Introduction
Renal cell carcinoma (RCC) is one of the most
prevalent cancers, accounting for 2% of adult malignancies
(1); its incidence is steadily rising
by approximately 2% per year (2).
Although the rate of early diagnosis of RCC has increased with the
development of diagnostic techniques in recent years, up to 30% of
patients are diagnosed with metastasized RCC, and one-third of
local RCCs eventually recur and metastasize after surgical
resection (3,4). Currently, surgery still plays a dominant
role in the first-line treatment of RCC. However, metastatic RCC
(mRCC) cannot be treated with radical surgery and is generally not
sensitive to chemotherapy or radiotherapy. Combined treatment
(cytoreductive nephrectomy, cytokine therapy, and targeted
therapeutic agent treatment) may prolong survival. Despite the
improvements in most treatment modalities, mRCC patients still have
extremely poor outcomes, with an overall median survival of <1
year (4,5). Hence, there is an urgent need to enhance
our understanding of the biological signaling pathways involved in
RCC and to identify novel therapeutic agents.
The phosphoinositide 3-kinase (PI3K)/protein kinase
B (Akt)/mammalian target of rapamycin (mTOR)-signaling pathway
plays a pivotal role in cell survival and growth via its effect on
multiple signaling pathways and is frequently disturbed in the
majority of human cancers (6,7). Recent researches have revealed that the
activation of Akt requires the phosphorylation of Thr308 in Akt1
and phosphorylation within the carboxy terminus at Ser473 (8,9).
Inactivated Akt protein promotes cell survival via the
phosphorylation and inactivation of several targets, including
Caspase-3 and caspase-9, and regulates cell cycle progression
through a reduction in the expression of cyclin-dependent kinase
inhibitors p21waf/cip1 and p27 (10). Indeed, studies have shown that
PI3K/Akt/mTOR can regulate hypoxia-inducible factor-1α (HIF-1α)
(11), a critical mediator of the
physiological response to hypoxia, and its dysregulation can
promote tumor angiogenesis and metastasis (12).
mTOR kinase forms two distinct kinase complexes,
mTORC1 and mTORC2. mTORC1 controls cellular growth and metabolic
processes by inactivating P70S6K and 4EBP1, and mTORC2 controls
cellular survival through the phosphorylation of Akt at Ser473
(13). Considering that enhanced
activity of the PI3K/Akt/mTOR pathway is frequently observed in
malignant cells, the inhibition of mTOR is an attractive strategy
to treat cancer. Currently, there are two methods of targeted
therapeutic agents for mRCC: Tyrosine kinase inhibitors targeting
tumor angiogenesis and mTOR inhibitors. However, the emergence of
drug resistance may ultimately limit the utility of mTOR inhibitors
(14).
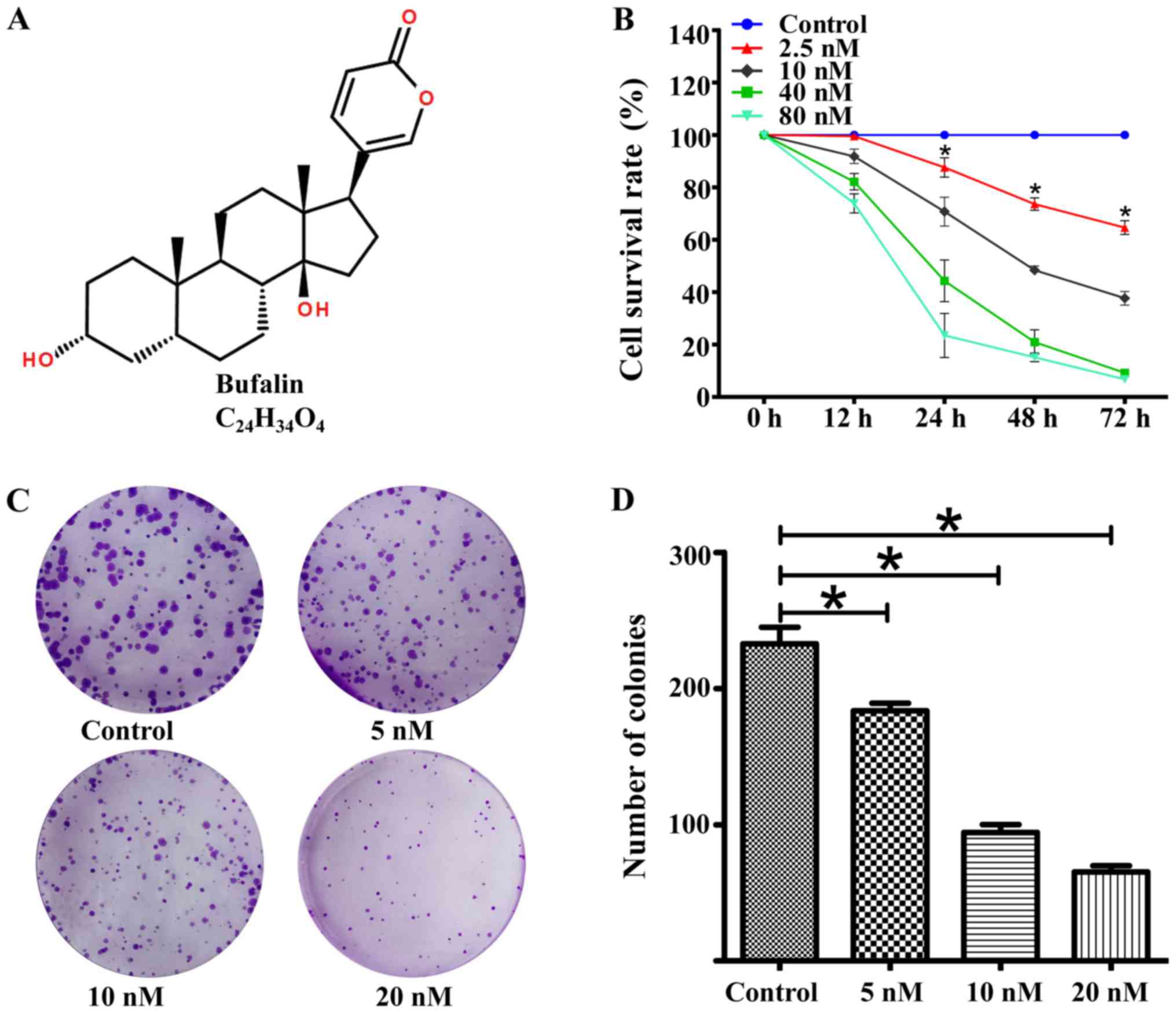
Bufalin (Fig. 1A), an
active component of the traditional Chinese drug Chan Su (15,16), has
been reported to have anti-tumor effects on various types of
cancers, including breast, lung, liver, prostate, and cervical
cancer (17–21). These studies have suggested that
bufalin is a potential therapeutic agent for combined chemotherapy.
However, the effect of bufalin on RCC has not yet been thoroughly
evaluated. In this study, we sought to illustrate the anti-tumor
effects and potential basic mechanisms of bufalin in the human RCC
ACHN cell line.
Materials and methods
Cell lines and chemicals
