Introduction
In Japan, esophageal squamous cell carcinoma (ESCC)
is located in the middle thoracic esophagus (Mt) region (51.6%),
lower thoracic esophagus (Lt) region (24.2%), upper thoracic
esophagus (Ut) region (13.4%), abdominal thoracic esophagus (Ae)
region (4.5%), and cervical esophagus (Ce) region (4.0%) (1). There have been only a few reports of the
distribution of lymph node metastases in cases of lower thoracic
ESCC (LtESCC).
LtESCC leads to significantly higher lymph node
metastases to the abdomen than to any other location (2). A previous study reported that
three-field lymph node dissection improved 5-year overall survival
(OS) rates in LtESCC patients (3).
However, another study questioned whether three-field lymph node
dissection contributed to better OS in whole LtESCC patients
(4). In LtESCC patients, metastases
to para-aortic lymph nodes are also observed in 25.5% of cases.
Therefore, para-aortic lymph node dissection should have been
considered in cases of positive perigastric lymph node metastases
(5). Furthermore, poor patient
prognosis has been observed in LtESCC patients with positive
recurrent laryngeal nerve (RLN) lymph node metastases (6).
We have been committed to developing novel potent
chemotherapeutic regimens to control early treatment failures in
clinical stage (cStage) II/III ESCC by adding Docetaxel (DTX) to
Cisplatin (CDDP) and 5-fluorouracil (together referred to as DCF).
In our hospital, DCF neoadjuvant chemotherapy (NAC) had a better
prognosis than CF NAC (7,8). Describing the status of lymph node
metastases or recurrence with the latest treatment strategy will be
useful for developing future therapeutic strategies.
In this study, patients with LtESCC were assessed
with regard to the rates of lymph node metastases allowing for
recurrence in the context of the latest multimodality treatments,
as well as their influence on patient prognosis. These data could
be important for determining the optimal range of lymph node
dissection in LtESCC.
Materials and methods
Patients
A prospective database of 464 patients with thoracic
ESCC, provided by the esophageal Cancer Board of Kitasato
University of Medicine, Departments of Gastroenterology, Surgery,
and Radiology (Sagamihara, Japan), between January 1, 2009 and
March 31, 2016, was analyzed. Among 163 patients who had undergone
surgery at Kitasato University Hospital (Sagamihara, Japan), 41
patients diagnosed with LtESCC, excluding two cases of cStage IV
cancer, were included in the study. The median follow-up was 34.3
months (range, 2.3–87.4 months).
Staging
The tumor stage was classified according to the 6th
edition of the Union for International Cancer Control TNM (UICC
TNM) for esophageal cancer. The definition of positive lymph node
metastasis was a short diameter of 1 cm on CT. Until 2010, cases
were diagnosed as positive by a radiologist, but after 2011, a
short diameter of 1 cm was defined as positive. We examined the
patients limited to surgery alone (n=62) with regard to diagnostic
accuracy, after we ruled out those with NAC to allow for downstage
of the disease. As a result, the diagnosis rate of preoperative
lymph node metastasis was proved to be sensitivity of 100% (8/8)
and specificity of 68.5% (37/54), if we used the cut-off size of
lymph node swelling with 1 cm.
NAC Treatments in cStage II/III
ESCC
Patients received 3 cycles of DCF NAC every 3 weeks.
The regimen consisted of DTX 70–75 mg/m2 given as a 1-h
intravenous infusion on day 1, CDDP 70–75 mg/m2 as a 2-h
intravenous infusion on day 1, and 5-FU 750 mg/m2 as a
continuous 24-h peripheral infusion on days 1–5. From February
2011, the starting doses of DTX and CDDP were increased to 75
mg/m2 CF NAC with CDDP plus 5-FU was repeated twice
every 3 weeks.
In patients whose response to the first or second
course of chemotherapy was progressive disease or who could not
tolerate the side effects of DCF or CF NAC, the next course of
chemotherapy was not given. Non-responders with stable disease
received scheduled cycles of NAC.
Surgical Treatment
A standard esophagectomy was performed according to
the McKeown method (i.e., right thoracotomy followed by laparotomy
and neck incision with cervical anastomosis), and three-field
(thoracoabdominal and cervical) lymph node dissection was also
performed if indicated. After surgery, the lymph nodes were
separated from the resected esophagus and adjacent tissue, and they
were assigned specific numbers indicating the location of the lymph
nodes, in accordance with the guideline of the Japanese Society for
Esophageal Disease (JSED) (9). R0,
R1, and R2 surgical resections were defined as microscopically and
curatively resected, microscopically remnant, and macroscopically
remnant cancer cells, respectively.
Statistical analyses
All statistical analyses were performed using
JMP® 11 software (SAS Institute, Inc., Cary, NC, USA).
Frequency tables were analyzed using the χ2 test, with
the likelihood ratio or Fisher's exact test used to determine the
significance of differences between categorical variables. OS was
measured from the date of death or censored at the date of the last
follow-up evaluation. Survival was estimated by a life table using
the Kaplan-Meier method and compared by the log-rank test.
Differences between results of comparative tests were considered
significant if the two-sided P-value was less than 0.05.
