Introduction
Anti-angiogenic therapy, initially proposed in
Folkman's hypothesis, is applied with the aim of reducing tumor
angiogenesis and growth (1). However,
excessive anti-angiogenic treatment can increase areas of hypoxia
and decrease blood perfusion in tumors, processes partly
accountable for the development of radio- or chemotherapy
resistance (2). Thus, although the
concept of anti-angiogenesis appears to have conflicting results, a
new hypothesis proposed that there is a window of opportunity for
the transient normalization of abnormal structures and functions of
tumor vessels during anti-angiogenic treatment (3,4). These
‘re-normalized’ vessels improved hypoxic conditions within the
tumor by increasing blood perfusion and decreasing vascular
leakage, rendering chemo- and radiotherapy more effective (5–7).
Monitoring of vessel normalization is required for the development
of improved combination therapies and for strengthening the
antitumor effect of anti-angiogenic therapies. So far, data have
proposed that by tracking improvements to intratumoral areas of
hypoxia during anti-angiogenic therapy,
18F-fluoromisonidazole-PET could identify vascular
normalization (8,9). However, high costs and technical
requirements continue to limit the wide clinical application of
18F-fluoromisonidazole-PET. Therefore, the aim was to
discover reliable and easily detected biomarkers for monitoring the
window of tumor vascular normalization during anti-angiogenic
therapy in clinical practice.
Anterior gradient 2 (AGR2) is a human ortholog of
the Xenopus laevis cement gland protein and a member of the
protein disulfide isomerase (PDI) family (10). AGR2 is highly expressed and secreted
in malignant cancer types, including breast carcinomas, pancreatic
cancer, glioblastoma, prostate cancer and metastatic colorectal
cancer (11–18). Additionally, high levels of plasma
AGR2 are associated with a poor prognosis in numerous cancer types
(19–22). It has been reported that a hypoxic
microenvironment induces AGR2 expression in tumor cells, hypoxia
inducible factor-1α (HIF-1α) regulated intracellular expression and
extracellular secretion of AGR2. Furthermore, overexpressed AGR2
stabilizes HIF-1α in tumor cells (18,23).
Gold nanoparticles (AuNPs) are a promising
anti-angiogenic agent with activity against VEGF and VEGF receptor
2 (VEGFR2). Our previous study showed that, in vitro, AuNPs
could inhibit VEGF-induced activation of phosphorylated AKT in
endothelial cells and disrupt the proliferation or migration of
endothelial cells in tumor cell-conditioned medium, which further
indicated that AuNPs could lessen the angiogenic tendency of
endothelial cells in the tumor microenvironment. Furthermore, it
was confirmed that AuNPs are biocompatible and stable, with low
cytotoxicity (24–26). Further studies showed that AuNPs would
muster in solid tumors, and could penetrate directly into tumor
vessels (27–29). Further research ascertained that
AuNPs, as a nanoparticle-based drug-delivery system, could deliver
other anti-angiogenic agents and reengineer abnormal vessels,
resulting in improvements to areas of hypoxia in the tumor and more
effective chemotherapy (30).
Because abnormal vasculature cannot supply
sufficient oxygen to the tumor, the interstitial tissue remains
under hypoxic conditions (5–7). Anti-angiogenic agents reduce hypoxia
during the window of tumor vascular normalization; however,
extended anti-angiogenic therapy causes excess tumor vessel
reduction, which eventually creates more hypoxic areas within the
tumor (31). It is hypothesized that
AGR2 could detect internal tumor hypoxia, which offered an
opportunity to track vascular abnormality and vascular
‘re-normalization’ during anti-angiogenic treatment. In the present
study, to elucidate the role of AGR2 as a potential biomarker of
vascular normalization during anti-angiogenic treatment, it was
analyzed whether AuNPs could normalize tumor vasculature in nude
mice that were ectopically transplanted with human metastatic
colorectal cancer (SW620) cells, and how AGR2 secretion and
expression profiles changed in plasma and tumor microenvironments
during treatment.
Materials and methods
Cell line, mouse xenografts and
treatment design
SW620 metastatic colorectal cancer cells were
purchased from Shanghai Zzbio Co., Ltd. (Shanghai, China). Cells
were maintained at 37°C with 5% CO2 and 95% humidity,
and cultured with Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin (HyClone; GE Healthcare Life Sciences,
Logan, UT, USA). AuNPs were purchased from Shanghai Jie Ning
Biotechnology Co., Ltd. (Shanghai, China), stored in
light-resistant containers at 4°C, and the hydrodynamic size
distribution of AuNPs was 58.2±7.1 nm. Female BALB/c nude mice
(n=57, 18–22 g weight, 6–8 weeks old) were purchased from the
Guangdong Medical Laboratory Animal Center (Guangdong, China) and
maintained under pathogen-free conditions (temperature, 23±2°C;
light/dark cycle, 12/12 h; and relative humidity, 50±10%. Food and
water were provided ad libitum. All animal study protocols were
approved and conducted in accordance with the guidelines of the
Laboratory Animal Ethics Committee of Jinan University (Guangzhou,
Guangdong, China). Mouse xenografts were constructed by suspending
SW620 metastatic colorectal cancer cells (4×106/100
µl/mouse) in saline and subcutaneously injecting the solution into
the flank. The day when the volume of xenografts reached 175–200
mm3 was designated as day 0. In the group receiving
treatment with AuNPs, the mice were subcutaneously given an
injection of 1.3 mg/kg and the treatment was repeated daily from
day 1–6, and then repeated every other day from day 8–14. Mice were
weighed and the tumor volume was measured daily. The tumor volumes
were measured in two dimensions with calipers and calculated with
the formula (L × W2) × 0.5 (L is the length and W is the
width of tumor). Mice were anesthetized with lidocaine
(intraperitoneal injection, 10 mg/kg). Tumors were harvested and
examined on day 0, 4, 6, 9 and 14 (n=3/time point). Blood samples
were collected from the retro-orbital plexus at day 0, 2, 4, 6, 9,
12 and 14 (n=6/time point). Control mice were injected with
saline.
Plasma AGR2 ELISA
Blood samples were collected in test tubes and
immediately centrifuged at 2,000 × g for 15 min at 4°C to obtain
plasma samples, following which they were stored at −40°C. An ELISA
kit for AGR2 (MEXN-H0280; Meixuan Biological Technology Co., Ltd.;
Shanghai, China) (URL: http://www.mexnbio.com/mxbio-Products-19629906/) was
used to measure the level of serum AGR2, according to the
manufacturer's instructions.
Immunofluorescence
The mice were sacrificed and tumors were harvested.
The tumor tissues (10–20 µm thick) were fixed in 4%
paraformaldehyde for 24 h at 4°C, paraffin-embedded and sectioned.
