Introduction
Type 1 diabetes mellitus, or insulin-dependent
diabetes, is a form of diabetes mellitus which results from
considerable destruction of the insulin-producing β cells in the
islets of the pancreas by autoimmune disorders (1). In general, islet transplantation and
engraftment is believed to be a potential radical cure for diabetes
mellitus. However, the requirement for human donors is an
inevitable limiting factor to obtain sufficient tissues to meet the
demand for islet transplantation treatment (2). Immunosuppressive therapy and technology
for preserving the isolated islets from the pancreas during
surgical treatment have emerged as useful approaches to improve
patient survival (3).
The microenvironment of the islets in the pancreas
is considered to be critical for the maintenance of cell viability
for implantation. For example, proinflammatory cytokines, activated
neutrophils and neutrophil elastase (NE) released from these
neutrophils in the microenvironments of surgically isolated islets
surrounding pancreatic tissues may directly cause injury to islet
grafts. Therefore, technology to protect isolated islets from
deleterious damage is required to increase the viability of grafts
and naturally improve surgical outcomes of islet transplantation
(4). Previous studies have indicated
that the modulation of proinflammatory cytokines, including tumor
necrosis factor α and interleukin 6, which are markedly increased
at the end of warm digestion during islet isolation and exhibit
direct cytotoxic activity against the islets causing their
apoptosis, significantly contribute to the yield of islets
(4). In addition, treatment with
sivelestat, an NE inhibitor, resulted in an improvement of the
viability of islet grafts in a mouse allotransplant model (4). The survival and insulin function of an
islet graft was enhanced by the combined transplantation of
pancreatic islets and adipose tissue-derived stem cells (ADSCs)
(5), suggesting that the
microenvironment of islets serves a function in the survival of
islets in transplantation. Indeed, ADSCs exhibit anti-inflammatory
properties (6).
Substantial effort has been invested into the
development of cellular reprogramming of differentiated cells,
including ADSCs (7). The defined
Yamanaka factors, octamer binding transcription factor 3/4,
Sex-determining region Y-box 2, Kruppel-like factor 4, and v-myc
avian myelocytomatosis viral oncogene homolog, have been
demonstrated to induce cellular reprogramming into a pluripotent
state (8). ADSCs possess the
multipotential for differentiation and are prone to induction
(7). Eventually, the expression of
cluster of differentiation 90, or Thy-1, was revealed to be useful
for the selection of reprogramming-prone cells to improve the
efficiency of ADSC induction into pancreatic cells (9). Although the Yamanaka factors elicit full
reprogramming into a pluripotent state, several tissues (including
pancreatic β-like cells) are proposed to be induced not via the
pluripotent state, but directly from differentiated cells via
pancreas/duodenum homeobox protein 1 (Pdx1), neurogenin-3 and V-maf
musculoaponeurotic fibrosarcoma oncogene homolog (10,11). In
addition, other studies have demonstrated that small molecules
(12) and microRNA-302 (13), which is a non-coding RNA, were able to
induce pancreatic β-like cells. Several microRNAs, including
microRNA-302, were previously demonstrated able to induce the
cellular reprogramming of ADSCs (14). Therefore, to elucidate the methods
required to induce pancreatic β-like cells by defined factors or
the development of a simple technology would be beneficial in a
clinical setting for the purposes of regenerative medicine for
insulin-dependent diabetes.
In the present study, a simple method to address
this problem was investigated using the culture of ADSCs in
cell-free conditioned medium (CM) from insulinoma MIN6, which are
insulin-producing tumor cells. The culture of ADSCs in the MIN6-CM
resulted in an increase in insulin expression. Whole-genome
epigenetic profiling data indicated that the culture of ADSCs in
cell-free CM from MIN6 resulted in the induction of epigenetic
modification toward an insulin-producing transcriptional phenotype.
Cell culture in the MIN6-CM may have resulted in the cell-to-cell
transmission of the phenotype of insulin-producing β cells and
provides additional rationale to study this protocol for use in
regenerative medicine.
