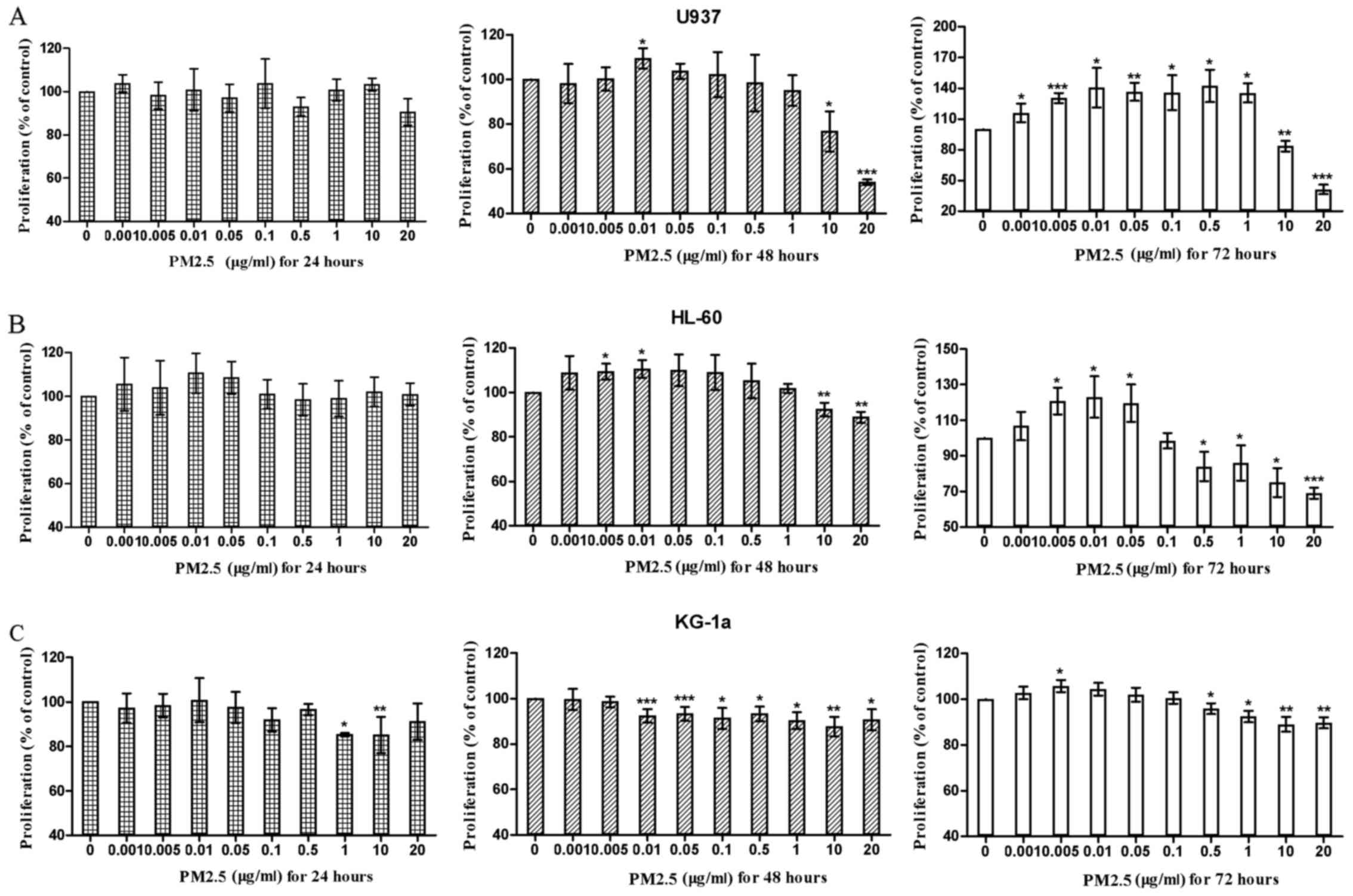
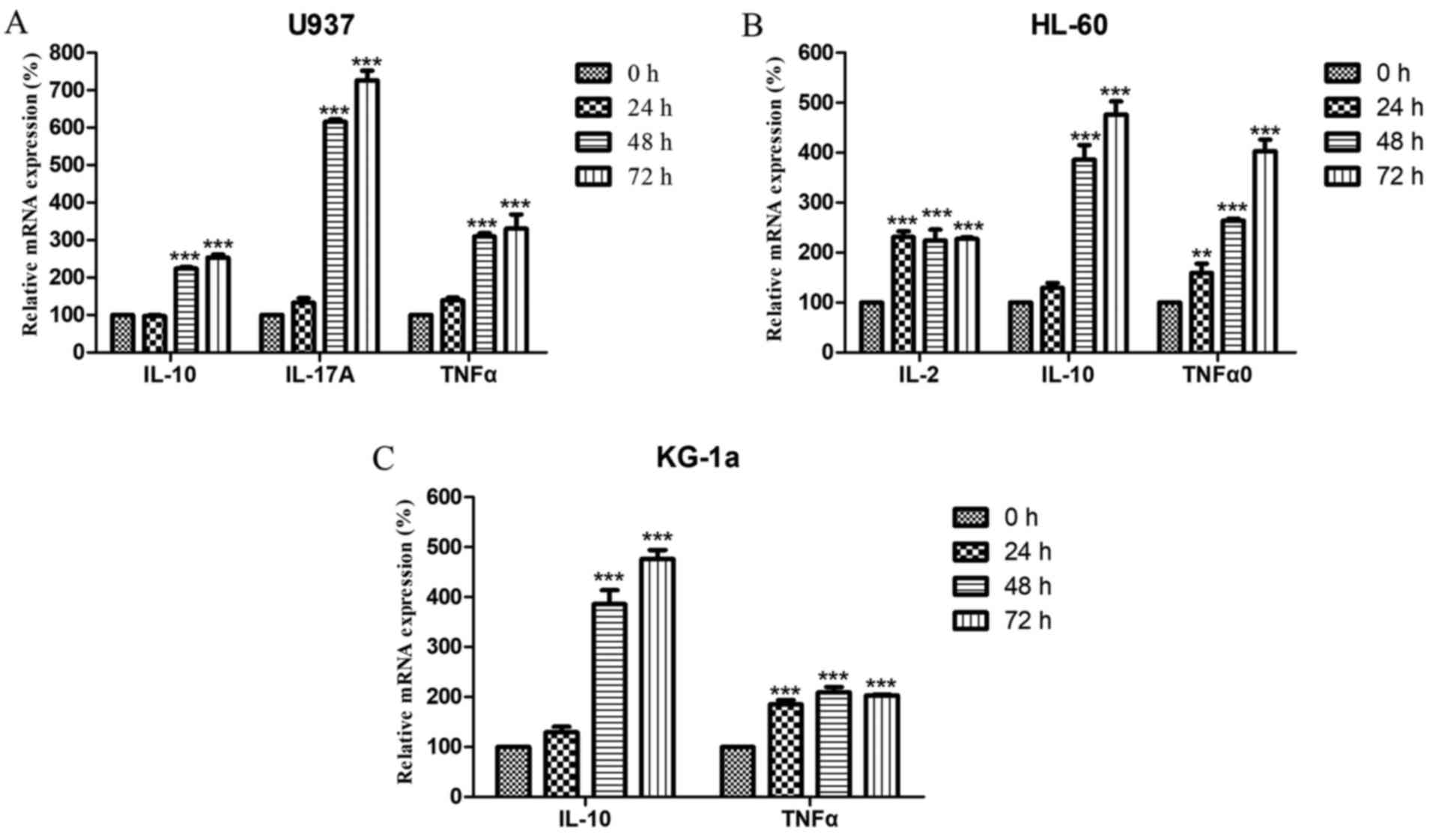