Following on, the tissues were dewaxed in Xylene and rehydrated
with graded alcohol. Antigen retrieval was conducted in citric acid
buffer (PH 6.0) for 10 min at 98°C. Tumor sections were blocked in
2% normal goat serum (1:200; ProteinTech Group, Inc., Chicago, IL,
USA) for 1 h at room temperature. Then, the sections were incubated
overnight at 4°C with an anti-cluster of differentiation (CD)-31
antibody (1:500; ab28364; Abcam, Cambridge, UK), an α-smooth muscle
actin (SMA) antibody (1:100; 14395-1-AP; ProteinTech Group, Inc.),
an anti-VEGFR2 antibody (1:100; ab2349; Abcam) and an anti-AGR2
antibody (1:500; EPR20164-278; Abcam). Then those sections were
washed and incubated with rhodamine-conjugated goat anti-rabbit
IgG-FITC (1:200; sc-2359; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) for 40 min at room temperature. The nuclei were
counterstained using DAPI for 15 min at room temperature
(00-4959-52; Invitrogen; Thermo Fisher Scientific, Inc.), and the
tissues were visualized using a fluorescent microscope (×20 to
×1,000; Leica DM6000B; Leica Microsystems GmbH, Wetzlar, Germany)
at magnification ×20. For each section, a total of 8 images were
taken of randomly selected fields of view.
Histology and
immunohistochemistry
Tumors were dissected and fixed in 10% formalin
solution, embedded in paraffin and sectioned into 3-mm-thick
sections. The remaining tissue sections were cryopreserved in
isopentane at −70°C. Hematoxylin and eosin (H&E) staining was
performed on 3-mm paraffin-embedded sections by staining nuclei
with alum hematoxylin (2 min), washing in running tap water for 5
min, differentiating with 0.3% acid alcohol for 1 min, rinsing with
tap water for min, staining with eosin for 2 min, dehydrating
through 95% alcohol for 10 min and then 2 incubations with absolute
alcohol for 5 min each. All H&E procedures were performed at
room temperature. A Hypoxyprobe™-1 Plus kit (Hypoxyprobe Inc.,
Burlington, MA) was used for pimonidazole staining. Mice were
intraperitoneally injected with pimonidazole at a dose of 60 mg/kg,
and sacrificed after 1 h to obtain tumor samples. Hypoxyprobe™-1
adducts were examined with an affinity-purified rabbit IgG
polyclonal antibody (1:100; ab208280; Abcam) conjugated with
horseradish peroxidase according to the manufacturer's
instructions. The images were obtained using a Leica DM6000 B
fluorescent microscope (Leica DM6000B) at magnification ×20. For
each section, a total of 8 images were taken of randomly selected
fields of view.
Image analysis
To evaluate vessel density, vessel maturity, vessel
integrity, hypoxic areas, necrotic areas and AGR2 expression in
tumor tissues, images were obtained of 8 random fields per tumor
section at ×20 magnification and analyzed with ImageJ software
(version 1.48; National Institutes of Health, Bethesda, MD, USA).
Vessel density was measured by counting CD31-positive areas per
field. The pericyte coverage, one of the most important indicators
of vessel maturity was quantified as α-SMA staining area/CD31
positive area. The vessel integrity was quantified as the radio of
the vessels surrounded by VEGFR2-positive area per field and
presented as the VEGFR2 staining area/CD31 positive area. Hypoxic
areas were measured by pimonidazole staining areas per field.
Necrotic areas were characterized by unclear cell structure and
nuclear pyknosis, and calculated as the percentage of necrotic
regions compared to the total tumor areas per field by H&E
staining. The necrotic and total areas were quantified by
CellProfiler software (version 3.0.0; http://cellprofiler.org/).
Protein extraction and
western-blotting assay
RIPA lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China) was used to lyse the tumor tissues.
BCA protein Assay kits (Takara Biotechnology Co., Ltd., Dalian,
China) were used to detect protein concentrations. Proteins (40
µg/lane) were separated via 12% SDS-PAGE and transferred onto
polyvinylidene difluoride membranes. A western-blot assay was
conducted for the detection of HIF-1α and AGR2, using a HIF-1α
antibody (1:100; 20960-1-AP; ProteinTech Group, Inc.) and an
anti-AGR2 antibody (1:500; EPR20164-278; Abcam) as the primary
antibody overnight at 4°C. The internal control protein was used to
detect the protein concentrations, along with a pro-β-actin
antibody (1:1,000; ab8226; Abcam). Detection was performed using an
enhanced chemiluminescent kit (20148; GE Healthcare Life Sciences).
An enhanced chemiluminescence detection system (ChemiDoc XRS;
Bio-Rad Laboratories, Inc., Hercules, CA, USA) and Quantity One
software (version 4.62; Bio-Rad Laboratories, Inc.) were used to
visualize and quantify the resultant bands.
Statistical analysis
All of the data were displayed as the mean ±
standard error. A Student's t-test was the principal statistical
test for two-group comparisons; one-way analysis of variance with a
Bonferroni test for pairwise comparison was used for multiple group
comparisons. All statistical analyses were performed using GraphPad
Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). A value of
P<0.05 was considered to indicate a statistically significant
difference.
Results
AuNPs delays tumor growth
To examine whether treatment with AuNPs could result
in vessel normalization in a SW620 xenograft tumor model, mice were
inoculated with cancer cells and injected subcutaneously with 1.3
mg/kg AuNPs when the tumor volume reached 175–200 mm3.
The treatments were repeated daily from day 1 to day 6, and then
repeated every other day from days 8–14. Plasma collection was
conducted at days 0, 2, 4, 6, 9, 12 and 14, and tumor harvest was
conducted at days 0, 4, 6, 9 and 14 (Fig.
1A). There was no significant different in the body weights of
mice treated with AuNPs, compared with those of mice treated with
saline (Fig. 1B); whereas, tumors in
AuNPs-treated mice exhibited a considerably slower growth pattern
as compared with those in saline-treated mice, indicating that
AuNPs have an antitumor effect on the SW620 xenograft tumor models
(P=0.02; Fig. 1B).