Materials and methods
Cell culture and exosome
isolation
Mouse ADSCs (RIKEN BioResource Centre, Tsukuba,
Japan) were cultured in Dulbecco's modified Eagle's medium (DMEM;
Nacalai Tesque, Inc., Kyoto, Japan) containing 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) in a CO2 incubator at 37°C. MIN6 cells, which were
supplied by Professor J. Miyazaki, and the subclones, m9 and m14,
derived from parental MIN6 cells, which were from Professor T.
Miki, were cultured in DMEM containing 10% FBS and
2-mercapteethanol in a CO2 incubator at 37°C (15). ExoQuick-TC (System Biosciences, Palo
Alto, CA, USA) was used for exosome isolation according to the
manufacturer's protocol.
Pancreatic differentiation
Preparation of MIN6-CM was performed as previously
described (9). ADSCs were cultured in
the MIN6-CM for 72 h, and non-conditioned DMEM with 10% FBS was
used as the control.
Chromatin immunoprecipitation (ChIP)
sequencing
Cells were trypsinized and homogenized in 10 ml PBS,
were resuspended in 1% formaldehyde in PBS, and cross-linked at
room temperature for 5 min. The reaction was stopped by adding 0.2
M glycine. The cells were washed twice with cold PBS and
resuspended in ice-cold cell lysis buffer (10 mM NaCl; 10 mM
Tris-HCl, pH 8.0; 0.5% NP-40). The samples were washed with cell
lysis buffer and resuspended in nuclear lysis buffer (1% SDS; 10 mM
EDTA; 10 mM Tris-HCl, pH 8.0). The samples were incubated for 10
min at 4°C and added to a ChIP buffer (50 mM Tris-HCl, pH 8.0; 167
mM NaCl; 1.1% TritonX-100; 0.11% sodium deoxycholate; protease
inhibitor mix). Chromatin was sonicated to 300–500 bp, followed by
standard immunoprecipitation analysis with the following antibody:
a 1:1,000 dilution of mouse Pdx1 (cat. no., LS-C145426, LSBio,
Seattle, WA, USA). Total DNA was sequenced using HiSeq 2000
(Illumina, Inc., San Diego, CA, USA).
RNA expression study
For microarray experiments, following assessment of
quality by electrophoresis in gel at 100 V, 500 ng extracted total
RNA, extracted using a TRIzol kit (Thermo Fisher Scientific, Inc.)
as labeled with Cyanine-3 (Cy3) using the Low Input Quick Amp
Labeling kit (Agilent Technologies, Inc., Santa Clara, CA, USA)
according to the manufacturer's protocol. Dye incorporation and
cRNA yield were checked with the NanoDrop ND-2000 Spectrophotometer
(230 and 260 nm; Thermo Fisher Scientific, Inc.). The labeled RNAs
were hybridized onto the Agilent Mouse Microarray (Agilent
Technologies, Inc.) for 17 h at 65°C in a rotating Agilent
hybridization oven (Agilent Technologies, Inc.). Following
hybridization, microarrays were washed for 1 min at room
temperature with GE Wash Buffer 1 (Agilent Technologies, Inc.) and
for 1 min at 37°C with GE Wash Buffer 2 (Agilent Technologies,
Inc.), then dried immediately by brief centrifugation at room
temperature. Following washes with GE Wash Buffer 1 and GE Wash
Buffer 2 for 1 min each, the fluorescent signals were scanned with
the Agilent DNA Microarray Scanner (G2565CA; Agilent Technologies,
Inc.) and analyzed using Feature Extraction Software v.10.10
(Agilent Technologies, Inc.). For reverse
transcription-quantitative polymerase chain reaction (PCR), total
RNA were reverse transcribed using Rever Tra Ace RT kit (FSQ-101;
Toyobo Life Science, Osaka, Japan) with amplification by
Thunderbird® Syber qPCR Mix kit (QPS-101; Toyobo Life
Science) using a Light Cycler (Roche Diagnostics, Tokyo, Japan)
(16). The conditions were: 95°C for
10 sec; 60°C for 10 sec; and 72°C for 10 sec, which was repeated 40
times. The primers used were: Pdx1, forward,
AGCAGTCTGAGGGTGAGCGGGTCT, and reverse, AGCAGTCTGAGGGTGAGCGGGTCT;
and Ins2, forward, TCCGCTACAATCAAAAACCAT, and reverse,
GCTGGGTAGTGGTGGGTCTA. For reference, we used primer for Gapdh,
forward, ACCACAGTCCATGCCATCAC, and reverse,
TCCACCACCCTGTTGCTGTA.
