Introduction
Nasopharyngeal carcinoma (NPC) is a unique head and
neck cancer as the pathogenesis of cancer is closely linked with
Epstein-Barr virus (EBV). Guangdong province of southern China has
the highest NPC incidence and EBV-associated NPC is the major
histological type (1). EBV infection
could be found in the normal individuals. In immunocompetent EBV
carrier, EBV is in the latency 0 which does not trigger innate
immune response (2). When the virus
enters lytic phases, many viral gene products could interfere with
the innate and adaptive response which protects the cells from
detection and elimination by the immune effectors (3). EBV in NPC is in latency II stages. The
characteristic viral gene products in EBV latency II can exert
immunomodulatory functions and contribute to the immunoevasion to
the host.
Natural killer (NK) cell is a cytotoxic effector in
human innate system with the capability to kill tumor cells.
Evasion from NK cell surveillance enables cancer to proliferate and
spread at early stage (4). NK cells
mediate target specific cytolysis by activation of the triggering
receptors on their cell surface. Of which, the communication
between immunoreceptor natural killer group 2, member D (NKG2D) and
major histocompatibility complex class I chain-related peptide A
(MICA) is a key regulatory axis. NKG2D is a c-type lectin-like
receptor expressed on the NK cell surface (5). MICA is the NKG2D ligand expressed on
transformed cells surface. NKG2D is an activating receptor of NK
cells. Binding of MICA will trigger the activating signals and
antitumor response of NK cells (6).
In NPC, it has been shown that the cytotoxic
activity of NK cells in NPC patients were reduced in comparison
with the normal individuals (7). NK
cells can recognize cancer with high surface MICA expression.
However, the cancer cells can suppress MICA expression which
reduces their susceptibility to NK cell cytolysis. For NPC, the
underlying mechanism remains unclear. MICA polymorphism (MICA-A9
and A5.1) is associated with the NPC risk in the Southern China
male NPC patients (8,9). In liver cancer, it has been shown that
MICA expression is regulated by microRNA (10). MicroRNA is short non-coding RNA which
functions as post-transcriptional regulators to specific gene
expression. In view of the close association between EBV and NPC,
we speculate that EBV-encoded microRNA has a regulatory role on
MICA expression in NPC. It has been shown that MICA expression
could be upregulated by transforming growth factor β 1 (TGFβ1)
(11). As our previous findings
indicate that the EBV-encoded microRNA BART7 (ebv-miR-BART7) is a
functional TGFβ1 suppressor (12), we
hypothesized that NPC expressing ebv-miR-BART7 has lower MICA
expression and has reduced sensitivity to NK cell cytolysis.
Materials and methods
Cell lines
NPC cell line HK1 was maintained in PRMI-1640 medium
supplemented with 10% fetal bovine serum, 200 Unit/ml penicillin G
sodium, 20 µg/ml streptomycin sulfate, and 0.5 µg/ml amphotericin B
(all Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
NK92MI cells were incubated in Alpha Minimum Essential medium
supplemented with 12.5% fetal bovine serum, 12.5% horse serum (both
Gibco; Thermo Fisher Scientific, Inc.), 0.2 mM inositol, 0.1 mM
2-mercaptoethanol, 0.02 mM folic acid (all Sigma, St. Louis, MO,
USA), 200 Unit/ml penicillin G sodium, 20 µg/ml streptomycin
sulfate, and 0.5 µg/ml amphotericin B (all Gibco; Thermo Fisher
Scientific, Inc.).
Recombinant TGFβ1 treatment
HK1 cells were treated by recombinant TGFβ1 (Thermo
Fisher Scientific, Inc.) as previously described (12). HK1 was incubated with 0.5 ng/ml
recombinant TGFβ1 for 72 h.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted with TRIzol (Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. RNA was
converted to cDNA using High Capacity cDNA Reverse Transcription
kit (Thermo Fisher Scientific, Inc.) following the supplier's
guideline. RT-qPCR was carried out using FastStart Universal Probe
Master on a LightCycler® 480 (both Roche Applied
Science, Penzberg, Germany). The primers and probes were designed
from Universal ProbeLibrary Assay Design Center (http://www.roche-applied-science.com/).
Primers and probes were as follows: MICA-forward,
5′-GGCATCTTCCCTTTTGCAC-3′; MICA-reverse,
5′-GGACAGCACCGTGAGGTTAT-3′; MICA-probe, cat. no. 24;
c-Myc-forward, 5′-GCTGCTTAGACGCTGGATTT-3′; c-Myc-reverse,
5′-TAACGTTGAGGGGCATCG-3′; c-Myc-probe, cat. no. 66;
TGFβ1-forward, 5′-ACTACTACGCCAAGGAGGTCAC-3′; TGFβ1-reverse,
5′-TGCTTGAACTTGTCATAGATTTCG-3′; TGFβ1-probe, cat. no.
27. qPCR reaction was carried out at 95°C for 10 min followed by 45
cycles of 95°C for 15 sec and 60°C for 1 min. Expression levels
were calculated using the comparative threshold cycle method
(2−ΔΔCT).
BART7 mimic transfection
HK1 cells were transfected with 3 nM ebv-miR-BART7
mimic or negative control siRNA using Hiperfect transfection
reagent (all Qiagen Inc., Valencia, CA, USA). After 72 h, the
expression levels of TGFβ1, MICA and c-Myc were determined.
