Introduction
Colorectal cancer is a malignancy that occurs in the
gut, and the incidence is second only to the stomach and esophageal
cancer, and it is a common malignancy in the gastrointestinal
tract. In addition to genetics, behavioral and environmental causes
are also closely related to its incidence (1–3). It is one
of the main causes of cancer-related deaths worldwide, especially
in Western countries (4–7). Due to high recurrence rates and
metastases, the mortality rate caused by colorectal cancer remains
high despite the treatment of colorectal cancer has improved with
development of technology (8,9). Thus, new promising targets for therapy
of colorectal cancer are urgently needed.
Rho GTPase-activating protein 24 (ARHGAP24), belongs
to Rho GTPase-activating proteins (RHOGAPs) family, is a protein
containing 748 amino acids involved in cell cycle, apoptosis and
invasion and other cell processes. As for tumor research, several
RHOGAP proteins were found implicated in human tumors. For example,
it is revealed that upregulation of ARHGAP10 had an inhibitory
effect on tumorigenicity of ovarian cancer cells (10), and ARHGAP10 also acted as a tumor
suppressor in lung cancer (11).
Besides ARHGAP10, ARHGAP35 and ARHGAP8 were also reported as
candidate tumor suppressors functionig in colorectal cancer
(12,13). A study has related the function of
ARHGAP24 to the cell apoptosis and invasion of renal cell carcinoma
(14). However, there is little
research on the function of ARHGAP24 in colorectal cancer, so here
we aim to investigated the functions of ARHGAP24 in colorectal
cancer.
Additionally, the studies of tumor usually focus on
cell apoptosis and the cell cycle process, which are associated
with p53. p53, mutated in ~50% of all malignant neoplasm, is a
well-known tumor suppressor gene of human cancer. It is reported
that the mutation of p53 can cause alteration of growth arrest and
deficient apoptosis (15).
In the present study, low expression of ARHGAP24 and
p53 was noted in tumor tissues of colorectal cancer patients
revealed that ARHGAP24 may function in colorectal cancer via p53.
In vitro, upregulation of ARHGAP24 in colorectal cancer cell
lines inhibited the cell ability, in contrast, apoptotic cells were
significantly increased accompanied with high expression of
apoptosis-related proteins p53, p21 and Bax. The addition of p53
inhibitor PFT-α antagonized the induction of ARHGAP24 on cell
ability of colorectal cancer cells. The protein level of p21 and
Bax were correspondingly declined by PFT-α. Based on the above, we
conjectured that ARHGAP24 may affect colorectal cancer via the
regulation of p53, p21 and Bax. Targeting ARHGAP24 may provide a
potential promising direction for research and therapy of
colorectal cancer.
Materials and methods
Colorectal cancer and adjacent normal
tissues
With written informed consent, thirty colorectal
cancer and paired adjacent normal tissues were collected from the
colorectal cancer patients treated in The Seventh People's Hospital
of Shanghai University of Traditional Chinese Medicine (Shanghai,
China) who volunteered to participate in the study. Another four
pairs of colorectal cancer and adjacent normal tissues were also
collected. Before used, the tissue samples were stored in liquid
nitrogen. The study was approved by the Ethics Committee of The
Seventh People's Hospital of Shanghai University of Traditional
Chinese Medicine.
Cell culture
LoVo and HCT116, two human colorectal cancer cell
lines, were obtained from Cell Bank of Chinese Academy of Science
(Shanghai, China). Generally, in a 37°C, 5% CO2
incubator (Thermo Fisher Scientific, Inc., Waltham, MA, USA), LoVo
and HCT116 cells were respectively cultured in 1640 medium
(HyClone; GE Healthcare Life Sciences, Logan, UT, USA) and DMEM
high glucose medium (HyClone; GE Healthcare Life Sciences) both
containing 10% fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc.) and 1% antibiotic (admixture of penicillin and streptomycin;
Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China). The medium was replaced according to the cell growth state
during incubation.
The construction of lentivirus
First, vector pLVX-Puro was constructed according to
the experimental requirements. The 2247 bp length coding DNA
sequence (CDS) region of ARHGAP24 containing cleavage sites of EcoR
I and BamH I was then synthesized by Genewiz Company (Shanghai,
China), which was then inserted into EcoR I/BamH I sites of
pLVX-Puro and confirmed by DNA sequencing (Majorbio, Shanghai,
China). Next, through Lipofectamine™ 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.), lentivirus core plasmid
pLVX-Puro-ARHGAP24 (Clontech Laboratories, Inc., Mountainview, CA,
USA) and two packaging plasmids psPAX2 and pMD2G (Addgene, Inc.,
Cambridge, MA, USA) were co-tranfected into 293 T cells. After 48 h
the virus particles in the supernatant were collected by
ultracentrifugation (16).
Experimental group
LoVo cells were divided into three groups to infect
with ARHGAP24 lentivirus. Grouped as follows, LoVo cells were
counted and infected with lentivirus of ARHGAP24 overexpression
(oeARHGAP24)/empty vectors (Vector). Wild-type LoVo cells only
cultured with medium were used as control. On the other hand,
HCT116 cells were also grouped following the above. Subsequently,
RT-qPCR and western blot analysis were carried out to detect the
overexpression efficiency of ARHGAP24. The assays of the cell
ability and apoptosis were also performed.
In addition, to investigate the effect of p53 on
oeARHGAP24-induced LoVo cells, cells were grouped as follows.
Vector, LoVo cells were infected with empty vectors; oeARHGAP24,
LoVo cells were infected with ARHGAP24 overexpression lentivirus;
oeARHGAP24+PFT-α, LoVo cells were treated with oeARHGAP24 and PFT-α
(20 µg/l). Next, cell ability assay and western blot analysis were
carried out.