Results
As it was hypothesized that insulinoma cell
line-derived CM may induce insulin expression in murine ADSCs,
insulin expression was assessed using a DNA gene array to explore
the mechanistic insight of the CM (data not shown). Pdx1 is a
critical pancreatic transcription factor upstream of several genes.
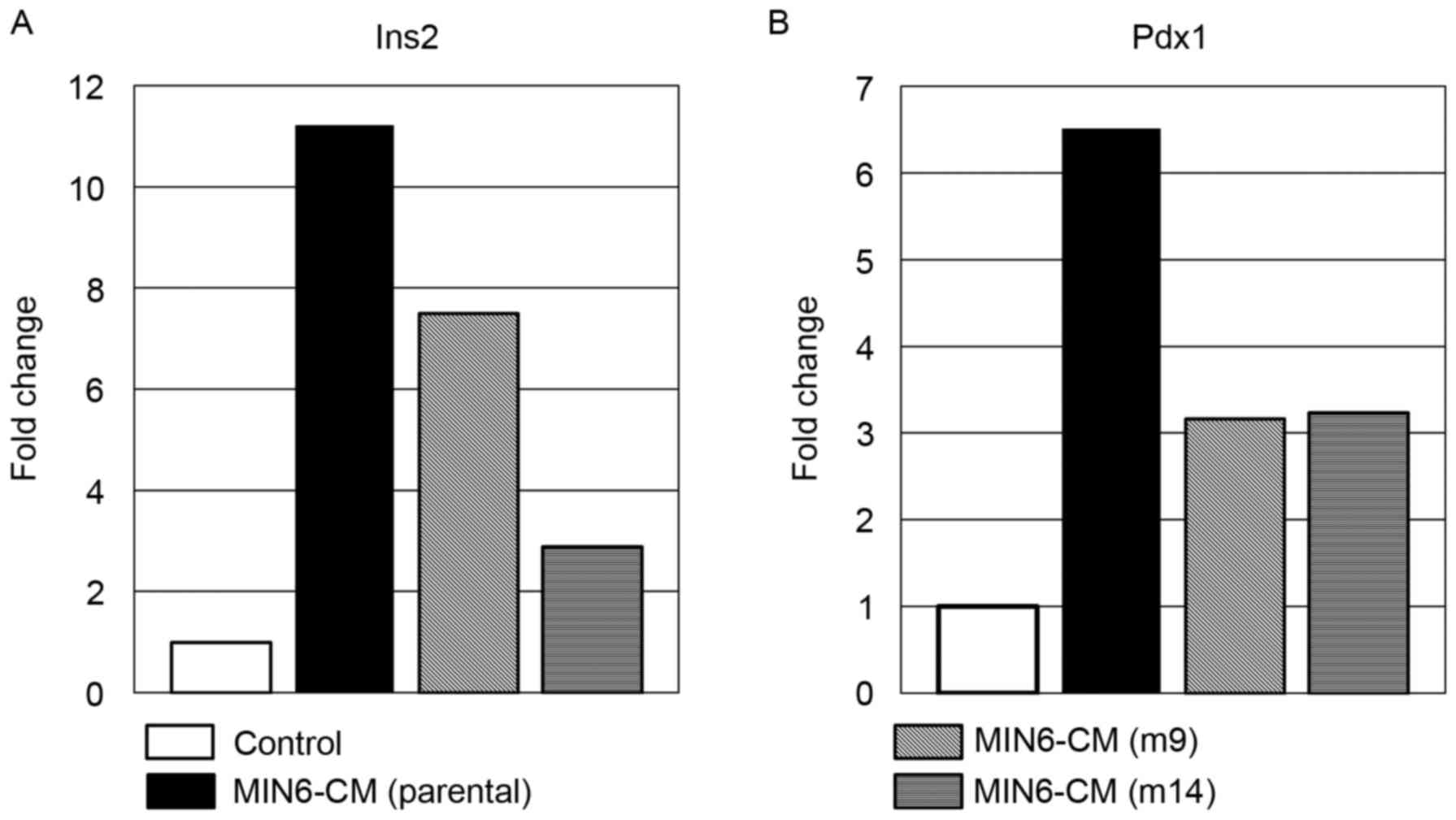
The expression of insulin II (Fig.