Immunocytochemistry (ICC)
HK1 cells were seed on chamber slides and treated
with recombinant TGFβ1 or transfected with ebv-miR-BART7 mimic. NPC
cells were washed with PBS and fixed with 4% paraformaldehyde.
Then, NPC cells were incubated with anti-MICA antibodies (Abcam,
Cambridge, UK), followed by the addition of CF™488A
Secondary Antibody Conjugates (Biotium, Inc., Freemont, CA, USA).
Blue-fluorescent 4′,6-Diamidino-2-Phenylindole (DAPI; Thermo Fisher
Scientific, Inc.) was used to label nucleus.
Chromatin immunoprecipitation (ChIP)
assay
ChIP assay was performed using Magna
ChIP™ A Chromatin Immunoprecipitation kit (EMD Millipore
Billerica, MA, USA) following the manufacturer's protocol. In
brief, HK1 cells were fixed with 1% paraformaldehyde and were lysed
by cell lysis buffer. Then, cells were sonicated to shear
cross-linked DNA to approximately 200–1,000 base pairs. The cell
lysates were incubated overnight at 4°C with protein A magnetic
beads and anti-c-Myc antibodies or normal mouse IgG. Protein/DNA
complex were eluted from the beads and free DNA was obtained by
reverse cross-links of protein/DNA complex. The precipitated MICA
promoter DNA was detected by RT-PCR. The primers spanning the c-Myc
binding site of MICA promoter were as follows: Forward,
5′-GGTGGGATAGGGTGAGGAGA-3′; reverse, 5′-CCCCATCTGCTGAATGTCAC-3′.
The RT-PCR products were analyzed by QIAxcel Advanced system
(Qiagen Inc.), a capillary electrophoresis device.
NK cell cytotoxicity test
NK cell cytotoxicity test was performed using the
xCELLigence system (13). HK1 cells
were transfected with ebv-miR-BART7 mimic or negative control siRNA
followed by seeded on the E16 plate. Then, NK92MI cells were added
to NPC cells and survival of NPC cells was continuously monitored.
The percentage of cytotoxicity was calculated from the formula:
Percentage of cytotoxicity=[(cell index no effector-cell
index effector)/cell index no effector]
x100%.
Statistical analysis
All results were shown as mean ± SD from 3
independent experiments. Student's t test was used to evaluate the
differences between control and experimental groups. P<0.05 was
considered statistically significant.
Results
TGFβ1 was implicated in MICA
expression in NPC
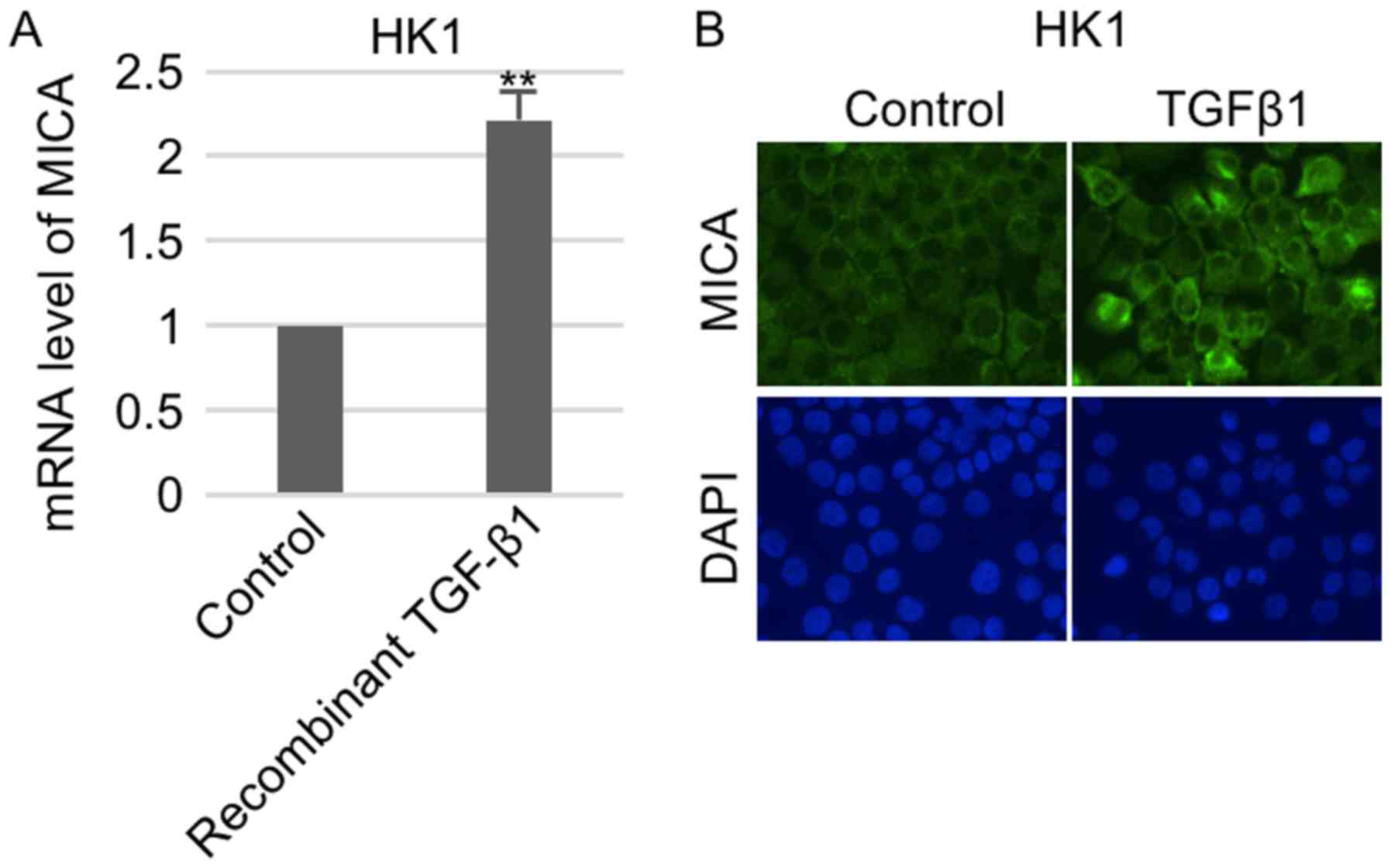
To study whether TGFβ1 regulated MICA expression,
NPC cells were first treated with recombinant TGFβ1. RT-qPCR
analysis showed that MICA expression was transcriptionally
un-regulated in HK1 cells (Fig. 1A).
