Introduction
Bladder cancer was the ninth most common cancer type
globally and the second most common urogenital malignancy in 2012
(1). More than 60% of bladder cancer
cases occur in less well-developed countries, including China, and
75% of these cases occur in men (2).
Furthermore, bladder cancer has a high recurrence rate (50%), and
15–40% of cases develop into a muscle-invasive form of the disease
(3,4).
Therefore, early diagnosis and consistent follow-up are necessary
in order to improve patient quality of life.
Previously, the primary methods used to detect and
follow up bladder cancer were cystoscopy and cytology (5). Cystoscopy is able to identify the
majority of papillary and solid lesions and is therefore widely
used. Cystoscopy combined with pathological biopsy is the gold
standard for the diagnosis and follow-up of bladder cancer
(6). However, it is not only an
invasive procedure but also has limited accuracy in detecting
certain lesions, particularly small areas of carcinoma in
situ (7). While cytology has a
specificity of >90%, its sensitivity is <44%, particularly in
highly-differentiated tumor types (stages G1-G2) (8–10).
Therefore, the invasive nature of cystoscopy and the low
sensitivity of cytology limit the early diagnosis of bladder cancer
in clinical practice. Consequently, a non-invasive, highly
sensitive and specific alternative test is urgently required.
To identify a better method to diagnose bladder
cancer, various urine-based tumor markers have been extensively
investigated (7). These markers,
including human complement factor H, cytokeratin 19 fragments and
nuclear matrix protein 22, generally demonstrate a higher
sensitivity but lower specificity compared with cytology (9,11,12). Biomarker diagnosis has not yet been
recommended in the European Association of Urology guidelines
(13). Recently, a novel non-invasive
qualitative immunochromatographic test has been launched to
identify the urinary bladder cancer antigen. The UBC®
Rapid Test (Concile GmbH, Freiburg, Germany) is a point-of-care
test, compliant with International Organization for Standardization
22870:2016, which may quantitatively measure fragments of
cytokeratins 8 and 18 (14–16). These cytokeratins are located in the
cytoskeleton of epithelial cells and tend to be overexpressed in
urothelial tumor types including bladder cancer (17–19). Based
on this, several trials have been performed to investigate the
efficacy of the UBC® Rapid Test in the detection and
follow-up of bladder cancer (20–22).
However, differences in the design and enrollment of these studies
have resulted in inconsistent conclusions, so its diagnostic
accuracy remains unclear.
In the present study, these previous studies were
systematically reviewed to assess the diagnostic value of the
UBC® Rapid Test in the detection and follow-up of
bladder cancer.
Materials and methods
Search strategy
The following databases were comprehensively
searched for studies published between January 1, 1990 and June 1,
2017: Pubmed, Embase, the Cochrane Central Register of Controlled
Trials (CENTRAL), Web of Science, China (WANFANG) and the China
CNKI database. The search was performed using the following
keywords in combination: (‘UBC’ OR ‘Cytokeratin 8’ OR ‘Cytokeratin
18’) AND (‘Bladder cancer’ OR ‘urinary bladder neoplasm’) as
medical subject headings. Furthermore, the reference lists of all
studies included in the meta-analysis were also reviewed for
possible inclusion.
Inclusion and exclusion criteria
The inclusion criteria were as follows: i)
Case-control or cohort design; ii) sufficient data for
meta-analysis [true positive (TP), false positive (FP), false
negative (FN) and true negative (TN)]; iii) if data or subsets of
data were used in more than one article, the most recent article or
the one with greater detail was selected; and iv) written in
English or Chinese. The exclusion criteria were as follows: i)
Reviews, case reports and letters to editors; ii) duplicate
publications; iii) studies in languages other than English or
Chinese; and iv) studies with insufficient data to construct a 2×2
table. All records were independently reviewed by Dr Pei Lu and Dr
Rijin Song (Department of Urology, The First Affiliated Hospital of
Nanjing Medical University, Nanjing, China). Consensus was normally
reached for each eligible study and any disagreements were resolved
by consultation with a third reviewer (Dr Min Gu; Department of
Urology, The First Affiliated Hospital of Nanjing Medical
University).
Data extraction and quality
assessment
Relevant data were extracted from the full text of
the included studies and included: First author, publication year,
ethnicity, sample size, mean age, sex, specific details of index
test used, sensitivity and specificity, TP, FP, FN and TN for
various grades of bladder tumor types.
The quality assessment of diagnostic accuracy
studies 2 (QUADAS-2) scale was used to evaluate the quality of the
eligible studies (23). This contains
four domains including patient selection, index test, reference
standard and flow and timing. All domains were evaluated for the
potential risk of bias and the first three were mainly concerned
with applicability.
Statistical analysis
The accuracy indicators included pooled sensitivity,
pooled specificity, positive likelihood ratio (PLR), negative
likelihood ratio (NLR), diagnostic odds ratio (DOR) and their 95%
confidence intervals (CIs). These were calculated using the random
effects model (24). The summary
receiver operative curve (SROC), which reveals the association
between sensitivity and the false positive rate, was used to
evaluate the consistency of results between all studies in addition
to the accuracy of the test (25).
The area under the curve (AUC) was also calculated. Heterogeneity
was measured using a Q test and the inconsistency index
(I2) (26). P<0.05 or
I2>50% was considered to indicate significant
heterogeneity and therefore the random effects model was applied
(27); otherwise the fixed-effect
model was used. One of the most important causes of heterogeneity
in diagnostic tests is the threshold effect. This occurs when the
sensitivity and specificity are negatively correlated (or
sensitivity is positively correlated with 1-specificity), resulting
in a typical ‘shoulder arm’ of the ROC plane distribution. A
Spearman correlation analysis was performed. Subsequently,
meta-regression and subgroup analyses were conducted to explore
potential sources of inter-study heterogeneity. Furthermore, Deeks'
funnel plots were used to detect any publication bias (28). All statistical analysis was conducted
using Meta-Disc 1.4 software (Hospital Universitario Ramon y Cajal,
Madrid, Spain) and STATA 12.0 software (StataCorp, LLC, College
Station, TX, USA) (29,30).