1A) and Pdx1 (Fig. 1B) was
upregulated in MIN6-CM-cultured ADSC. Expression in parental
MIN6-CM-cultured murine ADSCs was further increased compared with
ADSCs cultured in m9 or m14 MIN6-CM. These results demonstrated
that there was variability across these subclones. As Pdx1 mRNA
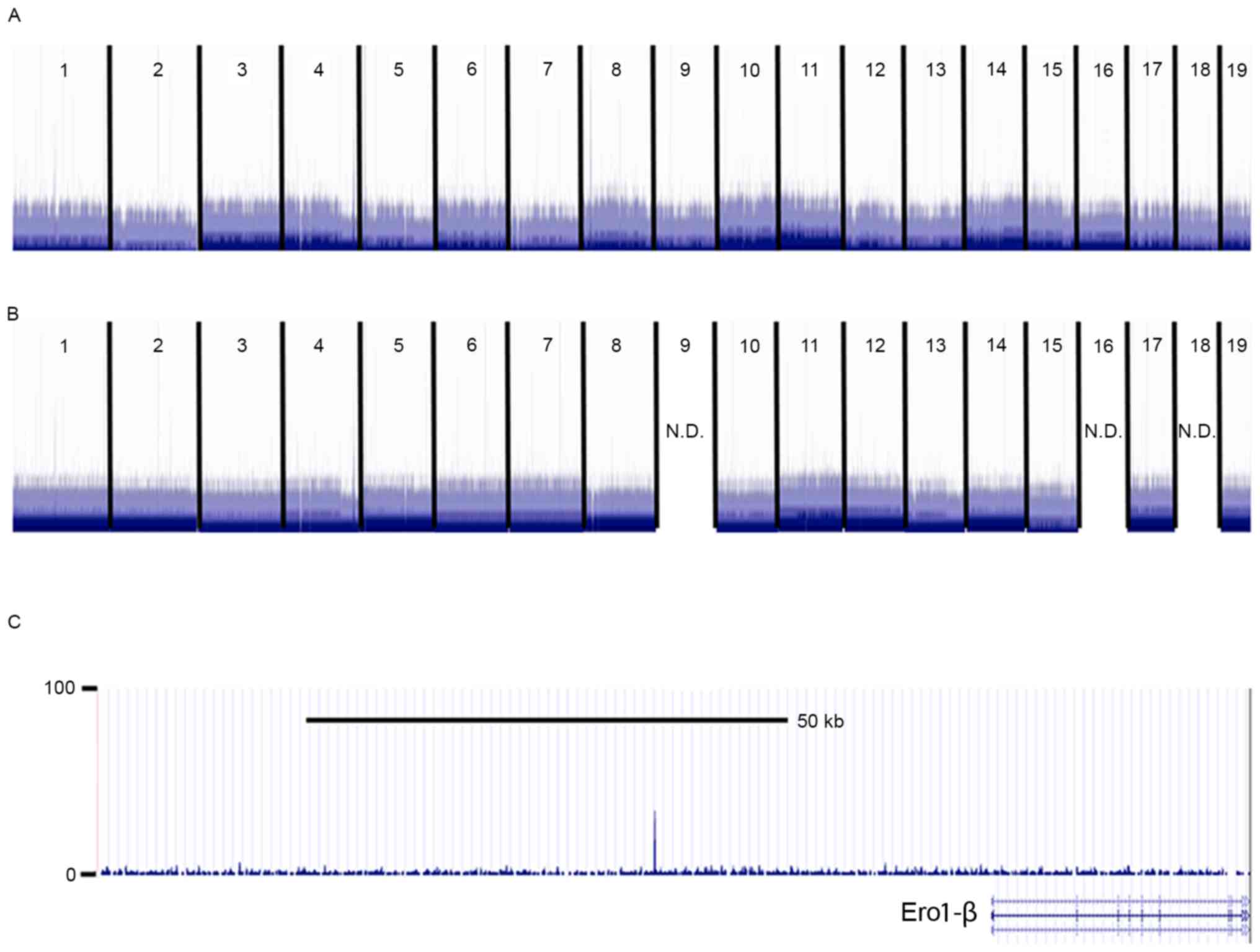
should exist in exosomes isolated from CM, the potential existence
of Pdx1 mRNA in the MIN6-CM cell line of the present study was
assessed using global sequence analysis. The read number was ~0.2,
