Introduction
A variety of elastography techniques have been
developed to visualize and quantify the mechanical properties of
biological tissues, including thyroid, breast and liver tissues
(1,2).
In previous years, virtual touch tissue imaging and quantification
(VTIQ), a novel two-dimensional shear wave speed (SWS) measurement
technique, has been introduced into clinical practice, which not
only provides qualitative but also quantitative measurements of the
tissue stiffness (3–5). VTIQ is based on the acoustic radiation
force imaging method, which transmits ultrasonic pulses in the
targeted tissue, resulting in a tissue displacement of 1–20 µm,
thereby attenuating the tissues to establish elasticity (6). Elasticity is a biological and mechanical
property, which represents the stiffness of the tumor tissue
(7). This characteristic may be
associated with the physiological or pathological processes of the
tissue structure (8). Therefore, the
evaluation of elasticity in tumor-bearing mice may be a tool to
monitor the development of hepatocellular carcinoma (HCC) and
putatively detect the physiological and pathological variation in
the tissues during liver carcinogenesis.
HCC, a major type of primary liver cancer, is one of
the most prevalent human malignancies globally (9). In a number of cases, the disease may
remain undiagnosed until patients reach terminal-stage disease, and
are therefore not eligible for curative surgical resection
(10). In addition, HCC has a high
recurrence rate, frequent metastasis events and several
postoperative complications post-surgery (11). Therefore, identification of novel
biomarkers for its early diagnosis and specific targets for therapy
is essential. At present, liver carcinogenesis is considered to be
stimulated by the accumulation of various genetic and epigenetic
alterations (12). Compared with
conventional treatment methods, including chemotherapy and surgery,
gene therapy may kill tumor cells accurately and efficiently with
fewer side effects (13,14).
RNA interference (RNAi) is an effective tool for
inhibiting gene expression. It may silence the expression of
targeted genes by binding to specific mRNA and triggering their
degradation (15). This selective
knockdown of specific gene sequences supports RNAi as a promising
therapeutic strategy. Neuroepithelial cell-transforming gene 1
(NET-1) protein is characterized by the presence of 4 hydrophobic
domains and belongs to the tetraspanin superfamily (TM4SF)
(16). It has been demonstrated that
the NET-1 gene is involved in the division, proliferation
and carcinogenesis of HCC cells (17–19). The
level of NET-1 expression exhibits a significant association with
HCC pathological grading and clinical stages (20,21).
Therefore, the present study selected the NET-1 gene
sequence as a rational target for HCC therapy (22,23).
Glypican-3 (GPC3) is one of the glypican families of heparin
sulfate proteoglycans, and is highly expressed on the surface of
HCC cells but not in normal tissue (24,25).
Therefore, GPC3 may be a promising biomarker for initial diagnosis,
immunological therapy and assessing the risk of HCC recurrence
(26–28). Furthermore, compared with
Lipofectamine® 2000 (Lipo2000), ultrasound-targeted
microbubble destruction (UTMD) may enhance gene permeability and
retention effect (29). It is
reported that the nanobubbles with 30–200 nm in hydrodynamic
diameter accumulate with high efficiency in many solid tumors by
EPR effect (30). At present, several
studies have utilized a nanobubbles-mediated targeted RNAi delivery
system as a novel therapeutic method for the treatment of HCC
(31–33). The UTMD delivery method has a number
of advantages, including low cytotoxicity, low immunogenicity and
dual role of ultrasound imaging and ultrasound-mediated therapy
(34).
In the present study, we hypothesized that the UTMD
delivery system may increase the efficacy of gene transfection, and
VTIQ was used to measure the changes in elasticity in the tumor
tissues that received NET-1 small interfering (si)RNA
treatment.
Materials and methods
Xenograft model
All animal experiments were approved by the
Institutional Animal Care and Use Committee of Harbin Medical
University Cancer Hospital (Harbin, China). Surgical procedures
were performed under isoflurane anesthesia inhalation (1–2% in 100%
oxygen; RWD Life Science Co., Ltd. Shenzhen, China).
A total of 30 female BALB/c nude mice with a mean
weight of 19.3 g, 5–6-weeks-old, purchased from Beijing Vital River
Laboratory Animal Technology Co., Ltd. (Beijing, China), were
housed in individually ventilated cages with sawdust on a 12-h
day/night cycle under specific pathogen-free conditions and room
temperature for 1 week prior to experiments. During the experiment,
the nude mice were allowed ad libitum access to food and
water. SMMC-7721 cells (2×106), were gifted from the
Institute of Cancer Research affiliated to Harbin Medical
University (Harbin, China), were suspended in 100 µl PBS and
injected subcutaneously into the lower back of the mice. The
maximum diameter of the tumor was representative of tumor growth
and was measured twice/week using Vernier calipers. The
experimental procedures were conducted in the xenograft models when
the tumor diameters reached >5 mm. However, the tumor only
reached the pre-determined experimental size in 24 mice. When the
maximum diameter of the tumor reached ~20 mm, or at 60 days
following the first injection, the animals were sacrificed
following the institutional ethical guidelines.
Preparation and characterization of
NET-1 small interfering RNA (siRNA)-conjugated targeted
nanobubbles
The NET-1 siRNA was conjugated with biotin; the
compound was synthesized by Shanghai GenePharma Co., Ltd.
(Shanghai, China). The BLAST analysis (http://www.ncbi.nlm.nih.gov/blast/) ensured that the
siRNA specifically binds the targeted gene (accessed 6 September
2017). According to the manufacturer's recommendations, the NET-1
siRNA was covalently labeled with Cy3 using the Label IT kit (Mirus
Bio, LLC Madison, WI, USA). The sequences of NET-1 siRNA and
negative control are described in Table
I.
 | Table I.Sequences of NET-1 and negative
control siRNA. |
Table I.
Sequences of NET-1 and negative
control siRNA.
| Gene | Sequences |
|---|
| NET-1 siRNA | Sense:
5′-GGGCAUCCUUUCUGAAGAUTT-3′ |
|
| Antisense:
5′-AUCUUCAGAAAGGAUGCCCTT-3′ |
| Negative
control | Sense:
5′-UUCUCCGAACGUGUCACGUTT-3′ |
|
| Antisense:
5′-ACGUGACACGUUGGGAGAATT-3′ |
Based on our previous study (35),
1,2-Distearoyl-sn-glycero-3-phosphocholine,
1,2-Distearoyl-sn-glycero-3-phospho ethanolamine (DSPE) and
DSPE-PEG2000-biotin were utilized at a 9:0.5:0.5 molar ratio to
formulate the lipid nanobubbles. Firstly, the mixture was
solubilized in 99.7% chloroform, which was subsequently evaporated
for 5 min by vacuum rotary evaporation at 1,000 × g at 37°C. This
resulted in the formation of a lipid film that was resolubilized in
5 ml PBS for 3 min at 40°C. Next, the air was exhausted from the
sealed vials using a syringe, and 99.90% perfluoropropane (Research
Institute of Physical and Chemical Engineering of Nuclear Industry,
Tianjin, China) was added. Then, the mixture was mechanically
vibrated for 45 sec in a dental amalgamator with 50 Hz at 37°C
(Hubei YJT Technology Co., Ltd., Shanghai, China), and the
resulting solution was sonicated with a 20 kHz probe (Sonics &
Materials, Inc., Newtown, CT, USA). Finally, the nanoparticles were
washed with sterile PBS and centrifuged at 800 × g for 5 min at
37°C. The biotinylated GPC3 (ab218874; 1:1,000; Abcam, Cambridge,
MA, USA) antibody, NET-1 siRNA and nanobubbles were gently agitated
in PBS, and the mixture was incubated at 4°C overnight to form the
NET-1 siRNA-TNBs (targeted nanobubbles) complexes.
The particle size was measured using a Zetasizer
Nano ZS90 analyzer (Malvern Instruments Ltd., Malvern, UK). The
NET-1 siRNA conjugated with TNBs was detected by confocal laser
scanning microscopy (×200, magnification) (Olympus Corporation,
Tokyo, Japan).