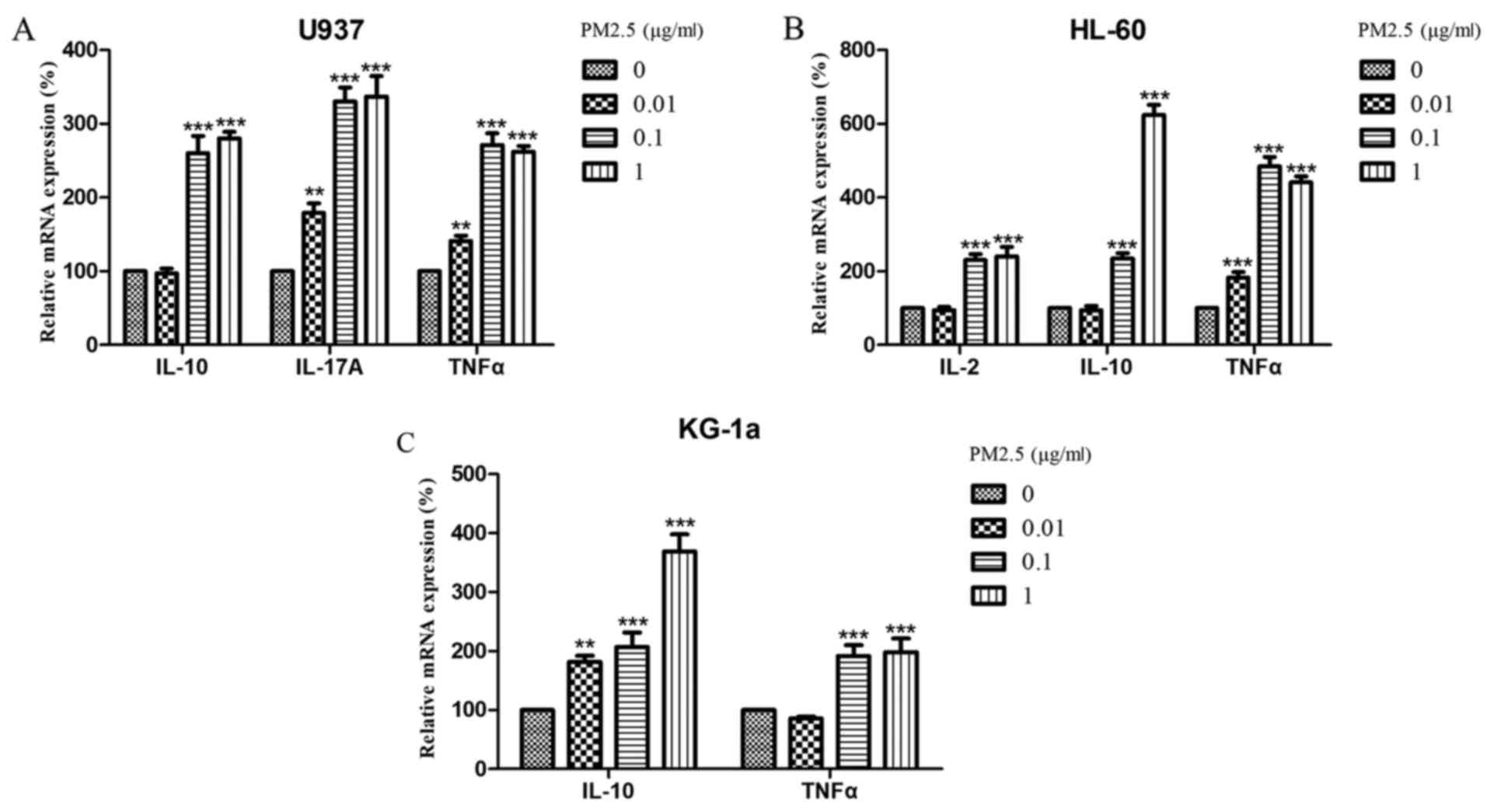
|
1
|
Henriquez G and Urrea C: Association
between air pollution and emergency consultations for respiratory
diseases. Rev Med Chil. 145:1371–1377. 2017.(In Spanish).