suggesting that the expression level of Pdx1 mRNA was low in
purified exosomes (Fig. 2).
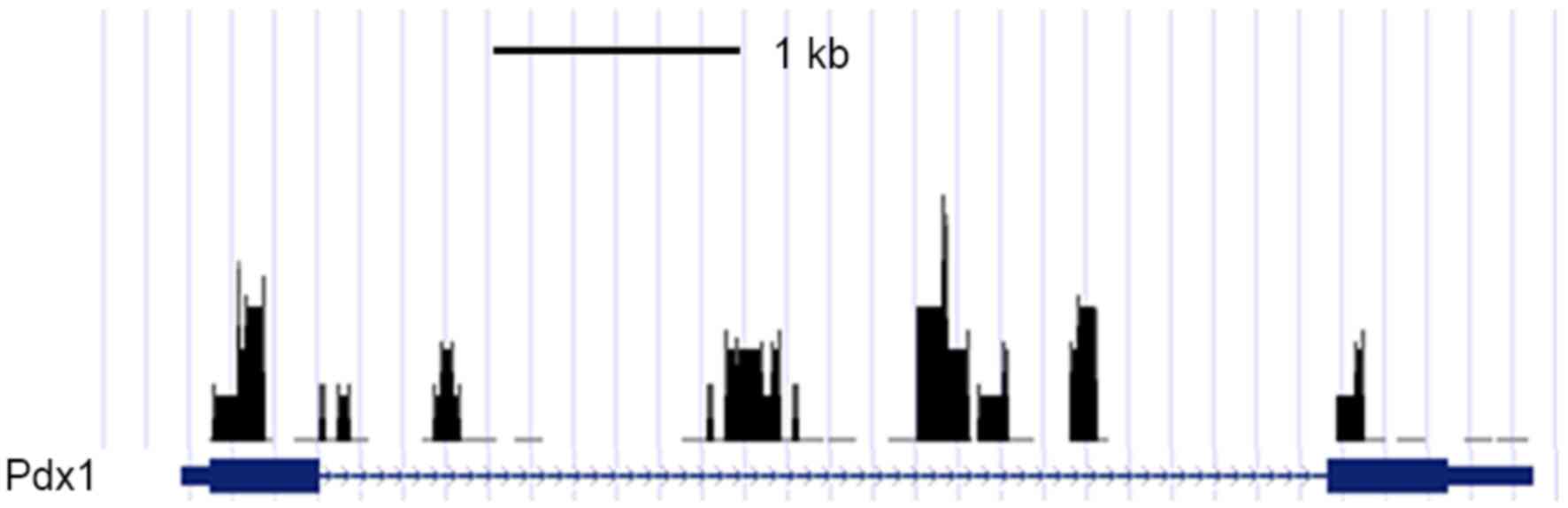
The binding of Pdx1 was subsequently assessed using
a ChIP assay. The ChIP analysis indicated that, compared with the
control cells, multi-methylated forms of histone H3 lysine 4 were
preferentially associated with the promoter sequence of the
Pdx1 genes in CM-cultured ADSCs (Fig. 3). In addition, the ChIP assay also
indicated that Pdx1 was bound to the promoter region of the ER
oxidoreductase 1 (Ero1)-β gene (Fig.