Immunohistochemical detection
The embedded and fixed tissues were cut into 4–7 µm
slices. The slices were grilled in a 65°C constant temperature oven
for 30 min, soaked in xylene I (Sinopharm Chemical Reagent Co.,
Ltd., Shanghai, China) for 15 min, and then soaked in xylene II for
15 min. The dewaxed-slices were soaked with gradient concentrations
of ethanol (100, 95, 85, and 75%) and each gradient for 5 min,
followed by 10 min flushing of tap water. After antigen retrieval
with 0.01 M citrate buffer solution for 15 min, the slices were
incubated in wet-box with 0.3% H2O2 for 10
min. Later, following incubation with rabbit polyclonal ARHGAP24
antibody (1:200; cat. no. Ab203874; Abcam, Cambridge, UK) in
wet-box at room temperature for 1 h, the slices were incubated with
secondary goat anti-rabbit (HRP) IgG antibody (1:2000; cat. no.
ab6721; Abcam) at room for 20–30 min. Subsequently, the slices were
treated with DAB, 3 min staining of hematoxylin (714094; Baso
Diagnostic, Inc., Wuhan, China), 1% hydrochloric acid for alcohol
differentiation. After 10 min flushing of tap water, the slices
were grilled, made transparent and closed. Finally, the images were
observed under microscopy (CX41; Olympus Corporation, Tokyo, Japan)
and analyzed by IMS image analysis system (Shanghai Jierdun Biotech
Co., Ltd., Shanghai, China).
Cell Counting Kit-8 (CCK-8) assay
Logarithmic growth phase cells after digested by
0.25% trypsin were seeded in 96-well plates with 100 µl of cell
suspension (3×104 cells/ml) added to each well, then
cultured overnight. Cells were treated according to the above
protocol, and then 100 µl mixtures of 10% CCK-8 (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany Inc.) solution in serum-free medium
were added to each well with 1 h of incubation. After that, a
microplate reader (Perlong, Beijing, China) was applied to measure
the absorbance value (OD) of each well at 450 nm.
Reverse transcription-quantitative PCR
(RT-qPCR) assay
Total RNA was extracted from LoVo and HCT116 cells
by using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), and then confirmed by 1% agarose gel electrophoresis after
quantification. Subsequently, through reverse transcriptase kit
(Fermentas; Thermo Fisher Scientific, Inc.), cDNA was synthesized
from the isolated RNA. After that, following the procedure of
RT-qPCR reactions, the gene expression was analyzed by a machine of
ABI Prism 7300 (ABI; Thermo Fisher Scientific, Inc.) using
SYBR-Green PCR kit (Thermo Fisher Scientific, Inc.). The mRNA
expression of ARHGAP24 was analyzed by application of
2−ΔΔCq method with GAPDH as an internal
control (17). The primers were as
follows: ARHGAP24, 5′-AACTCCTGTCGCTCTTCTACC-3′ and
5′-GCTGTTGCCCACAAATGTCTC-3′; p53, 5′-CCACCATCCACTACAACTAC-3′ and
5′-AAACACGCACCTCAAAGC −3′; GAPDH, 5′-CACCCACTCCTCCACCTTTG-3′ and
5′-CCACCACCCTGTTGCTGTAG-3′. The RT-qPCR was conducted as the
following procedure: cycle 1, 95°C for 10 min; cycle 2 with 40
repeated cycles of 95°C for 15 sec, 60°C for 45 sec, and then 95°C
for 15 sec, 60°C for 1 min for one cycle; 95°C for 15 sec, 60°C for
15 sec for one cycle (18,19).
Western blot analysis
Treated cells were washed with cold
phosphate-buffered saline (PBS) twice and then lysed in a RIPA
buffer (Solarbio Science & Technology Co., Ltd.) which
contained protease and phosphatase inhibitors for ~30 min on ice. A
pre-cooled centrifuge was applied to centrifuge the lysates and the
protein in the supernatant was obtained. Subsequently, the isolated
protein was calculated by BCA method (Thermo Fisher Scientific,
Inc.). Every 15 µl extracted protein used for one sample were
subjected to 15 and 10% SDS-PAGE (JRDUN Biotechnology Co., Ltd,
Shanghai, China) and semi-dry transferred onto polyvinylidene
fluoride (PVDF) membranes (EMD Millipore, Billerica, MA, USA).
Following blocking with 5% skim milk (BD Biosciences, San Jose, CA,
USA) in PBST, the membranes were incubated with primary antibodies,
ARHGAP24 (cat. no. Ab203874; 1:200; Abcam, Cambridge, UK), Bax
(cat. no. Sc-493; 1:300, Santa Cruz Biotechnology, Inc., Dallas,
TX, USA), p21 (1:1,000; cat. no. 2947), GAPDH (1:2,000; cat. no.
5174) and p53 (cat. no. 2524; 1:1,000) all from Cell Signaling
Technology, Inc., (Danvers, MA, USA) at 4°C overnight with gentle
shaking (at room for 2 h), followed by washing with PBST 6 times.
After incubation of secondary goat anti-rabbit (HRP) IgG antibody
(1:2,000; cat. no. ab6721; Abcam) for 2 h at at 37°C in the dark,
the membranes were washed with PBST 6 times again. Finally, using a
chemiluminescence detection reagent (Millipore), the blots were
visualized by an instrument of ECL chemiluminescence (Tanon Science
and Technology Co., Ltd., Shanghai, China).
Cell cycle detection
Colorectal cancer cells of LoVo and HCT116 were
infected with oeARHGAP24 lentivirus. After 48 h of infection, the
cells were centrifuged at 1,000 × g for 5 min, and then resuspended
with 300 µl PBS containing 10% fetal bovine serum (Gibco; Thermo
Fisher Scientific, Inc.). Then 700 µl of absolute ethanol was added
for fixing at −20°C overnight. The next day, the fixed-cells were
centrifuged at 3,000 × g for 30 sec and washed twice with 1 ml
pre-cooled PBS. The cell pellets were resuspended with 100 µl of 1
mg/ml RNase A solution and incubated at 37°C. Subsequently, the
cells were stained with a 400 µl of 50 µg/ml propidium iodide (PI)
solutions (Shanghai Beiyi Bioequip Information Co., Ltd.) in the
dark for 10 min. Finally, the numbers of LoVo and HCT116 cells in
each phase of the cell cycle were analyzed by BD flow
cytometry.