Human RCC ACHN cells were purchased from the Cell
Bank of the Chinese Academy of Sciences (Shanghai, China) and
cultured at 37°C in 5% CO2 in RPMI-1640 medium
supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) and antibiotics (100 µg/ml of
streptomycin and 100 U/ml of penicillin). Bufalin, with a purity of
up to 98%, was purchased from the Shanghai Yuanye Biological
Technology Company (Shanghai, China); dissolved in dimethyl
sulfoxide (DMSO) to a concentration of 50 µmol/l; and stored at
−20°C. The anti-cyclin B1 (1:1,000 dilution, no. 12231), anti-cdc2,
also known as anti-CDK1 (1:1,000 dilution, no. 28439),
anti-p21waf/cip1 (1:1,000 dilution, no. 2947),
anti-E-cadherin (1:1,000 dilution, no. 3195), anti-N-cadherin
(1:1,000 dilution, no. 13116), anti-Akt (1:1,000 dilution, no.
4691), anti-p-Akt (Ser473) (1:1,000 dilution, no. 4060) anti-mTOR
(1:1,000 dilution, no. 2983), and anti-p-mTOR (Ser2448) antibodies
(1:1,000 dilution, no. 5536) were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). The antibodies against GAPDH
(1:1,000 dilution, no. WL01114), HIF-1α (1:700 dilution, no.
WL01607), and PI3K (1:700 dilution, WL01169) were obtained from
Wanlei Biological Technology Co. (Shenyang, China).
Cell viability assay
ACHN cell viability was detected using the Cell
Counting Kit-8 assay (CCK-8; Dojindo Laboratories, Kumamoto,
Japan). Briefly, ACHN cells (5×103 per well) were seeded
in 96-well plates and cultured for 24 h. Then, the cells were
treated with the designated concentrations of bufalin for 12, 24,
48, or 72 h. After incubation, 10 µl of CCK-8 solution was added to
each well and incubated at 37°C in 5% CO2 for an
additional 1 h. The optical density value of absorbance at 450 nm
was detected using a microplate reader (ELx808; BioTek Instruments,
Inc., Winooski, VT, USA). The experiments were conducted three
times.
Colony-forming assay
A total of 5×102 cells were seeded in
6-well plates at single-cell density and treated with different
concentrations of bufalin for 24 h. Then, the complete medium was
added to allow cell growth for 10 days at 37°C in 5%
CO2. Colonies with >50 cells were photographed and
counted after staining with crystal violet (Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China).
Cell-cycle and apoptosis analyses
ACHN cells were treated with different
concentrations of bufalin for 24 h and then harvested, washed twice
with phosphate-buffered saline (PBS), and re-suspended in 200 µl of
PBS. The cells were fixed in 2 ml of ice-cold 70% ethanol at 4°C
overnight. The precipitate was obtained by centrifugation and added
to 450 µl of propidium iodide (PI; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) and 50 µl of RNase (MP Biomedicals, Solon, OH,
USA) in a 37°C water bath for 15–20 min and then analyzed within 4
h. Apoptosis analysis was performed similarly except that the cells
were stained with 5 µl of Annexin V (eBioscience; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) for 15 min in dark conditions
at room temperature, and 10 µl PI was added prior to immediate
analysis. The cell status was detected by flow cytometry (BD
FACSVerse, USA), and then the cell cycle data were analyzed using
ModFit LT (for Windows, version 3.0) software; the apoptosis data
were analyzed using BD FACSuite software. The results are expressed
as the mean values from three independent determinations.