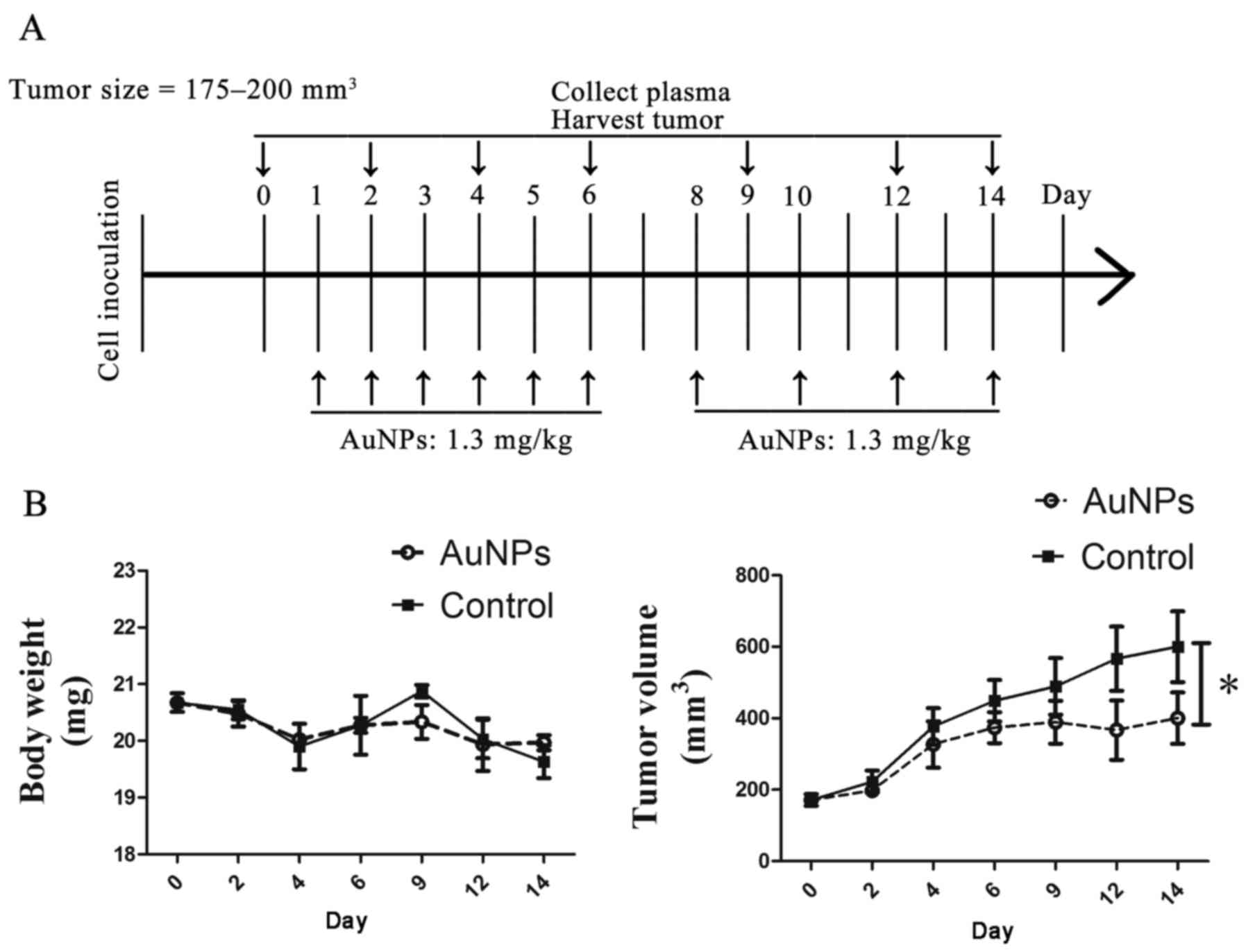 | Figure 1.AuNPs delayed tumor growth. (A)
Schematic of the study design. SW620-bearing mice were treated
daily from day 1–6, and then every other day from day 8–14, with
AuNPs (1.3 mg/kg) when the tumor size reached 175–200
mm3 (designated as day 0 and control mice). Control mice
were injected with saline. Tumors were harvested and examined at
days 0, 4, 6, 9 and 14 (n=3 per time point). Blood samples were
collected from the retro-orbital plexus at days 0, 2, 4, 6, 9, 12
and 14 (n=6 per time point). (B) Quantitative evaluation of body
weight and tumor growth after treatment with AuNPs and saline.
AuNPs treatment allowed retention of comparable body weights
(P>0.05) but delayed tumor growth (*P<0.05). AuNPs, gold
nanoparticles. |
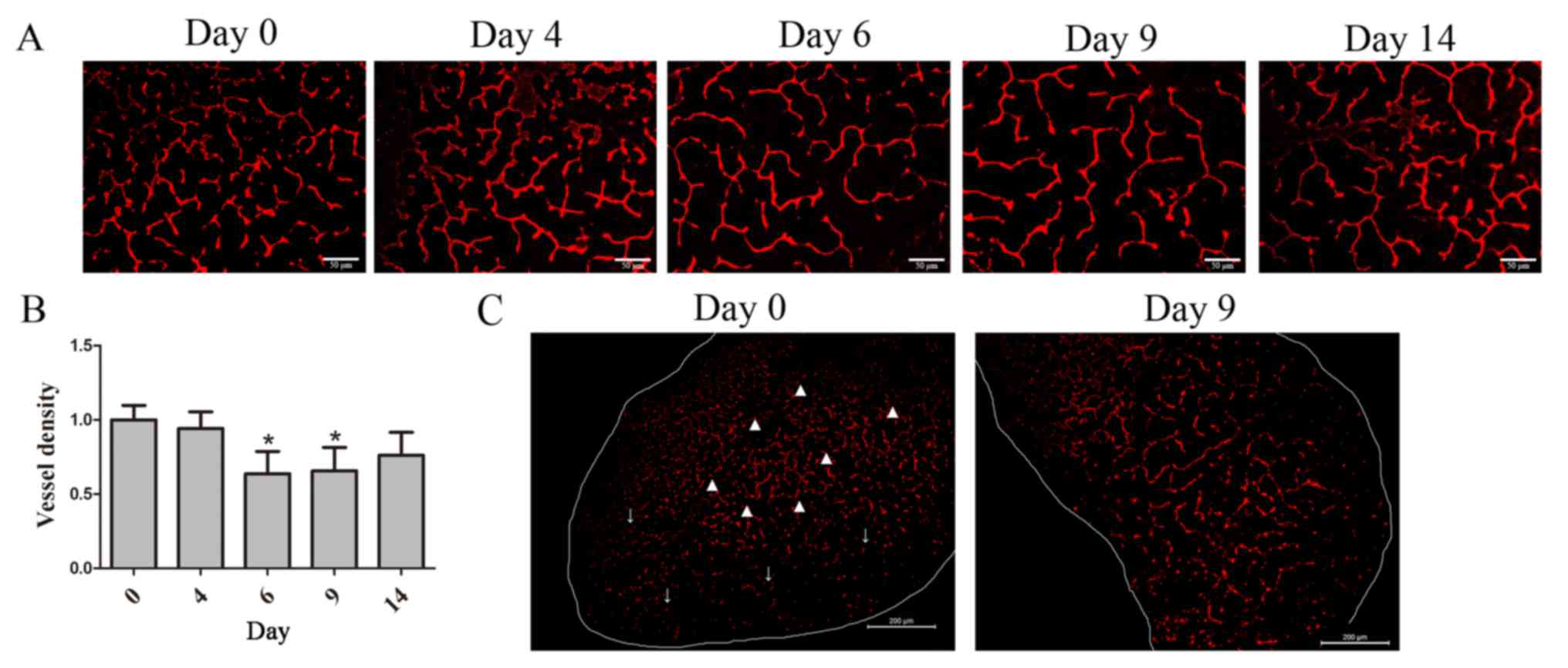
AuNPs decreases vessel density and
reconstructs vessel morphology
To demonstrate the tumor vessel density after
treatment with AuNPs, tumor tissues were stained with the
endothelial cell marker CD31. A significant decrease was exhibited
in AuNPs-treated tumor vessel density at day 6 (P=0.031) and 9
(P=0.042) (Fig. 2A). It was revealed
that vessel density decreased with AuNP treatment by day 9
(P=0.019; Fig. 2B); however,
AuNPs-treated tumor vessels reversely increased at day 14 and had
no statistic significant difference with day 0 (Fig. 2B). Furthermore, CD31 staining also
revealed that vessel in saline-treated tumor was collapsed on the
peripheral region, but dilated and discontinued in the central
region, compared with vessel in AuNPs-treated tumor at day 9, which
was well-organized and -structured (Fig.