Gene transfection
As summarized in Table
II, 24 xenograft mice were randomized into four groups. The
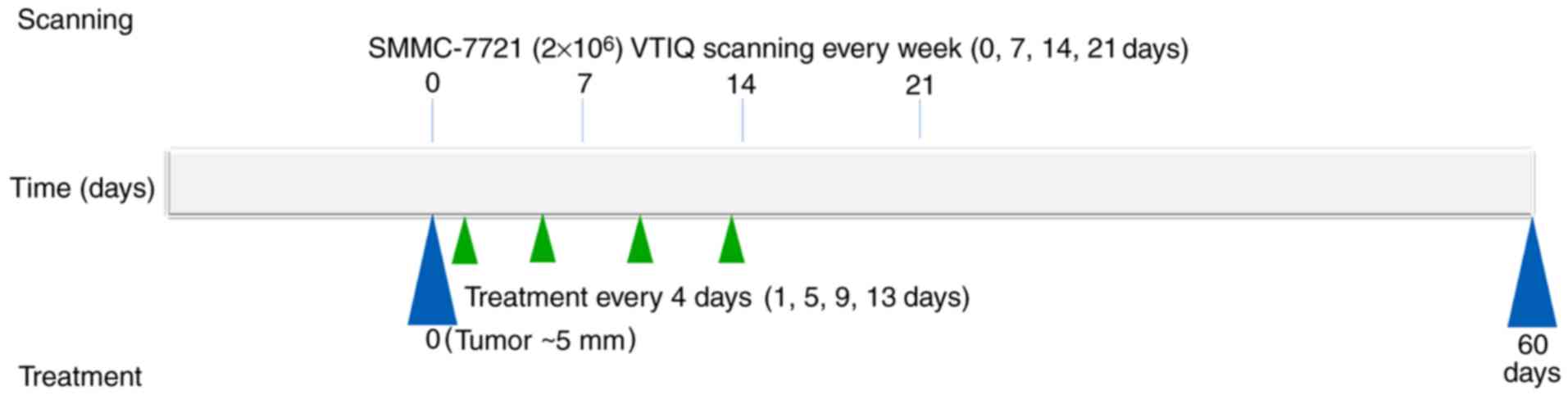
timeline of the experiment is demonstrated in Fig. 1. For every group (A-D), the
experimental injection was administered 4 times (days 1, 5, 9 and
13). For group D, the NET-1 siRNA-conjugated TNBs (100 mg/kg) were
injected into BALB/c nude mice via the tail vein. For group C,
Lipo2000 (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) was used as a control for the delivery of NET-1 siRNA into
nude mice. The Lipo2000 (100 mg/kg) was also injected via the tail
vein, according to the manufacturer's protocol. Nude mice in all
groups underwent ultrasound examination, which was performed for 45
sec with a Low-Frequency Ultrasound Transfection Instrument
(Institute of Ultrasound Imaging, Second Affiliated Hospital of
Chongqing Medical University, Chongqing, China) at the following
settings: Frequency of 1 MHz, pulse repetition frequency of 1 kHz,
50% duty cycle, and 1 W/cm2 intensity.
 | Table II.Experimental groups and corresponding
treatments. |
Table II.
Experimental groups and corresponding
treatments.
| Group | Experimental
treatment | Abbreviations |
|---|
| A | Normal saline | Blank control |
| B | Targeted
nanobubbles + negative control RNA | TNBs + NC |
| C |
Lipofectamine® 2000 + NET-1
siRNA | Lipo2000 +
siRNA |
| D | NET-1
siRNA-conjugated targeted nanobubbles | TNBs-siRNA |
VTIQ measurements
Ultrasound scanning was performed 4 times (days 0,
7, 14 and 21). VTIQ was performed by a radiologist (Harbin Medical
University Cancer Hospital, Harbin, China) with >2 years of
experience performing ultrasound scanning elastography with Siemens
Acuson S3000 Device (Siemens Medical Solutions, Mountain View, CA,
USA), which was equipped with a 9L4 linear array transducer
(frequency range, 4–9 MHz). Nude mice were anesthetized and
positioned on the bed. Subsequently, the transducer was placed
perpendicular to the target tumor. The operator held the transducer
as lightly as possible, in order to minimize the pre-compression
distortion.
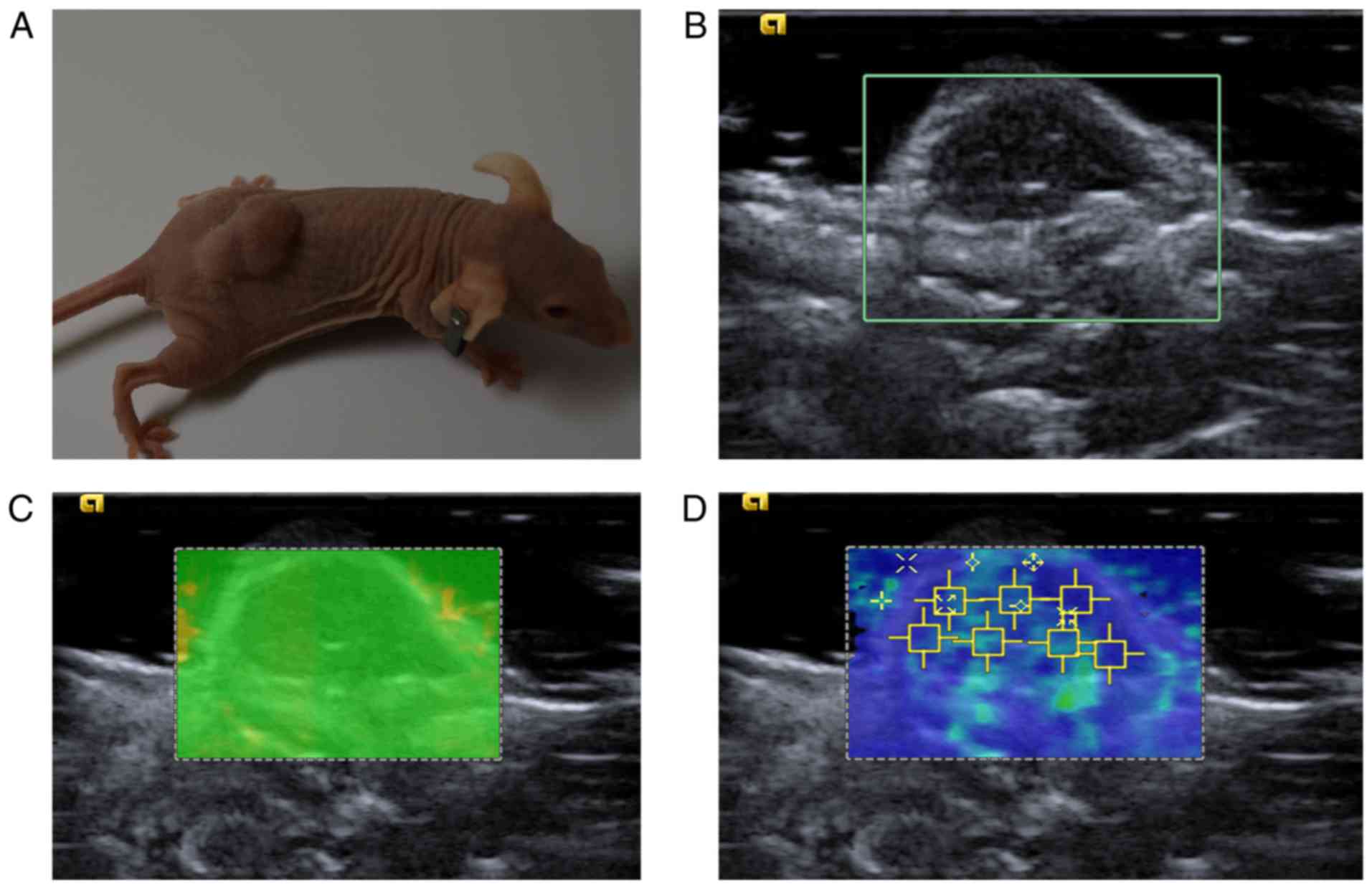
As demonstrated in Fig.