Wound-healing assay
ACHN cells were seeded in 6-well plates and grown to
80–90% confluence. A wound was scratched with a 200-µl pipette tip
and washed twice with PBS. Then, the cells were treated with
various concentrations of serum-free medium containing bufalin for
24 h. Wound closure was photographed at 0 and 24 h with an inverted
microscope (Carl Zeiss AG, Oberkochen, Germany). The results are
presented as the migration index, which is the ratio of the cell
migration area at 24 h to the scratched area at 0 h.
Cell migration and invasion assay
A cell migration assay, the Transwell assay, was
performed. The chambers (BD Biosciences, Franklin Lakes, NJ, USA)
with 8-µm (pore size) polycarbonate filters were precoated with
Matrigel BD Biosciences). ACHN cells were treated with bufalin at
different concentrations for 12 h; they were then harvested, washed
with serum-free medium, re-suspended to a final concentration of
5×104 cells in 100 µl serum-free medium, and placed in
the upper chamber. The lower chamber contained 0.5 ml of medium
containing 20% fetal bovine serum. Cells were allowed to invade at
37°C for 24 h. Invaded cells were stained with crystal violet and
manually counted under a microscope. The cell invasion assay was
performed similarly, except that 50 µl of Matrigel diluted to a
concentration of 1:8 with serum-free medium was added to each well
for 2 h at 37°C before the cells were seeded into the upper
chamber.
Western blot analysis
The treated cells were washed twice with cold PBS
and lysed with RIPA lysis buffer (ComWin Biotech, Beijing, China)
containing 1% phosphatase inhibitor (ComWin Biotech) and 1%
protease inhibitor (ComWin Biotech). The total protein
concentration was determined by the BCA protein assay kit (Beyotime
Institute of Biotechnology, Shanghai, China). Equal amounts (30 µg
per load) of protein samples were electrophoresed using sodium
dodecyl sulfate-polyacrylamide gel electrophoresis and were
transferred onto polyvinylidene fluoride membranes (EMD Millipore,
Billerica, MA, USA). The blots were blocked in 5% non-fat milk for
1 h, and the membranes were washed and incubated with the primary
antibody overnight at 4°C, after shaking. After washing with TBS-T,
the membranes were incubated with secondary antibody at room
temperature for 1 h. The proteins were visualized using an ECL Kit
(EMD Millipore), and signal detection was performed in a Bio-Rad
Universal Hood 2 Electrophoresis Imaging Cabinet.
Immunofluorescence staining assay
ACHN cells treated with or without bufalin for 24 h
were fixed with 4% paraformaldehyde for 15 min at room temperature.
After being washed with PBS three times, the cells were
permeabilized with 0.5% Triton X-100 for 30 min, followed by
another three washes with PBS. The cells were blocked in goat serum
for 1 h and then incubated with various primary antibodies
overnight. The next day, after being washed with PBS three times,
the cells were incubated with the appropriate secondary antibody
(Thermo Fisher Scientific, Inc.) for 1 h, and
4,6-diamidino-2-pheylindole (DAPI) was used to stain the nucleus.
All fluorescence signals were photographed with a fluorescence
microscope.
Statistical analysis
The data in this study were calculated using
GraphPad Prism (version 5.01 for Windows) and expressed as mean ±
standard deviation. Significance levels were assessed with
Student's t-tests or one-way ANOVA followed by Bonferroni post-hoc
comparison test (in cases of equal variances) or Welch and
Brown-Forsythe tests (in cases of unequal variances). All
statistical analyses were performed using SPSS software (version
20.0 for Windows; IBM Corp., Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Bufalin suppresses ACHN cell
proliferation
To investigate the anti-cancer effects of bufalin on
ACHN, the cells were treated with bufalin (0, 2.5, 10, 40, and 80
nM) for 12, 24, 48, or 72 h. As shown in Fig. 1B, bufalin had a significant time-and
dose-dependent inhibitory effect on ACHN cell proliferation. The
IC50 values of bufalin for ACHN cells at 12, 24, 48, and 72 h were
228.08±9.64, 29.41±2.60, 10.49±0.79, and 6.7±0.97 nM, respectively.
We then tested whether bufalin could attenuate the ACHN cell colony
formation ability. As shown in Fig. 1C
and D, the numbers and sizes of colonies formed were
significantly reduced in a dose-dependent manner, from 233.00±12.12
to 183.67±5.69, 94.33±5.86, and 65.33±4.51 after treatment with
bufalin (concentrations of 0, 5, 10 and 20 nM, respectively).