2C). On day 9 after AuNPs treatment, vessel density in
AuNPs-treated tumor was less abundant than that in saline-treated
tumor (P=0.042; Fig. 2C). These
results suggested that AuNPs normalized vascular growth, as
demonstrated by a reduction in the number of abnormal tumor vessels
and vessel density.
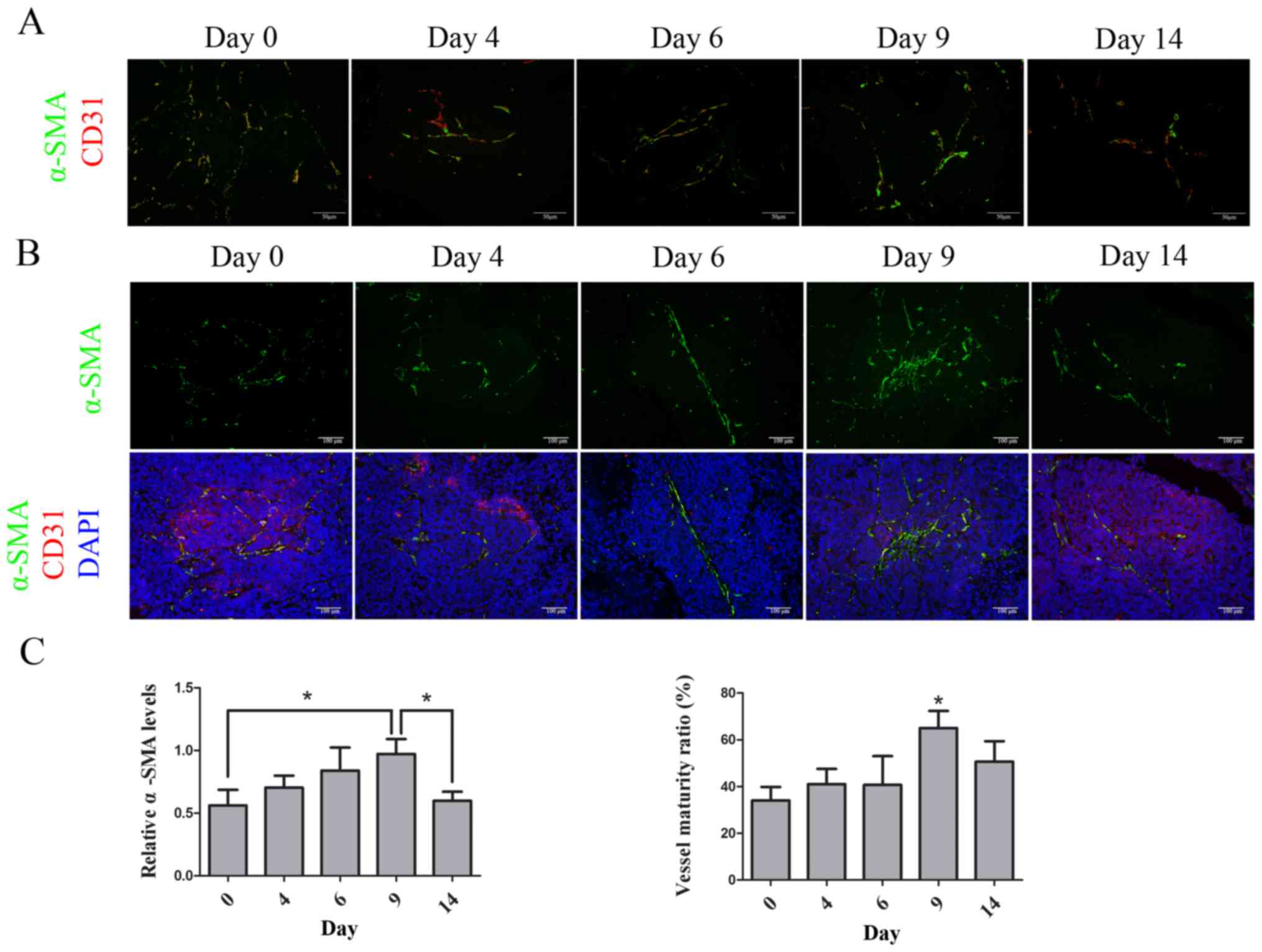
AuNPs increases pericyte adhesion and
maintains vessel maturity
To evaluate whether AuNPs could normalize tumor
vessel morphology, vessel maturity in AuNPs-treated tumors was
studied. Tumor tissues were double-stained with CD31 and the
pericyte marker α-SMA. There were α-SMA-positive cells observed at
the CD31-positive area in SW620 xenograft tumors prior to the AuNPs
treatment (day 0). After treatment with AuNPs, the α-SMA-positive
cells appeared to be constantly distributed and had closer contact
with CD31-positive areas in the tumor at days 6 and 9; however, at
day 14, as the CD31-positive area decreased, the number of
α-SMA-positive cells also decreased (Fig.
3A). Mature vessels were defined as those with α-SMA-positive
cell adhesion, and the vessel maturity ratio was calculated as the
α-SMA-positive area to the CD31-positive area, at the same scale
(32). The quantity of α-SMA-positive
cells increased at days 6 (P=0.39) and 9 (P=0.027), but decreased
again at day 14 (P=0.037) during the treatment with AuNPs.
Furthermore, the vessel maturity ratio was significantly increased,
due to the decrease of CD31 staining and increase of α-SMA staining
at day 9 (P=0.019), although it was not statistically significant
at day 14 (Fig. 3B and C). These
results indicated that AuNPs stimulated the expression of
α-SMA-positive cells that accumulated near endothelial cells and
resulted in the maturity of tumor vessels.