Results
Prognosis of LtESCC who underwent
surgery with curative intent
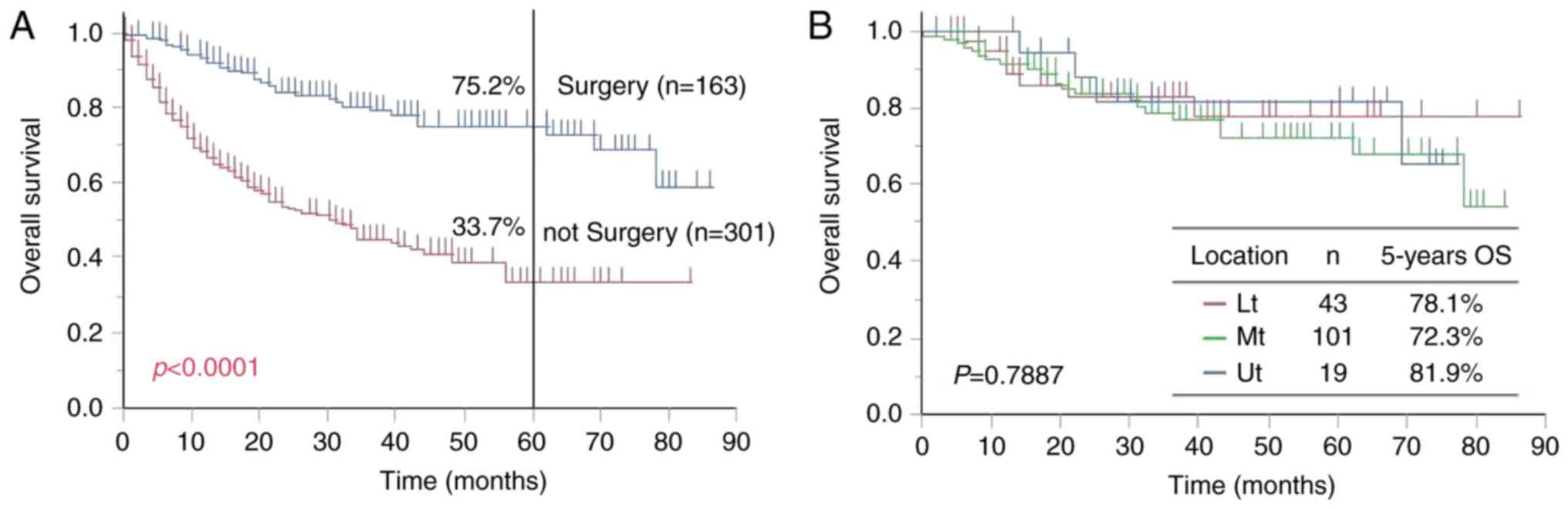
Among the 464 thoracic esophageal cancer,
esophagectomy was recommended to be performed with curative intent
in 163 cases (35.1%) of the thoracic ESCC cases; the remaining 301
cases (64.9%) did not have surgical treatment. Salvage surgery
after curative definitive chemoradiotherapy (dCRT) was not
considered surgical treatment. The 5-year OS rate was 75.2 and
33.7% for the surgical and non-surgical treatment groups,
respectively, and this difference was significant (P<0.0001;
Fig. 1A). Cancer of the Ut, Mt, and
Lt was found in 19, 101, and 43 cases, respectively, and 5-year OS
rates for these patients were 81.9, 72.3, and 78.1%, respectively.
There was no significant difference in prognosis among these groups
(P=0.7887; Fig. 1B).
Prognosis of Lt ESCC with
esophagectomy and lymph node dissection
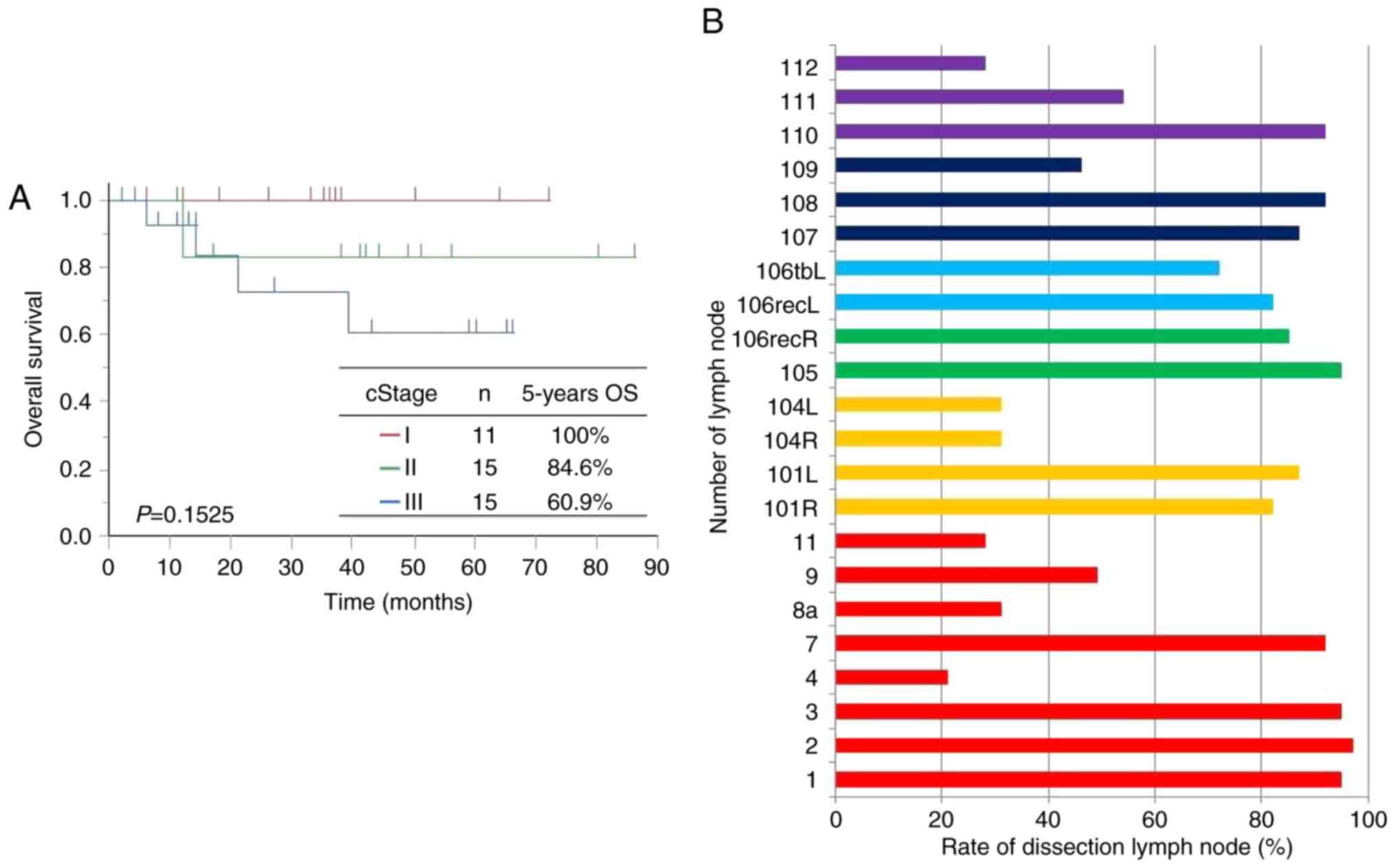
When the data were stratified by clinical stage for
patients with LtESCC, the 5-year OS rates were 100% in cStage I
patients, 84.6% in cStage II patients, and 60.9% in cStage III
patients. While there was no significant difference in prognosis
among these patients (P=0.1525), OS decreased with higher cStage
(Fig. 2A).