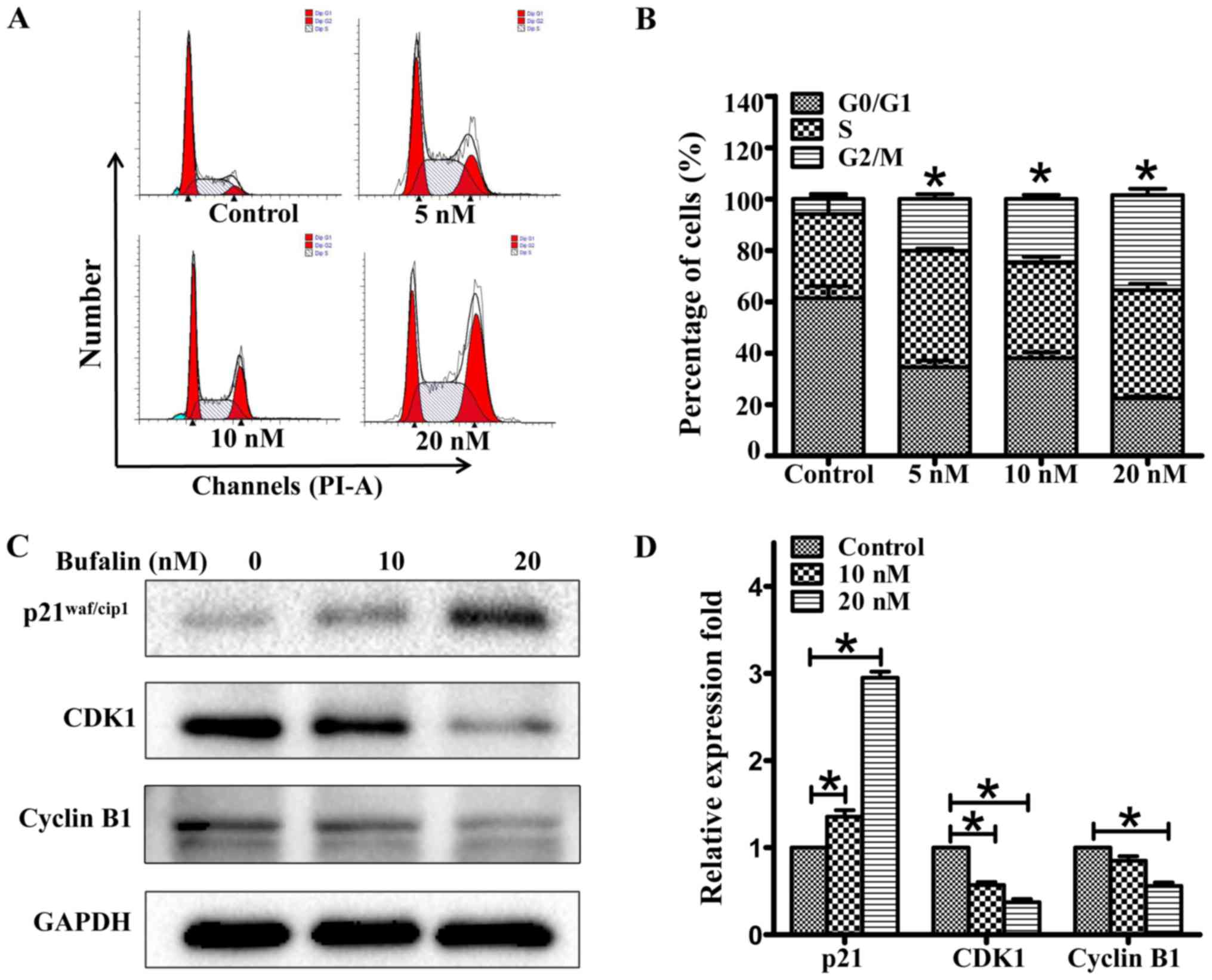
Bufalin induces G2/M phase arrest in
ACHN cells by regulating the expression of p21, cyclin B1, and CDK1
in ACHN cells
To explore the potential mechanism of its
anti-proliferative effect, we carried out cell cycle and apoptosis
analyses. As shown in Fig. 2A and B,
bufalin induced G2/M phase arrest in ACHN cells. The percentage of
ACHN cells in the G2/M phase increased from 7.86±0.96 to
15.49±3.51, 23.38±2.98, and 37.07±7.05% after treatment with
bufalin (concentrations of 0, 5, 10, and 20 nM, respectively) for
24 h. Under similar conditions, apoptotic cells were also assayed
using the Annexin V-PI staining kit. Bufalin did not induce ACHN
cell apoptosis at a dose of 20 nM but induced apoptosis at a high
dose of 80 nM. To unveil the mechanisms of the cycle arrest by
bufalin, we analyzed cell cycle progression-related proteins by
western blotting. As shown in Fig. 2C and
D, p21waf/cip1 expression was dose-dependently
increased, and cyclin B1 and CDK1 expression was decreased in ACHN
cell lines after bufalin treatment for 24 h. GAPDH was used as a
loading control.