2, the measurement of SWS on each tumor was repeated 7 times.
The 7 SW regions of interest (SW-ROI) were placed arbitrarily on
the lesions with homogeneous SW distribution. In the case of
lesions with heterogeneous SW distribution, 2 SW-ROIs were placed
on the highest and lowest stiffness areas, respectively,
corresponding to the SW distribution on the SW-velocity map, and
the other 5 SW-ROIs were placed in the remaining areas at random.
Although large tumors are easy to manipulate, visualize and
analyze, they often form a necrotic core (36). Cystic, calcified and necrotic areas
corresponding with low or marginal quality on SW-quality map were
avoided while placing the SW-ROI on the SW-velocity map, as these
factors may affect the measurements (37). Using SW-ROI, the SWS may be
quantitatively measured in m/s. The scale of SWS ranged from 0.5–10
m/s and was not adjusted during the entire process. As a result, 7
values were obtained to compute the value of SWSmax and
SWSmean for the subsequent comparisons between
groups.
Histological analysis and toxicity
test
When nude mice were sacrificed, all tumors were
rapidly harvested, fixed in 10% formalin at room temperature for 24
h and embedded in paraffin using a Leica EG 1160 embedder (Leica
Microsystems GmbH, Wetzlar, Germany). Tumor tissues were cut into
4-µm thick slices using a Leica CM1850 cryostat (Leica Microsystems
GmbH). Tissue sections were dewaxed in xylene and re-hydrated
through graded alcohol concentrations using standard procedures
(anhydrous ethanol; 95, 90, 85 and 80%; 10 min for each step) at
37°C. After washing in PBS (thrice for 5 min), peroxidase was
blocked by incubation in 3% H2O2 (Beyotime
Institute of Biotechnology, Haimen, China) for 10 min at room
temperature. The slides were washed twice in PBS and incubated for
24 h with rabbit anti-human NET-1 polyclonal antibody (ab113202;
1:1,000; Abcam, Shanghai, China) diluted in 0.01M PBS containing
0.3% Triton X-100 and 0.5% bovine serum albumin (BSA; Boster
Biological Technology, Pleasanton, CA, USA). Following incubation,
sections were washed with PBS (thrice for 5 min) and subsequently
incubated with goat anti-rabbit monoclonal secondary antibody
(ab79006; 1:100; Abcam) at 37°C for 30 min. Finally, tissue slides
were stained with 3,3′-diaminobenzidine (Shanghai Mingrui
Biological Technology Co., Ltd., Shanghai, China) at room
temperature for 5 min and counterstained with hematoxylin (Beyotime
Institute of Biotechnology) at room temperature for 10 min. All
incubations were performed at room temperature (8).
For the toxicity assessment of NET-1
siRNA-conjugated TNBs, standard Harris hematoxylin (5 mg/ml) and
eosin (10 mg/ml) (H&E) staining was performed on the liver and
kidney samples (38). The liver and
kidney tissues were immersed in 10% formalin for 24 h. Then the
slides were placed in processing cassettes, dehydrated through a
serial alcohol gradient (50, 70, 80, 85, 90 and 95% anhydrous
ethanol twice; 10 min for each step) at 37°C, and embedded in
paraffin wax blocks. 5-µm thick live and kidney tissue sections
were dewaxed in xylene, rehydrated through decreasing
concentrations of ethanol (anhydrous ethanol; 95, 90, 85 and 80%;
10 min for each step) at 37°C, and washed in dilled water. The
slides were then stained in hematoxylin solution for 1 min at 37°C.
Excessive chromatin, which was bound to the tissues, was removed
from tissues with 1% hydrochloric alcohol solution for 5 sec at
room temperature. The slides were washed in dilled water for 1 min
and stained with eosin for 30 sec at 37°C. Finally, the slides were
dehydrated in alcohol (80, 85, 90 and 95% anhydrous ethanol; 3 min
for each step) at 37°C and mounted with Eukitt quick-hardening
mounting medium (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany). An
experienced pathologist belonged to the department of pathology,
Harbin Medical University Cancer Hospital, evaluated hepatic and
renal toxicity using a light microscope. The images of the tissue
slides were obtained by montage imaging via the automated tiling
function of the light microscope (magnification, ×100). The
quantified analysis of NET-1 protein in the tumor tissue was done
using ImageJ software (1.46; National Institutes of Health,
Bethesda, MD, USA) (39).
Statistical analysis
All statistical analysis were performed using
GraphPad Prism 5.01 software (GraphPad Software Inc., La Jolla, CA,
USA). An unpaired Student's t-test was performed. A two-way
analysis of variance (ANOVA) analyzed the effect of treatment type
and duration on tumors, and Bonferroni post hoc tests were used to
compare the control and the treated groups at each time point. The
log-rank test and Kaplan-Meier analysis were performed to assess
the survival rates. Pearson's correlation coefficient was used to
compare the maximum diameter of the tumor and tumor elasticity. The
measurement data are expressed as mean ± standard deviation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Characterization of the TNBs-blank and
siRNA-conjugated TNBs
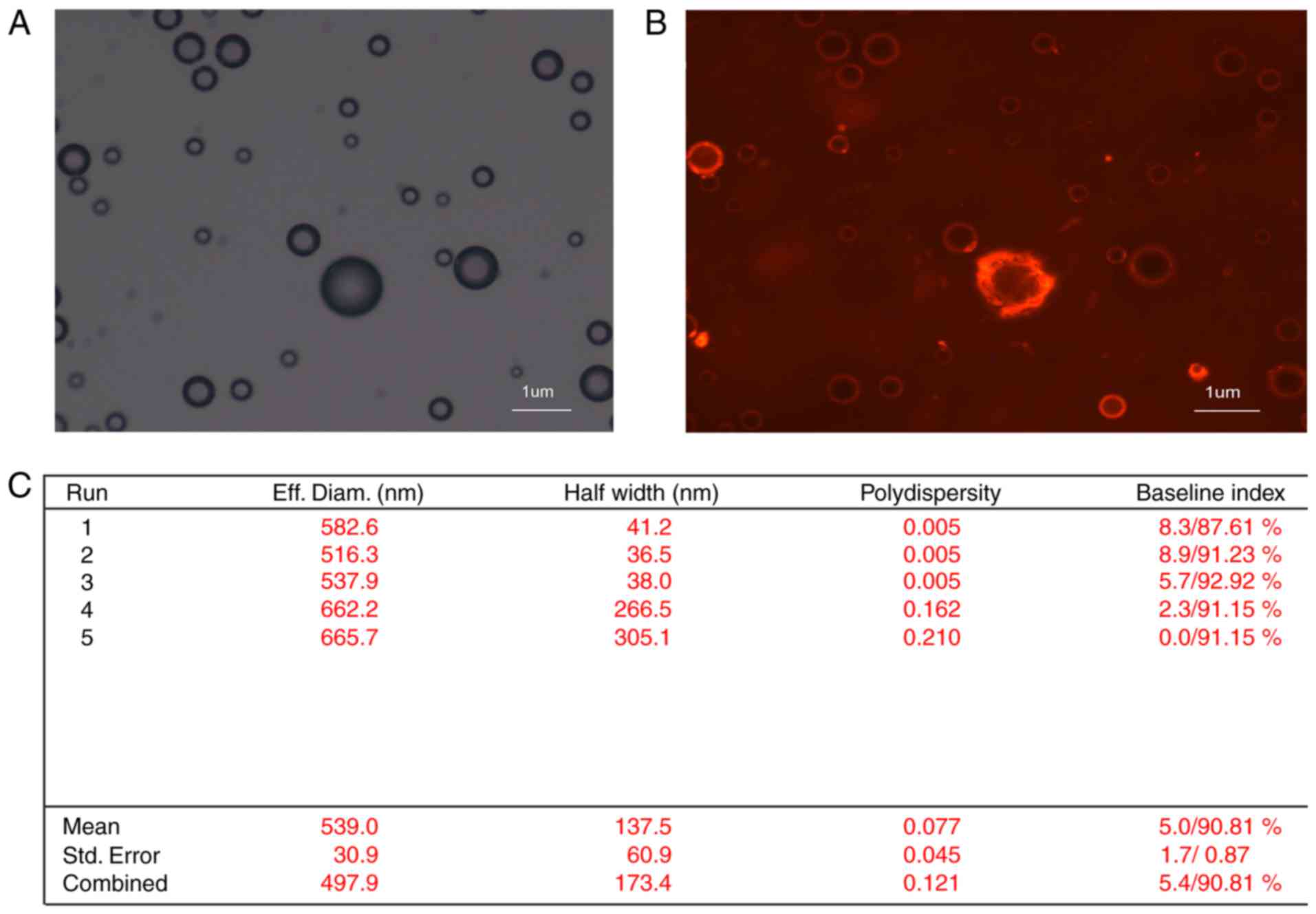
The combination between NET-1 siRNA and TNBs was
established using light microscope and confocal laser scanning
microscope, as demonstrated in Fig.