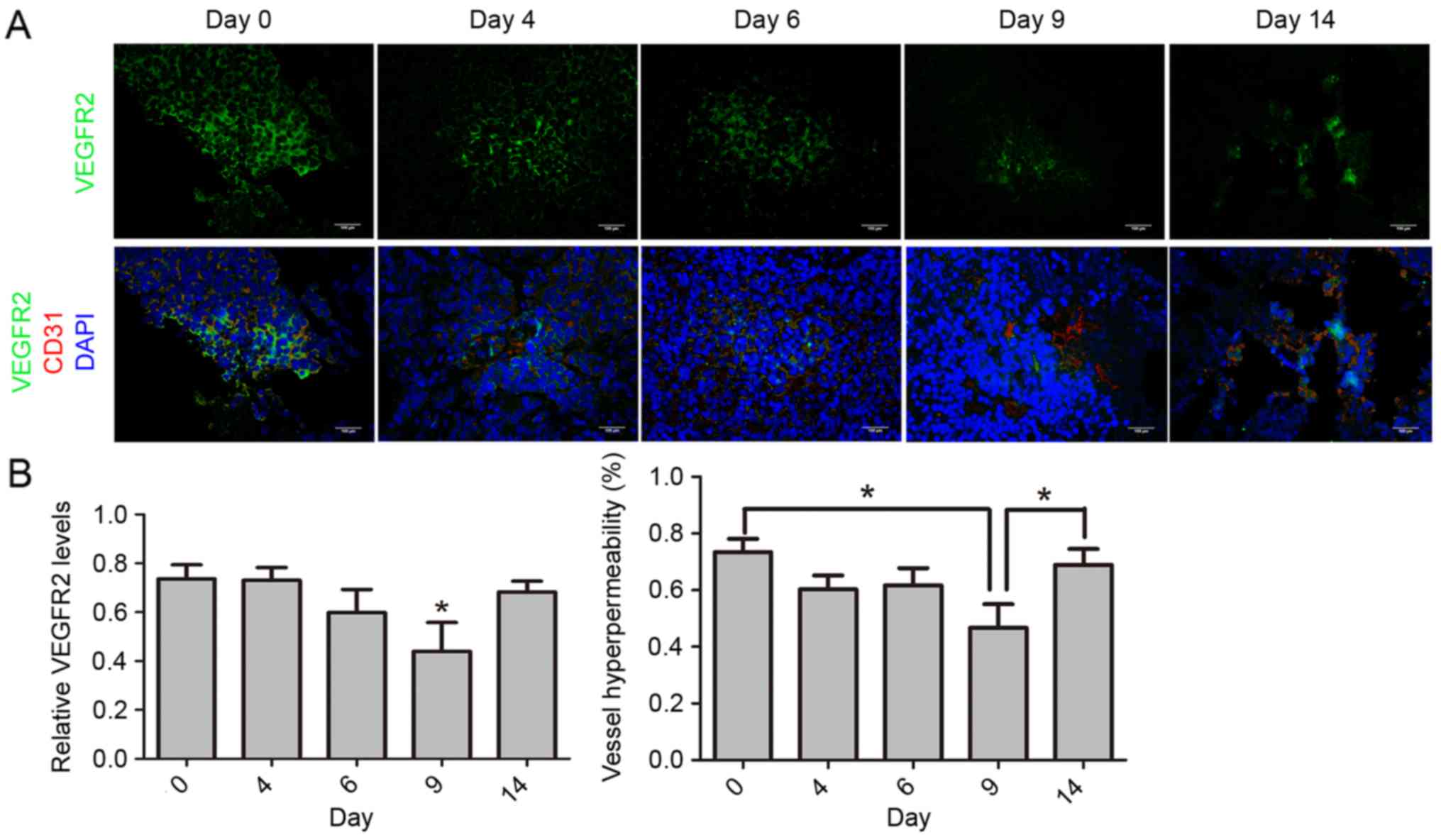
AuNPs reduces VEGFR2 expression and
alleviates vessel hyperpermeability
To further explore the morphological changes of
tumor blood vessels during AuNP treatment, vessel hyperpermeability
was also studied. It has been reported that VEGFR2 expression
levels in endothelial cells are associated with vessel
hyperpermeability, and that high expression of VEGFR2 is
responsible for tumor vessel leakage (33). It is considered that vessel
hyperpermeability is represented by the ratio of VEGFR2-positive
areas to CD31-positive areas, at the same scale. The expression
level of VEGFR2 was diminished after treatment with AuNPs at day 9
(P=0.042), but increased again at day 14 (P=0.026), remaining at a
high level. Additionally, the ratio of the VEGFR2-positive area to
the CD31-positive area was lower at day 9 (P=0.046) and became
significantly higher again at day 14 (P=0.019; Fig. 4A and B). These results indicated that
AuNPs inhibit VEGFR2 expression in tumor endothelial cells and
alleviate vessel hyperpermeability.
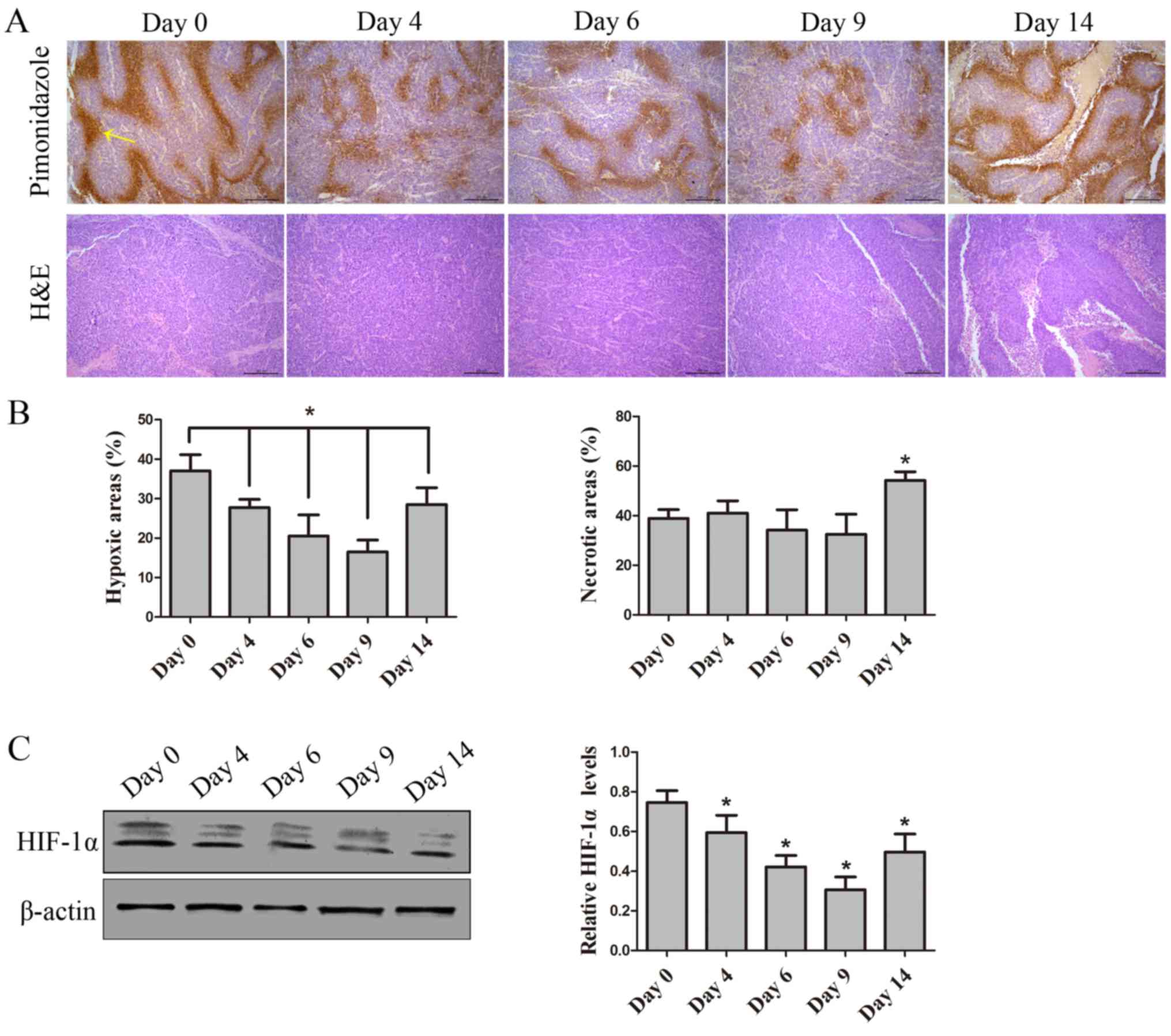
AuNPs improves areas of tumor hypoxia
and preserves tissue viability
To verify functional changes to tumor vessels that
followed morphological changes induced by AuNP treatment, tumor
oxygenation as an assessment for vessel function was utilized.