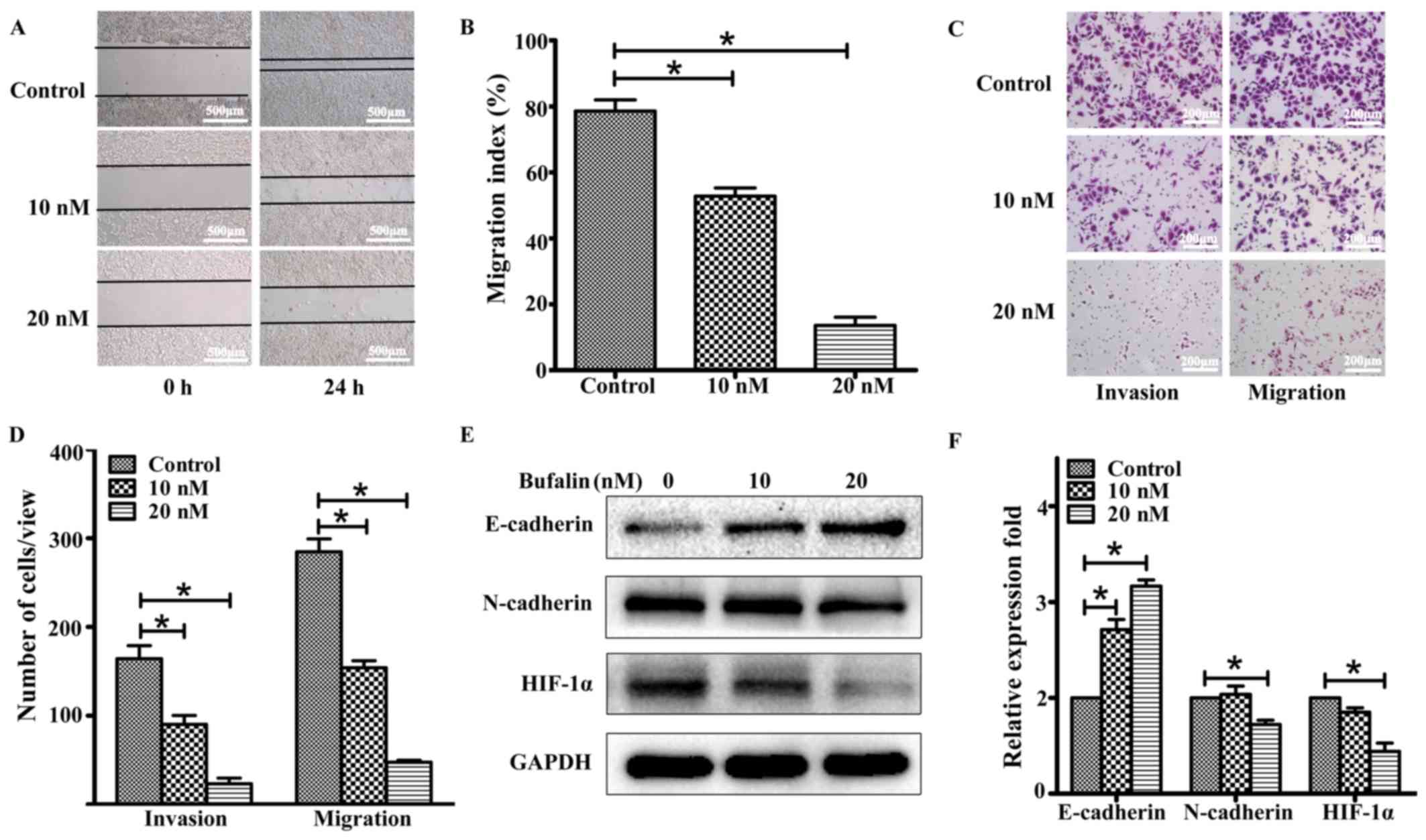
Bufalin inhibits ACHN cell migration
and invasion by regulating the expression of HIF-1α, E-cadherin,
and N-cadherin in ACHN cells
We then assessed whether bufalin could inhibit ACHN
cell migration and invasion. Wound-healing assays were performed to
detect the cell migration speed. As shown in Fig. 3A and B, bufalin decreased the
migration speed of ACHN cells in a dose-dependent manner. Our
analysis also determined that bufalin significantly reduced ACHN
cell invasion through Matrigel and migration through the membrane
in the bottom chamber (Fig. 3C and
D); these findings indicated that bufalin may inhibit ACHN cell
migration and invasion. To determine the differences in
epithelial-to-mesenchymal transition (EMT)-associated protein
expression levels in ACHN cells before and after treatment with
bufalin, further studies were performed using western blotting.
Compared with the control group, after 24 h of treatment with
bufalin, the expression level of N-cadherin decreased, whereas that
of E-cadherin increased in a dose-dependent manner in the
bufalin-treatment group (Fig. 3E and
F). The results of immunofluorescence staining confirmed the
upregulation of E-cadherin in ACHN cells treated with bufalin
(Fig. 4A). The results also showed
that bufalin inhibited the expression of HIF-1α, a central player
that accelerates invasion and metastasis in solid cancers.