PubMed/NCBI
|
|
2
|
Rabiei K, Hosseini SM, Sadeghi E,
Jafari-Koshki T, Rahimi M, Shishehforoush M, Lahijanzadeh A,
Sadeghian B, Moazam E, Mohebi MB, et al: Air pollution and
cardiovascular and respiratory disease: Rationale and methodology
of CAPACITY study. ARYA Atheroscler. 13:264–273. 2017.PubMed/NCBI
|
|
3
|
Trnjar K, Pintarić S, Mornar Jelavić M,
Nesek V, Ostojić J, Pleština S, Šikić A and Pintarić H: Correlation
between occurrence and deterioration of respiratory diseases and
air pollution within the legally permissible limits. Acta Clin
Croat. 56:210–217. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Faridi S, Shamsipour M, Krzyzanowski M,
Künzli N, Amini H, Azimi F, Malkawi M, Momeniha F, Gholampour A,
Hassanvand MS and Naddafi K: Long-term trends and health impact of
PM2.5 and O3 in Tehran, Iran, 2006–2015. Environ Int. 114:37–49.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kolpakova AF, Sharipov RN and Kolpakov FA:
Air pollution by particulate matter as the risk factor for the
cardiovascular diseases. Gig Sanit. 96:133–137. 2017.PubMed/NCBI
|
|
6
|
Stachyra K, Kiepura A and Olszanecki R:
Air pollution and atherosclerosis-a brief review of mechanistic
links between atherogenesis and biological actions of inorganic
part of particulate matter. Folia Med Cracov. 57:37–46.
2017.PubMed/NCBI
|
|
7
|
Hüls A, Vierkötter A, Sugiri D, Abramson
MJ, Ranft U, Krämer U and Schikowski T: The role of air pollution
and lung function in cognitive impairment. Eur Respir J.
51:17019632018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim H, Kim J, Kim S, Kang SH, Kim HJ, Kim
H, Heo J, Yi SM, Kim K, Youn TJ and Chae IH: Cardiovascular effects
of long-term exposure to air pollution: A population-based study
with 900 845 person-years of follow-up. J Am Heart Assoc.
6:e0071702017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brunekreef B and Holgate ST: Air pollution
and health. Lancet. 360:1233–1242. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li R, Kou X, Geng H, Xie J, Tian J, Cai Z
and Dong C: Mitochondrial damage: An important mechanism of ambient
PM2.5 exposure-induced acute heart injury in rats. J Hazard Mater.
287:392–401. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang C, Tu Y, Yu Z and Lu R: PM2.5 and
cardiovascular diseases in the elderly: An overview. Int J Environ
Res Public Health. 12:8187–8197. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dabass A, Talbott EO, Venkat A, Rager J,
Marsh GM, Sharma RK and Holguin F: Association of exposure to
particulate matter (PM2.5) air pollution and biomarkers of
cardiovascular disease risk in adult NHANES participants
(2001–2008). Int J Hyg Environ Health. 219:301–310. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang A, Janssen NA, Brunekreef B, Cassee
FR, Hoek G and Gehring U: Children's respiratory health and
oxidative potential of PM2.5: The PIAMA birth cohort study. Occup
Environ Med. 73:154–160. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brosselin P, Rudant J, Orsi L, Leverger G,
Baruchel A, Bertrand Y, Nelken B, Robert A, Michel G, Margueritte
G, et al: Acute childhood leukaemia and residence next to petrol
stations and automotive repair garages: The ESCALE study (SFCE).
Occup Environ Med. 66:598–606. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Steffen C, Auclerc MF, Auvrignon A,
Baruchel A, Kebaili K, Lambilliotte A, Leverger G, Sommelet D,
Vilmer E, Hémon D and Clavel J: Acute childhood leukaemia and
environmental exposure to potential sources of benzene and other
hydrocarbons; a case-control study. Occup Environ Med. 61:773–778.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Raaschou-Nielsen O, Ketzel M, Poulsen
Harbo A and Sørensen M: Traffic-related air pollution and risk for
leukaemia of an adult population. Int J Cancer. 138:1111–1117.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chiarini F, Lonetti A, Evangelisti C,
Buontempo F, Orsini E, Evangelisti C, Cappellini A, Neri LM,
McCubrey JA and Martelli AM: Advances in understanding the acute
lymphoblastic leukemia bone marrow microenvironment: From biology
to therapeutic targeting. Biochim Biophys Acta. 1863:449–463. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kumar B, Garcia M, Murakami JL and Chen
CC: Exosome-mediated microenvironment dysregulation in leukemia.