The characteristics of the 41 patients with LtESCC,
with cStage I to cStage III, are presented in Table I. One patient in cStage III had
inoperable cancer. Video-Assisted Thoracic Surgery (VATS)
esophagectomy was performed more often in the earlier stage than in
the more advanced stage (P=0.0007). Regarding the range for lymph
node dissection, no significant differences were observed between
two-field and three-field dissection methods (P=0.4294). DCF NAC
was performed in 66.7 and 60.0% of patients with cStages II and III
LtESCC, respectively. Patients with cStage I LtESCC who underwent
CRT (S1+Radiation) prior to surgery had concomitant advanced
hypopharyngeal carcinoma (Table
I).
 | Table I.Patients' characteristics. |
Table I.
Patients' characteristics.
| Variable | Number (n=41) | cStage I (n=11) | cStage II (n=15) | cStage III
(n=15)a | P-value |
|---|
| Age (years) |
|
|
|
| 0.9049 |
|
<69 | 21 | 6 | 7 | 8 |
|
| ≥69 | 20 | 5 | 8 | 7 |
|
| Sex |
|
|
|
| 0.0793 |
| Male | 34 | 11 | 12 | 11 |
|
|
Female | 7 | 0 | 3 | 4 |
|
| pT factor |
|
|
|
| 0.0030 |
| pT0 | 4 | 0 | 2 | 2 |
|
| pT1 | 15 | 10 | 5 | 0 |
|
| pT2 | 4 | 0 | 2 | 2 |
|
| pT3 | 16 | 1 | 6 | 9 |
|
| pT4 | 1 | 0 | 0 | 1 |
|
| Lymph vessels
invasion |
|
|
|
| 0.1261 |
|
Presence | 18 | 5 | 4 | 9 |
|
|
Absence | 22 | 6 | 11 | 5 |
|
| Blood vessels
invasion |
|
|
|
| 0.0686 |
|
Presence | 19 | 3 | 6 | 10 |
|
|
Absence | 21 | 8 | 9 | 4 |
|
| Pathological
stage |
|
|
|
| 0.0487 |
| I | 12 | 8 | 4 | 0 |
|
| IIA | 6 | 0 | 2 | 4 |
|
| IIB | 5 | 2 | 2 | 1 |
|
| III | 11 | 1 | 5 | 5 |
|
|
IVA | 1 | 0 | 0 | 1 |
|
|
IVB | 1 | 0 | 0 | 1 |
|
| CR | 4 | 0 | 2 | 2 |
|
| Procedure of
esophagectomy |
|
|
|
| 0.0007 |
|
Open | 14 | 0 | 3 | 11 |
|
|
VATS | 26 | 10 | 12 | 4 |
|
|
Transhiatal approach | 1 | 1 | 0 | 0 |
|
| Field of lymph node
dissection |
|
|
|
| 0.4294 |
|
Two | 25 | 7 | 11 | 7 |
|
|
Three | 15 | 4 | 4 | 7 |
|
| Preoperative
therapy |
|
|
|
| 0.0002 |
| Surgery
alone | 16 | 10 | 1 | 5 |
|
|
Cisplatin+5-FU | 5 | 0 | 4 | 1 |
|
|
Docetaxel+Cisplatin+5-FU | 19 | 0 | 10 | 9 |
|
|
Chemoradiotherapy | 1 | 1 | 0 | 0 |
|
| Reccurence of lymph
node |
|
|
|
| 0.0867 |
|
Presence | 5 | 0 | 1 | 4 |
|
|
Absence | 36 | 11 | 14 | 11 |
|
Lymph node dissection rates are shown in Fig. 2B. In cervical lymph nodes representing
yellow color, No. 101 was dissected in more than 80% of patients,
but No. 104 was dissected in 30.8% of patients. In upper
mediastinal lymph nodes representing green and light blue colors,
Nos. 105 and 106recR lymph nodes along the right RLN (green) were
dissected in 94.9 and 84.6% of patients, respectively, and lymph
nodes along the left RLN lymph nodes (Nos. 106recL and 106tbL)
(light blue) were dissected in 82.1 and 71.8% of patients,
respectively. In middle mediastinal lymph node representing dark
blue colors, No. 109 was dissected in only 46% of patients, whereas
Nos. 107 and 108 were dissected in 87 and 92% of patients,
respectively. This is putatively because No. 109 was often removed
with No. 107. In the lower mediastinum representing purple color,
No. 110 was dissected in 92% of patients, but Nos. 111 and 112 were
dissected in only 54 and 28% of patients, respectively. This is
also because Nos. 111 and 112 were often removed with No. 110. In
the abdomen representing red color, lymph nodes were dissected
around the cardia, the small curvature, and the celiac artery in
about 90% of patients. However, lymph nodes Nos. 4, 8a, 9, and 11
were dissected in 20.5, 30.8, 48.7, and 28.2% of patients,
respectively. No. 9 may be dissected with No. 7.