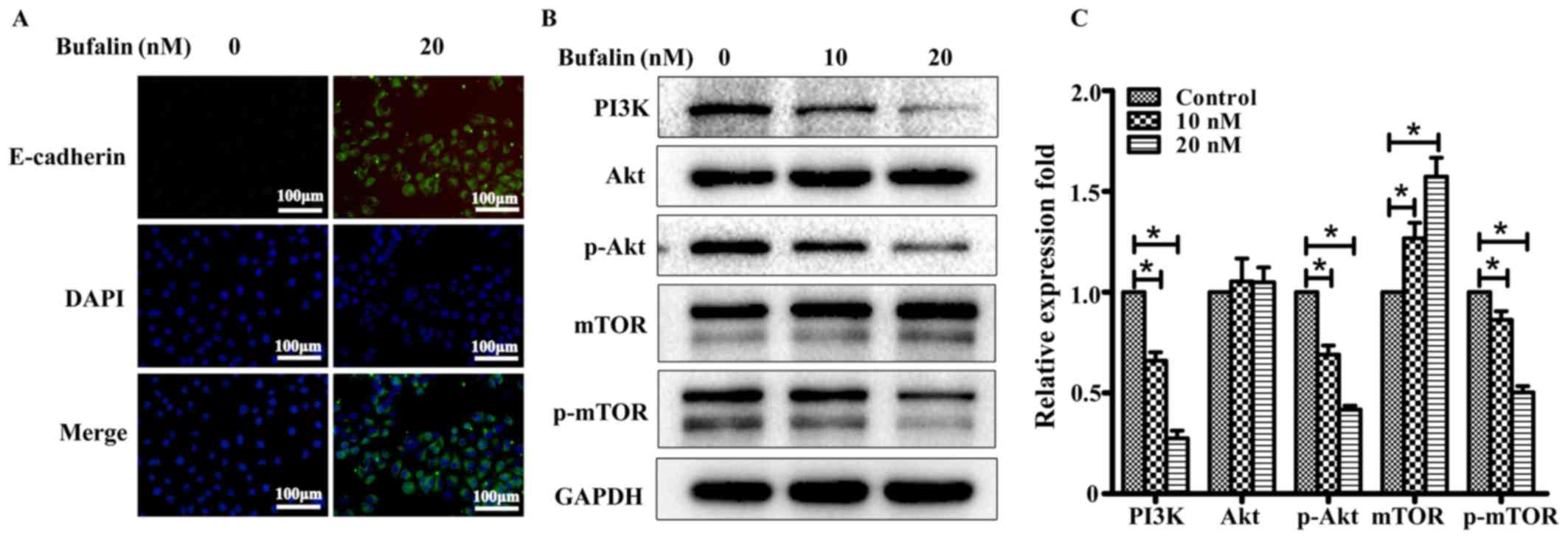 | Figure 4.Bufalin suppressed the expression of
p-Akt Ser473 in ACHN cells. (A) Two-color immunofluorescence
analysis showing the expression of E-cadherin (green) and DAPI
(blue) in ACHN cells treated with bufalin (concentrations of 0 or
20 nM) (magnification, ×200). (B) PI3K/Akt signaling
pathway-related protein expression was detected by Western blot
analysis after treatment with bufalin (concentrations of 0, 10, and
20 nM) for 24 h. GAPDH was considered as an internal standard. (C)
Bufalin treatment downregulated the expression of p-Akt, PI3K, and
p-mTOR in both the 10- and 20-nM treatment groups compared with the
DMSO control. The test was repeated three times, and the data are
presented as mean ± SD. *P<0.05. SD, standard deviation; PI3K,
phosphoinositide 3-kinase; Akt, protein kinase B; mTOR, mammalian
target of rapamycin. |
Bufalin exerts its anti-tumor effects
by regulating the expression of PI3K/Akt in ACHN cells
In this study, we demonstrated that bufalin exerts
its anti-tumor effects by inducing ACHN cell cycle arrest and
inhibiting metastasis. Furthermore, we determined that bufalin
disrupted the PI3K/Akt signaling pathway. As shown in Fig. 4B and C, there were no significant
changes in Akt protein expression. However, phospho-Akt (Ser473)
and phospho-mTOR levels were reduced in both the 10- and 20-nM
treatment groups compared with the blank control group; this
finding implies that bufalin mediated anti-tumor responsevia
phosphorylation. Correspondingly, PI3K protein expression decreased
significantly in both the 10- and 20-nM treatment groups compared
with the blank control group.
Discussion
Although mRCC is one of the most treatment-resistant
malignancies, there are new choices on the horizon (4,22).
Currently, surgical management (metastasectomy and cytoreductive
nephrectomy) remains an important component of the management of
mRCC, and targeted therapies are beginning to demonstrate promising
results (5,23). In a large phase 3 trial of mRCC
patients, Escudier et al (24)
assessed the efficacy and safety of sorafenib and demonstrated that
it significantly improved progression-free survival compared with a
placebo. Thus, targeted therapeutic agents may play an increasingly
important role in mRCC, and further studies are needed to
understand the potential mechanisms.
The cytotoxic effect of bufalin has been
demonstrated in various cancers. For example, the upregulation of
p53 and induction of Fas-mediated cell apoptosis were shown to
mediate the bufalin-induced death of prostate cancer cells
(25). Bufalin was shown to induce
cervical cell apoptosis and suppress the integrin
α2/β5/FAK-signaling pathway (21).