Results
Study selection and
characteristics
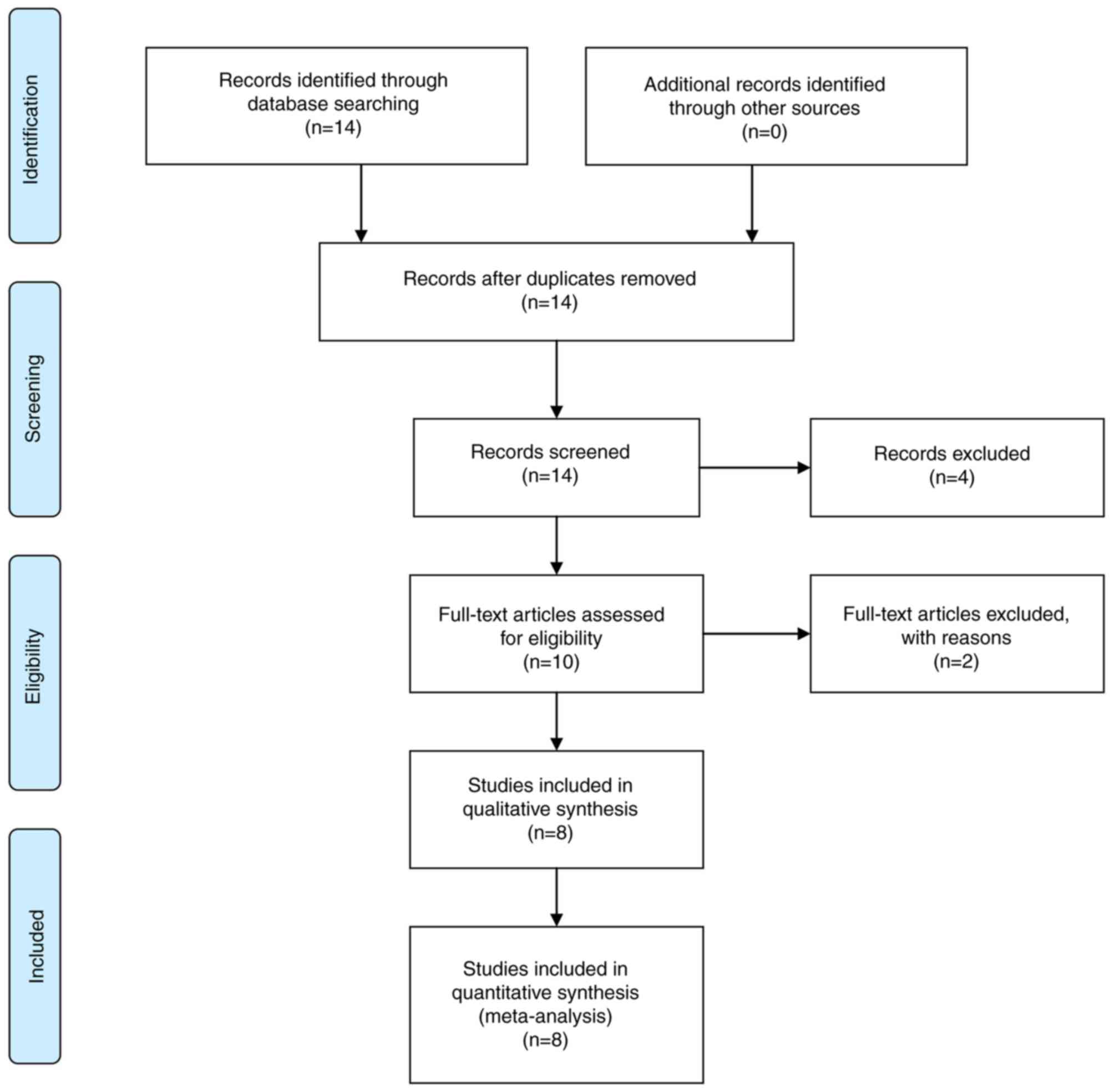
As presented in the flow chart (Fig. 1), a total of 14 potential relevant
articles were identified initially, of which four were removed
subsequent to reading the titles and abstracts in further detail.
Following a full-text review, two studies were eliminated due to
lack of sufficient data, leaving eight studies (14–16,22,31–34).
The basic characteristics of the studies are summarized in Table I.
 | Table I.Characteristics of the eight included
studies in the present meta-analysis. |
Table I.
Characteristics of the eight included
studies in the present meta-analysis.
| Study | Country | Year | Design | Blinding | Ethnicity | Mean age
(years) | Male:Female | Sample size | TP | FP | FN | TN | (Refs.) |
|---|
| Mian et al,
2000 | Austria | 2000 | Retrospective | Yes | Caucasian | 65.8 | NA | 180 | 36 | 13 | 17 | 114 | (31) |
| Babjuk et
al, 2002 | Czech | 2001 | Retrospective | Yes | Caucasian | 66.3 | 141:77 | 107 | 38 | 6 | 40 | 23 | (32) |
| Schroeder et
al, 2004 | Germany | 2004 | Prospective | Yes | Caucasian | 64.3 | 80:35 | 135 | 21 | 19 | 38 | 57 | (33) |
| Hakenberg et
al, 2004 | Germany | 2004 | Prospective | Yes | Caucasian | 68.5 | 87:25 | 112 | 58 | 8 | 32 | 14 | (34) |
| Ritter et