3. The nanobubble surfaces appeared red under confocal laser
scanning microscopy, indicating that the cy3-labeled NET-1 siRNA
was packaged on the TNB surfaces. The concentration of nanobubbles
was 3×108/ml. The average diameter of the particle was
593±30.9 nm, which is able to pass the capillary wall and reach the
tumor tissue (40).
Elastic properties of tumor
Firstly, prior to treatment, no significant
difference was observed in the SWSmax or
SWSmean (day 0) among the four groups (P>0.05). Also,
no significant difference was noted in the 4 SWS measurements,
including SWSmax and SWSmean, between the
blank and negative control groups (P>0.05). Next, the two-way
ANOVA indicated that the duration and types of treatment
significantly affected the SWS values. The effect of NET-1 siRNA on
tumor elasticity was evaluated by VTIQ measurement. As demonstrated
in Fig. 4, SWSmean (days
7, 14 and 21) in the TNBs-siRNA group was significantly different
compared with the blank group (P<0.001). The difference was also
observed in SWSmax at days 14 and 21. In addition,
SWSmean (days 14 and 21) in the TNBs-siRNA group was
statistically different from the Lipo2000 group (P<0.05 and
P<0.01). However, no significant difference was observed in the
4 SWSmax measurements between groups C and D
(P>0.05). The last VTIQ value (day 21) for the
SWSmean in groups A, B, C and D was 3.64±0.24,
3.88±0.30, 3.22±0.11 and 2.81±0.20 m/s, respectively (Table III).
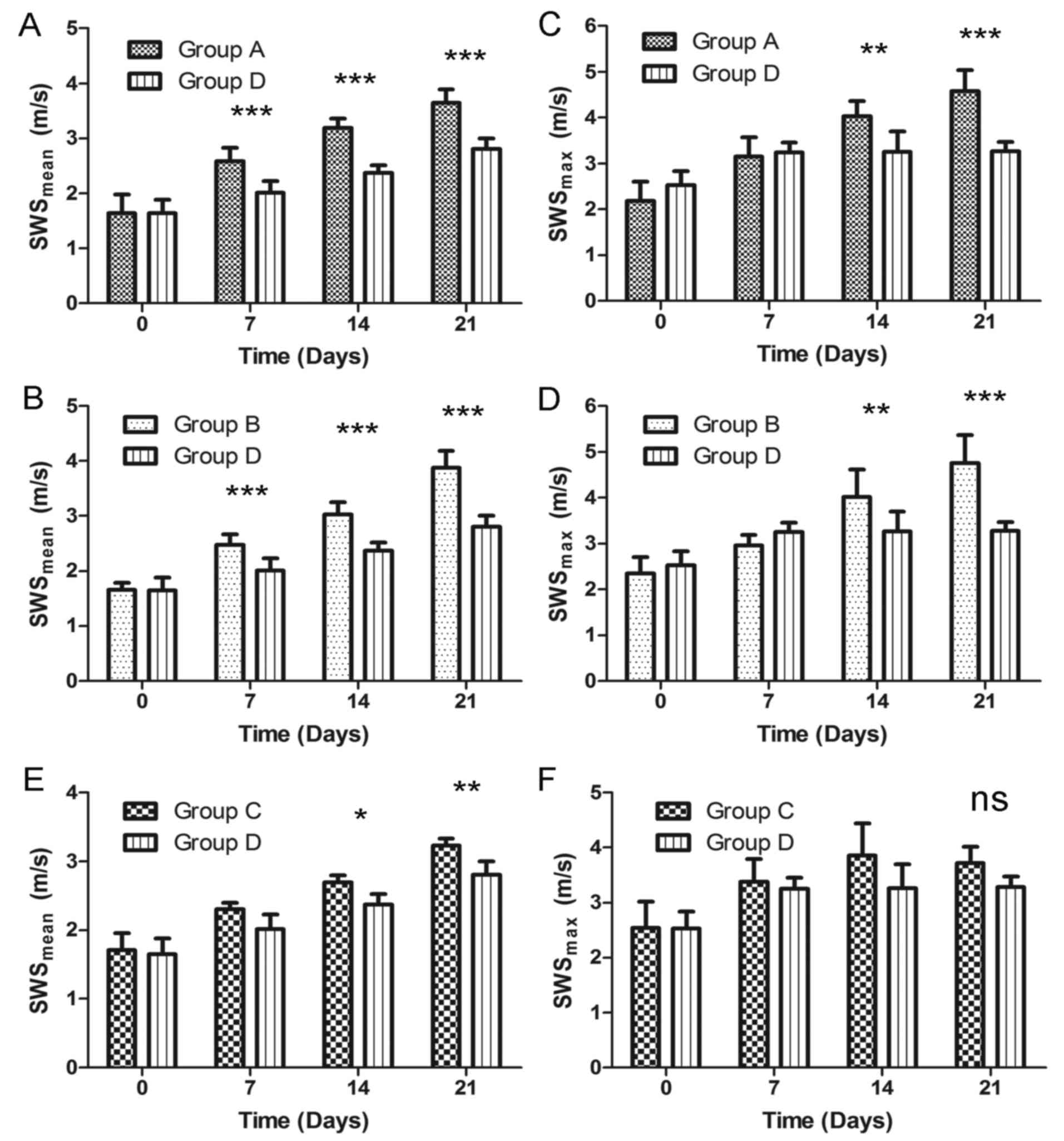 | Figure 4.Comparison of SWSmean and
SWSmax between groups. For SWSmean
measurement, the treated group D was statistically different from
control groups including (A) group A and (B) group B except to
before the experiment. Similarly, the significant difference in
SWSmax (day 7, 14 and 21) was also shown between group D
and in (C) group A, (D) group B. (E) Compared with group C,
SWSmean in group D was the same on day 0, 7 but
different on day 14, 21. (F) There was no significant different in
four time SWSmax measurement between group C and D.
Bonferroni post-hoc tests were used for comparisons at each time
point after the two-way analysis of variance (ANOVA). *P<0.05,
**P<0.01 and ***P<0.001. SWS, shear wave speed; ns, not
significant. |
 | Table III.The result of SWS measurement of the
four groups. SWSmean and SWSmax in four
groups were expressed as mean ± SD (m/s) and measured by virtual
touch tissue imaging and quantification on days 0, 7, 14 and
21. |
Table III.