Flow cytometry (FCM) analysis
Apoptotic cells were analyzed by FCM (BD
Biosciences) using Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) double staining (Shanghai Beiyi
Bioequip Information Co., Ltd.), as follows. After washing,
digestio, resuspension and counting,
~5×105−1×106 of the treated cells was
obtained after centrifugation for 5 min at 1,000 × g. Resuspended
cells were precipitated with 195 µl of Annexin V-FITC binding
buffer, 5 µl Annexin V-FITC was added to incubate the cells at 4°C
for 15 min in the dark. Under the same conditions, the cells were
then incubated with 5 µl PI for 5 min, while a tube without Annexin
V-FITC and PI was a control. Finally, cell apoptosis rates were
determined by BD flow cytometry.
Statistical analysis
Software of GraphPad prism 7.0 (GraphPad Software,
Inc., La Jolla, CA, USA) was used to express all the statistical
analyses. The differences of each two groups were evaluated by
Student's t-test, while three and more comparisons were presented
by one way analysis of vari-ance (ANOVA) followed by post hoc test
(Least Significant Difference). All values are mean ± standard
deviation of at least three independent experiments. P<0.05 was
considered to indicate a statistically significant difference.
Results
ARHGAP24 and p53 low expression in
tumor tissues of colorectal cancer patients
Thirty cancer tissues and paired adjacent normal
tissues were collected from thirty colorectal cancer patients who
volunteered to participate in this study. Further four pairs of
these tissues were also obtained for protein detection. As shown in
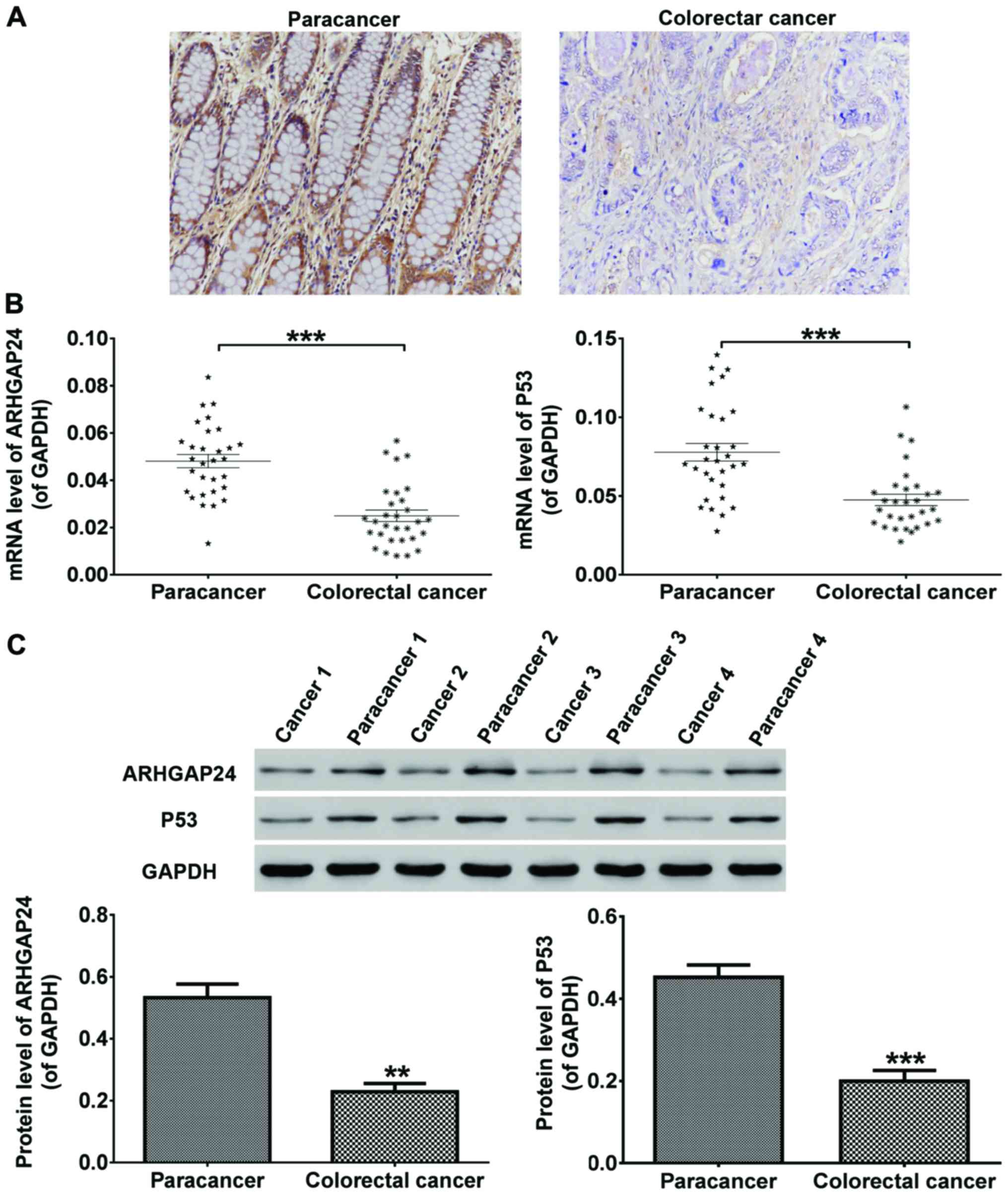
Fig. 1, immunohistochemical
detections of tissues revealed that ARHGAP24 expression was
significantly low in tumors of colorectal cancer patients (Fig. 1A). After RNA and proteins extraction,
the mRNA and protein expression of ARHGAP24 and p53 was
respectively detected by RT-qPCR and western blot analysis. We
discovered that in colorectal cancer patients, the mRNA (Fig. 1B) as well as protein (Fig. 1C) level of ARHGAP24 and p53 was
reduced obviously in cancer tissues compared to adjacent normal
tissues. From this, ARHGAP24 and p53 were considered likely to be
involved in colorectal cancer.