Pimonidazole staining was conducted to evaluate tumor hypoxia,
which demonstrated that AuNPs reduced tumor hypoxia by days 4 and
9, particularly by day 9; however, the tumor hypoxia increased
again after treatment for 14 days (P=0.31). Furthermore, H&E
staining demonstrated that, at days 0, 4, 6 and 9, there was no
significant difference in viability among the AuNP-treated tumor
tissues; however, compared with day 0, there was an increase in the
size of necrotic areas by day 14 (P=0.028; Figs. 5A and B). Hypoxia is mediated by
HIF-1α (34); thus to further
identify improvements to hypoxia, expression of HIF-in AuNP-treated
tumor tissue was assessed. The data demonstrated that expression of
HIF-1α decreased by days 6 and 9, but increased by day 14 following
AuNPs treatment (P=0.043; Fig. 5C).
Reduced tumor hypoxia indicated that AuNPs can normalize tumor
vessels and improve vessel function.
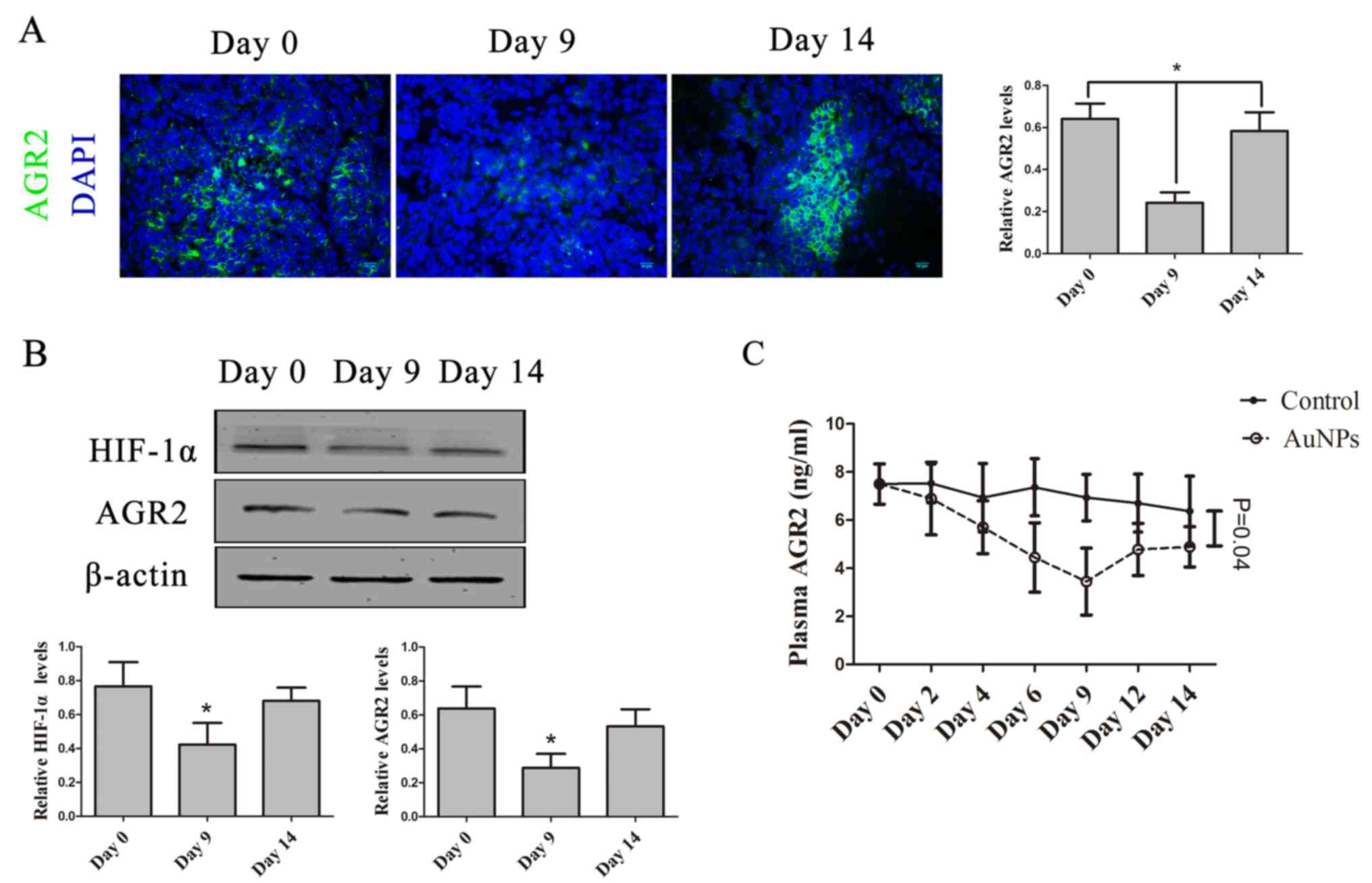
AuNPs abates AGR2 expression during
the window of vessel normalization
AGR2 was reported to increase in tumor cells under
hypoxia, and be regulated by HIF-1α (18,23). In
the present study, tumor hypoxia observably improved. To
investigate whether AGR2 could be used as biomarker to detect
AuNP-treated tumor hypoxia improvement during vessel normalization,
the AGR2 expression changes in tumor tissue and plasma following
AuNP-treatment was assessed. AGR2 protein expression in tumor
tissue decreased by day 9, but increased again by day 14 (P=0.027;
Fig. 6A). There was significant
decrease in AGR2 protein expression by day 9, similar to changes in
HIF-1α protein levels in tumor tissues. AGR2 protein expression
increased again by day 14, after AuNP-treatment (P=0.046; Fig. 6B). Furthermore, plasma AGR2 levels
were significantly decreased by day 9, though increased again by
day 14 (P=0.044; Fig. 6C). These data
demonstrate that AGR2, regulated by HIF-1α, could be a supervisory
biomarker to detect vessel normalization induced by AuNPs.
Discussion
Anti-angiogenic agents induce a transient window of
vascular normalization where both chemotherapy and radiotherapy
achieve a better curative effect (3,4). Direct
morphological changes were observed using histological methods,
vessel function detection by PET-CT and dynamic MRI, particularly
applied to the monitoring of tumor vessel normalization (6,8); however,
these methods were neither invasive or expensive, which restricted
the combination of anti-angiogenic therapy and chemotherapy in
clinic. A reliable and easily detected biomarker was required
during anti-angiogenic therapy to identify a suitable time for
antitumor treatment.
In the present study, the practicability of AGR2 as
a marker to detect tumor vascular normalization after treatment
with AuNPs was analyzed. Previous studies indicated that AuNPs
interrupted angiogenesis via various pathways, including VEGF and
VEGFR2 in vitro (3,4). AuNPs were also reported to be
biocompatible and stable, with low cytotoxicity (24–26). In
the present study, mice were treated with 1.3 mg/kg AuNPs daily
from day 0–6, which was then repeated every other day from day
8–14. It was revealed that an SW620-inoculated xenograft model was
adequate for monitoring the vascular normalization induced by
AuNPs. It was demonstrated that AGR2 could be used as a potential
marker for observing vessel normalization during AuNP-mediated
anti-angiogenesis.