Consistently, subsequent immunostaining indicated that surface
expression of MICA in HK1 were remarkably increased in response to
TGFβ1 treatment (Fig. 1B).
C-Myc bound to the promoter region of
MICA
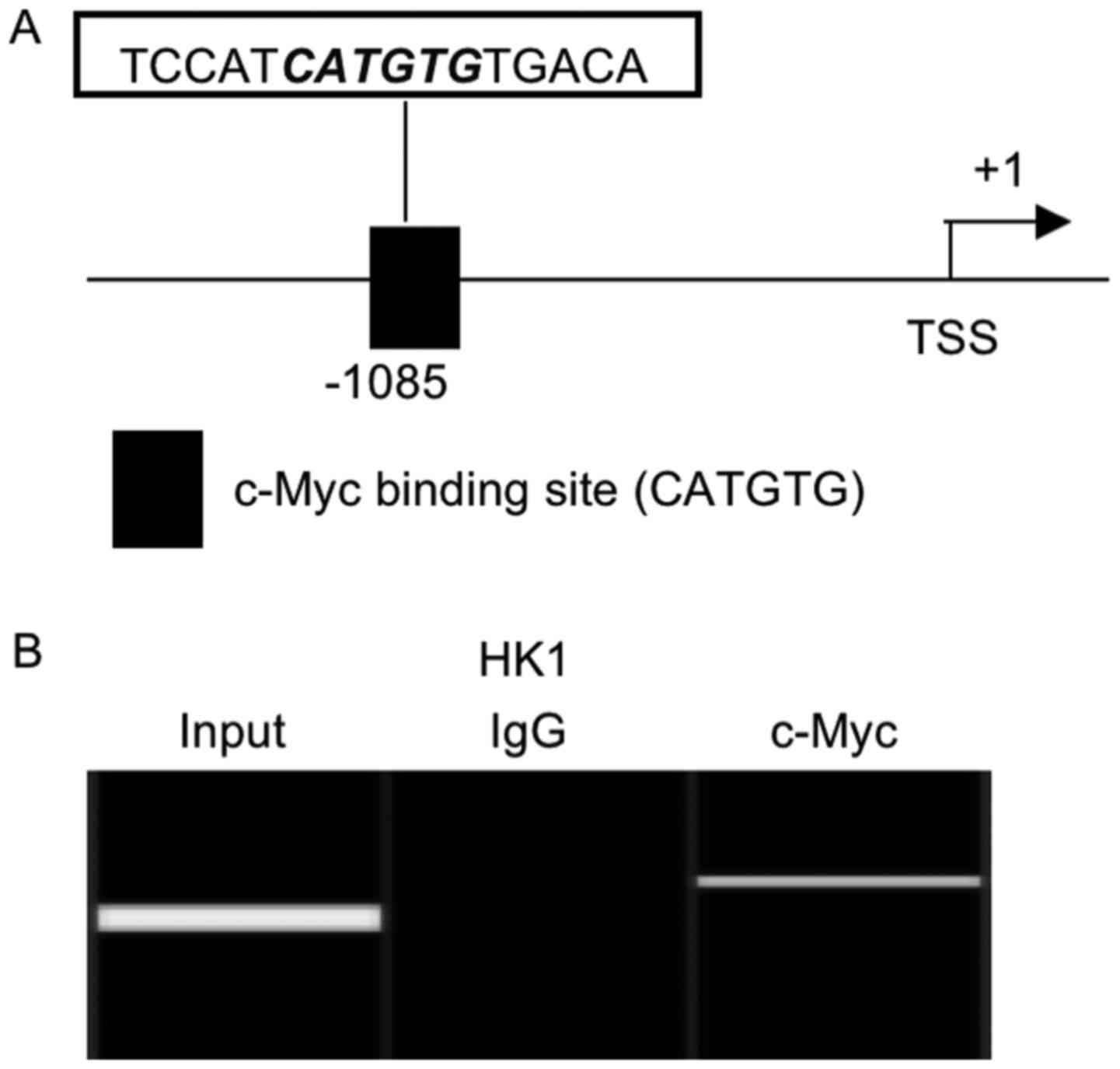
To investigate the regulatory mechanism of MICA
transcription, we performed promoter analysis and identified
potential c-Myc binding site (CATGTG) in the promoter region of
MICA gene (Fig. 2A). As shown in the
ChIP assay, c-Myc protein could bind to the predicted c-Myc binding
site located upstream of the transcription start site of MICA gene
(Fig. 2B).