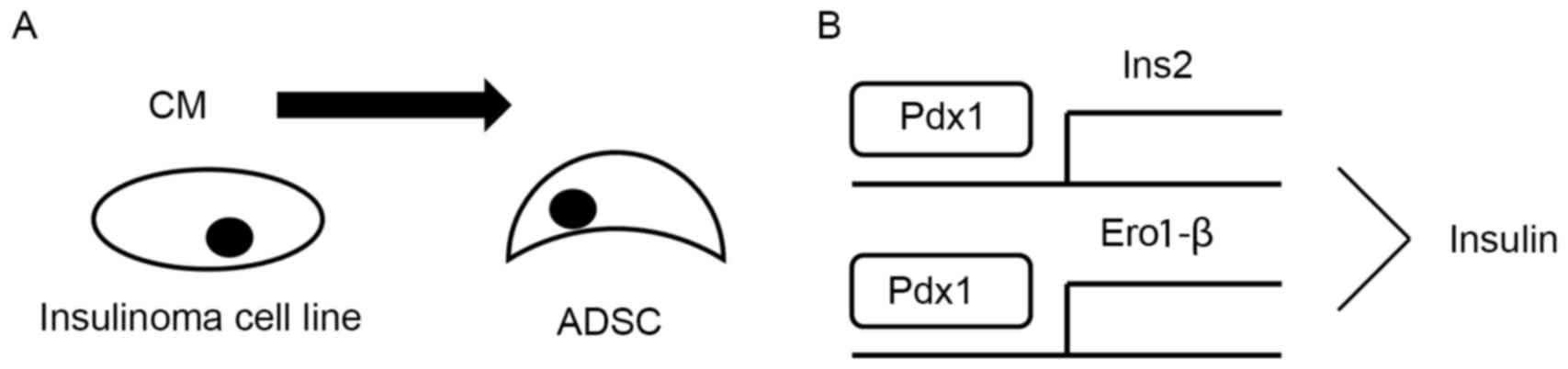
3). Finally, the putative mechanism underlying the effect of CM
was proposed (Fig. 4).
Discussion
The present study indicated that the culture of
mouse ADSCs in cell-free, MIN6-CM resulted in the induction of
insulin and Pdx1 gene expression (Fig. 1). Considering that gene expression is
under tight regulation by epigenetic modifications, including DNA
methylations and histone modifications, the epigenetic profiling
was investigated using a ChIP assay. This assay was performed with
an anti-Pdx1 antibody, followed by next generation sequencing for
mouse ADSCs in cell-free MIN6-CM. The results of the ADSCs were
compared with those of the the original MIN6. Pancreatic
development is regulated by multiple transcription factors. Pdx1 is
a critical factor for the determination of differentiation in
insulin-producing β cells (17).
Ero1 was initially identified in yeast. Yeast
possesses a single copy of the Ero1 gene, whereas mammal
cells have two Ero1-like genes. The present study also demonstrated
that the expression of Pdx1 was minimal in CM-derived exosomes.