The result of SWS measurement of the
four groups. SWSmean and SWSmax in four
groups were expressed as mean ± SD (m/s) and measured by virtual
touch tissue imaging and quantification on days 0, 7, 14 and
21.
| A,
SWSmean |
|---|
|
|---|
|
| Group A | Group B | Group C | Group D |
|---|
|
|
|
|
|
|
|---|
| Day | Mean ± SD | n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | n |
|---|
| 0 | 1.65±0.33 | 6 | 1.66±0.12 | 6 | 1.71±0.24 | 6 | 1.64±0.24 | 6 |
| 7 | 2.59±0.24 | 6 | 2.47±0.20 | 6 | 2.30±0.10 | 6 | 2.01±0.22 | 6 |
| 14 | 3.19±0.17 | 5 | 3.02±0.22 | 6 | 2.69±0.11 | 6 | 2.37±0.15 | 6 |
| 21 | 3.64±0.24 | 3 | 3.88±0.30 | 3 | 3.22±0.11 | 5 | 2.81±0.20 | 6 |
|
| B,
SWSmax |
|
|
| Group A | Group B | Group C | Group D |
|
|
|
|
|
|
| Day | Mean ±
SD | n | Mean ±
SD | n | Mean ±
SD | n | Mean ±
SD | n |
|
| 0 | 2.18±0.42 | 6 | 2.34±0.35 | 6 | 2.54±0.48 | 6 | 2.53±0.31 | 6 |
| 7 | 3.15±0.42 | 6 | 2.95±0.24 | 6 | 3.38±0.42 | 6 | 3.25±0.20 | 6 |
| 14 | 4.04±0.33 | 5 | 4.01±0.60 | 6 | 3.85±0.59 | 6 | 3.26±0.44 | 6 |
| 21 | 4.59±0.46 | 3 | 4.76±0.62 | 3 | 3.72±0.30 | 5 | 3.28±0.19 | 6 |
Pearson's correlation analysis established a
significantly positive correlation (r=0.9806, P=0.0194) between the
maximum diameter of tumor and SWSmean for group D.
However, SWSmax was not significant (r=0.8825,
P=0.1175).
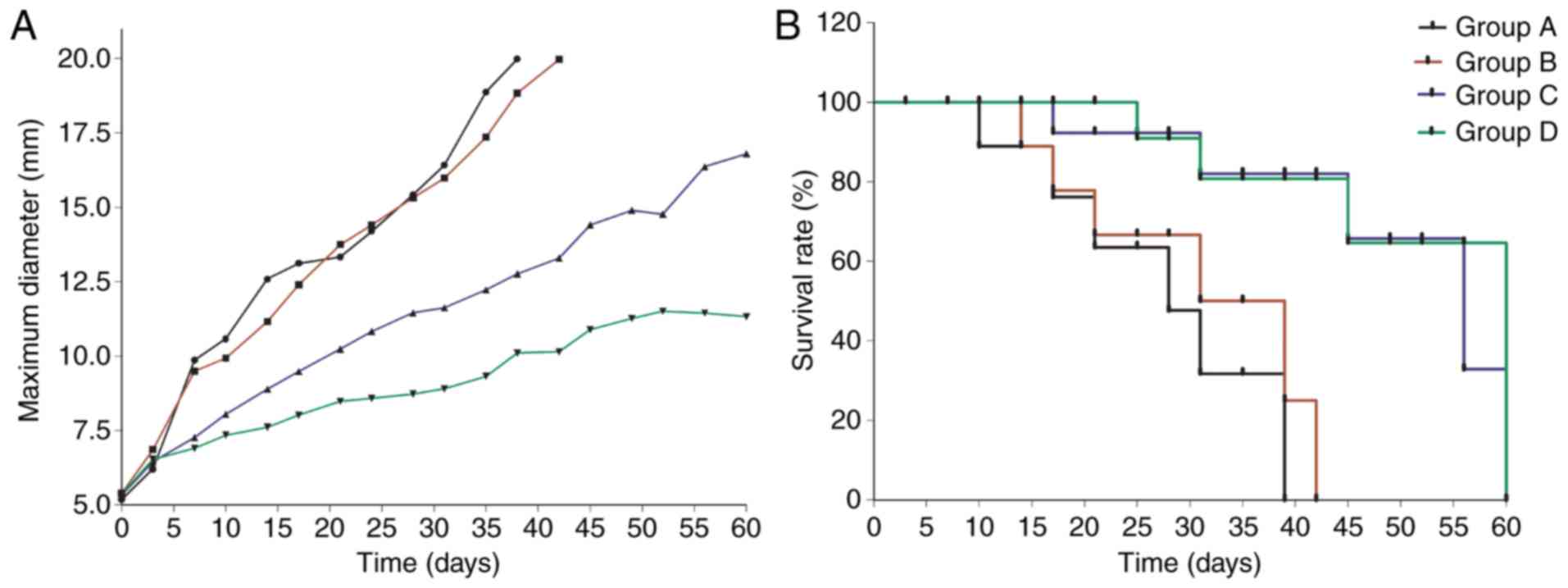
Antitumor effect
As observed in Fig. 5,
the survival times and tumor growth of every group were evaluated.
In the two treated groups delivering NET-1 siRNA with TNBs or
Lipo2000, the survival rates were increased compared with the
control groups. The median survival rates of groups A, B, C and D
were 28, 35, 56 and 60 days, respectively. Although all the tumors,
with or without treatment, increased in size, the tumor growth rate
in group D was significantly decreased compared with that in the
other groups. Silencing the NET-1 gene inhibited the tumor
growth, thereby increasing the rate of survival.
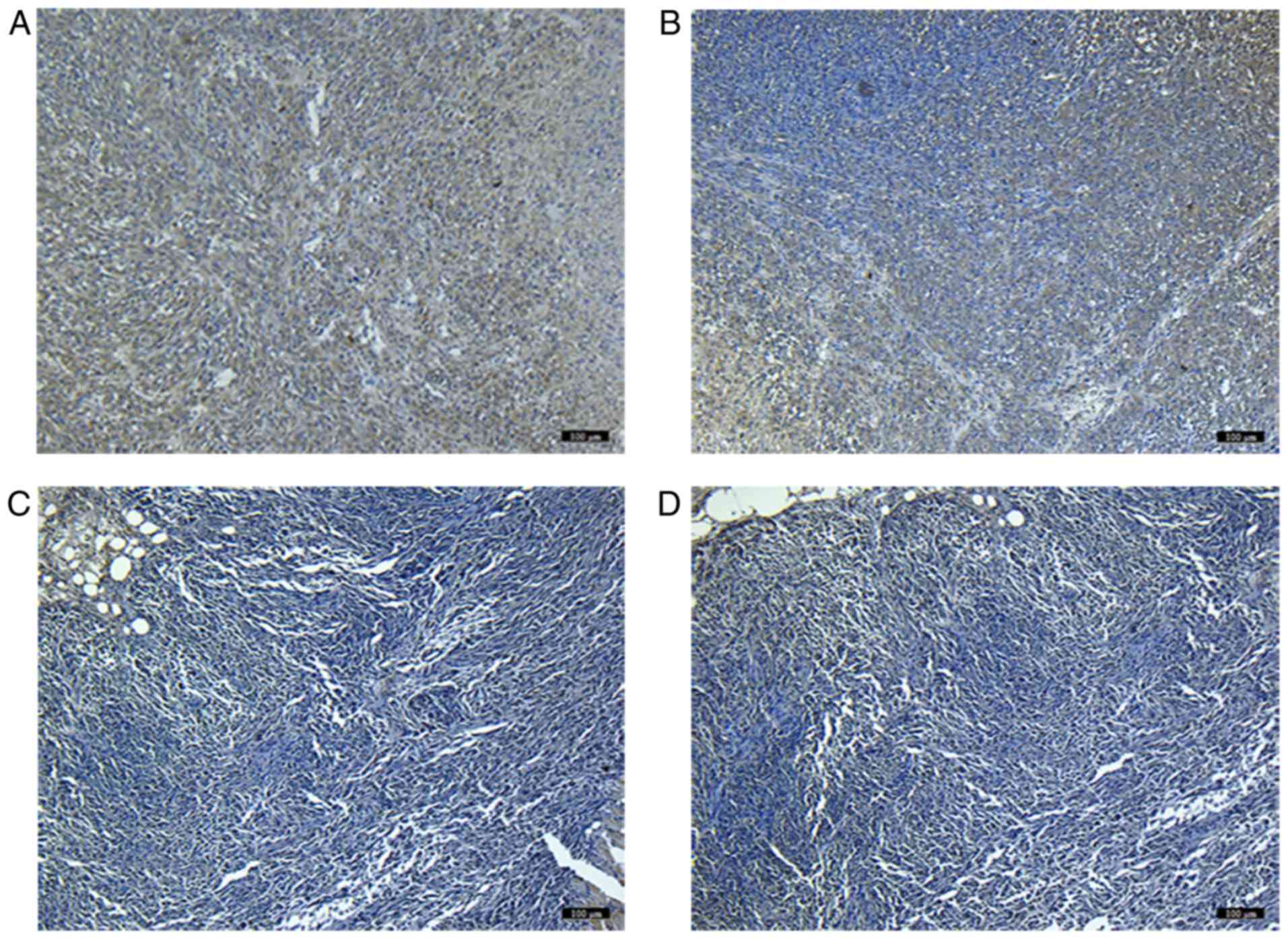
Expression of NET-1 in tumor tissue
and hepatic and renal toxicity
Immunohistochemical staining indicated that the
level of NET-1 expression in the treated groups (C and D) was
downregulated compared with the blank controls by quantified
analysis. In addition, the level of NET-1 protein in the TNBs-siRNA
group was significantly different from that of the Lipo2000 group
(P<0.05). No difference was observed between the blank and
negative controls (Fig. 6).