TGFβ1 activated c-Myc expression and
the TGFβ1/c-Myc regulatory axis in NPC was inhibited by
ebv-miR-BART7
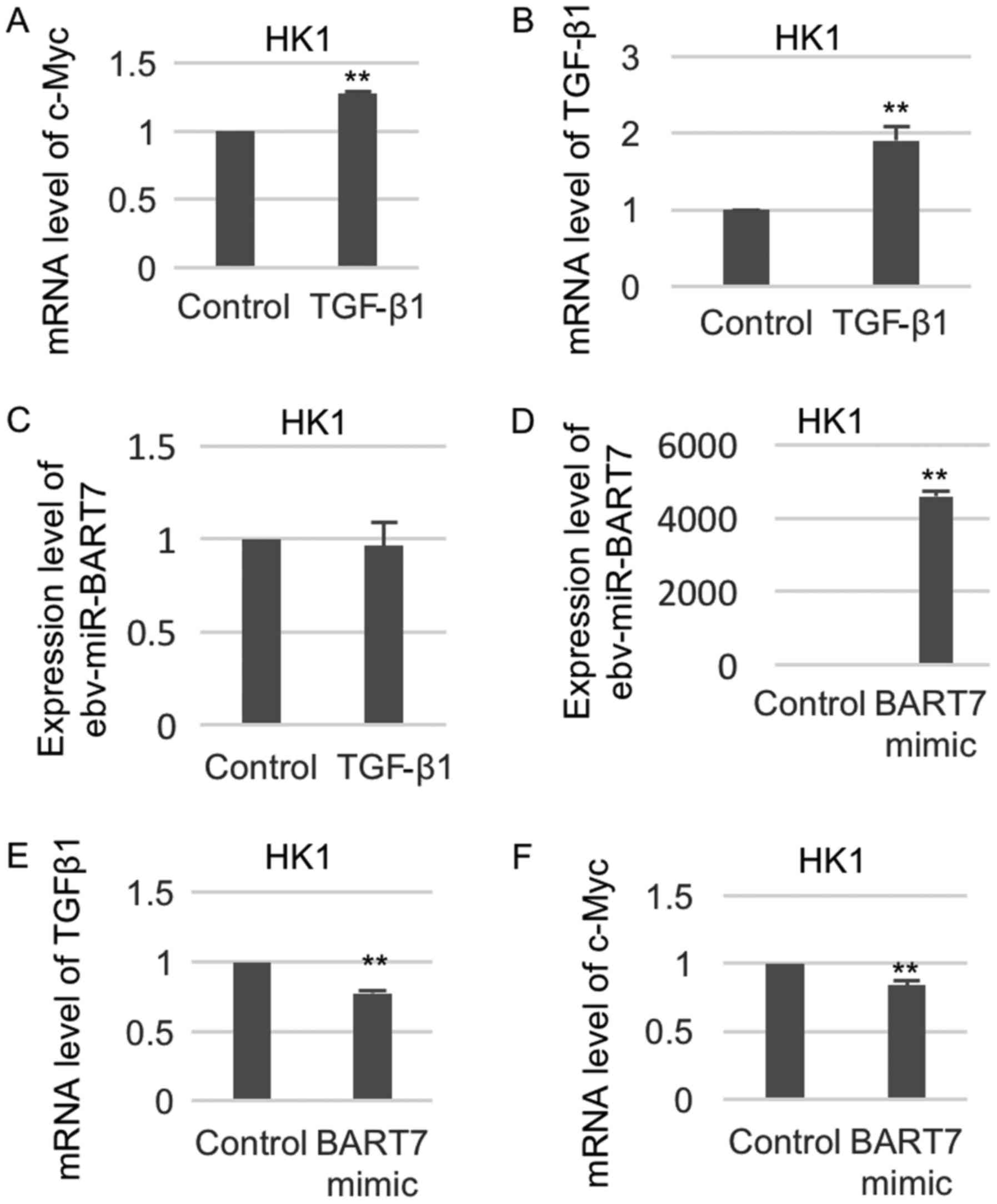
To examine whether TGFβ1 could regulate c-Myc at
transcriptional level, we examined the change of c-Myc expression
in the TGFβ1-treated NPC cell lines. As shown in Fig. 3A, c-Myc expression was significantly
increased in HK1 cells after treatment with recombinant TGFβ1. The
expression of TGFβ1 was increased in HK1 cells treated with
recombinant TGFβ1 (Fig. 3B).