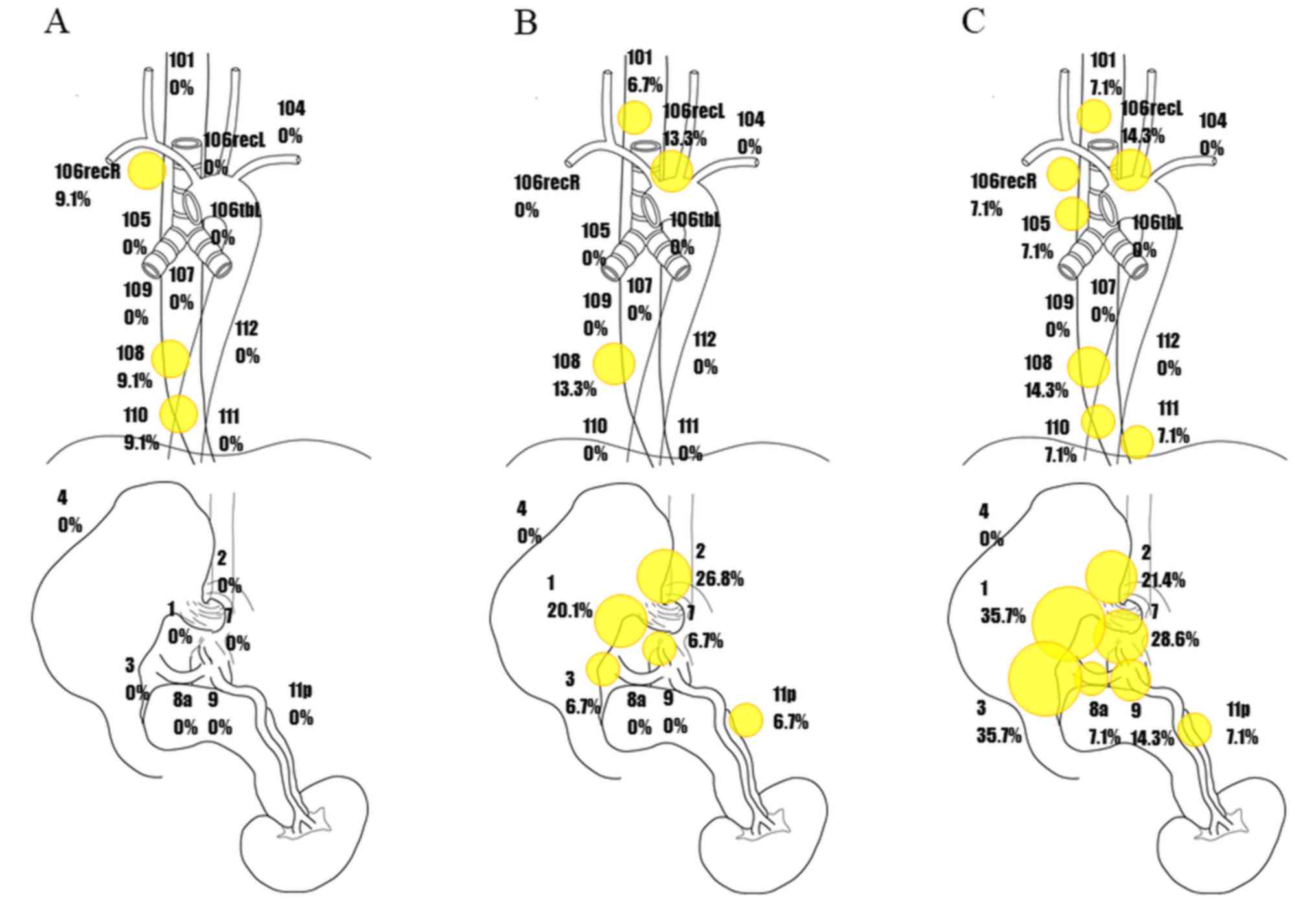
Distribution of pathological lymph
node metastasis and metastatic rates in LtESCC with esophagectomy
according to cStage
The rate of metastasis for each lymph node,
according to cStage, is shown in Fig.
3. In cStage I patients, the rates of metastases to lymph node
Nos. 106recR, 108, and 110 were 9.1, 9.1, and 9.1%, respectively,
with no lymph node metastases observed to the abdominal cavity
(Fig. 3A). In cStage II patients, the
rate of metastases to cervical lymph node No. 101 was 6.7%, whereas
the rates of metastases to thoracic lymph node Nos. 106recL and 108
were 13.3 and 13.3%, respectively. On the other hand, the rates of
metastases to abdominal lymph node Nos. 1, 2, 3, 7, and 11p were
20.1, 26.8, 6.7, 6.7, and 6.7%, respectively (Fig. 3B). In cStage III patients, rates of
metastases to lymph node Nos. 101, 106recR, 105, 106recL, and
106tbL were 7.1, 7.1, 7.1, 14.3, and 0%, respectively, whereas
rates for lymph node Nos. 108, 110, 111, and 112 were 14.3, 7.1,
7.1, and 0%, respectively. High rates of lymph node metastases were
observed to abdominal lymph node Nos. 1, 2, 3, 7, 8 a, 9, and 11 p
(35.7, 21.4, 35.7, 28.6, 7.1, 14.3, and 7.1%, respectively;
Fig. 3C). These results suggest that
abdominal lymph node metastases may increase rapidly with
progression.