This observation suggested that other mechanisms, including
proteins or microRNAs, may have a potential function in the CM
mechanism. Currently, the specific factors involved in this process
had not been clarified and additional investigation is required.
Zito et al (18) suggested
that homozygosity for a disrupting allele of Ero1-L-β
selectively compromises the oxidative folding of proinsulin and
promotes glucose intolerance in mutant mice. Exosomes are now
considered to be novel mediators of endocrine signaling via
cell-to-cell communication. Exosomes may deliver proteins and RNA
to recipient cells. These results suggested that additional
investigation would be beneficial to establish the mechanisms of
β-like cell differentiation. Ero1-L-α has been indicated to
serve a key function in a hypoxia-inducible factor 1-mediated
pathway for the induction of disulfide bond formation and vascular
endothelial growth factor secretion under hypoxia (19).
The data from the ChIP assay of the present study
indicated that Pdx1 was bound to the promoter region of the
Ero1-β gene (a pancreatic-specific disulfide oxidase), which
has been demonstrated to promote insulin biogenesis and glucose
homeostasis (18). Considering that
the in vivo function of Ero1-β in oxidative protein folding
in insulin-producing cells is required for glucose homeostasis
(18), the expression of Ero1-β may
be transmissible from cell-to-cell via the serum in vitro
and the microenvironment in vivo. The results of the present
study suggested a promising candidate for this method, to be used
in a clinical setting for the induction of insulin-producing β-like
cells.
Acknowledgements
The authors would like to thank Professor J.
Miyazaki (Department of Stem Cell Regulation Research, Osaka
University, Japan) for providing the MIN6 cells, and Professor T.
Miki (Department of Medical Physiology, Chiba University, Japan)
for providing the subclones.
Funding
The present study was financially supported by: A
Grant-in-Aid for Scientific Research from the Ministry of
Education, Culture, Sports, Science, and Technology (to M. Mori and
H. Ishii); a Grant-in-Aid from D-DIRECT (to H. Ishii); a
Grant-in-Aid from the Ministry of Health, Labor, and Welfare (to M.
Mori); grants from the National Institute of Biomedical Innovation
and Osaka University Drug Discovery Funds (to M. Mori); and grants
from the Kobayashi Cancer Research Foundation (to H. Ishii);
Princess Takamatsu Cancer Research Fund (to H. Ishii); Takeda
Science and Medical Research Foundation (to H. Ishii); Suzuken
Memorial Foundation (to M. Konno); Yasuda Medical Foundation and
the Pancreas Research Foundation, Japan (to K. Kawamoto). Professor
Hideshi Ishii received partial support from EBM Research Center,
Inc. (to Osaka, Japan), and Taiho Pharmaceutical Co., Ltd. (Tokyo,
Japan) through institutional endowments. These funding agencies
served no role in the main experimental equipments, supplies,
expenses, study design, data collection and analysis, decision to
publish or preparation of the manuscript.
Author's contributions
The experiments were performed by KK, TO, MK, NN,
the analysis of data was performed by KK, JK, HM, DS, TK. The
manuscript was written by KK, MK, HI. The design the study was by
HE, TS, YD, MM, HI.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kasper DL, Fauci AS, Hauser SL, Longo DL,
Jameson JL and Loscalzo J: Harrison's principles of internal
medicine 19/E. 1 & 2:2015.
|
|
2
|
Park CG, Bottino R and Hawthorne WJ:
Current status of islet xenotransplantation. Int J Surg.
23:261–266. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tomimaru Y, Ito T, Kawamoto K, Hama N,
Wada H, Kobayashi S, Eguchi H, Tanemura M, Mori M, Doki Y and
Nagano H: Clinical outcome of pancreas transplantation from
marginal donors in Japan. Transplant Proc. 46:954–957. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Machida T, Tanemura M, Ohmura Y, Tanida T,
Wada H, Kobayashi S, Marubashi S, Eguchi H, Ito T, Nagano H, et al:
Significant improvement in islet yield and survival with modified
ET-Kyoto solution: ET-Kyoto/Neutrophil elastase inhibitor. Cell
Transplant. 22:159–173. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ohmura Y, Tanemura M, Kawaguchi N, Machida
T, Tanida T, Deguchi T, Wada H, Kobayashi S, Marubashi S, Eguchi H,
et al: Combined transplantation of pancreatic islets and adipose
tissue-derived stem cells enhances the survival and insulin
function of islet grafts in diabetic mice. Transplantation.