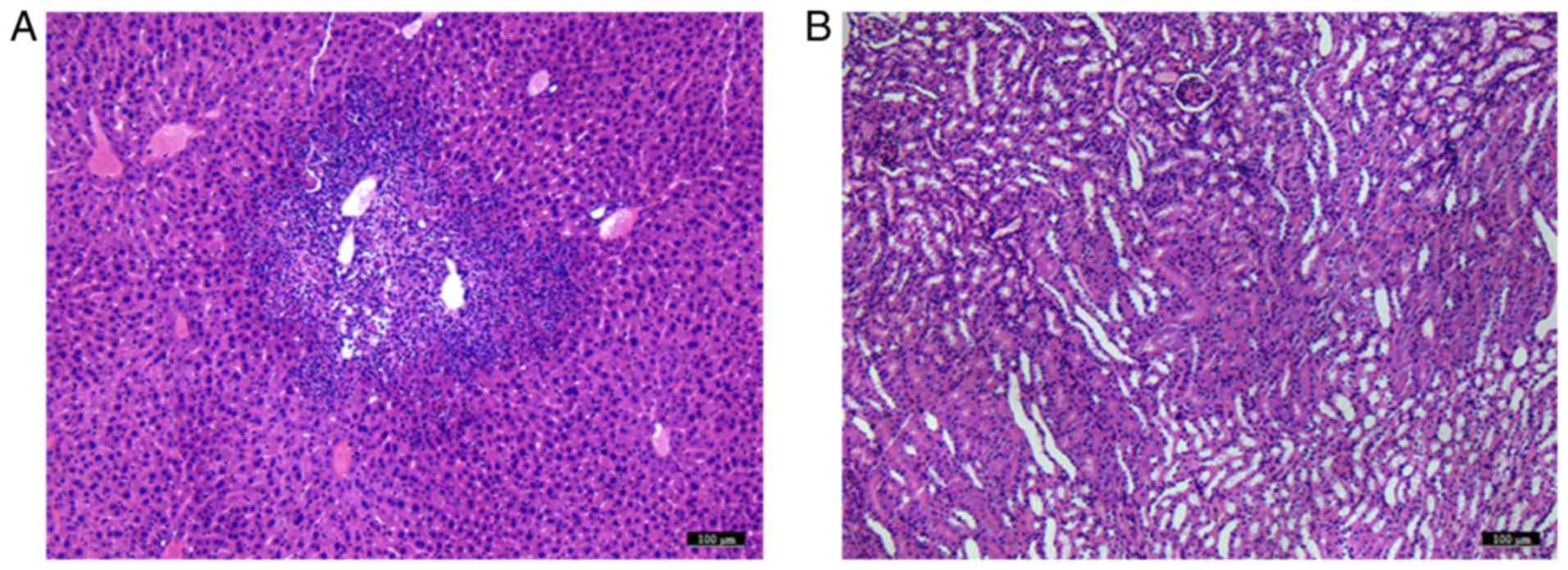
Using microscopy, tumor metastasis was identified in
liver samples from groups A and B, which was not observed in groups
C and D. No marked pathological changes were observed in kidney
samples from either of the groups (Fig.
7).
Discussion
In the present study, the antitumor effect of gene
transfection was initially evaluated using VTIQ. As a clinical
diagnostic tool, VTIQ assesses the stiffness of the tissue, which
aids in clinical differentiation between benign and malignant
nodules (41). Previous studies have
demonstrated the changes in the value of tumor stiffness during
disease progression (42,43). Quantitative elastography techniques,
including virtual touch tissue quantification and VTIQ, provide
valuable information for assessing the stiffness of the tumor
tissue (44). In the present study,
the VTIQ was measured once/week, based on the treatment frequency
and data obtained from a previous study (45). SWSmean and
SWSmax values from the four groups were compared at each
time point. A two-way ANOVA investigated the effect of treatment
and time on SWS measurement. It was identified that VTIQ was able
to track the alterations in elastic modules in response to gene
therapy. SWS and stiffness in tumor tissue increased gradually with
the development of the tumor. This result was consistent with that
of a previous study (46). A
significant difference was observed in SWS between the control and
treated groups, suggesting that the rate of hardening of the tumor
was decreased by gene treatment. In addition, SWSmean in
the Lipo2000 group was significantly different from that in the
TNBs-siRNA group; this demonstrated the improved effect of
UTMD-mediated gene transfection compared with that of Lipo2000. The
SWSmean values were markedly correlated with the tumor
growth curve induced by NET-1 siRNA. However, it was also noted
that comparison of the SWSmax values between the two
treated groups did not indicate a significant difference, which may
be attributed to the location of the 7 ROIs that avoided the
cystic, calcified and necrotic areas, thereby affecting the result
(36,47). Furthermore, SWSmean
achieved the highest diagnostic performance compared with the other
measured values.
As a new member of the TM4SF family, the
NET-1 gene was identified to be overexpressed in certain
tumors, including HCC, gastric cancer, colorectal adenocarcinoma
and skin squamous cell carcinoma (21,48). The
present study used siRNA to inhibit NET-1 gene expression in
a SMMC-7721 xenograft model; each group was treated four times.
Subsequently, the level of NET-1 protein in the two treated groups
was decreased compared with that in the control groups. The tumor
growth curve demonstrated that in the NET-1 siRNA group, the
maximum tumor diameter was observed post-treatment; it was
significantly decreased compared with that without treatment,
additionally illustrating that the overexpression of NET-1 in HCC
was involved in cancer cell proliferation (35). NET-1 siRNA-conjugated TNBs, with an
average size of 593 nm, were produced using a membrane hydration
combined with mechanical oscillation method. Due to the binding of
NET-1 siRNA to TNBs, a statistically significant difference in the
level of NET-1 protein was observed between the Lipo2000 and
TNBs-siRNA groups. In addition, the tumor growth curve demonstrated
a maximum diameter of tumor in the TNBs-siRNA group that was
significantly decreased compared with that of the Lipo2000 group.
However, the difference in the survival time between the two
treated groups was small and did not reach statistical
significance. These results may be ascribed to the short
observation time (60 days). Therefore, additional studies should
investigate the delivery system of the nanobubbles at different
exposure conditions and concentrations of siRNA, in order to
maximize the gene transfection efficiency for tumor therapy in
vivo.
Furthermore, one of the major concerns associated
the use of these enhancers in clinical studies is the safety of the
therapeutic system (38). Non-viral
vectors are being increasingly used for gene therapy instead of
viral vectors, owing to the concerns about the potential induction
of immune responses against viral proteins, endogenous virus
recombination and oncogenic effects (49). However, the conventional delivery
methods of the non-viral vectors including liposomes and polymeric
systems are less than satisfactory, due to the lack of tissue
specificity and potential toxicity (50). In the present study, it was identified
that the UTMD delivery method exhibited potential in improving gene
transfection efficiency. The toxicity of the nanobubbles and the
ultrasound radiation have also raised crucial safety issues in
clinical studies (38). Therefore,
liver and kidney samples were collected from each group of nude
mice, and H&E staining was performed to evaluate the safety of
the treatment. In the control groups, malignant pathology was
identified in liver samples but not kidney samples, which was
uncommon in a previous study (38).