Recombinant TGFβ1 treatment did not affect the expression level of
ebv-miR-BART7 (Fig. 3C). We have
previously shown that ebv-mir-BART7 expression had a significant
negative regulatory function on TGFβ1 expression in NPC (12). Thus, we anticipated that ebv-miR-BART7
expression in the NPC cells will also suppress c-Myc expression. To
confirm our hypothesis, we transfected the NPC cell line with
synthetic ebv-miR-BART7 microRNA and measured the change of
TGFβ1/c-Myc expression. The expression of ebv-miR-BART7 was
significantly increased in HK1 cells transfected with BART7 mimic,
indicating the successful overexpression of ebv-miR-BART7 (Fig. 3D). NPC cells expressing ebv-miR-BART7
showed significant reduction of TGFβ1/c-Myc as compared with the
mock transfectant (Fig. 3E and
F).
MICA expression was remarkably reduced
in ebv-miR-BART7-expressing NPC
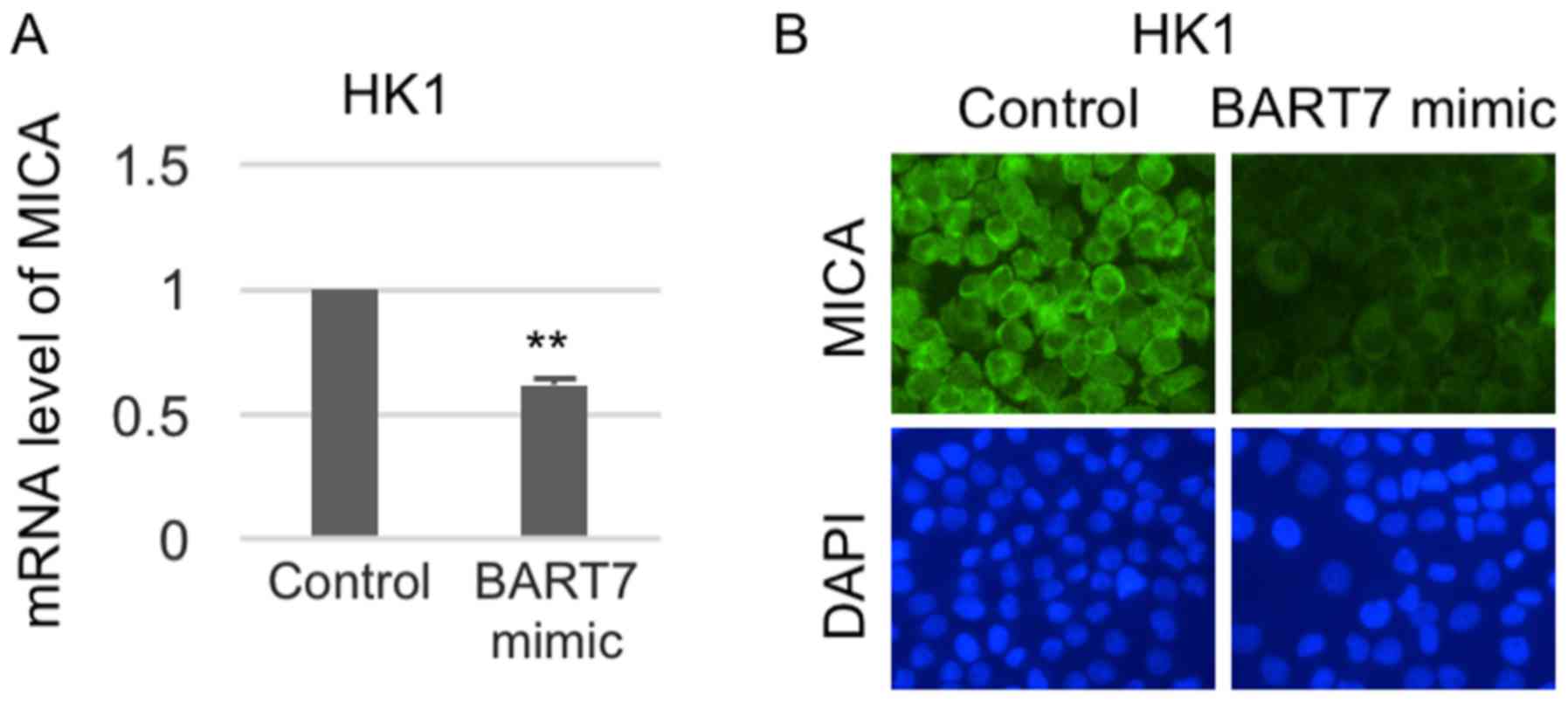
To confirm the importance of ebv-miR-BART7 on MICA
expression, changes of MICA expression in ebv-miR-BART7-expressing
NPC cell line were performed. As shown in Fig. 4, ebv-miR-BART7 had a significant
suppressive effect on MICA mRNA and protein expression in the NPC
cell line (Fig. 4).