al, 2014 | Germany | 2013 | Prospective | Yes | Caucasian | 70 | 151:47 | 198 | 37 | 41 | 24 | 96 | (14) |
| Ecke et al,
2015 | Germany | 2015 | Prospective | No | Caucasian | 73 | 97:28 | 125 | 49 | 3 | 43 | 30 | (15) |
| Styrke et
al, 2017 | Sweden | 2017 | Prospective | No | Caucasian | 70 | 224:46 | 270 | 120 | 39 | 49 | 62 | (16) |
| Ecke et al,
2017 | Sweden | 2017 | Prospective | No | Caucasian | 72 | 78:31 | 109 | 45 | 2 | 42 | 20 | (22) |
All eight studies were conducted in a European
population, and the majority of the patients were male and >50
years old (Table I). Urinary sediment
was used as a specimen, and cytology or cystoscopy was considered
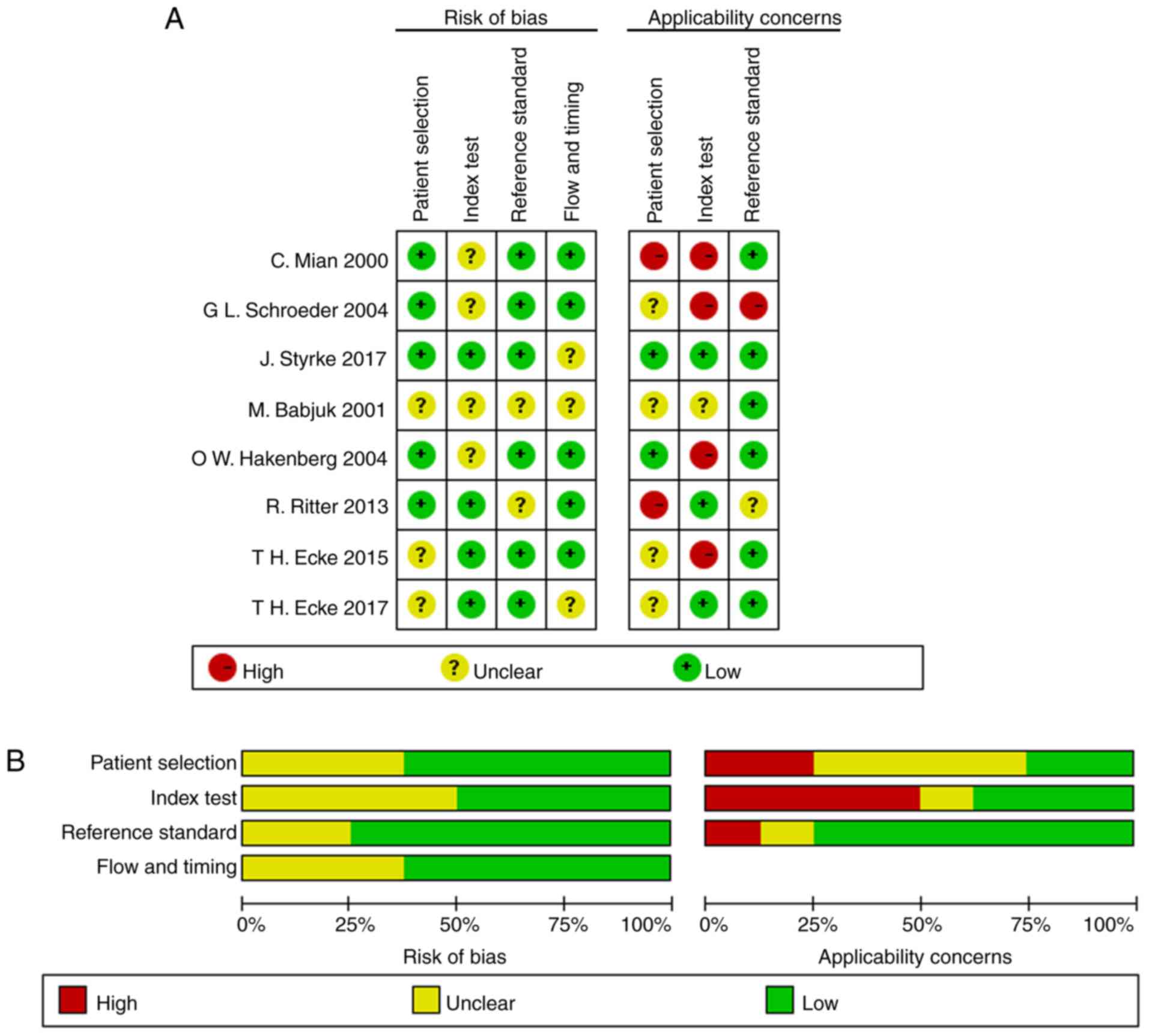
as the gold standard. The results of the quality assessment are
presented in Fig. 2. The majority of
articles included the majority of the QUADAS-2 domains, indicating
that the overall quality of the included studies was moderate to
high.
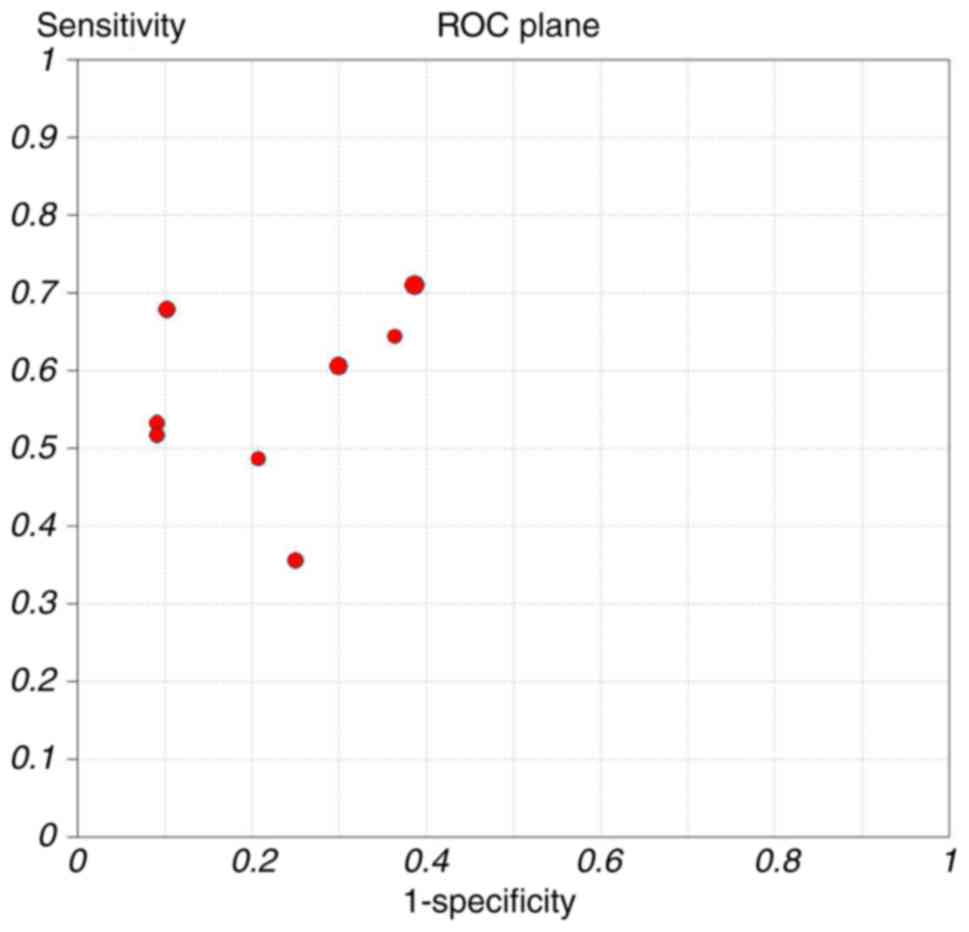
Threshold effect
The ROC curve of sensitivity against the specificity
of each study (Fig. 3) revealed a
non-typical shoulder arm appearance, indicating that there was no
threshold effect. In addition, the calculated Spearman correlation
coefficient value was 0.44 (P=0.27), also indicating no threshold
effect.