Biochim Biophys Acta. 1863:464–470. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jin XT, Chen ML, Li RJ, An Q, Song L, Zhao
Y, Xiao H, Cheng L and Li ZY: Progression and inflammation of human
myeloid leukemia induced by ambient PM2.5 exposure. Arch Toxicol.
90:1929–1938. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-delta delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Castro-Jimenez MÁ and Orozco-Vargas LC:
Parental exposure to carcinogens and risk for childhood acute
lymphoblastic leukemia, Colombia, 2000–2005. Prev Chronic Dis.
8:A1062011.PubMed/NCBI
|
|
22
|
McHale CM, Zhang L and Smith MT: Current
understanding of the mechanism of benzene-induced leukemia in
humans: Implications for risk assessment. Carcinogenesis.
33:240–252. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Filippini T, Heck JE, Malagoli C, Del
Giovane C and Vinceti M: A review and meta-analysis of outdoor air
pollution and risk of childhood leukemia. J Environ Sci Health C
Environ Carcinog Ecotoxicol Rev. 33:36–66. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sharma A, Rajappa M, Satyam A and Sharma
M: Cytokines (TH1 and TH2) in patients with advanced cervical
cancer undergoing neoadjuvant chemoradiation: Correlation with
treatment response. Int J Gynecol Cancer. 19:1269–1275. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Becker Y: Molecular immunological
approaches to biotherapy of human cancers-a review, hypothesis and
implications. Anticancer Res. 26:1113–1134. 2006.PubMed/NCBI
|
|
26
|
Min G: Interleukin-2 and its application
in the treatment of patients with acute myelogenous leukemia. J
Leukemia Lymphoma. 17:152–155. 2008.(In Chinese).
|
|
27
|
Shouval DS, Ouahed J, Biswas A, Goettel
JA, Horwitz BH, Klein C, Muise AM and Snapper SB: Interleukin 10
receptor signaling: Master regulator of intestinal mucosal
homeostasis in mice and humans. Adv Immunol. 122:177–210. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qing Yang ZL: Interleukin family cytokines
and stem cell mobilization. Chin J Comp Med. 21:62–65. 2011.(In
Chinese).
|
|
29
|
Lobo-Silva D, Carriche GM, Castro AG,
Roque S and Saraiva M: Balancing the immune response in the brain:
IL-10 and its regulation. J Neuroinflammation. 13:2972016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
de Waal Malefyt R, Haanen J, Spits H,
Roncarolo MG, te Velde A, Figdor C, Johnson K, Kastelein R, Yssel H
and de Vries JE: Interleukin-10 (IL-10) and viral IL-10 strongly
reduce antigen-specific human T cell proliferation by diminishing
the antigen-presenting capacity of monocytes via downregulation of
class II major histocompatibility complex expression. J Exp Med.
174:915–924. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mumm JB, Emmerich J, Zhang X, Chan I, Wu
L, Mauze S, Blaisdell S, Basham B, Dai J, Grein J, et al: IL-10
elicits IFNγ-dependent tumor immune surveillance. Cancer Cell.
20:781–796. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Y, Ma Y, Fang Y, Wu S, Liu L, Fu D
and Shen X: Regulatory T cell: A protection for tumour cells. J
Cell Mol Med. 16:425–436. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tanikawa T, Wilke CM, Kryczek I, Chen GY,
Kao J, Núñez G and Zou W: Interleukin-10 ablation promotes tumor
development, growth, and metastasis. Cancer Res. 72:420–429. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mocellin S, Marincola F, Rossi CR, Nitti D
and Lise M: The multifaceted relationship between IL-10 and
adaptive immunity: Putting together the pieces of a puzzle.