To investigate whether AuNPs have the ability to
modify vessel structure, the vessel density, pericyte coverage and
vessel hyperpermeability after AuNPs treatment were studied. The
results demonstrated that tumor vessel density was significantly
reduced at day 6 and 9 after AuNPs treatment. Pericyte coverage
increased by day 9 in AuNPs-treated tumors. The vessel maturity
ratio significantly increased by day 9, probably due to reduction
of abnormal tumor vessels and increases in pericyte coverage after
AuNPs treatment. It was also demonstrated that AuNPs significantly
inhibited VEGFR2 expression at day 9, resulting in lower vessel
hyperpermeability in the tumor tissue. To investigate the
functional improvement that followed structure changes in the tumor
vessels after AuNPs treatment, tumor hypoxia changes were analyzed.
Tumor hypoxia was reduced at days 4, 6 and 9 after AuNPs treatment,
with improvement was observed at day 9. In addition, there was no
significant difference in the necrosis rate of the tumor tissue at
days 0, 4, 6 and 9, but there was a slight increase observed at day
14. The data corroborated that tumor vessel normalization could be
induced by anti-angiogenic treatment (3,4).
Using histology and functional methodology, it was
demonstrated that anti-angiogenic treatment with AuNPs provided
vascular normalization in the tumor at day 9. Based on these
results, the expression levels of AGR2 in tumor tissue were
investigated. It appeared that AGR2 had lower expression, along
with a decreased hypoxic area in the tumor tissue after treatment
with AuNPs for 9 days, and, at day 14, an increase in AGR2
expression and hypoxia was observed in the tumor tissue. These
results are concordant with those of previous studies, which
identified that AGR2 was overexpressed in hypoxic areas and was
regulated by HIF-1α (3,4). Furthermore, plasma AGR2 levels in mice
treated with AuNPs were monitored. AGR2 levels decreased following
treatment with AuNPs and reached the lowest point at day 9;
however, plasma AGR2 levels increased again by day 14, parallel to
the development of tumor hypoxia. The data illustrate that
AuNPs-mediated AGR2 expression could monitor the improvement of
hypoxia in anti-angiogenic therapy and plasma AGR2 levels could be
a supervisory marker for the window of vascular normalization.
Overall, the data illustrate that treatment with
AuNPs provides a transient time window of vascular normalization,
which improved pericyte coverage, vessel hyperpermeability and
tumor hypoxia. Furthermore, AuNPs may suppress AGR2 expression in
the window of vascular normalization via improving the hypoxic
tumor microenvironment. Excessive treatment with AuNPs indirectly,
through hypoxia regulation, stimulated AGR2 to be expressed once
again in the tumor; therefore, AGR2 may be a potential monitor of
vascular normalization during AuNPs treatment. A study could be
conducted to develop a more practical and rational application for
AuNPs treatment combined with chemotherapeutic agents; however,
whether AuNPs are directly involved in regulating AGR2 expression
remains unknown, so further study is required to analyze the
reliability of AGR2 expression levels in monitoring vessel
normalization induced by other anti-angiogenic agents, including
bevacizumab, dovitinib and endostatin. Furthermore, plasma AGR2
levels are known to be a blood-based biomarker for metastasis and
recurrence in numerous malignant tumors, including ovarian and
pancreatic cancer, and lung adenocarcinoma (35,36).
Peripheral blood AGR2 levels in patients with breast cancer,
papillary thyroid carcinoma or metastatic colorectal cancer are
associated with tumor progression and prognosis (12,37–40).
Therefore, the clinical applications of AGR2 expression for
identifying vessel normalization in tumors during anti-angiogenic
treatment are viable for future work.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural
Science Foundation of China (grant no., 81472849), the Guangdong
Natural Science Research (grant no., 2014A030313383) and the
Guangdong High-level University Construction Fund for Jinan
University (grant no., 88016013034).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
FP, WL and WY conducted the study and performed the
statistical analysis. FP and YP performed the study design and
drafted the manuscript. All the authors participated in the
discussion, and read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures involving animals were approved by
the Ethics Committee of Jinan University. All animal studies also
comply with the ARRIVE guidelines and the AVMA euthanasia
guidelines 2013.
Patient consent for publication
Not applicable.
Patient consent for publication
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AGR2
|
anterior gradient 2
|
|
AuNPs
|
gold nanoparticles
|
References
|
1
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jain RK: Anti-angiogenesis strategies
revisited: From starving tumors to alleviating hypoxia. Cancer
Cell. 26:605–622. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jain RK: Normalization of tumor
vasculature: an emerging concept in antiangiogenic therapy.
Science. 307:58–62. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jain RK: Normalizing tumor vasculature
with anti-angiogenic therapy: A new paradigm for combination
therapy. Nat Med. 7:987–989. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cobleigh MA, Langmuir VK, Sledge GW,
Miller KD, Haney L, Novotny WF, Reimann JD and Vassel A: A phase
I/II dose-escalation trial of bevacizumab in previously treated
metastatic breast cancer. Semin Oncol. 30 5 Suppl 16:S117–S124.
2003. View Article : Google Scholar
|
|
6
|
Goel S, Duda DG, Xu L, Munn LL, Boucher Y,
Fukumura D and Jain RK: Normalization of the vasculature for
treatment of cancer and other diseases. Physiol Rev. 91:1071–1121.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carmeliet P and Jain RK: Molecular
mechanisms and clinical applications of angiogenesis. Nature.
473:298–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hernández-Agudo E, Mondejar T,
Soto-Montenegro ML, Megías D, Mouron S, Sanchez J, Hidalgo M,
Lopez-Casas PP, Mulero F, Desco M, et al: Monitoring vascular
normalization induced by antiangiogenic treatment with
18F-fluoromisonidazole-PET. Mol Oncol. 10:704–718. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bao X, Wang MW, Luo JM, Wang SY, Zhang YP
and Zhang YJ: Optimization of early response monitoring and
prediction of cancer anti-angiogenesis therapy via noninvasive PET
molecular imaging strategies of multifactorial bioparameters.
Theranostics. 6:2084–2098. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park SW, Zhen GH, Verhaeghe C, Nakagami Y,
Nguyenvu LT, Barczak AJ, Killeen N and Erle DJ: The protein
disulfide isomerase AGR2 is essential for production of intestinal
mucus. Proc Natl Acad Sci USA. 106:6950–6955. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu D, Rudland PS, Sibson DR,
Platt-Higgins A and Barraclough R: Human homologue of cement gland
protein, a novel metastasis inducer associated with breast
carcinomas. Cancer Res. 65:3796–3805. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fritzsche FR, Dah E, Pahl S, Burkhardt M,
Luo J, Mayordomo E, Gansukh T, Dankof A, Knuechel R, Denkert C, et
al: Prognostic relevance of AGR2 expression in breast cancer. Clin
Cancer Res. 12:1728–1734. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Innes HE, Liu D, Barraclough R, Davies MP,
O'Neill PA, Platt-Higgins A, de Silva Rudland S, Sibson DR and
Rudland PS: Significance of the metastasis-inducing protein AGR2
for outcome in hormonally treated breast cancer patients. Br J
Cancer. 94:1057–1065. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Z, Hao Y and Lowe AW: The
adenocarcinoma-associated antigen, AGR2, promotes tumor growth,
cell migration, and cellular transformation. Cancer Res.
