
Giles FJ, Dey A, Estrov Z, Koller CA, Kurzrock R, Thomas DA, Faderl
S, et al: The clinical significance of tumor necrosis factor-alpha
plasma level in patients having chronic lymphocytic leukemia.
Blood. 100:1215–1219. 2002.PubMed/NCBI
|
|
57
|
Kupsa T, Vasatova M, Karesova I, Zak P and
Horacek JM: Baseline serum levels of multiple cytokines and
adhesion molecules in patients with acute myeloid leukemia: Results
of a pivotal trial. Exp Oncol. 36:252–257. 2014.PubMed/NCBI
|
|
58
|
Fung FY, Li M, Breunis H, Timilshina N,
Minden MD and Alibhai SM: Correlation between cytokine levels and
changes in fatigue and quality of life in patients with acute
myeloid leukemia. Leuk Res. 37:274–279. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hess P, Pihan G, Sawyers CL, Flavell RA
and Davis RJ: Survival signaling mediated by c-Jun NH(2)-terminal
kinase in transformed B lymphoblasts. Nat Genet. 32:201–205. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tsai HJ, Kobayashi S, Izawa K, Ishida T,
Watanabe T, Umezawa K, Lin SF and Tojo A: Bioimaging analysis of
nuclear factor-κB activity in Philadelphia chromosome-positive
acute lymphoblastic leukemia cells reveals its synergistic
upregulation by tumor necrosis factor-α-stimulated changes to the
microenvironment. Cancer Sci. 102:2014–2021. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Volk A, Li J, Xin J, You D, Zhang J, Liu
X, Xiao Y, Breslin P, Li Z, Wei W, et al: Co-inhibition of NF-κB
and JNK is synergistic in TNF-expressing human AML. J Exp Med.
211:1093–1108. 2014. View Article : Google Scholar : PubMed/NCBI
|