Overexpression of ARHGAP24 in LoVo and
HCT116 cell lines
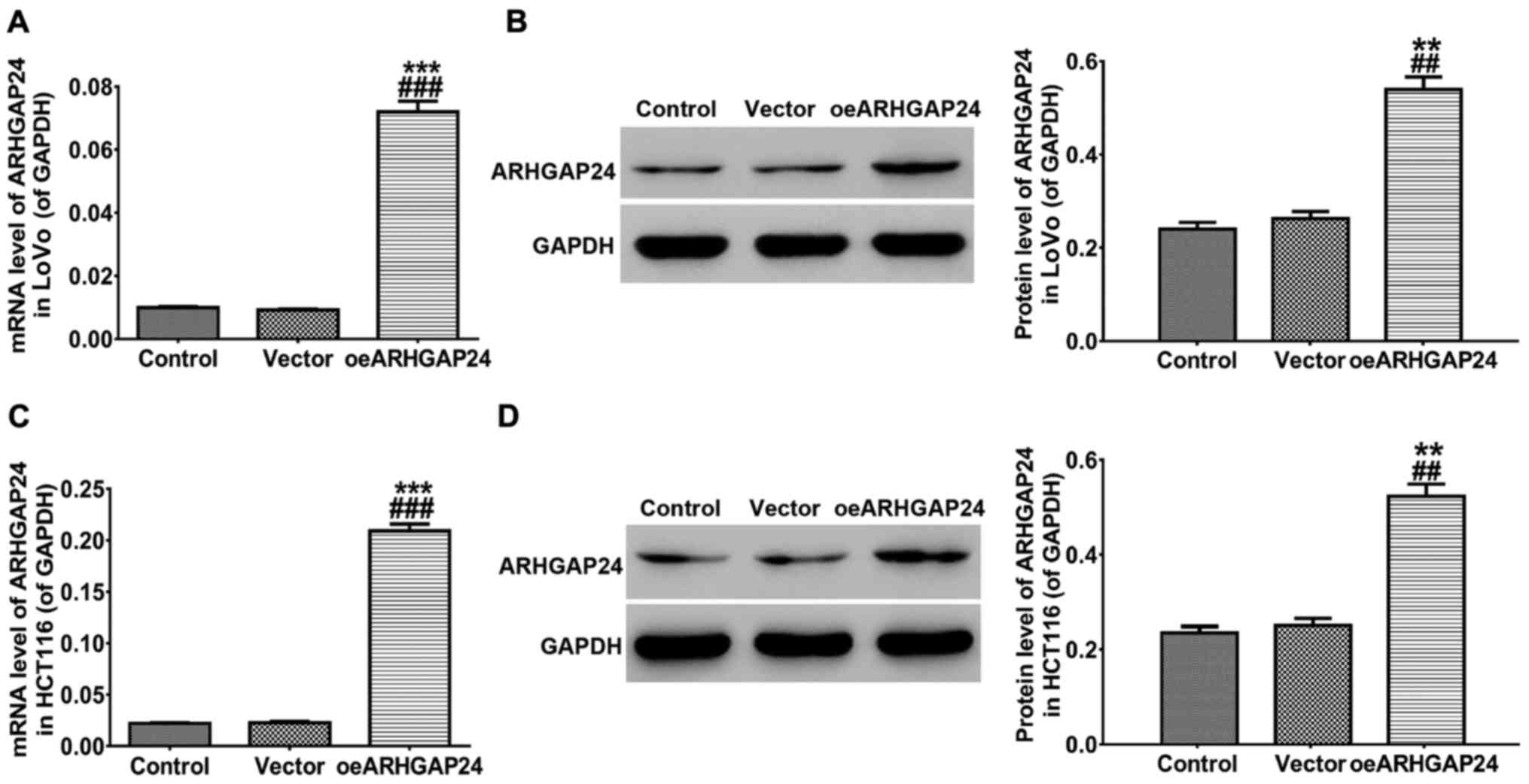
To further study the function of ARHGAP24 exerted in
colorectal cancer, LoVo and HCT116 cell lines were infected with
ARHGAP24 overexpressed lentivirus, respectively. After 48 h of
infection, RT-qPCR and western blot analysis were carried to detect
the overexpression efficiency of ARHGAP24. As presented in Fig. 2, both at the transcription and protein
translation level, the expression of ARHGAP24 was significantly
increased in LoVo (Fig. 2A and B) and
HCT116 (Fig. 2C and D) cells.
Therefore, ARHGAP24 overexpressed lentivirus was chosen to use in
subsequent experiments.
Upregulation of ARHGAP24 inhibits the
cell ability of colorectal cancer cells via p53, p21 and Bax
expression
For exploring the effect of ARHGAP24 on cell ability
of colorectal cancer, after infected with oeARHGAP24 lentivirus,
counted LoVo and HCT116 cells were cultured with CCK-8 mixture for
0, 24, 48, and 72 h. Subsequently, the cell ability of LoVo and
HCT116 cells were measured by a machine of microplate reader
(Bio-Rad, Hercules, CA, USA). The results in Fig. 3 showed that ARHGAP24 overexpression
inhibited the cell ability of LoVo cells in a time-dependent manner
and had an obvious effect after 48 h of CCK-8 treatment (Fig. 3A). On the other hand, in HCT116 cells,
overexpression of ARHGAP24 performed a similar effect (Fig. 3B). Further, ARHGAP24-infected LoVo
cells were treated with PFT-α, an inhibitor of p53, for 48 h. We
found that the addition of PFT-α significantly antagonized the
effect of oeARHGAP24 on the cell ability of LoVo cells (Fig. 3C). Simultaneously, ARHGAP24-induced
the expression of p53, p21 and Bax expression was significantly
decreased by PFT-α (Fig. 3D). These
results demonstrated that ARHGAP24 regulated colorectal cancer cell
proliferation probably through modulating p53, p21 and Bax
expression.