This observation may be associated with the highly aggressive and
hepatogenic properties of the SMMC-7721 cell line (51). Also, no marked pathological changes
were observed in the kidney samples of the TNBs-siRNA group, which
was in agreement with a previous study (38).
Nevertheless, the present study had certain
limitations. Firstly, the histological development and tumor
elasticity during tumor progression was not compared. Therefore, an
association between SWS and pathological processes of the tumors
was not able to be established. However, this was not the aim of
the present study, as previous studies have demonstrated that the
difference in the stiffness is associated with tissue
reorganization, particularly fibrosis and necrosis (2,47,52). Secondly, the results of these
experiments are not directly applicable to human liver cancer
tissues: The present tumor model was implanted under the skin of
the nude mice. Although central necrosis is not commonly identified
in small human liver cancer, it was observed in the present model
as the blood supply to the skin tissue may be decreased compared
with that in human liver tissue (53).
In summary, the NET-1 siRNA-TNBs synthesized in the
present study were effective in vivo, including the
nanoscale size, low toxicity and high transfection efficiency in
the tumor. The present study not only described an effective gene
vehicle, but also identified a novel elastography technique for
investigation of the stiffness of HCC tissues.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81571682 and
81371568), and the Fundamental Research for the Provincial
Universities (grant no. 2017LCZX91).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BW prepared and characterized of NET-1 small
interfering RNA (siRNA)-conjugated targeted nanobubbles. HS was in
charge of the xenograft model and gene transfection. XL performed
VTIQ measurement and was a major contributor in writing the
manuscript. XH contributed to the analysis of the VTIQ measurement.
HJ and YS analyzed and interpreted IHC and toxicity test. YS was in
charge of revising the article. WC contributed to study design. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were approved by the
Institutional Animal Care and Use Committee of Harbin Medical
University Cancer Hospital (Harbin, China). All procedures were
performed according to the Guidelines for Tumor Induction in Mice
and Rats (updated May 2013).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yao MH, Wu R, Xu G, Zhao LX, Liu H, Pu H
and Fang Y: A novel two-dimensional quantitative shear wave
elastography to make differential diagnosis of breast lesions:
Comprehensive evaluation and influencing factors. Clin Hemorheol
Microcirc. 64:223–233. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Özkan MB, Bilgici MC, Eren E, Caltepe G,
Yilmaz G, Kara C and Gun S: Role of point shear wave elastography
in the determination of the severity of fibrosis in pediatric liver
diseases with pathologic correlations. J Ultrasound Med.
36:2337–2344. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tozaki M, Saito M, Benson J, Fan L and
Isobe S: Shear wave velocity measurements for differential
diagnosis of solid breast masses: A comparison between virtual
touch quantification and virtual touch IQ. Ultrasound Med Biol.
39:2233–2245. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ianculescu V, Ciolovan LM, Dunant A, Vielh
P, Mazouni C, Delaloge S, Dromain C, Blidaru A and Balleyguier C:
Added value of virtual touch IQ shear wave elastography in the
ultrasound assessment of breast lesions. Eur J Radiol. 83:773–777.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ben Z, Gao S, Wu W, Chen S, Fu S, Zhang J
and Chen Y: Clinical value of the VTIQ technology in the
differential diagnosis of superficially enlarged lymph nodes. Acta
Radiol. 59:836–844. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yeh CC, Horng HC and Wang PH: Acoustic
radiation force imaging (ARFI): A new powerful tool of ultrasound.
J Chin Med Assoc. 80:681–682. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Y, Luo L and Luo Q: Identification
of benign and malignant endometrial cancer with transvaginal
ultrasonography combined with elastography and tissue hardness
analysis. J Biol Regul Homeost Agents. 29:905–912. 2015.PubMed/NCBI
|
|
8
|
Seguin J, Mignet N, Ossa Latorre H, Tanter
M and Gennisson JL: Evaluation of antivascular combretastatin A4 P
efficacy using supersonic shear imaging technique of ectopic colon
carcinoma CT26. Ultrasound Med Biol. 43:2352–2361. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sherman M: Hepatocellular carcinoma:
Epidemiology, surveillance, and diagnosis. Semin Liver Dis.
30:3–16. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Le Grazie M, Biagini MR, Tarocchi M,
Polvani S and Galli A: Chemotherapy for hepatocellular carcinoma:
The present and the future. World J Hepatol. 9:907–920. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
van't Veer LJ and Bernards R: Enabling
personalized cancer medicine through analysis of gene-expression
patterns. Nature. 452:564–570. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xue Y, Yang G, Wang C, Li X and Du G:
Effects of shRNA-mediated SOX9 inhibition on cell proliferation and
apoptosis in human HCC cell line Hep3B mediated by
ultrasound-targeted microbubble destruction (UTMD). Cell Biochem
Biophys. 73:553–558. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Avila MA, Berasain C, Sangro B and Prieto
J: New therapies for hepatocellular carcinoma. Oncogene.
25:3866–3884. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gomes-da-Silva LC, Fonseca NA, Moura V, de
Lima Pedroso MC, Simões S and Moreira JN: Lipid-based nanoparticles
for siRNA delivery in cancer therapy: Paradigms and challenges. Acc
Chem Res. 45:1163–1171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu YY, Chen L, Wang GL, Zhang YX, Zhou JM,
He S, Qin J and Zhu YY: Inhibition of hepatocellular carcinoma
growth and angiogenesis by dual silencing of NET-1 and VEGF. J Mol
Histol. 44:433–445. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang GL, Chen L, Wei YZ, Zhou JM, Wu YY,
Zhang YX, Qin J and Zhu YY: The effect of NET-1 on the
proliferation, migration and endocytosis of the SMMC-7721 HCC cell
line. Oncol Rep. 27:1944–1952. 2012.PubMed/NCBI
|
|
18
|
Qiu DM, Wang GL, Chen L, Xu YY, He S, Cao
XL, Qin J, Zhou JM, Zhang YX and E Q: The expression of beclin-1,
an autophagic gene, in hepatocellular carcinoma associated with
clinical pathological and prognostic significance. BMC Cancer.
14:3272014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ye K, Wang Z, Zhang G and Liang S:
Prognostic significance of neuroepithelial transforming protein 1
in hepatocellular carcinoma. J Invest Surg. 23:163–169. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen L, Wang Z, Zhan X, Li DC, Zhu YY and
Zhu J: Association of NET-1 gene expression with human
hepatocellular carcinoma. Int J Surg Pathol. 15:346–353. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li T, Xue Y, Wang G, Gu T, Li Y, Zhu YY
and Chen L: Multi-target siRNA: Therapeutic strategy for
hepatocellular carcinoma. J Cancer. 7:1317–1327. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Han X, Cheng W, Jing H, Zhang JW and Tang
LL: Neuroepithelial transforming protein 1 short interfering
RNA-mediated gene silencing with microbubble and ultrasound
exposure inhibits the proliferation of hepatic carcinoma cells in
vitro. J Ultrasound Med. 31:853–861. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He S, Wei YZ, Wang GL, Xu YY, Zhou JM,
Zhang YX and Chen L: Study of RNA interference targeting NET-1
combination with sorafenib for hepatocellular carcinoma therapy in
vitro and in vivo. Gastroenterol Res Pract. 2013:6851502013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tsuchiya N, Sawada Y, Endo I, Saito K,
Uemura Y and Nakatsura T: Biomarkers for the early diagnosis of
hepatocellular carcinoma. World J Gastroenterol. 21:10573–10583.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jing JS, Ye W, Jiang YK, Ma J, Zhu MQ, Ma
JM, Zhou H, Yu LQ, Yang YF and Wang SC: The value of GPC3 and GP73
in clinical diagnosis of hepatocellular carcinoma. Clin Lab.