Diagnostic accuracy
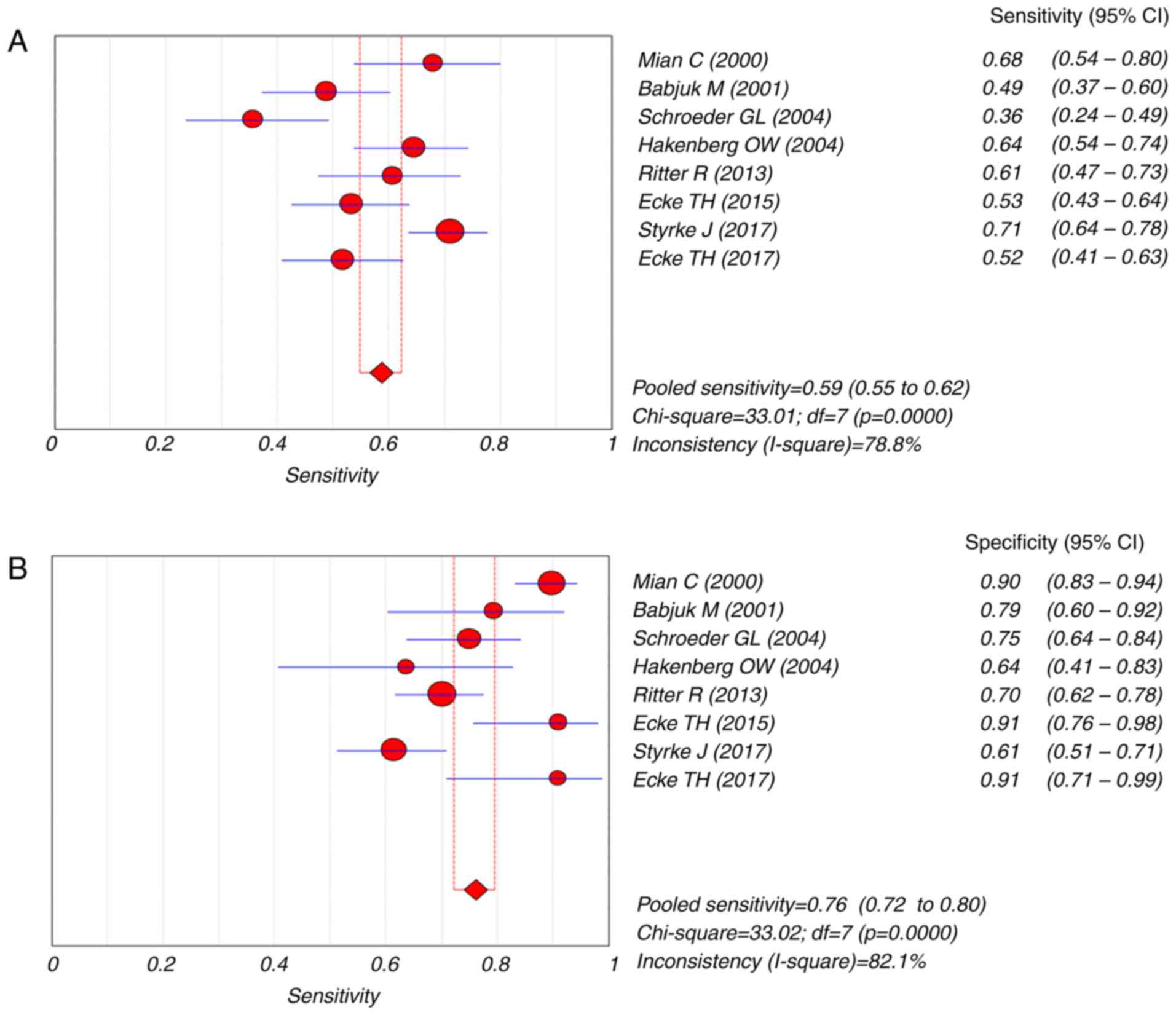
Overall, the sensitivity of the pooled data was 0.59
(95% CI=0.55–0.62) and the specificity was 0.76 (95% CI=0.72–0.80)
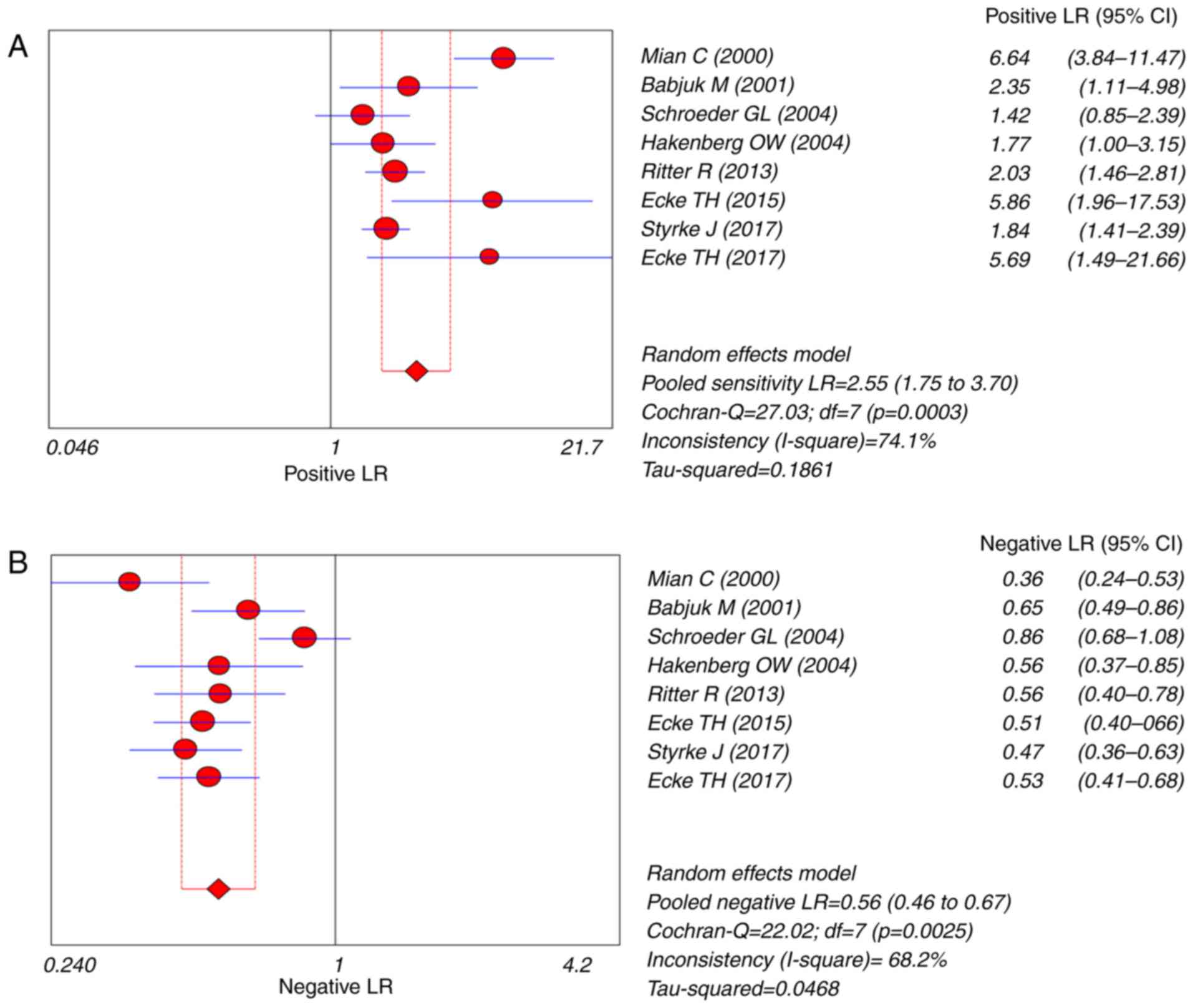
(Fig. 4). The pooled PLR was 2.55
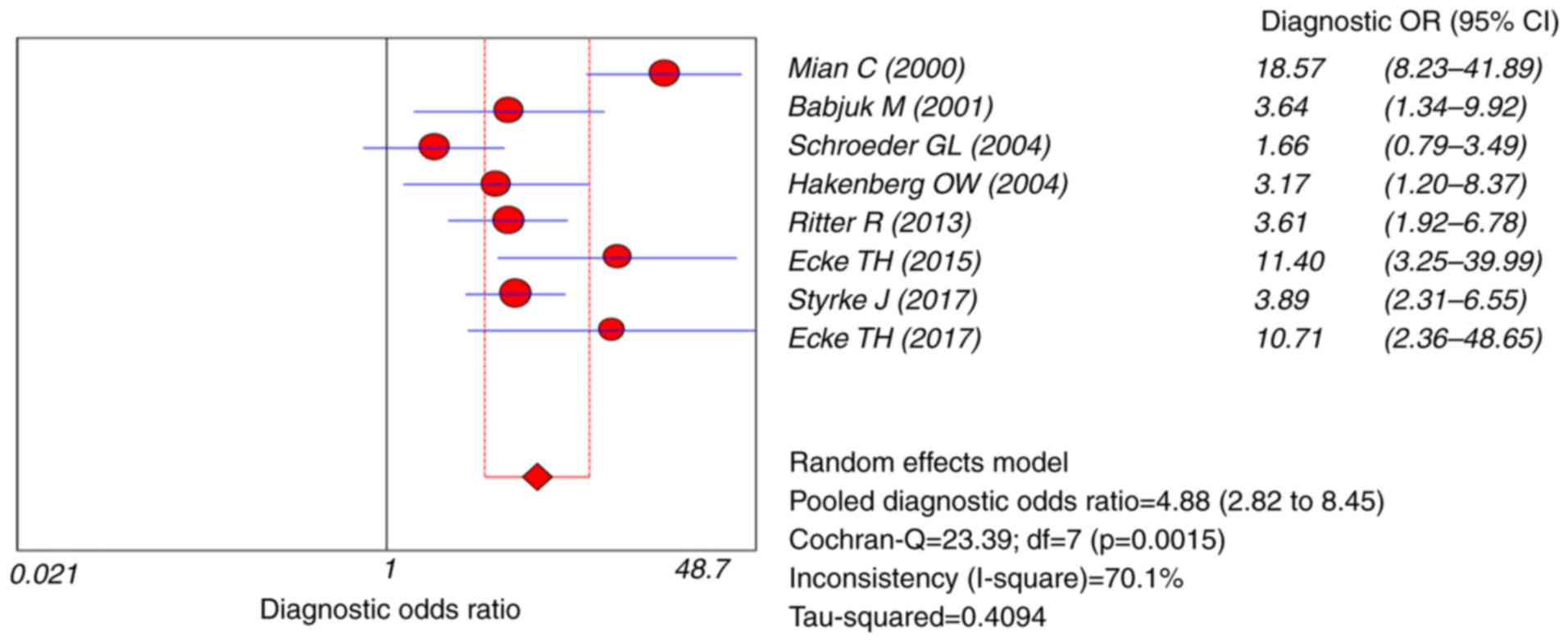
(95% CI=1.75–3.70), the NLR was 0.56 (95% CI=0.46–0.67) and the DOR
was 4.88 (95% CI=2.82–8.45) (Figs. 5
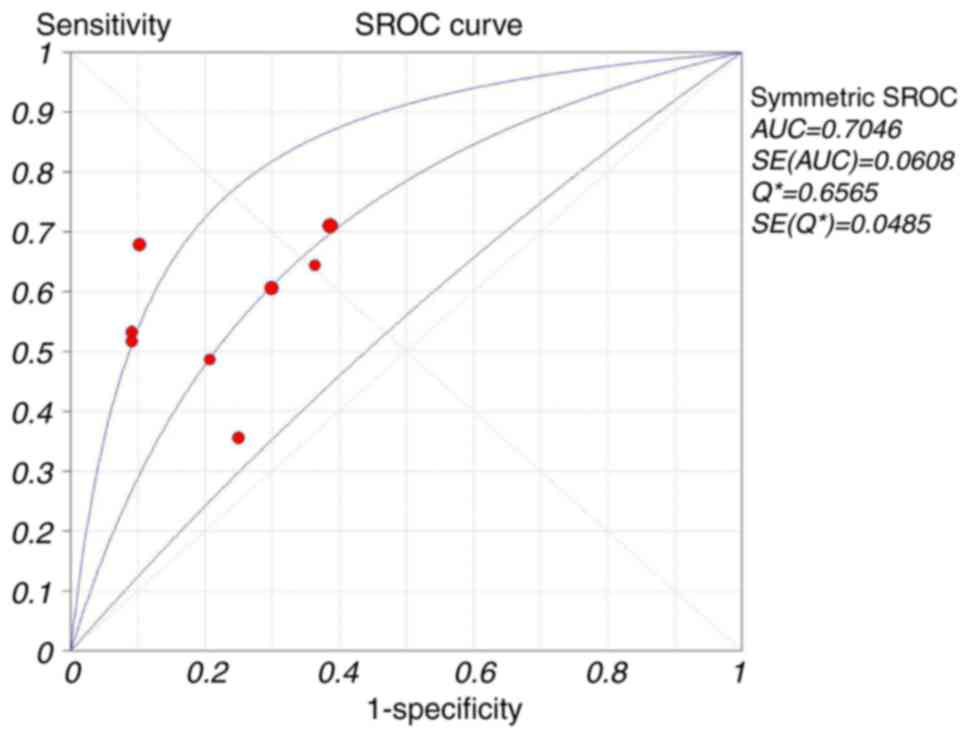
and 6). The SROC curve for the eight
studies is presented in Fig. 7. The
overall AUC of the UBC® Rapid Test was 0.70 (95%
CI=0.85–0.91). Significant heterogeneity was identified for pooled
sensitivity (I2=78.8%, P<0.001; Fig. 4) and specificity (I2=82.1%,
P<0.001; Fig. 4) so the random
effects model was applied for further analysis.
Meta-regression and subgroup
analysis
Heterogeneity was identified in the estimates of
sensitivity, specificity, PLR, NLR and DOR. Therefore,
meta-regression was used to explore the source of heterogeneity on
the basis of study design, double blinding and sample size.
However, none of the above covariates were heterogeneous (all
P>0.05; Table II). Although
subgroup analysis, including design, blind and sample size, were
performed, there was no difference in the diagnostic efficacy of
this test, indicating none of the parameters were identified to be
a source of heterogeneity (Table
III).
 | Table II.Results of the multivariable
meta-regression model for the characteristics with backward
regression analysis. |
Table II.