However, the role of bufalin in RCC remains unclear. Our study is
the first to demonstrate the suppression of p-Akt in
bufalin-induced RCC cell cycle arrest and bufalin-reduced
metastasis.
Our results showed that at a low dose of 5 nM,
bufalin inhibited ACHN cell proliferation by blocking the cell
cycle in the G2/M phase. Further study revealed that bufalin
blocked the ACHN cell cycle via the upregulation of
p21waf/cip1. However, bufalin did not induce apoptosis
at an effective dose of 20 nM, but it induced cell apoptosis at a
high dose of 80 nM. Interestingly, bufalin did not inhibit the
proliferation of HK-2 cells, a normal renal proximal tubular cell
line, at a high dose of 80 nM; this finding suggests that the
effect of bufalin is specific to cancer cells.
To date, numerous studies have suggested that EMT is
a key process in tumor metastasis. Hypoxia induces EMT via the
HIF-dependent upregulation of transcription repressors of
E-cadherin (12). Meanwhile,
increasing evidence has addressed the molecular mechanisms
underlying the reversal of EMT to exert anti-metastasis effects
(26). Consistent with these studies,
our results revealed an upregulation of the epithelial marker
E-cadherin and a downregulation of the mesenchymal marker
N-cadherin, with reduced expression of HIF-1α after treatment with
bufalin. Thus, we tentatively suggest that bufalin inhibits RCC
invasion and metastasis (Fig. 3) by
regulating the HIF-1α expression to reverse EMT.
Our study detected that bufalin treatment decreased
the levels of p-Akt and its downstream signaling member,
phospho-mTOR. By contrast, no significant changes in Akt protein
expression were observed in any of the groups. The data indicated
that bufalin exhibits significant anti-tumor activities, not only
via reducing the expression of phospho-mTOR but also via the
regulation of phospho-Akt. However, mutations in mTOR or PTEN and
the activation of PI3K/Akt were observed in different cell lines
after treatment with mTOR inhibitors (27). Thus, we believe that further studies
on other types of RCC lines and in an in vivo metastatic
model are required to better assess the therapeutic potential of
bufalin.
In conclusion, to our knowledge, our study is the
first to show that bufalin induces RCC ACHN cell cycle arrest and
suppresses metastasis via disruption of the PI3K/Akt/mTOR signaling
pathway. Our results indicate that bufalin is a potential
therapeutic agent for RCC.
Acknowledgements
Not applicable.
Funding
This study was supported by scientific research
grants from the Science and Technology Planning Project of the
Guangdong Province (grant no. 2016A020215109) and The Ministry of
Education, Culture, Sports, Science and Technology of Japan (grant
no. 17K1113809).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PH and CL conceived and designed the experiments.
JX, WL, LH and NX performed the experiments. WL, AX, BC, JX and MW
analyzed the data. JX, NX and PH wrote the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ANOVA
|
analysis of variance
|
|
DAPI
|
4,6-diamidino-2-pheylindole
|
|
DMSO
|
dimethyl sulfoxide
|
|
EMT
|
epithelial-to-mesenchymal
transition
|
|
PBS
|
phosphate-buffered saline
|
|
PI
|
propidium iodide
|
|
RCC
|
renal cell carcinoma
|
|
SD
|
standard deviation
|
References
|
1
|
Hirata H, Hinoda Y, Ueno K, Majid S, Saini
S and Dahiya R: Role of secreted frizzled-related protein 3 in
human renal cell carcinoma. Cancer Res. 70:1896–1905. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tan X, Liu Y, Hou J and Cao G: Targeted
therapies for renal cell carcinoma in Chinese patients: Focus on
everolimus. Onco Targets Ther. 8:313–321. 2015.PubMed/NCBI
|
|
3
|
Pei X, Li M, Zhan J, Yu Y, Wei X, Guan L,
Aydin H, Elson P, Zhou M, He H and Zhang H: Enhanced IMP3
Expression Activates NF-кB pathway and promotes renal cell
carcinoma progression. PLoS One. 10:e01243382015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sanchez-Gastaldo A, Kempf E, Del Alba
Gonzalez A and Duran I: Systemic treatment of renal cell cancer: A
comprehensive review. Cancer Treat Rev. 60:77–89. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chandrasekar T, Klaassen Z, Goldberg H,
Kulkarni GS, Hamilton RJ and Fleshner NE: Metastatic renal cell
carcinoma: Patterns and predictors of metastases-A contemporary
population-based series. Urol Oncol. 35:661.e7–661.e14. 2017.
View Article : Google Scholar
|
|
6
|
Xiang RF, Wang Y, Zhang N, Xu WB, Cao Y,
Tong J, Li JM, Wu YL and Yan H: MK2206 enhances the cytocidal
effects of bufalin in multiple myeloma by inhibiting the AKT/mTOR
pathway. Cell Death Dis. 8:e27762017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Martini M, De Santis MC, Braccini L,
Gulluni F and Hirsch E: PI3K/AKT signaling pathway and cancer: an
updated review. Ann Med. 46:372–383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alessi DR, Andjelkovic M, Caudwell B, Cron
P, Morrice N, Cohen P and Hemmings BA: Mechanism of activation of
protein kinase B by insulin and IGF-1. EMBO J. 15:6541–6551.