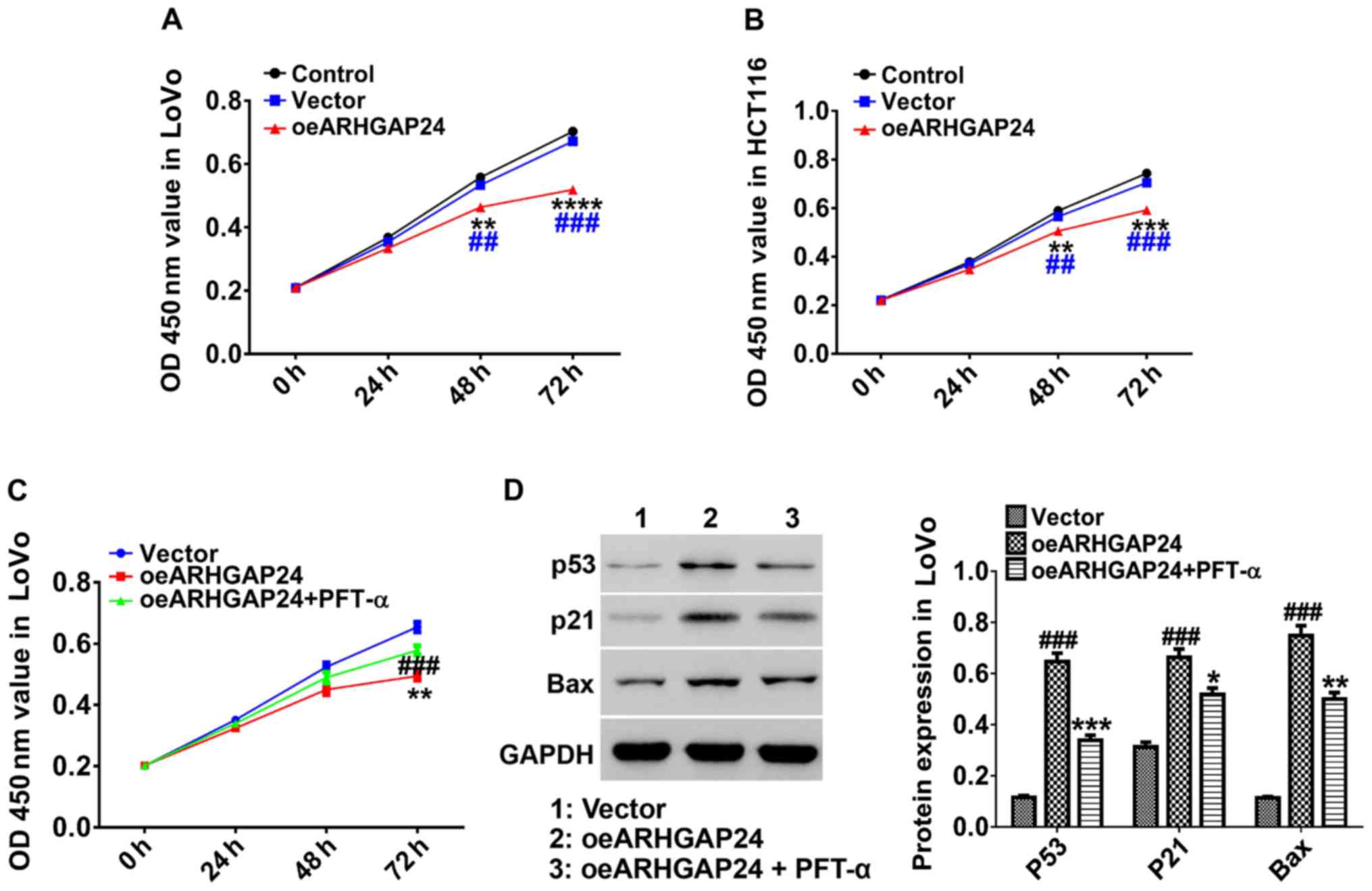 | Figure 3.Upregulation of ARHGAP24 inhibits the
cell ability of colorectal cancer cells via p53, p21 and Bax
expression LoVo and HCT116 cells were infected with lentivirus for
0, 24, 48, and 72 h before treated with CCK-8. (A and B) The cell
ability of ARHGAP24-infected LoVo and HCT116 cells was evaluated by
a microplate reader at 450 nm. (C) ARHGAP24-infected LoVo cells
were treated with p53 inhibitor PFT-α and then the cell ability was
assessed. (D) The protein levels of p53, p21 and Bax were detected.
Data are mean ± standard deviation. *P<0.05, **P<0.01,
***P<0.001, ****P<0.0001 compared to control;
##P<0.01, ###P<0.001 compared to
vector. ARHGAP24, Rho GTPase-activating protein 24; CCK-8, Cell
Counting Kit-8. |
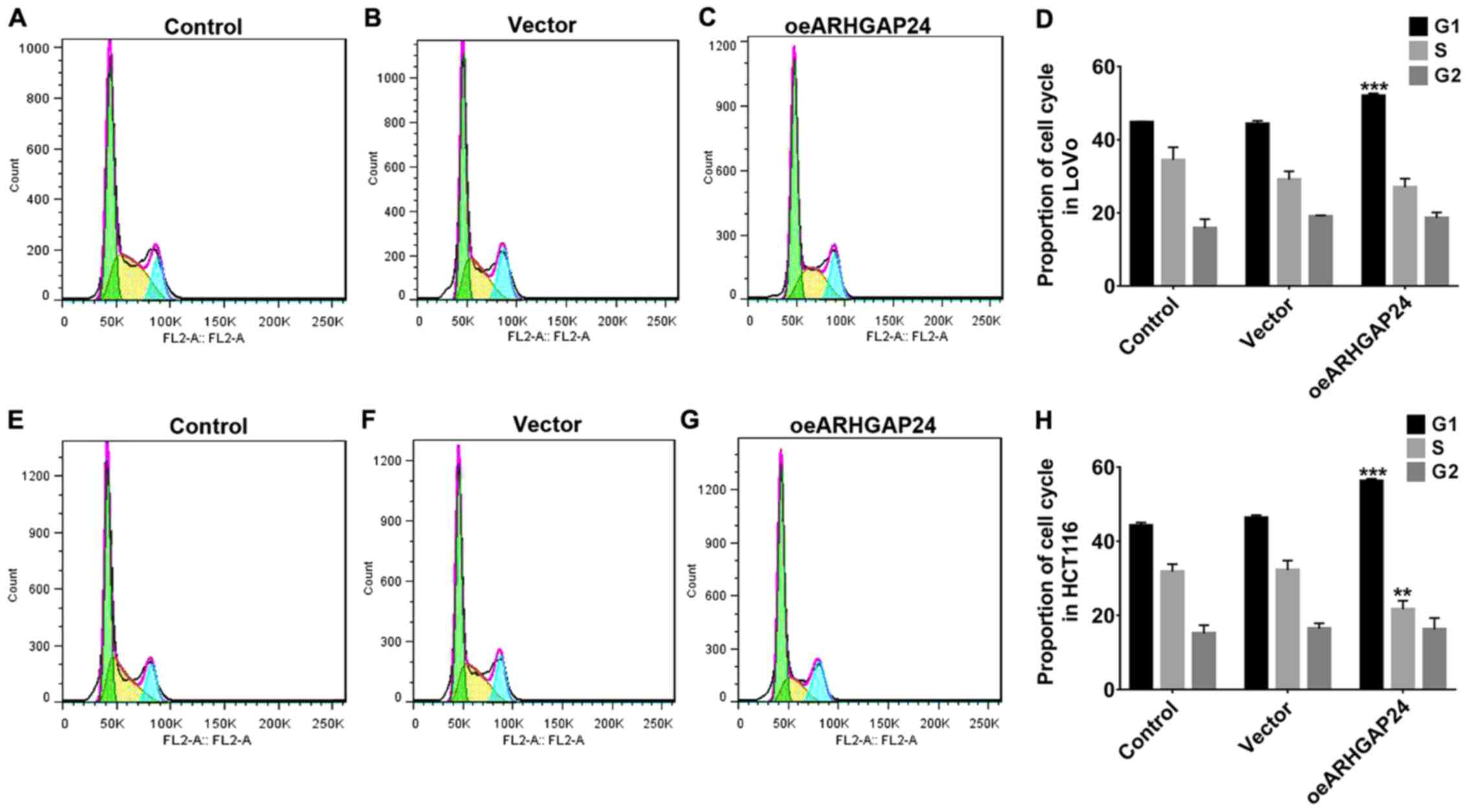
Upregulation of ARHGAP24 inhibited
cell cycle arrest of colorectal cancer cells
For further investigation, after infected with
oeARHGAP24, the cell cycle arrest of LoVo and HCT116 cells were
detected. As shown in Fig. 5,
upregulation of ARHGAP24 in LoVo cells significantly arrested the
cell cycle at G1 phase, therefore reducing the proportion of cells