Results of the multivariable
meta-regression model for the characteristics with backward
regression analysis.
| Variables | Coefficient | Standard error | P-value | RDOR | 95% confidence
interval |
|---|
| Cte. | 1.179 | 0.7577 | 0.2174 | – | – |
| S | −0.404 | 0.3753 | 0.3603 | – | – |
| Design | 0.798 | 0.7540 | 0.3673 | 2.22 | (0.20–24.49) |
| Blinding | −0.750 | 0.6327 | 0.3210 | 0.47 | (0.06–3.54) |
| Sample size | 0.693 | 0.6791 | 0.3829 | 2.00 | (0.23–17.35) |
 | Table III.Summary results of diagnostic
accuracy of UBC test for bladder cancer. |
Table III.
Summary results of diagnostic
accuracy of UBC test for bladder cancer.
| Subgroup | No. of studies | Sensitivity (95%
CI) | Specificity (95%
CI) | PLR (95% CI) | NLR (95% CI) | DOR (95% CI) | AUC |
|---|
| Design |
|
|
|
|
|
|
|
|
Retrospective | 2 | 0.56 (0.48,
0.65) | 0.88 (0.82,
0.93) | 4.08 (1.47,
11.33) | 0.49 (0.25,
0.94) | 8.45 (1.70,
42.05) | – |
|
Prospective | 6 | 0.59 (0.55,
0.63) | 0.71 (0.67,
0.76) | 2.0 (1.52,
2.64) | 0.58 (0.47,
0.70) | 3.81 (2.37,
6.12) | 0.6945 |
| Sample size |
|
|
|
|
|
|
|
|
>150 | 3 | 0.68 (0.62,
0.74) | 0.75 (0.70,
0.79) | 2.78 (1.41,
4.03) | 0.47 (0.37,
0.59) | 6.11 (2.46,
15.16) | 0.7271 |
|
≤150 | 5 | 0.52 (0.47,
0.57) | 0.79 (0.72,
0.85) | 2.39 (1.49,
5.17) | 0.62 (0.50,
0.76) | 4.09 (1.97,
8.49) | 0.6392 |
| Blinding |
|
|
|
|
|
|
|
|
Yes | 5 | 0.61 (0.56,
0.67) | 0.72 (0.64,
0.79) | 2.39 (1.44,
3.96) | 0.59 (0.43,
0.79) | 4.18 (1.87,
9.31) | 0.7058 |
| No | 3 | 0.56 (0.48,
0.65) | 0.88 (0.82,
0.93) | 3.47 (1.26,
9.58) | 0.51 (0.44,
0.59) | 6.30 (2.82,
14.06) | 0.7369 |
|
Total | 8 | 0.59 (0.55,
0.62) | 0.76 (0.72,
0.80) | 2.55 (1.75,
3.70) | 0.56 (0.46,
0.67) | 4.88 (2.82,
8.45) | 0.7046 |
Publication bias
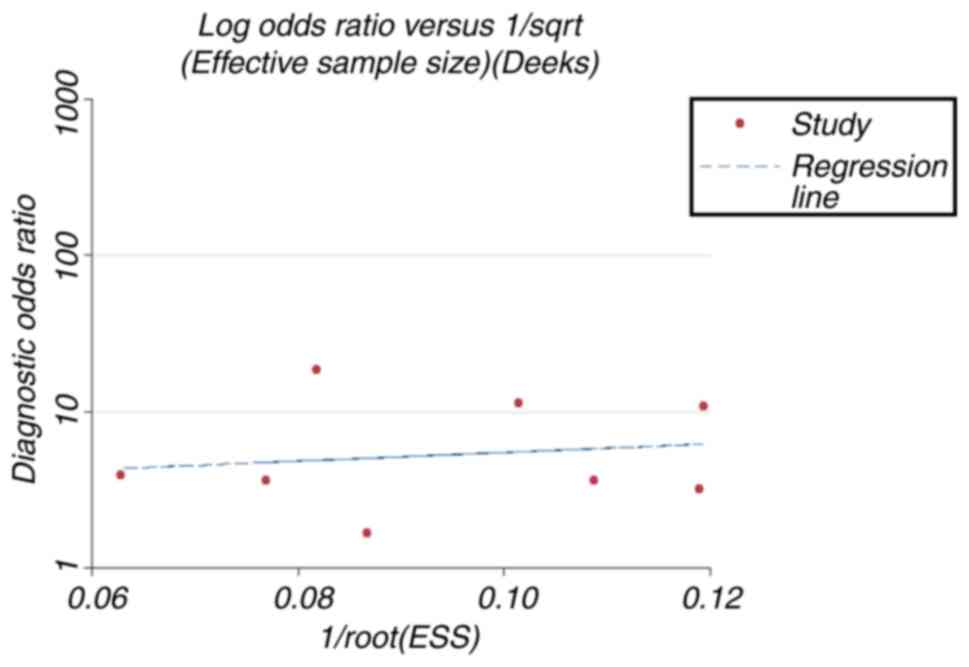
Deeks' funnel plot demonstrated no significant
publication bias (P=0.70; Fig.
8).
Discussion
To date, cystoscopy has been considered the gold
standard for detecting bladder cancer and for following up patients
who have undergone tumor resection (35). However, it is an invasive and
expensive tool. Another test widely used in clinical practice is
urine cytology; however, its low sensitivity limits its use
(36). Therefore, it is necessary to
identify a viable, reliable and minimally-invasive method to detect
new or recurrent bladder cancer.
The UBC® Rapid Test is a quantitative
method to determine the levels of urinary fragments of cytokeratin
8 and 18, and has recently been developed as a tumor marker to
detect bladder cancer (37). In the
present analysis, the pooled AUC of the UBC® Rapid test
indicated that it was a better diagnostic tool compared with
cystoscopy and cytology. The DOR value, the ratio of correct to
false diagnosis, is a comprehensive indicator of the diagnostic
efficiency index (38). The pooled
DOR in the present study suggested that the UBC® Rapid
Test is reliable compared with the overall accuracy of bladder
cancer diagnosis.
The likelihood ratio, including PLR and NLR, is also
a strong performance indicator for diagnostic experiments (39). Generally considered, a PLR>10
indicates the presence of disease, and a NLR<0.1 may rule out
the possibility of disease. However, the present study revealed
that the pooled PLR and NLR for the UBC® Rapid Test were
2.55 and 0.56, respectively. This suggests that the probability of
the test providing a positive result in patients with bladder
cancer was 2.55 times higher compared with patients without bladder
cancer; and the probability of negative results was 0.56 times
higher compared with in non-patients. Therefore, the performance of
the UBC® Rapid Test in terms of pooled PLR and NLR did
not meet clinical practice requirements and should be further
modified prior to clinical use.