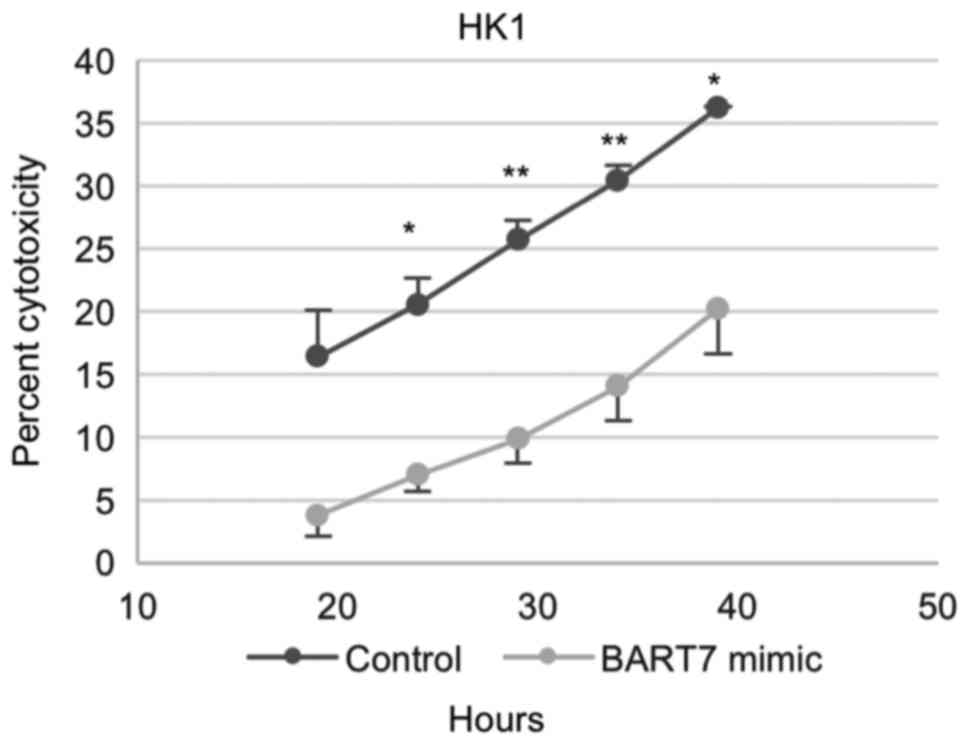
Ebv-miR-BART7-expressing NPC cells
exhibited reduced sensitivity to NK cell cytolysis
To explore whether ebv-miR-BART7 expression will
reduce the sensitivity of NPC cells to NK cell lysis,
ebv-miR-BART7-expressing NPC cells and the mock transfectants were
co-cultured with NK cell line NK92MI. When NPC cells reached their
logarithmic growth phase, NK92MI cells were added to NPC cells at
effector to target (E:T) ratio of 5:1. For HK1 cells, a significant
lysis of target cells was observed at 20 h after the addition of
NK92MI cells (Fig. 5).
Ebv-miR-BART7-expressing HK1 cells were less lysed by NK92MI cells
in comparison with control cells (Fig.
5), indicating that forced expression of ebv-miR-BART7 reduced
the sensitivity of NPC cells to NK92MI cells.
Discussion
Reduced expression of major histocompatibility
complex MHC class I protein on the cell surface is associated with
the oncogenic transformation (14).
MICA belongs to the MHC class I family and impairment of MHC class
I expression is common in viral infected cancers. MICA can
specifically bind to surface NKG2D on different immune cells such
as NK cells and CD8αβ T cells and activates their immune functions
(15,16). Therefore, suppressing MICA expression
could prevent the viral infected cancer cells from immune
attack.
TGFβ singling is important for maintaining the
epithelial homeostasis and immunity (17). Disruption of TGFβ signaling is
implicated in the pathogenesis of head and neck cancers (18). Recently, it was reported that TGFβ1
could inhibit mTOR activity of NK cells, leading to reduced
metabolic activity and impaired cytotoxic activity of NK cells
(19). TGFβ1 also reduced the
expression of NK cell surface receptors including CD122 and
IL-15RB, which might confer decreased metabolic activity (19). To identify the soluble factors
released by B-acute lymphoblastic leukaemia (ALL) blasts that could
impair function of NK cells, Rouce et al compared the levels
of known soluble factors upregulated in other cancers between ALL
blasts cultured with NK cells and healthy donor B cells cultured
with NK cells (20). They found that
TGFβ1 was the most notably increased soluble factor. Moreover,
TGFβ1 suppressed the expression of activating receptors including
NKG2D, NKp30 and NKp46 and enhanced the expression of inhibitory
receptor NKG2A of NK cells, resulting in ALL-mediated NK cell
dysfunction (20). We speculate that
TGFβ can interrupt the interaction of NKG2D (on NK cell surface)
and MICA in cancer patients in view of the fact that elevated TGFβ1
and reduced NKG2D expression on the NK cell surface is accompanying
with cancer patients (21). It has
been shown that TGFβ1 can suppress NKG2D expression on NK cells
(22). Whether TGFβ1 has similar
suppressing effects on MICA expression in NPC remains unclear.
Promoter analysis indicated that MICA contains the binding site for
the known TGFβ1-regulated transcriptional factor c-Myc (23). We first examined the effects of TGFβ1
on MICA expression in NPC. Our results demonstrated that TGFβ1 is a
potent activator of MICA in NPC. Thus, suppressing TGFβ1 expression
in NPC might provide protection against and evasion from NK cells.
It has been reported that c-Myc could affect the response of tumor
cells to the killing effect of NK cells. In tumor cell, c-Myc
enhanced the expression of B7-H6 which was the surface expressed
ligand for NKp30, an activating receptor of NK cells. Suppression
of c-Myc impaired the cytotoxic activity of NK cells activated by
NKp30 (24). Likewise, we found that
c-Myc could enhanced the expression of MICA, a ligand for another
activating receptor NKG2D of NK cells.