68:492–497. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ramachandran V, Arumugam T, Wang HM and
Logsdon CD: Anterior gradient 2 is expressed and secreted during
the development of pancreatic cancer and promotes cancer cell
survival. Cancer Res. 68:7811–7818. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang YX, Ali TZ, Zhou H, D'Souza DR, Lu
Y, Jaffe J, Liu Z, Passaniti A and Hamburger AW: ErbB3 binding
protein 1 represses metastasis-promoting gene anterior gradient
protein 2 in prostate cancer. Cancer Res. 70:240–248. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gao H, Xu XC, Chen B, Wang F, Zhang W,
Geng H and Wang Y: Anterior gradient 2: A new target to treat
colorectal cancer. Med Hypotheses. 80:706–708. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hong XY, Wang J and Li Z: AGR2 expression
is regulated by HIF-1 and contributes to growth and angiogenesis of
glioblastoma. Cell Biochem Biophys. 67:1487–1495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chevet E, Fessart D, Delom F, Mulot A,
Vojtesek B, Hrstka R, Murray E, Gray T and Hupp T: Emerging roles
for the pro-oncogenic anterior gradient-2 in cancer development.
Oncogene. 32:2499–2509. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brychtova V, Vojtesek B and Hrstka R:
Anterior gradient 2: A novel player in tumor cell biology. Cancer
Lett. 304:1–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Valladares-Ayerbes M, Blanco-Calvo M,
Reboredo M, Lorenzo-Patiño MJ, Iglesias-Díaz P, Haz M, Díaz-Prado
S, Medina V, Santamarina I, Pértega S, et al: Evaluation of the
adenocarcinoma-associated gene AGR2 and the intestinal stem cell
marker LGR5 as biomarkers in colorectal cancer. Int J Mol Sci.
13:4367–4387. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xue H, Lü B, Zhang J, Wu M, Huang Q, Wu Q,
Sheng H, Wu D, Hu J and Lai M: Identification of serum biomarkers
for colorectal cancer metastasis using a differential secretome
approach. J Proteome Res. 9:545–555. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Z, Zhu Q, Hu L, Chen H, Wu Z and Li D:
Anterior gradient 2 is a binding stabilizer of hypoxia inducible
factor-1α that enhances CoCl2-induced doxorubicin resistance in
breast cancer cells. Cancer Sci. 106:1041–1049. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pan Y, Wu Q, Liu R, Shao M, Pi J, Zhao X
and Qin L: Inhibition effects of gold nanoparticles on
proliferation and migration in hepatic carcinoma-conditioned
HUVECs. Bioorg Med Chem Lett. 24:679–684. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pan Y, Wu Q, Qin L, Cai J and Du B: Gold
nanoparticles inhibit VEGF165-induced migration and tube formation
of endothelial cells via the Akt pathway. Biomed Res Int.
2014:4186242014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pan Y, Ding H, Qin L, Zhao X, Cai J and Du
B: Gold nanoparticles induce nanostructural reorganization of
VEGFR2 to repress angiogenesis. J Biomed Nanotechnol. 9:1746–1756.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ernsting MJ, Murakami M, Roy A and Li SD:
Factors controlling the pharmacokinetics, biodistribution and
intratumoral penetration of nanoparticles. J Control Release.
172:782–794. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Y, Lian Y, Zhang LT, Aldousari SM,
Hedia HS, Asiri SA and Liu WK: Cell and nanoparticle transport in
tumour microvasculature: The role of size, shape and surface
functionality of nanoparticles. Interface Focus. 6:201500862016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Baban DF and Seymour LW: Control of tumour
vascular permeability. Adv Drug Deliv Rev. 34:109–119. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li W, Zhao X, Du B, Li X, Liu S, Yang XY,
Ding H, Yang W, Pan F, Wu X, et al: gold nanoparticle-mediated
targeted delivery of recombinant human endostatin normalizes tumour
vasculature and improves cancer therapy. Sci Rep. 6:306192016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Franco M, Man S, Chen L, Emmenegger U,
Shaked Y, Cheung AM, Brown AS, Hicklin DJ, Foster FS and Kerbel RS:
Targeted anti-vascular endothelial growth factor receptor-2 therapy
leads to short-term and long-term impairment of vascular function
and increase in tumor hypoxia. Cancer Res. 66:3639–3648. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yonenaga Y, Mori A, Onodera H, Yasuda S,
Oe H, Fujimoto A, Tachibana T and Imamura M: Absence of smooth
muscle actin-positive pericyte coverage of tumor vessels correlates
with hematogenous metastasis and prognosis of colorectal cancer
patients. Oncology. 69:159–166. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gille H, Kowalski J, Li B, LeCouter J,
Moffat B, Zioncheck TF, Pelletier N and Ferrara N: Analysis of
biological effects and signaling properties of Flt-1 (VEGFR-1) and
KDR (VEGFR-2). A reassessment using novel receptor-specific
vascular endothelial growth factor mutants. J Biol Chem.
276:3222–3230. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Facciabene A, Peng X, Hagemann IS, Balint
K, Barchetti A, Wang LP, Gimotty PA, Gilks CB, Lal P, Zhang L and
Coukos G: Tumour hypoxia promotes tolerance and angiogenesis via
CCL28 and Treg cells. Nature. 475:226–230. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kani K, Malihi PD, Jiang Y, Wang H, Wang
Y, Ruderman DL, Agus DB, Mallick P and Gross ME: Anterior gradient
2 (AGR2): Blood-based biomarker elevated in metastatic prostate
cancer associated with the neuroendocrine phenotype. Prostate.
73:306–315. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen R, Pan S, Duan X, Nelson BH, Sahota
RA, de Rham S, Kozarek RA, McIntosh M and Brentnall TA: Elevated
level of anterior gradient-2 in pancreatic juice from patients with
pre-malignant pancreatic neoplasia. Mol Cancer. 9:1492010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chung K, Nishiyama N, Yamano S, Komatsu H,
Hanada S, Wei M, Wanibuchi H, Suehiro S and Kakehashi A: Serum AGR2
as an early diagnostic and postoperative prognostic biomarker of
human lung adenocarcinoma. Cancer Biomark. 10:101–107. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tohti M, Li J, Tang C, Wen G, Abdujilil A,
Yizim P and Ma C: Serum AGR2 as a useful biomarker for pituitary
adenomas. Clin Neurol Neurosurg. 154:19–22. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Di Maro G, Salerno P, Unger K, Orlandella
FM, Monaco M, Chiappetta G, Thomas G, Oczko-Wojciechowska M,
Masullo M, Jarzab B, et al: Anterior gradient protein 2 promotes
survival, migration and invasion of papillary thyroid carcinoma
cells. Mol Cancer. 13:1602014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mostert B, Sieuwerts AM, Bolt-de Vries J,
Kraan J, Lalmahomed Z, van Galen A, van der Spoel P, de Weerd V,
Ramírez-Moreno R, Smid M, et al: mRNA expression profiles in
circulating tumor cells of metastatic colorectal cancer patients.
Mol Oncol. 9:920–932. 2015. View Article : Google Scholar : PubMed/NCBI
|