Exploring heterogeneity is crucial to understanding
the factors that affect accurate estimates in addition to the
appropriateness of combining the accuracy of different studies
(40). Substantial heterogeneity was
identified in the present meta-analysis in terms of the pooled
sensitivity, specificity, PLR, NLR and DOR. The threshold effect
remains an important cause of heterogeneity in diagnostic trials
(41). In the present meta-analysis,
a significant threshold effect was not observed. To further explore
the source of heterogeneity, a meta-regression analysis was used
based on design, blinding and sample size. The results suggested
that none of these parameters were the cause, indicating that other
variables contributed to the heterogeneity across the studies;
these may have been publication and choice bias.
There are several limitations to the study. First,
despite the extensive literature search, the number of studies and
sample sizes included were small. Secondly, several papers
published in different languages were excluded from the review,
which may result in potential heterogeneity. Thirdly, all the
trials included in this meta-analysis were retrospective, which may
limit the conclusions due to the bias of choice.
In general, the present study suggests that the
UBC® Rapid Test may be beneficial for the diagnosis of
bladder cancer, since this non-invasive approach has a good overall
diagnostic performance. However, further prospective, large-scale
and multicenter assessments of clinical studies are required to
fully assess the diagnostic role of the UBC® Rapid Test
in patients with bladder cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81570676), the
Science and Education Health Project of Jiangsu Province for
Important Talent (grant no. RC2011055), the ‘333 High Level Talents
Project’ in Jiangsu Province, China [grant nos. BRA2015469 and
BRA2016514 (2015)], the Standardized Diagnosis and Treatment
Research Program of Key Diseases in Jiangsu Province, China (grant
no. BE2016791), the Open Project Program of Health Department of
Jiangsu Province, China (grant no. JSY-2-2016-099), the Jiangsu
Province Six Talents Peak from Department of Human Resources,
Social Security Office of Jiangsu Province, China (grant no.
2010WSN-56), the General Program of Health Department of Jiangsu
Province, China (grant no. H2009907) and the Priority Academic
Program Development of Jiangsu Higher Education Institutions (grant
no. JX10231801).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PL carried out the study design and preparation of
the manuscript. JC carried out the study design and statistical
analysis. KC performed the statistical analysis and preparation of
the manuscript. QL performed the statistical analysis. JZ performed
the study design and data collection. JT performed the statistical
analysis and data collection. ZH performed the statistical
analysis. WZ aided with the interpretation of data and the
preparation of the manuscript. RS carried out the study design and
statistical analysis. MG provided funding and study design.
Ethical approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Agarwal N, Pal SK, Hahn AW, Nussenzveig
RH, Pond GR, Gupta SV, Wang J, Bilen MA, Naik G, Ghatalia P, et al:
Characterization of metastatic urothelial carcinoma via
comprehensive genomic profiling of circulating tumor DNA. Cancer.
124:2115–2124. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Antoni S, Ferlay J, Soerjomataram I, Znaor
A, Jemal A and Bray F: Bladder cancer incidence and mortality: A
global overview and recent trends. Eur Urol. 71:96–108. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Amin MB, Smith SC, Reuter VE, Epstein JI,
Grignon DJ, Hansel DE, Lin O, McKenney JK, Montironi R, Paner GP,
et al: Update for the practicing pathologist: The international
consultation on urologic disease-European association of urology
consultation on bladder cancer. Mod Pathol. 28:612–630. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kang M, Jeong CW, Kwak C, Kim HH and Ku
JH: Preoperative neutrophil-lymphocyte ratio can significantly
predict mortality outcomes in patients with non-muscle invasive
bladder cancer undergoing transurethral resection of bladder tumor.
Oncotarget. 8:12891–12901. 2017.PubMed/NCBI
|
|
5
|
Liang B, He X, Shang D, Tian Y and Liu Z:
The link between FOXJ1 expression level in bladder carcinoma and
tumor recurrence. Oncol Lett. 15:1483–1486. 2018.PubMed/NCBI
|
|
6
|
Soria F, Gurioli A, Peraldo F, Oderda M,
Giona S, Ambrosini E, Frea B and Gontero P: Innovations in the
endoscopic management of bladder cancer: Is the era of white light
cystoscopy over. Urologia. 80:1–8. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schmitz-Dräger BJ, Droller M, Lokeshwar
VB, Lotan Y, Hudson MA, van Rhijn BW, Marberger MJ, Fradet Y,
Hemstreet GP, Malmstrom PU, et al: Molecular markers for bladder
cancer screening, early diagnosis, and surveillance: The WHO/ICUD
consensus. Urol Int. 94:1–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mowatt G, Zhu S, Kilonzo M, Boachie C,
Fraser C, Griffiths TR, N'Dow J, Nabi G, Cook J and Vale L:
Systematic review of the clinical effectiveness and
cost-effectiveness of photodynamic diagnosis and urine biomarkers
(FISH, ImmunoCyt, NMP22) and cytology for the detection and
follow-up of bladder cancer. Health Technol Assess. 14(1–331):
iii–iv. 2010.
|
|
9
|
Lotan Y and Roehrborn CG: Sensitivity and
specificity of commonly available bladder tumor markers versus
cytology: Results of a comprehensive literature review and
meta-analyses. Urology. 61:109–118. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schwalb DM, Herr HW and Fair WR: The
management of clinically unconfirmed positive urinary cytology. J
Urol. 150:1751–1756. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kamat AM, Hahn NM, Efstathiou JA, Lerner
SP, Malmström PU, Choi W, Lotan Y and Kassouf W: Bladder cancer.
388:2796–2810. 2016.
|
|
12
|
Dawam D: Biomarkers of bladder cancer in
urine: Evaluation of diagnostic and prognostic significance of
current and potential markersBladder Cancer-From Basic Science to
Robotic Surgery. Abdullah Canda: InTech Europe; Rijeka: 2012,
https://www.intechopen.com/books/bladder-cancer-from-basic-science-to-robotic-surgery/biomarkers-of-bladder-cancer-in-urine-evaluation-of-diagnostic-and-prognostic-significance-of-currenFebruary
1–2012 View
Article : Google Scholar
|
|
13
|
Witjes JA, Compérat E, Cowan NC, De Santis
M, Gakis G, Lebret T, Ribal MJ, Van der Heijden AG and Sherif A:
European Association of Urology: EAU guidelines on muscle-invasive
and metastatic bladder cancer: Summary of the 2013 guidelines. Eur
Urol. 65:778–792. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ritter R, Hennenlotter J, Kühs U, Hofmann
U, Aufderklamm S, Blutbacher P, Deja A, Hohneder A, Gerber V, Gakis
G, et al: Evaluation of a new quantitative point-of-care test
platform for urine-based detection of bladder cancer. Urol Oncol.