The expression of MICA was much more affected by
BART7 mimic in comparison with c-Myc and TGFβ1. This observation
might be due to the presence of other mechanisms responsible for
the regulatory role of ebv-miR-BART7 on MICA. Therefore,
ebv-miR-BART7 modulated MICA expression partially through
TGFβ1/c-Myc.
EBV infection is commonly found in the
undifferentiated NPC. The viral gene products could be detected in
the tumor cells with potent oncogenic functions in NPC development
(25). At present, how EBV confers
protection to NPC to escape from the attack of NK cells remains to
be clarified. However, it has been shown that EBV can express
different viral proteins to suppress the immune attack from other
immune cells such as cytotoxic T cells. EBV-infected cells in the
lytic phase could express BNLF2a to reduce transporter associated
with antigen processing (TAP) function and expression of human
histocompatibility leukocyte antigen (HLA) class I (26). EBV-expressed BGLF5 (EBV alkaline
exonuclease) can impair T-cell recognition by interfering HLA class
II immune responses (27).
Recent study has shown that EBV-encoded microRNA can
inhibit recognition and immune attack of EBV-specific
CD8+ T cells by targeting peptide transporter subunit
TAP2 (28). EBV-encoded microRNA
could also modulate CD4+ T cells response by targeting
cytokine and MHC class II family expression (28). Comparatively, how does the
EBV-infected cells evade from the immune attack of NK cells is less
well understood. We have previously shown that ebv-miR-BART7 can
suppress TGFβ1 in the NPC cells (12). Based on the new observation that TGFβ1
can un-regulate MICA expression in NPC, we speculate that
ebv-miR-BART7 could play a part in the NPC immunity by weakening
the interaction between NPC and NK cells. The NPC cell lines
employed in the current study does not harbor EBV. Transfection of
synthetic ebv-miR-BART7 results in a significant decrease in c-Myc
and MICA indicating that EBV-associated NPC might escape from the
surveillance of NK cells. This functional role is confirmed in the
NPC/NK cells co-cultures and the results demonstrate the
significance of ebv-miR-BART7 in NPC.
In conclusion, our results show that ebv-miR-BART7
can reduce the susceptibility of NPC cells to NK cell lysis by
reducing expression of MICA. As the activating role of MICA is
well-studied and confirmed, we speculate that targeting the
ebv-miR-BART7 expression in the NPC tissues can increase MICA and
thereby increase the susceptibility of NPC to NK cells. Further
studies are warranted to confirm the potential role of
ebv-miR-BART7 in NPC immunotherapy.
Acknowledgements
Not applicable.
Funding
The present study is supported by the Health and
Medical Research Fund from Food and Health Bureau, Hong Kong SAR
(grant no. 01121626), Seed Funding of Basic Research from The
University of Hong Kong, and S. K. Yee Medical Foundation
Grant.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TSW, WG and JYWC set up the hypothesis and designed
the experiments. SC and MJZ performed the experiments and acquired
the data. TSW, WG and JYWC analyzed and interpreted the data. TSW
and WG wrote the manuscript. All authors read and approve the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tsao SW, Yip YL, Tsang CM, Pang PS, Lau
VM, Zhang G and Lo KW: Etiological factors of nasopharyngeal
carcinoma. Oral Oncol. 50:330–338. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Young LS and Rickinson AB: Epstein-barr
virus: 40 years on. Nat Rev Cancer. 4:757–768. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ressing ME, van Gent M, Gram AM, Hooykaas
MJ, Piersma SJ and Wiertz EJ: Immune evasion by epstein-barr virus.
Curr Top Microbiol Immunol. 391:355–381. 2015.PubMed/NCBI
|
|
4
|
Trinchieri G: Biology of natural killer
cells. Adv Immunol. 47:187–376. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guillerey C, Huntington ND and Smyth MJ:
Targeting natural killer cells in cancer immunotherapy. Nat
Immunol. 17:1025–1036. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jinushi M, Takehara T, Tatsumi T,
Hiramatsu N, Sakamori R, Yamaguchi S and Hayashi N: Impairment of
natural killer cell and dendritic cell functions by the soluble
form of MHC class I-related chain A in advanced human
hepatocellular carcinomas. J Hepatol. 43:1013–1020. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lynn TC, Wang JH, Yang CS and Tu SM:
Natural killer cell activity in patients with nasopharyngeal
carcinoma. Zhonghua Min Guo Wei Sheng Wu Ji Mian Yi Xue Za Zhi.