Cytokine Growth Factor Rev. 15:61–76. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mittal SK and Roche PA: Suppression of
antigen presentation by IL-10. Curr Opin Immunol. 34:22–27. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Han L, Yang J, Wang X, Li D, Lv L and Li
B: Th17 cells in autoimmune diseases. Front Med. 9:10–19. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Housseau F, Wu S, Wick EC, Fan H, Wu X,
Llosa NJ, Smith KN, Tam A, Ganguly S, Wanyiri JW, et al: Redundant
innate and adaptive sources of IL17 production drive colon
tumorigenesis. Cancer Res. 76:2115–2124. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Patil RS, Shah SU, Shrikhande SV, Goel M,
Dikshit RP and Chiplunkar SV: IL17 producing γδ T cells induce
angiogenesis and are associated with poor survival in gallbladder
cancer patients. Int J Cancer. 139:869–881. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Benevides L, da Fonseca DM, Donate PB,
Tiezzi DG, De Carvalho DD, de Andrade JM, Martins GA and Silva JS:
IL17 promotes mammary tumor progression by changing the behavior of
tumor cells and eliciting tumorigenic neutrophils recruitment.
Cancer Res. 75:3788–3799. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Numasaki M, Fukushi J, Ono M, Narula SK,
Zavodny PJ, Kudo T, Robbins PD, Tahara H and Lotze MT:
Interleukin-17 promotes angiogenesis and tumor growth. Blood.
101:2620–2627. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee EJ, Park HJ, Lee IJ, Kim WW, Ha SJ,
Suh YG and Seong J: Inhibition of IL-17A suppresses enhanced-tumor
growth in low dose pre-irradiated tumor beds. PLoS One.
9:e1064232014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ju X, Ijaz T, Sun H, Ray S, Lejeune W, Lee
C, Recinos A III, Guo DC, Milewicz DM, Tilton RG and Brasier AR:
Interleukin-6-signal transducer and activator of transcription-3
signaling mediates aortic dissections induced by angiotensin II via
the T-helper lymphocyte 17-interleukin 17 axis in C57BL/6 mice.
Arterioscler Thromb Vasc Biol. 33:1612–1621. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kumar P: Natarajan K and Shanmugam N: High
glucose driven expression of pro-inflammatory cytokine and
chemokine genes in lymphocytes: Molecular mechanisms of IL-17
family gene expression. Cell Signal. 26:528–539. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen XW and Zhou SF: Inflammation,
cytokines, the IL-17/IL-6/STAT3/NF-κB axis, and tumorigenesis. Drug
Des Devel Ther. 9:2941–2946. 2015.PubMed/NCBI
|
|
45
|
Hu Z, Luo D, Wang D, Ma L, Zhao Y and Li
L: IL-17 activates the IL-6/STAT3 signal pathway in the
proliferation of hepatitis B virus-related hepatocellular
carcinoma. Cell Physiol Biochem. 43:2379–2390. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang JP, Yan J, Xu J, Pang XH, Chen MS,
Li L, Wu C, Li SP and Zheng L: Increased intratumoral
IL-17-producing cells correlate with poor survival in
hepatocellular carcinoma patients. J Hepatol. 50:980–989. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Du JW, Xu KY, Fang LY and Qi XL:
Interleukin-17, produced by lymphocytes, promotes tumor growth and
angiogenesis in a mouse model of breast cancer. Mol Med Rep.
6:1099–1102. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mucida D, Park Y, Kim G, Turovskaya O,
Scott I, Kronenberg M and Cheroutre H: Reciprocal TH17 and
regulatory T cell differentiation mediated by retinoic acid.