32:337–344. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ecke TH, Arndt C, Stephan C, Hallmann S,
Lux O, Otto T, Ruttloff J and Gerullis H: Preliminary results of a
multicentre study of the UBC rapid test for detection of urinary
bladder cancer. Anticancer Res. 35:2651–2655. 2015.PubMed/NCBI
|
|
16
|
Styrke J, Henriksson H, Ljungberg B, Hasan
M, Silfverberg I, Einarsson R, Malmström PU and Sherif A:
Evaluation of the diagnostic accuracy of UBC® Rapid in
bladder cancer: A Swedish multicentre study. Scand J Urol.
51:293–300. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Barak V, Goike H, Panaretakis KW and
Einarsson R: Clinical utility of cytokeratins as tumor markers.
Clin Biochem. 37:529–540. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sumi S, Arai K, Kitahara S and Yoshida KI:
Preliminary report of the clinical performance of a new urinary
bladder cancer antigen test: Comparison to voided urine cytology in
the detection of transitional cell carcinoma of the bladder. Clin
Chim Acta. 296:111–120. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Giannopoulos A, Manousakas T, Gounari A,
Constantinides C, Choremi-Papadopoulou H and Dimopoulos C:
Comparative evaluation of the diagnostic performance of the BTA
stat test, NMP22 and urinary bladder cancer antigen for primary and
recurrent bladder tumors. J Urol. 166:470–475. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gleichenhagen J, Arndt C, Casjens S,
Meinig C, Gerullis H, Raiko I, Brüning T, Ecke T and Johnen G:
Evaluation of a new survivin ELISA and UBC® Rapid for the detection
of bladder cancer in urine. Int J Mol Sci. 19:pii: E226. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pichler R, Tulchiner G, Fritz J, Schaefer
G, Horninger W and Heidegger I: Urinary UBC rapid and NMP22 test
for bladder cancer surveillance in comparison to urinary cytology:
results from a prospective single-center study. Int J Med Sci.
14:811–819. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ecke TH, Weiß S, Stephan C, Hallmann S,
Barski D, Otto T and Gerullis H: UBC® Rapid test for detection of
carcinoma in situ for bladder cancer. Tumour Biol.
39:10104283177016242017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schueler S, Schuetz GM and Dewey M: The
revised QUADAS-2 tool. Ann Intern Med. 156:323–324. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rosenblat MA, Perrotta AS and Vicenzino B:
Polarized vs. threshold training intensity distribution on
endurance sport performance: A systematic review and meta-analysis
of randomized controlled trials. J Strength Cond Res. May
30–2018.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hajian-Tilaki K: Receiver operating
characteristic (ROC) curve analysis for medical diagnostic test
evaluation. Caspian J Intern Med. 4:627–635. 2013.PubMed/NCBI
|
|
26
|
Bae JM: An overview of systematic reviews
of diagnostic tests accuracy. Epidemiol Health. 36:e20140162014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gopalakrishna G, Langendam MW, Scholten
RJ, Bossuyt PM and Leeflang MM: Defining the clinical pathway in
cochrane diagnostic test accuracy reviews. BMC Med Res Methodol.
16:1532016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
van Enst WA, Ochodo E, Scholten RJ, Hooft
L and Leeflang MM: Investigation of publication bias in
meta-analyses of diagnostic test accuracy: A meta-epidemiological
study. BMC Med Res Methodol. 14:702014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zamora J, Abraira V, Muriel A, Khan K and
Coomarasamy A: Meta-DiSc: A software for meta-analysis of test
accuracy data. BMC Med Res Methodol. 6:312006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Deeks JJ, Macaskill P and Irwig L: The
performance of tests of publication bias and other sample size
effects in systematic reviews of diagnostic test accuracy was
assessed. J Clin Epidemiol. 58:882–893. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mian C, Lodde M, Haitel A, Vigl Egarter E,
Marberger M and Pycha A: Comparison of two qualitative assays, the
UBC rapid test and the BTA stat test, in the diagnosis of
urothelial cell carcinoma of the bladder. Urology. 56:228–231.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Babjuk M, Kostírová M, Mudra K, Pecher S,
Smolová H, Pecen L, Ibrahim Z, Dvorácek J, Jarolím L, Novák J and
Zima T: Qualitative and quantitative detection of urinary human
complement factor H-related protein (BTA stat and BTA TRAK) and
fragments of cytokeratins 8, 18 (UBC rapid and UBC IRMA) as markers
for transitional cell carcinoma of the bladder. Eur Urol. 41:34–39.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schroeder GL, Lorenzo-Gomez MF, Hautmann
SH, Friedrich MG, Ekici S, Huland H and Lokeshwar V: A side by side
comparison of cytology and biomarkers for bladder cancer detection.
J Urol. 172:1123–1136. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hakenberg OW, Fuessel S, Richter K,
Froehner M, Oehlschlaeger S, Rathert P, Meye A and Wirth MP:
Qualitative and quantitative assessment of urinary cytokeratin 8
and 18 fragments compared with voided urine cytology in diagnosis
of bladder carcinom. Urology. 64:1121–1126. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Feber A, Dhami P, Dong L, de Winter P, Tan
WS, Martínez-Fernández M, Paul DS, Hynes-Allen A, Rezaee S, Gurung
P, et al: UroMark-a urinary biomarker assay for the detection of
bladder cancer. Clin Epigenetics. 9:82017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Burger M, Grossman HB, Droller M,
Schmidbauer J, Hermann G, Drăgoescu O, Ray E, Fradet Y, Karl A,
Burgués JP, et al: Photodynamic diagnosis of non-muscle-invasive
bladder cancer with hexaminolevulinate cystoscopy: A meta-analysis
of detection and recurrence based on raw data. Eur Urol.
64:846–854. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xylinas E, Kluth LA, Rieken M, Karakiewicz
PI, Lotan Y and Shariat SF: Urine markers for detection and
surveillance of bladder cancer. Urol Oncol. 32:222–229. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Glas AS, Lijmer JG, Prins MH, Bonsel GJ
and Bossuyt PM: The diagnostic odds ratio: A single indicator of
test performance. J Clin Epidemiol. 56:1129–1135. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sedighi I: Interpretation of diagnostic
tests: Likelihood ratio vs. Predictive value. Iran J Pediatr.
23:7172013.PubMed/NCBI
|
|
40
|
Liberati A, Altman DG, Tetzlaff J, Mulrow
C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J
and Moher D: The PRISMA statement for reporting systematic reviews
and meta-analyses of studies that evaluate healthcare
interventions: Explanation and elaboration. BMJ. 339:b27002009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rubia K, Alegria AA, Cubillo AI, Smith AB,
Brammer MJ and Radua J: Effects of stimulants on brain function in
attention-deficit/hyperactivity disorder: A systematic review and
meta-analysis. Biol Psychiatry. 76:616–628. 2014. View Article : Google Scholar : PubMed/NCBI
|