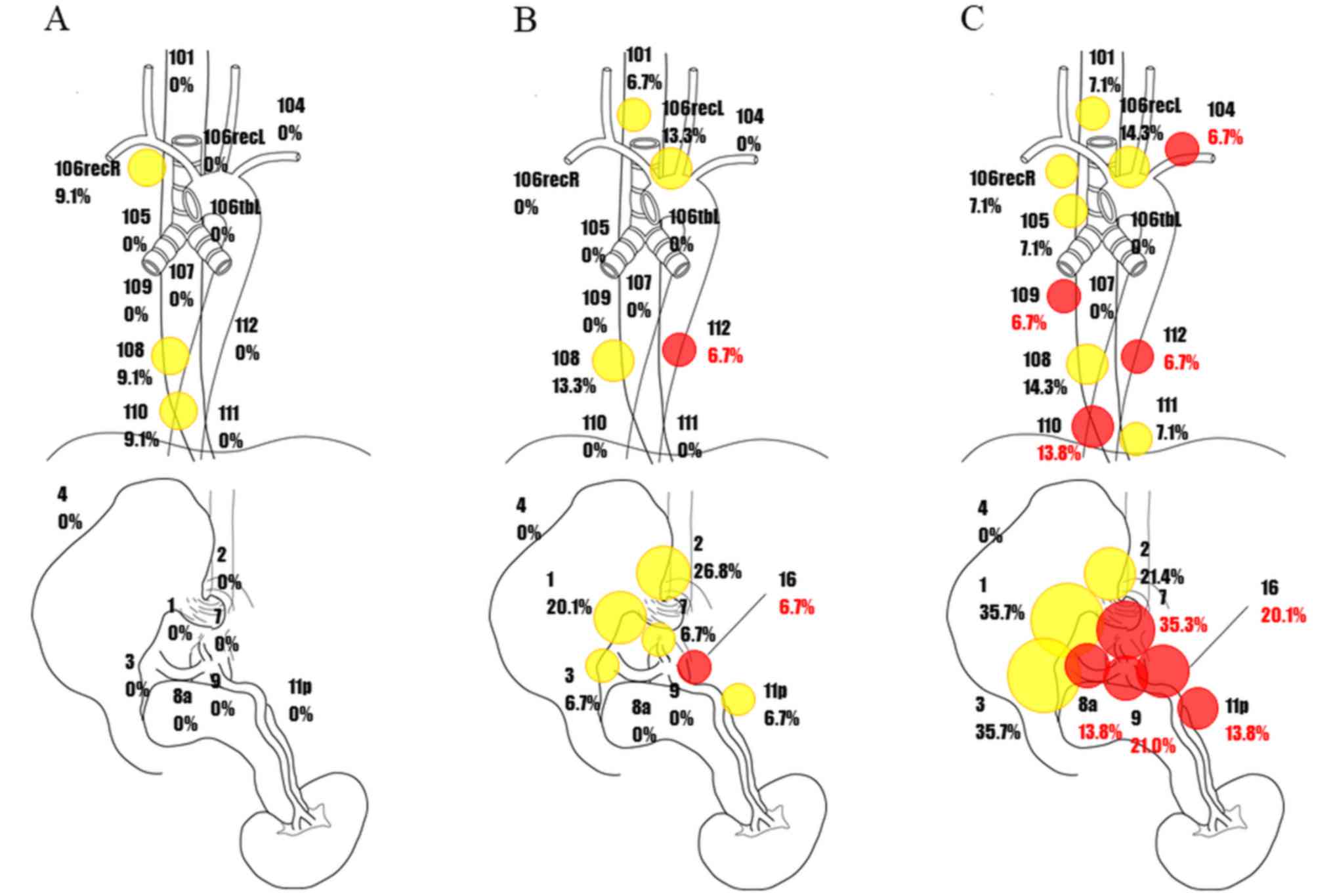
Distribution of potential lymph node
metastasis and metastatic rates in LtESCC with esophagectomy
according to cStage
Then, lymph node recurrence in combination with
pathological lymph node metastasis was defined as potential lymph
node metastasis, and the potential lymph node metastases are shown
Fig. 4. Red circles represent
recurrent lymph nodes, and the rate of the metastasis was
calculated after adding to pathological lymph node metastasis. This
figure therefore includes metastases that had not been removed by
surgery.
There were no cases of recurrent lymph node
metastases in cStage I patients (Fig.
4A). Recurrence of metastases to lymph node Nos. 112 and 16 was
added to the pathological lymph node metastases in cStage II
patients (Fig. 4B). In cStage III
patients, recurrence of metastases to lymph node Nos. 104, 109,
110, and 112 was observed in the cervical and thoracic areas. There
was also recurrence of metastases to lymph node Nos. 7, 8a, 9, 11p,
and 16 in the abdominal cavity (Fig.
4C).
Distribution of potential lymph node
metastasis and metastatic rates in LtESCC with esophagectomy
according to cStage
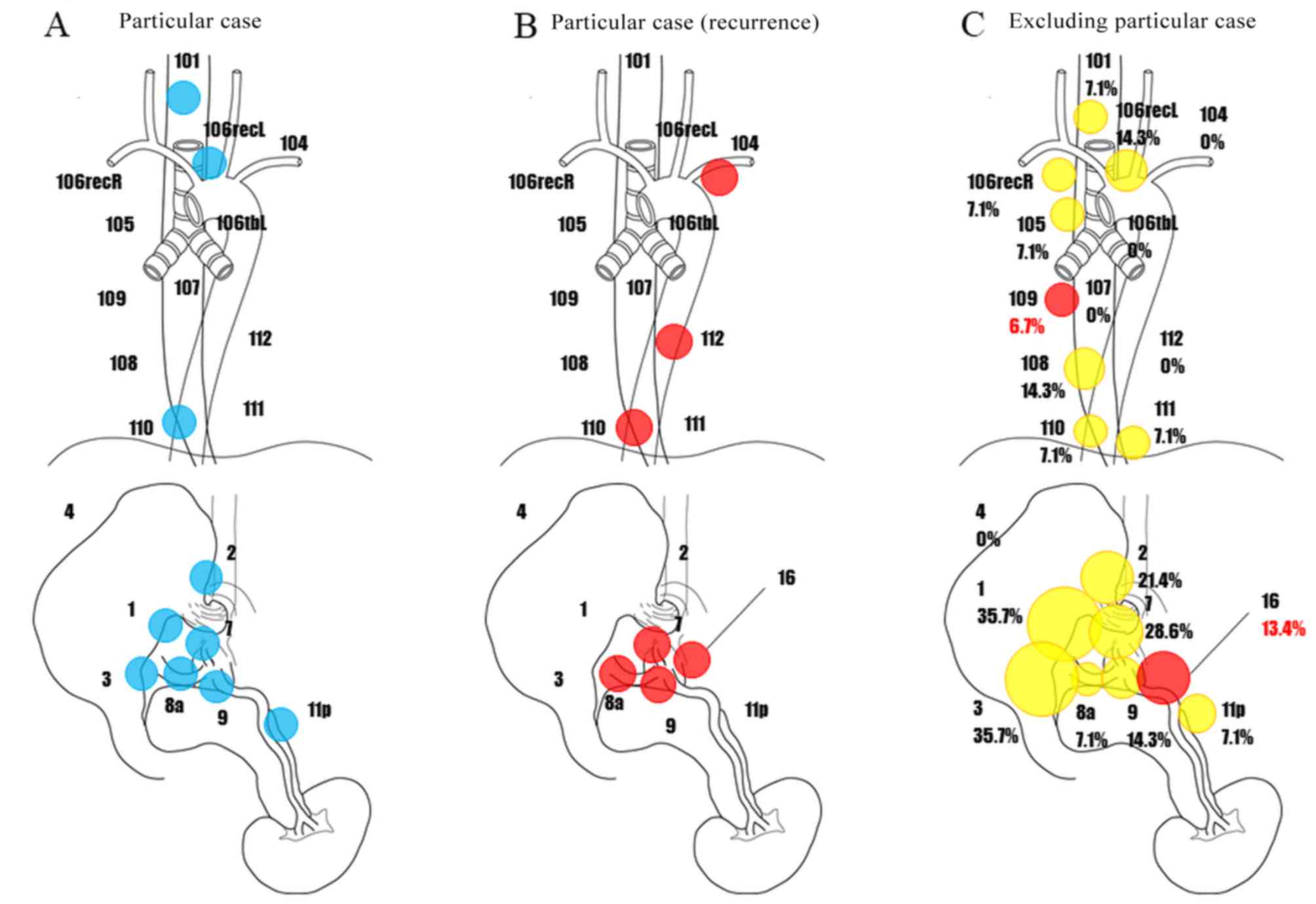
However, one case with cStage III uniquely had
robust lymph node metastases (Fig.