Science. 317:256–260. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wu C, Wang S, Wang F, Chen Q, Peng S,
Zhang Y, Qian J, Jin J and Xu H: Increased frequencies of T helper
type 17 cells in the peripheral blood of patients with acute
myeloid leukaemia. Clin Exp Immunol. 158:199–204. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Waters JP, Pober JS and Bradley JR: Tumour
necrosis factor and cancer. J Pathol. 230:241–248. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Gallipoli P, Pellicano F, Morrison H,
Laidlaw K, Allan EK, Bhatia R, Copland M, Jørgensen HG and Holyoake
TL: Autocrine TNF-α production supports CML stem and progenitor
cell survival and enhances their proliferation. Blood.
122:3335–3339. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sanchez-Correa B, Bergua JM, Campos C,
Gayoso I, Arcos MJ, Bañas H, Morgado S, Casado JG, Solana R and
Tarazona R: Cytokine profiles in acute myeloid leukemia patients at
diagnosis: Survival is inversely correlated with IL-6 and directly
correlated with IL-10 levels. Cytokine. 61:885–891. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Potapnev MP, Petyovka NV, Belevtsev MV,
Savitskiy VP and Migal NV: Plasma level of tumor necrosis
factor-alpha (TNF-alpha) correlates with leukocytosis and
biological features of leukemic cells, but not treatment response
of children with acute lymphoblastic leukemia. Leuk Lymphoma.
44:1077–1079. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Foa R, Massaia M, Cardona S, Tos AG,
Bianchi A, Attisano C, Guarini A, di Celle PF and Fierro MT:
Production of tumor necrosis factor-alpha by B-cell chronic
lymphocytic leukemia cells: A possible regulatory role of TNF in
the progression of the disease. Blood. 76:393–400. 1990.PubMed/NCBI
|
|
55
|
Lech-Maranda E, Grzybowska-Izydorczyk O,
Wyka K, Mlynarski W, Borowiec M, Antosik K, Cebula-Obrzut B,
Makuch-Lasica H, Nowak G, Klimkiewicz-Wojciechowska G, et al: Serum
tumor necrosis factor-alpha and interleukin-10 levels as markers to
predict outcome of patients with chronic lymphocytic leukemia in
different risk groups defined by the IGHV mutation status. Arch
Immunol Ther Exp (Warsz). 60:477–486. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ferrajoli A, Keating MJ, Manshouri T,
Giles FJ, Dey A, Estrov Z, Koller CA, Kurzrock R, Thomas DA, Faderl
S, et al: The clinical significance of tumor necrosis factor-alpha
plasma level in patients having chronic lymphocytic leukemia.
Blood. 100:1215–1219. 2002.PubMed/NCBI
|
|
57
|
Kupsa T, Vasatova M, Karesova I, Zak P and
Horacek JM: Baseline serum levels of multiple cytokines and
adhesion molecules in patients with acute myeloid leukemia: Results
of a pivotal trial. Exp Oncol. 36:252–257. 2014.PubMed/NCBI
|
|
58
|
Fung FY, Li M, Breunis H, Timilshina N,
Minden MD and Alibhai SM: Correlation between cytokine levels and
changes in fatigue and quality of life in patients with acute
myeloid leukemia. Leuk Res. 37:274–279. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hess P, Pihan G, Sawyers CL, Flavell RA
and Davis RJ: Survival signaling mediated by c-Jun NH(2)-terminal
kinase in transformed B lymphoblasts. Nat Genet. 32:201–205. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tsai HJ, Kobayashi S, Izawa K, Ishida T,
Watanabe T, Umezawa K, Lin SF and Tojo A: Bioimaging analysis of
nuclear factor-κB activity in Philadelphia chromosome-positive
acute lymphoblastic leukemia cells reveals its synergistic
upregulation by tumor necrosis factor-α-stimulated changes to the
microenvironment. Cancer Sci. 102:2014–2021. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Volk A, Li J, Xin J, You D, Zhang J, Liu
X, Xiao Y, Breslin P, Li Z, Wei W, et al: Co-inhibition of NF-κB
and JNK is synergistic in TNF-expressing human AML. J Exp Med.
211:1093–1108. 2014. View Article : Google Scholar : PubMed/NCBI
|