in the S/G2 phase (Fig. 4A-D).
Likewise, in HCT116 cells, ARHGAP24 upregulation showed a similar
effect on cell cycle (Fig. 4E-H).
These further evidenced the inhibitory effect of ARHGAP24
upregulation on the cell proliferation of colorectal cancer.
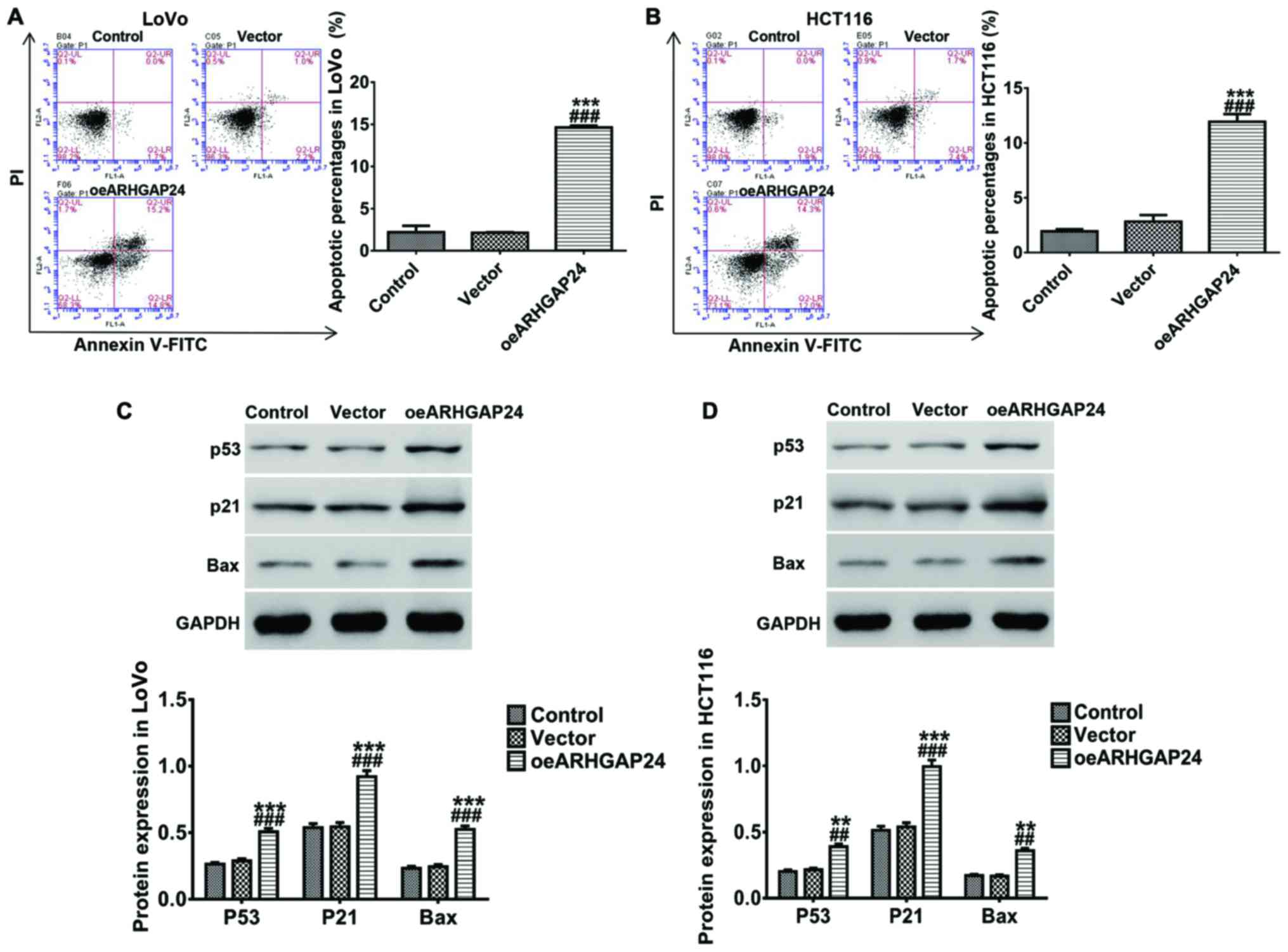
Upregulation of ARHGAP24 promotes
apoptosis of colorectal cancer cells in vitro
In addition, we also studied the effect of ARHGAP24
on apoptosis of colorectal cancer cells. After infected with
oeARHGAP24 lentivirus, LoVo and HCT116 cells were respectively
incubated with Annex V-FITC/PI double dyes. After that, apoptotic
cells were analyzed by flow cytometry. A remarkable increase of
apoptosis rates was noted in LoVo cells after ARHGAP24
overexpression (Fig. 5A), and the
protein levels of several apoptosis-associated proteins p53, p21
and Bax were increased with the increase of ARHGAP24 expression
(Fig. 5C). Moreover, changes similar
to those in LoVo cells also occurred in HCT116 cells (Fig. 5B and D). These indicated that
upregulation of ARHGAP24 enhanced apoptosis of colorectal cancer
cells which was likely to be associated with the expression of p53,
p21 and Bax.