1996.PubMed/NCBI
|
|
9
|
Wang C, Wang Q, Li X, Jin Z, Xu P, Xu N,
Xu A, Xu Y, Zheng S, Zheng J, et al: Lycorine induces apoptosis of
bladder cancer T24 cells by inhibiting phospho-Akt and activating
the intrinsic apoptotic cascade. Biochem Biophys Res Commun.
483:197–202. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Melnik BC: p53: Key conductor of all
anti-acne therapies. J Transl Med. 15:1952017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kitajima Y and Miyazaki K: The critical
impact of HIF-1a on gastric cancer biology. Cancers (Basel).
5:15–26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zong S, Li W, Li H, Han S, Liu S, Shi Q
and Hou F: Identification of hypoxia-regulated angiogenic genes in
colorectal cancer. Biochem Biophys Res Commun. 493:461–467. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thomas JD, Zhang YJ, Wei YH, Cho JH,
Morris LE, Wang HY and Zheng XF: Rab1A is an mTORC1 activator and a
colorectal oncogene. Cancer Cell. 26:754–769. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Carew JS, Kelly KR and Nawrocki ST:
Mechanisms of mTOR inhibitor resistance in cancer therapy. Target
Oncol. 6:17–27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Morishita S, Saito T, Mishima Y, Mizutani
A, Hirai Y and Kawakami M: Pharmacological studies of Senso (Ch'an
Su) containing drugs. Nihon Yakurigaku Zasshi. 87:361–378. 1986.(In
Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu Y, Chen M, Jin XF, Qian C, Xu XM and
Zhang X: Research progress of in vitro and in vivo anti-tumor
effects and formulation of bufalin. Zhongguo Zhong Yao Za Zhi.
39:2829–2833. 2014.(In Chinese). PubMed/NCBI
|
|
17
|
Tian X, Yin H, Zhang S, Luo Y, Xu K, Ma P,
Sui C, Meng F, Liu Y, Jiang Y and Fang J: Bufalin loaded
biotinylated chitosan nanoparticles: An efficient drug delivery
system for targeted chemotherapy against breast carcinoma. Eur J
Pharm Biopharm. 87:445–453. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao L, Liu S, Che X, Hou K, Ma Y, Li C,
Wen T, Fan Y, Hu X, Liu Y and Qu X: Bufalin inhibits TGF-β-induced
epithelial-to-mesenchymal transition and migration in human lung
cancer A549 cells by downregulating TGF-β receptors. Int J Mol Med.
36:645–652. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang H, Zhang C, Xu L, Zang K, Ning Z,
Jiang F, Chi H, Zhu X and Meng Z: Bufalin suppresses hepatocellular
carcinoma invasion and metastasis by targeting HIF-1α via the
PI3K/AKT/mTOR pathway. Oncotarget. 7:20193–20208. 2016.PubMed/NCBI
|
|
20
|
Yuan XF, Tian HY, Li J, Jin L, Jiang ST,
Liu KW, Luo C, Middleton DA, Esmann M, Ye WC and Jiang RW:
Synthesis of bufalin derivatives with inhibitory activity against
prostate cancer cells. Nat Prod Res. 28:843–847. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu F, Tong D, Li H, Liu M, Li J, Wang Z
and Cheng X: Bufalin enhances antitumor effect of paclitaxel on
cervical tumorigenesis via inhibiting the integrin α2/β5/FAK
signaling pathway. Oncotarget. 7:8896–8907. 2016.PubMed/NCBI
|
|
22
|
Li X, Xu P, Wang C, Xu N, Xu A, Xu Y,
Sadahira T, Araki M, Wada K, Matsuura E, et al: Synergistic effects
of the immune checkpoint inhibitor CTLA-4 combined with the growth
inhibitor lycorine in a mouse model of renal cell carcinoma.
Oncotarget. 8:21177–21186. 2017.PubMed/NCBI
|
|
23
|
Rodriguez-Vida A, Hutson TE, Bellmunt J
and Strijbos MH: New treatment options for metastatic renal cell
carcinoma. ESMO Open. 2:e0001852017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Escudier B, Eisen T, Stadler WM, Szczylik
C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA,
et al: Sorafenib in advanced clear-cell renal-cell carcinoma. N
Engl J Med. 356:125–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu CH, Kan SF, Pu HF, Chien Jea E and Wang
PS: Apoptotic signaling in bufalin- and cinobufagin-treated
androgen-dependent and -independent human prostate cancer cells.
Cancer Sci. 99:2467–2476. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hu M, Peng S, He Y, Qin M, Cong X, Xing Y,
Liu M and Yi Z: Lycorine is a novel inhibitor of the growth and
metastasis of hormone-refractory prostate cancer. Oncotarget.
6:15348–15361. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Thaiwong T, Sirivisoot S, Takada M,
Yuzbasiyan-Gurkan V and Kiupel M: Gain-of-function mutation in
PTPN11 in histiocytic sarcomas of Bernese Mountain Dogs. Vet Comp
Oncol. 16:220–228. 2018. View Article : Google Scholar : PubMed/NCBI
|