5A), which resulted in early lymph node recurrence after
surgery; this patient died due to rapid exacerbation of the
disease. In this patient, recurrence of metastases shortly occurred
to lymph node Nos. 104, 110, 112, 7, 8a, 9, 11p, and 16 (Fig. 5B). Since this case is considered a
unique entity in cStage III LtESCC, it was excluded from the
presentation of the sites of recurrent lymph node metastases of
cStage III LtESCC in Fig. 5C. As a
result, surgery was proved well to control recurrence of abdominal
lymph node metastases even in cStage III patients. In the thoracic
cavity, recurrence of metastases was confirmed to lymph node No.
109, and recurrence was only observed in abdominal lymph node No.
16 (extra-regional lymph node). In LtESCC patients, abdominal lymph
nodes had a high rate of metastases, but abdominal lymph nodes were
successfully dissected.
Lymph node dissection along right and
left RLN and prognosis in LtESCC
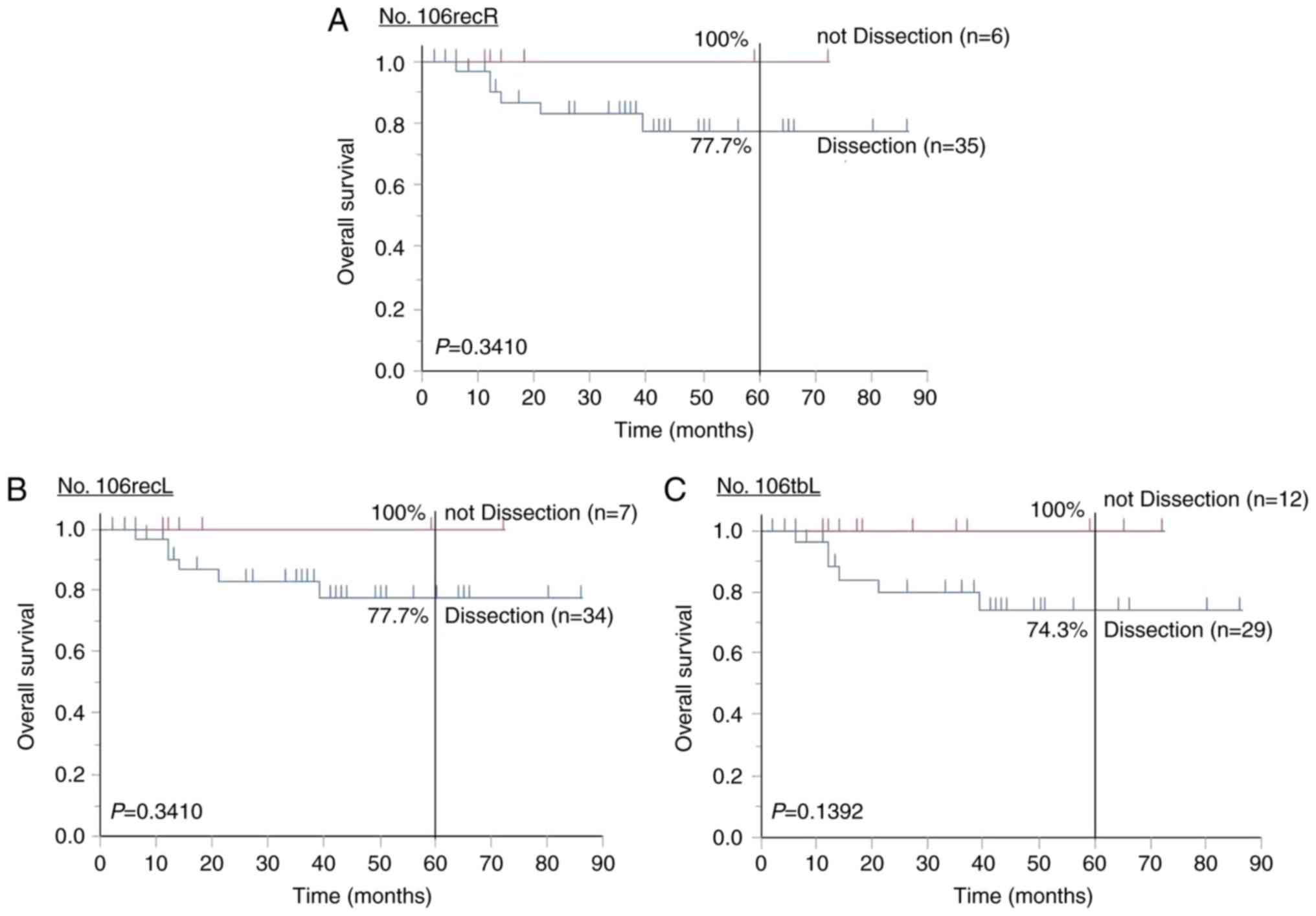
Finally, the prognostic relevance of lymph node
dissection around the RLN was examined. The 5-year OS rates for
cases undergoing RLN dissection of lymph node Nos. 106recR (n=6)
and 106recL (n=7) were 100 and 100%, respectively, while those for
cases not undergoing dissection were 77.7 and 77.7%, respectively
(P=0.3410; Fig. 6A and B). The 5-year
OS rates of the cases in which No. 106tbL was excised (n=29) and
not excised (n=12) were 100 and 74.3%, respectively (P=0.1392;
Fig. 6C). In LtESCC patients,
prognosis was not improved by dissection of lymph nodes around the
RLN.