Discussion
Colorectal cancer is a human malignant tumor with
high incidence and its incidence is on the rise. Due to high
relapse and metastasis rate, chemical resistance and other
characteristics, colorectal cancer is a serious threat to human
health. Thus, more novel promising targets for colorectal cancer
are urgently required to further investigate its pathogenesis. In
the present study, we found that in colorectal cancer patients, the
expression of ARHGAP24 and p53 in tumors was much lower than that
in normal tissues, and in vitro, overexpression of ARHGAP24
remarkably inhibited the cell ability of colorectal cancer cells,
arrested the cell cycle at G1 phase, reducing the proportion of
cells in S/G2 phase, and accelerated the cell apoptosis probably
through modulating p53, p21 and Bax expression.
Up to date, several members of RHOGAP proteins were
reported to be involved in colorectal cancer such as ARHGAP35, and
ARHGAP8. A previous study revealed that the methylation in the
promoter region of ARHGAP28 may affect in metastatic ability of
colorectal cancer (20). In our
study, declined ARHGAP24 and p53 in colorectal cancer tumor may be
associated with the progress of colorectal cancer which is likely
to be related to p53. Further in vitro, ARHGAP24
upregulation inhibited the cell ability of colorectal cancer cells
and arrested the cell cycle at G1 phase, whereas apoptotic cells
were increased, concurrent with an increase in p53, p21 and Bax
expression, which suggested that ARHGAP24 may be used as a tumor
suppressor in colorectal cancer through the regulation of p53, p21
and Bax. It is known that apoptosis and growth arrest are essential
to the process of various cancers that occur in human. Proteins
p53, p21 and Bax were more heavily acting in key roles in growth
arrest and apoptosis. p53 often plays an essential role in tumor
via the induction of apoptosis (21).
Similar to p53, another apoptosis-related protein, Bax, belonged to
Bcl2 family, is known as the main effecter of apoptosis and the
activity of Bax is enhanced in p53-induced tumor cell apoptosis
(22–24), thus, Bax is activated by p53 to take
part in multiple processes such as apoptotic program (25,26). In
addition, tumor growth suppressor p21, downstream of p53, is also
an effector gene activated by p53, which is implicated in cell
cycle and may participate in induction of p53-dependent apoptosis.
It is revealed that p21 can bind cyclin-dependent kinases to
repress the phosphorylation of cell cycle-required proteins such as
pRb, which may be induced by p53-dependent apoptosis (27–29). That
is to say, through regulation of p21 and Bax, p53 performed the
most important role in the regulation of the cell growth arrest and
cell apoptosis in the progress of cancers (30–32). It is
consistent with our results that the addition of p53 inhibitor
PFT-α showed an antagonistic effect on oeARHGAP24-induced cell
ability of colorectal cancer. The expression of p21 and Bax reduced
by PFT-α further demonstrated that p53 had an activation effect on
the expression of p21 and Bax, which indicated that the function of
p53 in the cell proliferation and apoptosis closely related to p21
and Bax.
In conclusion, this study demonstrated that
overexpression of ARHGAP24 may suppress the survival of colorectal
cancer cells by regulating the cell ability and apoptosis via the
modulation of p53, p21 and Bax. Therefore, ARHGAP24 may be
considered as a novel promising target for the further research of
colorectal cancer.
Acknowledgements
Not applicable.
Funding
The study was supported by grants from Key
disciplines Group Construction Project of Pudong Health Burea of
Shanghai (PWZxq2014-12), Natural Science Foundation of China (no.
81571718), Budgetary fund of Shanghai University of Traditional
Chinese Medicine (2016YSN67) and Talents Training Program of
Seventh People's Hospital of Shanghai University of TCM (grant no.
QMX2017-01).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SZ and LS were responsible for cell culture and
construction of lentivirus. JZ helped with immunohistochemical
detection. SH performed PCR. YS and YY contributed to CCK-8 assay
and western blot analysis. WX was in charge of Flow Cytometry
analysis. All authors read and approved the final study.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The Seventh People's Hospital of Shanghai University of Traditional
Chinese Medicine (Shanghai, China) and informed consents were
signed by the patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Center MM, DeSantis C and Ward
EM: Global patterns of cancer incidence and mortality rates and
trends. Cancer Epidemiol Biomarkers Prev. 19:1893–1907. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Favoriti P, Carbone G, Greco M, Pirozzi F,
Pirozzi RE and Corcione F: Worldwide burden of colorectal cancer: A
review. Updates Surg. 68:7–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marley AR and Nan H: Epidemiology of
colorectal cancer. Int J Mol Epidemiol Genet. 7:105–114.
2016.PubMed/NCBI
|
|
4
|
Gaedcke J, Grade M, Camps J, Søkilde R,
Kaczkowski B, Schetter AJ, Difilippantonio MJ, Harris CC, Ghadimi
BM, Møller S, et al: The rectal cancer microRNAome - microRNA
expression in rectal cancer and matched normal mucosa. Clin Cancer
Res. 18:4919–4930. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: The impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Merika E, Saif MW, Katz A, Syrigos K and
Morse M: Review. Colon cancer vaccines: An update. In Vivo.
24:607–628. 2010.PubMed/NCBI
|
|
7
|
Cunningham D, Atkin W, Lenz HJ, Lynch HT,
Minsky B, Nordlinger B and Starling N: Colorectal cancer. Lancet.