19:177–182. 1986.(In Chinese). PubMed/NCBI
|
|
8
|
Tian W, Luo QZ, Li LX, Jin HK, Wang F, Guo
SS and Cao Y: Polymorphism of short tandem repeat of exon 5 of MHC
class-I chain related gene A and association with nasopharyngeal
carcinoma in a southern Chinese population. Zhonghua Yi Xue Yi
Chuan Xue Za Zhi. 22:309–312. 2005.(In Chinese). PubMed/NCBI
|
|
9
|
Tian W, Zeng XM, Li LX, Jin HK, Luo QZ,
Wang F, Guo SS and Cao Y: Gender-specific associations between
MICA-STR and nasopharyngeal carcinoma in a southern Chinese Han
population. Immunogenetics. 58:113–121. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kishikawa T, Otsuka M, Yoshikawa T, Ohno
M, Takata A, Shibata C, Kondo Y, Akanuma M, Yoshida H and Koike K:
Regulation of the expression of the liver cancer susceptibility
gene MICA by microRNAs. Sci Rep. 3:27392013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Song H, Kim Y, Park G, Kim YS, Kim S, Lee
HK, Chung WY, Park SJ, Han SY, Cho D and Hur D: Transforming growth
factor-β1 regulates human renal proximal tubular epithelial cell
susceptibility to natural killer cells via modulation of the NKG2
ligands. Int J Mol Med. 36:1180–1188. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao W, Li ZH, Chen S, Chan JY, Yin M,
Zhang MJ and Wong TS: Epstein-Barr virus encoded microRNA BART7
regulates radiation sensitivity of nasopharyngeal carcinoma.
Oncotarget. 8:20297–20308. 2017.PubMed/NCBI
|
|
13
|
Moodley K, Angel CE, Glass M and Graham
ES: Real-time profiling of NK cell killing of human astrocytes
using xCELLigence technology. J Neurosci Methods. 200:173–180.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Seliger B, Harders C, Lohmann S, Momburg
F, Urlinger S, Tampé R and Huber C: Down-regulation of the MHC
class I antigen-processing machinery after oncogenic transformation
of murine fibroblasts. Eur J Immunol. 28:122–133. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bauer S, Groh V, Wu J, Steinle A, Phillips
JH, Lanier LL and Spies T: Activation of NK cells and T cells by
NKG2D, a receptor for stress-inducible MICA. Science. 285:727–729.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Groh V, Rhinehart R, Randolph-Habecker J,
Topp MS, Riddell SR and Spies T: Costimulation of CD8alphabeta T
cells by NKG2D via engagement by MIC induced on virus-infected
cells. Nat immunol. 2:255–260. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li MO, Wan YY, Sanjabi S, Robertson AK and
Flavell RA: Transforming growth factor-beta regulation of immune
responses. Annu Rev Immunol. 24:99–146. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
White RA, Malkoski SP and Wang XJ: TGFβ
signaling in head and neck squamous cell carcinoma. Oncogene.
29:5437–5446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Viel S, Marçais A, Guimaraes FS, Loftus R,
Rabilloud J, Grau M, Degouve S, Djebali S, Sanlaville A, Charrier
E, et al: TGF-β inhibits the activation and functions of NK cells
by repressing the mTOR pathway. Sci Signal. 9:ra192016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rouce RH, Shaim H, Sekine T, Weber G,
Ballard B, Ku S, Barese C, Murali V, Wu MF, Liu H, et al: The
TGF-β/SMAD pathway is an important mechanism for NK cell immune
evasion in childhood B-acute lymphoblastic leukemia. Leukaemia.
30:800–811. 2016. View Article : Google Scholar
|
|
21
|
Crane CA, Han SJ, Barry JJ, Ahn BJ, Lanier
LL and Parsa AT: TGF-beta downregulates the activating receptor
NKG2D on NK cells and CD8+ T cells in glioma patients. Neuro Oncol.
12:7–13. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee JC, Lee KM, Kim DW and Heo DS:
Elevated TGF-beta1 secretion and down-modulation of NKG2D underlies
impaired NK cytotoxicity in cancer patients. J Immunol.
172:7335–7340. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu X, Ozturk F, Liu C, Oakley GG and
Nawshad A: Transforming growth factor-β activates c-Myc to promote
palatal growth. J Cell Biochem. 113:3069–3085. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Textor S, Bossler F, Henrich KO,
Gartlgruber M, Pollmann J, Fiegler N, Arnold A, Westermann F,
Waldburger N, Breuhahn K, et al: The proto-oncogene Myc drives
expression of the NK cell-activating NKp30 ligand B7-H6 in tumor
cells. Oncoimmunology. 5:e11166742016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Young LS and Dawson CW: Epstein-Barr virus
and nasopharyngeal carcinoma. Chin J Cancer. 33:581–590.
2014.PubMed/NCBI
|
|
26
|
Hislop AD, Ressing ME, van Leeuwen D,
Pudney VA, Horst D, Koppers-Lalic D, Croft NP, Neefjes JJ,
Rickinson AB and Wiertz EJ: A CD8+ T cell immune evasion protein
specific to Epstein-Barr virus and its close relatives in Old World
primates. J Exp Med. 204:1863–1873. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zuo J, Thomas W, van Leeuwen D, Middeldorp
JM, Wiertz EJ, Ressing ME and Rowe M: The DNase of
gammaherpesviruses impairs recognition by virus-specific CD8+ T
cells through an additional host shutoff function. J Virol.
82:2385–2393. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Albanese M, Tagawa T, Bouvet M, Maliqi L,
Lutter D, Hoser J, Hastreiter M, Hayes M, Sugden B, Martin L, et
al: Epstein-Barr virus microRNAs reduce immune surveillance by
virus-specific CD8+ T cells. Proc Natl Acad Sci USA.
113:E6467–E6475. 2016. View Article : Google Scholar : PubMed/NCBI
|