63:1903–1909. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Montalbano M, Rastellini C, McGuire JT,
Prajapati J, Shirafkan A, Vento R and Cicalese L: Role of
Glypican-3 in the growth, migration and invasion of primary
hepatocytes isolated from patients with hepatocellular carcinoma.
Cell Oncol. 41:169–184. 2018. View Article : Google Scholar
|
|
27
|
Tsuchiya N, Yoshikawa T, Fujinami N, Saito
K, Mizuno S, Sawada Y, Endo I and Nakatsura T: Immunological
efficacy of glypican-3 peptide vaccine in patients with advanced
hepatocellular carcinoma. Oncoimmunology. 6:e13467642017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen JJ, Xie CM, Wang CR, Wan Y, Dong ZN,
Li M and Xu WW: Development of a time-resolved fluorescence
immunoassay for the diagnosis of hepatocellular carcinoma based on
the detection of glypican-3. J Fluoresc. 27:1479–1485. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li M, Zhang W, Wang B, Gao Y, Song Z and
Zheng QC: Ligand-based targeted therapy: A novel strategy for
hepatocellular carcinoma. Int J Nanomedicine. 11:5645–5669. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kievit FM and Zhang M: Cancer
nanotheranostics: Improving imaging and therapy by targeted
delivery across biological barriers. Adv Mater. 23:H217–H247. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang J, Hendricks W, Liu G, McCaffery JM,
Kinzler KW, Huso DL, Vogelstein B and Zhou S: A nanoparticle
formulation that selectively transfects metastatic tumors in mice.
Proc Natl Acad Sci USA. 110:14717–14722. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Eggen S, Fagerland SM, Mørch Ý, Hansen R,
Søvik K, Berg S, Furu H, Bøhn AD, Lilledahl MB, Angelsen A, et al:
Ultrasound-enhanced drug delivery in prostate cancer xenografts by
nanoparticles stabilizing microbubbles. J Control Release.
187:39–49. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Juffermans LJ, Meijering BD, Henning RH
and Deelman LE: Ultrasound and microbubble-targeted delivery of
small interfering RNA into primary endothelial cells is more
effective than delivery of plasmid DNA. Ultrasound Med Biol.
40:532–540. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Meng M, Gao J, Wu C, Zhou X, Zang X, Lin
X, Liu H, Wang C, Su H, Liu K, et al: Doxorubicin nanobubble for
combining ultrasonography and targeted chemotherapy of rabbit with
VX2 liver tumor. Tumour Biol. 37:8673–8680. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu B, Qiao Q, Han X, Jing H, Zhang H,
Liang H and Cheng W: Targeted nanobubbles in low-frequency
ultrasound-mediated gene transfection and growth inhibition of
hepatocellular carcinoma cells. Tumour Biol. 37:12113–12121. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang YP, Xu XH, Bo XW, Liu BJ, Guo LH, Xu
JM, Sun LP and Xu HX: Comparison of virtual touch tissue imaging
& quantification (VTIQ) and virtual touch tissue quantification
(VTQ) for diagnosis of thyroid nodules. Clin Hemorheol Microcirc.
65:137–149. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
He YP and Xu HX, Li XL, Li DD, Bo XW, Zhao
CK, Liu BJ, Wang D and Xu HX: Comparison of virtual touch tissue
imaging & quantification (VTIQ) and Toshiba shear wave
elastography (T-SWE) in diagnosis of thyroid nodules: Initial
experience. Clin Hemorheol Microc. 66:15–26. 2017. View Article : Google Scholar
|
|
38
|
Park DH, Jung BK, Lee YS, Jang JY, Kim MK,
Lee JK, Park H, Seo J and Kim CW: Evaluation of in vivo antitumor
effects of ANT2 shRNA delivered using PEI and ultrasound with
microbubbles. Gene Ther. 22:325–332. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lucero HA, Patterson S, Matsuura S and
Ravid K: Quantitative histological image analyses of reticulin
fibers in a myelofibrotic mouse. J Biol Methods. 3:e602016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Peng H, Birkett GR and Nguyen AV: Progress
on the surface nanobubble story: What is in the bubble? Why does it
exist? Adv Colloid Interface Sci. 222:573–580. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kapetas P, Pinker-Domenig K, Woitek R,
Clauser P, Bernathova M, Spick C, Helbich T and Baltzer PA:
Clinical application of acoustic radiation force impulse imaging
with Virtual Touch IQ in breast ultrasound: Diagnostic performance
and reproducibility of a new technique. Acta Radiol. 58:140–147.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chamming's F, Latorre-Ossa H, Le
Frère-Belda MA, Fitoussi V, Quibel T, Assayag F, Marangoni E,
Autret G, Balvay D, Pidial L, et al: Shear wave elastography of
tumour growth in a human breast cancer model with pathological
correlation. Eur Radiol. 23:2079–2086. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li J, Wu Y, Schimmel N, Al-Ameen MA and
Ghosh G: Breast cancer cells mechanosensing in engineered matrices:
Correlation with aggressive phenotype. J Mech Behav Biomed Mater.
61:208–220. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhou H, Zhou XL, Xu HX, Li DD, Liu BJ,
Zhang YF, Xu JM, Bo XW, Li XL, Guo LH and Qu S: Virtual touch
tissue imaging and quantification in the evaluation of thyroid
nodules. J Ultrasound Med. 36:251–260. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chamming's F, Le-Frère-Belda MA,
Latorre-Ossa H, Fitoussi V, Redheuil A, Assayag F, Pidial L,
Gennisson JL, Tanter M, Cuénod CA and Fournier LS: Supersonic shear
wave elastography of response to anti-cancer therapy in a xenograft
tumor model. Ultrasound Med Biol. 42:924–930. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kasai Y, Moriyasu F, Saito K, Hara T,
Kobayashi Y, Nakamura I and Sugimoto K: Value of shear wave
elastography for predicting hepatocellular carcinoma and
esophagogastric varices in patients with chronic liver disease. J
Med Ultrason. 42:349–355. 2015. View Article : Google Scholar
|
|
47
|
Sun CY, Lei KR, Liu BJ, Bo XW, Li XL, He
YP, Wang D, Ren WW, Zhao CK and Xu HX: Virtual touch tissue imaging
and quantification (VTIQ) in the evaluation of thyroid nodules: The
associated factors leading to misdiagnosis. Sci Rep. 7:419582017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ji ZJ, Wang JL and Chen L: Inhibition of
skin squamous cell carcinoma proliferation and promote apoptosis by
dual silencing of NET-1 and survivin. Oncol Rep. 34:811–822. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen ZY, Liang K, Lin Y and Yang F: Study
of the UTMD-based delivery system to induce cervical cancer cell
apoptosis and inhibit proliferation with shRNA targeting Survivin.
Int J Mol Sci. 14:1763–1777. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lee H, Lytton-Jean AK, Chen Y, Love KT,
Park AI, Karagiannis ED, Sehgal A, Querbes W, Zurenko CS, Jayaraman
M, et al: Molecularly self-assembled nucleic acid nanoparticles for
targeted in vivo siRNA delivery. Nat Nanotechnol. 7:389–393. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xu B, Yao M, Gu W, Yan M, Liu L and Zhang
J: Establishment and biological characteristics of mouse models
mimicking postoperative human carcinoma metastasis. Nan Fang Yi Ke
Da Xue Xue Bao. 27:1009–1011. 2007.(In Chinese). PubMed/NCBI
|
|
52
|
Elyas E, Papaevangelou E, Alles EJ, Erler
JT, Cox TR, Robinson SP and Bamber JC: Correlation of ultrasound
shear wave elastography with pathological analysis in a xenografic
tumour model. Sci Rep. 7:1652017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
von Ardenne M: Differences in induction of
the cellular switch mechanism of blood microcirculation in various
organs and tissues of the human body. Z Alternsforsch. 40:357–368.
1985.(In German). PubMed/NCBI
|