Curative treatment after recurrence in
cases with lymph node metastasis
Among the 41 LtESCC cases, recurrences of lymph node
alone were found only in 3 cases, and definitive CRT was performed
in 2 among the three. Both cases survived with no relapse. In the
remaining one case, chemotherapy of Nedaplatin and 5-FU was
performed and the patient survived with no relapse.
Discussion
There have been few reports describing distribution
of lymph node metastases including disease recurrences and the
optimal dissection range of lymph nodes in LtESCC. In this current
study, we have opinions with regard to efficacy of lymph node
dissection for i) cervical lymph nodes; ii) para-aortic lymph
nodes; and iii) lymph nodes along the RLN.
The have been clinical question with regard to
necessity of cervical lymph node dissection in Lt ESCC. One of the
important literature in terms of this point is a report by Fujita
et al that rates of metastases to cervical lymph nodes were
infrequent (Nos. 101R, 101L, 104R, and 104L were 3, 1, 3, and 1%,
respectively), which were even fewer than those with para-aortic
ones (4). This incidence of
metastatic cervical lymph nodes is consistent with our current
study. Nishimaki et al also reported that cervical lymph
node metastases in LtESCC are infrequent and do not affect
prognosis. Therefore, radical esophagectomy for LtESCC patients
should only be performed, limited to mediastinal and abdominal
lymphadenectomy (10). On the other
hand, Igaki et al critically reported that 3-field lymph
node dissection in LtESCC with metastases to the upper and middle
mediastinal lymph nodes results in better 5-year OS (30.0%) than
the use of two-field dissection (5.6%; P=0.005), suggesting that
cervical dissection might play a given role in improving prognosis
in the very limited LtESCC (3).
Nevertheless, Igaki et al also reported that 3-field lymph
node dissection did not improve survival in LtESCC with no
metastases to mediastinal lymph nodes (3). Allowing for these data, cervical lymph
node dissection may be necessary in the very limited LtESCC, eg.
cases with clinically lymph node metastases in the upper and/or
middle mediastinum.
In this current study, we experienced 1 unique stage
III LtESCC with cervical lymph node recurrence, however this case
is very unique in that there were robust metastases and recurrences
to multiple lymph nodes during the clinical curse. In addition,
there was a report describing that patient prognosis is better in
cervical lymph node recurrences to the neck (11). In cases of recurrent cervical lymph
node metastases alone, salvage surgery can be performed, or even
combined with CRT.
Rates of metastases were as high as 36, 26, 29, and
32% to abdominal lymph node Nos. 1, 2, 3, and 7 and more frequent
than those in cervical and mediastinal lymph nodes (4), which are again consistent with other's
(2) and our current study.
Importantly, in this study, regional lymph nodes could be well
controlled, and recurrences were few.
Unique case described in the cervical lymph nodes
only showed recurrence of the regional lymph nodes in the abdomen.
Except this unique 1 case, para-aortic lymph node metastases are
concerns in LtESCC. Shimada et al reported that para-aortic
lymph node metastases or recurrence were recognized in 25.5% of
LtESCC (5). In our hospital,
recurrence rates in para-aortic lymph nodes was relatively high:
6.7 and 20.1% of LtESCC patients with cStages II and III,
respectively. However, the recurrences of the para-aortic lymph
nodes in our study were diagnosed after surgery, and it was not
seen before surgery. Therefore, it is difficult to apply the NAC to
such cases before surgery. As it has been reported that recurrence
of para-aortic lymph nodes after surgery may be cured by performing
definitive CRT after recurrence (12), preventive lymph node dissection is
considered to be overtreatment for the para-aortic lymph nodes.
Lymph node dissection along RLN is another debate
issue in LtESCC. The rates of metastases to lymph nodes Nos.
106recR, 106recL, and 106tbL were 11, 11, and 3%, respectively,
which were further infrequent than those with metastatic
para-aortic lymph nodes (4). This
report result is re-capitulated in Ye's one, where the 3-year
survival rate was 29.3%, and prognosis was poor in patients with
metastases to RLN lymph nodes (6). In
cases of RLN lymph node metastases in our hospital, rates of
metastases to lymph node Nos. 106recR and 106recL were,
respectively, 9.1 and 0% in cStage I, 0 and 13.3% in cStage II, and
7.1 and 14.3 in cStage III, which were consistent with the previous
reports (4,6). More importantly, there were no
significant differences in the prognoses of patients with surgical
dissection of lymph nodes around RLN. Considering that RLN can be
caused by RLN lymph node dissection, this procedure should be
carefully considered in LtESCC patients; for example, this
procedures should not be mandatory in cStage I/II for high risk
patients such as elderly patients with low performance status or
patients with impaired critical organs.
Limitations
The limitation of this research is that the number
of cases is small. However, the survival rate of stage and tumor
location in this study showed the same trend as other reports. For
example, there is a report that the 5-year survival rate of each
stage in surgical cases was 84.9/80.5/62.2/38.8/22.8/14.2% in
cStage 0/I/II/III/IVA/IVB, respectively (13). The 5-year survival rate of tumor
location in surgical cases was 44.8/50.5/45.6% in Ut/Mt/Lt,
respectively, and there was no significant difference (14). Therefore, survival rates of stage and
tumor location in our research are thought to be consistent
outcomes despite the small number of cases. There are also other
limitations on lymph node dissection range in this current study
due to a retrospective study. According to the current clinical
guideline of the 7th UICC-TNM, the abdominal lymph node dissection
extent is limited to Nos. 1, 2, 3, 7, 9 lymph nodes. Nos. 4, 8, and
11 lymph nodes are out of range of dissection, and extra-regional.