90:1366–1373. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mizushima T, Takahashi H, Takeyama H,
Naito A, Haraguchi N, Uemura M, Nishimura J, Hata T, Takemasa I,
Yamamoto H, et al: A clinical trial of autologous adipose-derived
regenerative cell transplantation for a postoperative
enterocutaneous fistula. Surg Today. 46:835–842. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Konno M, Hamabe A, Hasegawa S, Ogawa H,
Fukusumi T, Nishikawa S, Ohta K, Kano Y, Ozaki M, Noguchi Y, et al:
Adipose-derived mesenchymal stem cells and regenerative medicine.
Dev Growth Differ. 55:309–318. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takahashi K and Yamanaka S: Induction of
pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors. Cell. 126:663–676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kawamoto K, Konno M, Nagano H, Nishikawa
S, Tomimaru Y, Akita H, Hama N, Wada H, Kobayashi S, Eguchi H, et
al: CD90-(Thy-1-) high selection enhances reprogramming capacity of
murine adipose-derived mesenchymal stem cells. Dis Markers.
35:573–579. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Akinci E, Banga A, Tungatt K, Segal J,
Eberhard D, Dutton JR and Slack JM: Reprogramming of various cell
types to a beta-like state by Pdx1, Ngn3 and MafA. PLoS One.
8:e824242013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Okita K, Mizuguchi T, Shigenori O, Ishii
M, Nishidate T, Ueki T, Meguro M, Kimura Y, Tanimizu N, Ichinohe N,
et al: Pancreatic regeneration: Basic research and gene regulation.
Surg Today. 46:633–640. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lumelsky N: Small molecules convert
fibroblasts into islet-like cells avoiding pluripotent state. Cell
Metab. 19:551–552. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu J, Dong H, Lin L, Wang Q, Huang L and
Tan J: miRNA-302 facilitates reprogramming of human adult
hepatocytes into pancreatic islets-like cells in combination with a
chemical defined media. Biochem Biophys Res Commun. 453:405–410.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Miyoshi N, Ishii H, Nagano H, Haraguchi N,
Dewi DL, Kano Y, Nishikawa S, Tanemura M, Mimori K, Tanaka F, et
al: Reprogramming of mouse and human cells to pluripotency using
mature microRNAs. Cell Stem Cell. 8:633–638. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miyazaki J, Araki K, Yamato E, Ikegami H,
Asano T, Shibasaki Y, Oka Y and Yamamura K: Establishment of a
pancreatic beta cell line that retains glucose-inducible insulin
secretion: Special reference to expression of glucose transporter
isoforms. Endocrinology. 127:126–132. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pedica F, Beccari S, Pedron S, Montagna L,
Piccoli P, Doglioni C and Chilosi M: PDX-1 (pancreatic/duodenal
homeobox-1 protein 1). Pathologica. 106:315–321. 2014.PubMed/NCBI
|
|
18
|
Zito E, Chin KT, Blais J, Harding HP and
Ron D: ERO1-beta, a pancreas-specific disulfide oxidase, promotes
insulin biogenesis and glucose homeostasis. J Cell Biol.
188:821–832. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
May D, Itin A, Gal O, Kalinski H,
Feinstein E and Keshet E: Ero1-L alpha plays a key role in a
HIF-1-mediated pathway to improve disulfide bond formation and VEGF
secretion under hypoxia: Implication for cancer. Oncogene.
24:1011–1020. 2005. View Article : Google Scholar : PubMed/NCBI
|