375:1030–1047. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xi ZW, Xin SY, Zhou LQ, Yuan HX, Wang Q
and Chen KX: Downregulation of rho-associated protein kinase 1 by
miR-124 in colorectal cancer. World J Gastroenterol. 21:5454–5464.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakamura Y, Nishi M, Fukuda Y, Ogino M and
Kosuga H: Case of the multiple liver metastases from colon cancer
obtained long-term disease-free survival with multimodality
therapy. Gan To Kagaku Ryoho. 39:2228–2230. 2012.(In Japanese).
PubMed/NCBI
|
|
10
|
Luo N, Guo J, Chen L, Yang W, Qu X and
Cheng Z: ARHGAP10, downregulated in ovarian cancer, suppresses
tumorigenicity of ovarian cancer cells. Cell Death Dis.
7:e21572016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Teng JP, Yang ZY, Zhu YM, Ni D, Zhu ZJ and
Li XQ: The roles of ARHGAP10 in the proliferation, migration and
invasion of lung cancer cells. Oncol Lett. 14:4613–4618. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choi EJ, Kim MS, Song SY, Yoo NJ and Lee
SH: Low frequent mutation of ARHGAP35, a candidate tumor suppressor
gene, in gastric and colorectal cancers. Pathol Oncol Res.
24:175–176. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Johnstone CN, Castellví-Bel S, Chang LM,
Bessa X, Nakagawa H, Harada H, Sung RK, Piqué JM, Castells A and
Rustgi AK: ARHGAP8 is a novel member of the RHOGAP family related
to ARHGAP1/CDC42GAP/p50RHOGAP: Mutation and expression analyses in
colorectal and breast cancers. Gene. 336:59–71. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu G, Lu X, Huang T and Fan J: ARHGAP24
inhibits cell cycle progression, induces apoptosis and suppresses
invasion in renal cell carcinoma. Oncotarget. 7:51829–51839.
2016.PubMed/NCBI
|
|
15
|
Muller PAJ, Vousden KH and Norman JC: p53
and its mutants in tumor cell migration and invasion. J Cell Biol.
192:209–218. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dang WQ, Tang H, Cao H, Wang L and Chen
TM: Construction of lentivirus-mediated short hairpin RNA targeting
human STAT3 gene. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi.
28:1204–1207. 2012.(In Chinese). PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-ΔΔC(T)) method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tan Z, Liu X, Yu E, Wang H, Tang L, Wang H
and Fu C: Lentivirus-mediated RNA interference of tripartite motif
68 inhibits the proliferation of colorectal cancer cell lines
SW1116 and HCT116 in vitro. Oncol Lett. 13:2649–2655. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu K, Guo J, Wang H and Yu W: FRAT1
expression regulates proliferation in colon cancer cells. Oncol
Lett. 12:4761–4766. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kasuya K, Nagakawa Y, Hosokawa Y, Sahara
Y, Takishita C, Nakajima T, Hijikata Y, Soya R, Katsumata K and
Tsuchida A: RhoA activity increases due to hypermethylation of
ARHGAP28 in a highly liver-metastatic colon cancer cell line.
Biomed Rep. 4:335–339. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kastan MB, Canman CE and Leonard CJ: P53,
cell cycle control and apoptosis: Implications for cancer. Cancer
Metastasis Rev. 14:3–15. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vaseva AV and Moll UM: The mitochondrial
p53 pathway. Biochim Biophys Acta. 1787:414–420. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Czabotar PE, Lessene G, Strasser A and
Adams JM: Control of apoptosis by the BCL-2 protein family:
Implications for physiology and therapy. Nat Rev Mol Cell Biol.
15:49–63. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim EM, Jung CH, Kim J, Hwang SG, Park JK
and Um HD: The p53/p21 complex regulates cancer cell invasion and
apoptosis by targeting Bcl-2 family proteins. Cancer Res.
77:3092–3100. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chipuk JE, Kuwana T, Bouchier-Hayes L,
Droin NM, Newmeyer DD, Schuler M and Green DR: Direct activation of
Bax by p53 mediates mitochondrial membrane permeabilization and
apoptosis. Science. 303:1010–1014. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao G, Zhu Y, Eno CO, Liu Y, Deleeuw L,
Burlison JA, Chaires JB, Trent JO and Li C: Activation of the
proapoptotic Bcl-2 protein Bax by a small molecule induces tumor
cell apoptosis. Mol Cell Biol. 34:1198–1207. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bukholm IK and Nesland JM: Protein
expression of p53, p21 (WAF1/CIP1), bcl-2, Bax, cyclin D1 and pRb
in human colon carcinomas. Virchows Arch. 436:224–228. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Katsumata K, Sumi T, Tomioka H, Aoki T and
Koyanagi Y: Induction of apoptosis by p53, bax, bcl-2, and p21
expressed in colorectal cancer. Int J Clin Oncol. 8:352–356. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
el-Deiry WS, Harper JW, O'Connor PM,
Velculescu VE, Canman CE, Jackman J, Pietenpol JA, Burrell M, Hill
DE, Wang Y, et al: WAF1/CIP1 is induced in p53-mediated G1 arrest
and apoptosis. Cancer Res. 54:1169–1174. 1994.PubMed/NCBI
|
|
30
|
Kanavaros P, Stefanaki K, Valassiadou K,
Vlachonikolis J, Mavromanolakis M, Vlychou M, Kakolyris S,
Gorgoulis V, Tzardi M and Georgoulias V: Expression of p53,
p21/waf, bcl-2, bax, Rb and Ki67 proteins in colorectal
adenocarcinomas. Med Oncol. 16:23–30. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Miyashita T, Krajewski S, Krajewska M,
Wang HG, Lin HK, Liebermann DA, Hoffman B and Reed JC: Tumor
suppressor p53 is a regulator of bcl-2 and bax gene expression
in vitro and in vivo. Oncogene. 9:1799–1805. 1994.PubMed/NCBI
|
|
32
|
Miyashita T and Reed JC: Tumor suppressor
p53 is a direct transcriptional activator of the human bax gene.
Cell. 80:293–299. 1995. View Article : Google Scholar : PubMed/NCBI
|