Recurrences at such extra-regional regions were hardly seen except
for particular cases, and then the lymph node metastasis rate would
not increase even if all cases are dissected of lymph nodes noted
in this study. Since No. 9 lymph node is dissected together with
No. 7, it may be contained as No. 7 in our cases. It is not
possible to discuss backwardly with No. 7 and No. 9 separately, and
the accuracy of the rates were not definitive.
In conclusion, rates of metastases to abdominal
lymph nodes were higher than those to thoracic and cervical lymph
nodes in LtESCC. In the context of the potent preoperative
chemotherapy and esophagectomy, lymph node dissection of cervical
areas, para-aortic areas, and even putative RLN areas are not
mandatory to all patients with LtESCC, while the best operation no
doubt should include all such procedures in patients with no
impaired conditions. Moreover, we should know existence of a rare
ESCC patient with uniquely aggressive lymph node disease, which
might not be indicated for esophagectomy. Biomarkers may select
such particular patients in the future.
Finally, from our study, we will describe the
rationale of lymph node dissection of LtESCC cancer. (1) Neck lymph node dissection is likely to be
optional. As a reason, the pathological metastasis rate and
recurrence rate of the cervical lymph nodes are extremely low.
(2) Prophylactic dissection of the
para-aortic lymph node is then considered overtreatment, because
recurrences at the para-aortic area were infrequent, and we have
alternate therapeutic option for cure-definitive CRT even after
recurrence (12). (3) Allowing for the Kaplan-Meier curve, it
could be possible to omit either of RLN dissection for elderly
patients and organ dysfunction cases with cStage I/II according to
the specific clinical situation.
Acknowledgments
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HH and KY designed the study and wrote the initial
draft of the manuscript. HH and KY analysed and interpreted the
data and assisted in the preparation of the manuscript. KH, HMo,
HMi, AE, MWas, YK and MWat contributed to the collection and
interpretation of the data and critically reviewed the manuscript.
All authors approved the final version of the manuscript and agree
to be accountable for all aspects of the work in ensuring that
questions related to the accuracy or integrity of any part of the
work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ozawa S, Tachimori Y, Baba H, Matsubara H,
Muro K, Numasaki H, Oyama T, Shinoda M, Takeuchi H, Tanaka O, et
al: Comprehesive registry of esophageal cancer in Japan, 2002.
Esophagus. 7:7–22. 2010. View Article : Google Scholar
|
|
2
|
Li H, Zhang Y, Cai H and Xiang J: Pattern
of lymph node metastases in patients with squamous cell carcinoma
of the thoracic esophagus who underwent three-field
lymphadenectomy. Eur Surg Res. 39:1–6. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Igaki H, Tachimori Y and Kato H: Improved
survival for patients with upper and/or middle mediastinal lymph
node metastasis of squamous cell carcinoma of the lower thoracic
esophagus treated with 3-field dissection. Ann Surg. 239:483–490.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fujita H, Sueyoshi S, Tanaka T and
Shirouzu K: Three-field dissection for squamous cell carcinoma in
the thoracic esophagus. Ann Thorac Cardiovasc Surg. 8:328–335.
2002.PubMed/NCBI
|
|
5
|
Shimada Y, Imamura M, Sato F, Maeda M,
Kaganoi J, Hashimoto Y, Kan T, Nagatani S and Li Z: Indications for
abdominal para-aortic lymph node dissection in patients with
esophageal squamous cell carcinoma. Surgery. 132:93–99. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ye K, Xu JH, Sun YF, Lin JA and Zheng ZG:
Characteristics and clinical significance of lymph node metastases
near the recurrent laryngeal nerve from thoracic esophageal
carcinoma. Genet Mol Res. 13:6411–6419. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Katada N, Yamashita K, Katada C, Moriya H,
Hosoda K, Mieno H, Higuchi K, Komori S, Ishiyama H, Hayakawa K, et
al: Neoadjuvant chemotherapy using concurrent Docetaxel/CDDP/5-FU
(DCF) in esophageal squamous cell carcinoma and its short-term
prognosis. Esophagus. 11:173–181. 2014. View Article : Google Scholar
|
|
8
|
Yamashita K, Katada N, Moriya H, Hosoda K,
Mieno H, Katada C, Koizumi W, Hoshi K and Watanabe M: Neoadjuvant
chemotherapy of triplet regimens of docetaxel/cisplatin/5-FU (DCF
NAC) may improve patient prognosis of cStage II/III esophageal
squamous cell carcinoma-propensity score analysis. Gen Thorac
Cardiovasc Surg. 64:209–215. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Japanese Society for Esophageal Disease:
Guideline for the clinical and pathological studies on carcinoma of
the esophagus. 10th edition. revised. Kanehara & Co., Ltd;
Tokyo: 2002
|
|
10
|
Nishimaki T, Suzuki T, Tanaka Y, Nakagawa
S, Aizawa K and Hatakeyama K: Evaluating the rational extent of
dissection in radical esophagectomy for invasive carcinoma of the
thoracic esophagus. Surg Today. 27:3–8. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Motoyama S, Kitamura M, Saito R, Maruyama
K, Okuyama M and Ogawa J: Outcome and treatment strategy for mid-
and lower-thoracic esophageal cancer recurring locally in the lymph
nodes of the neck. World J Surg. 30:191–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamashita K, Hosoda K, Moriya H, Katada C,
Sugawara M, Mieno H, Komori S, Katada N and Watanabe M: Prognostic
advantage of docetaxel/cisplatin/ 5-fluorouracil neoadjuvant
chemotherapy in clinical stage II/III esophageal squamous cell
carcinoma due to excellent control of preoperative disease and
postoperative lymph node recurrence. Oncology. 92:221–228. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tachimori Y, Ozawa S, Numasaki H, Ishihara
R, Matsubara H, Muro K, Oyama T, Toh Y, Udagawa H and Uno T:
Registration Committee for Esophageal Cancer of the Japan
Esophageal Society: Comprehensive registry of esophageal cancer in
Japan, 2010. Esophagus. 14:189–214. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang HX, Hou X, Liu QW, Zhang LJ, Liu JG,
Lin P and Fu JH: Tumor location does not impact long-term survival
in patients with operable thoracic esophageal squamous cell
carcinoma in China. Ann Thorac Surg. 93:1861–1866. 2012. View Article : Google Scholar : PubMed/NCBI
|