Introduction
Transfer ribonucleic acids (tRNAs) play a crucial
role in protein translation. The abnormal expression of proteins
modulating tRNA modification are closely related to diseases such
as maternally inherited mitochondrial disease, nervous system
disease, tumor and type II diabetes mellitus (1). Human tRNA methyltransferase 9-like
(hTRM9L, also known as KIAA1456) protein is a kind of enzyme that
catalyzes the swing of tRNA bases (2). The KIAA1456 gene contains two
exons, located at the end of chromosome 8. Its protein expression
level is the highest in adult brain tissue and relatively low in
skeletal muscle, testis and ovary. The content of KIAA1456 in fetal
brain tissue is overtly lower than that in adult human brain
tissue, and that in cerebellum of adult human brain tissue is the
highest (3).
KIAA1456 can catalyze the swing of tRNA bases to
make tRNA mature, so as to ensure the normal and orderly
translation of proteins. The biological function of KIAA1456
protein is closely correlated with the deoxyribonucleic acid (DNA)
damage response. After exogenous overexpression of KIAA1456, the
repair capacity of cells to DNA damage is significantly improved,
DNA damage in some cells can be completely repaired, but cells with
incompletely repaired DNA damage rapidly die (4,5). Previous
findings have shown that KIAA1456 is involved in the occurrence and
development of tumors, and KIAA1456 protein has evidently lower
expression in breast, colon, bladder, cervical and testicular
cancer (6). After overexpression of
KIAA1456, the migration and invasion abilities and the growth rate
of tumor cells are decreased to different extents (7). The abnormal expression of KIAA1456
protein in cells interferes with normal protein translation and DNA
damage repair, eventually leading to canceration and malignant
proliferation of cells (5).
Therefore, considering the important role of KIAA1456 in tRNA
modification, it is inferred that KIAA1456 is a potential
tumor suppressor gene, and the abnormal decrease or loss of
KIAA1456 expression may contribute to tumor formation and
development. At present, the expression of KIAA1456 in lung cancer
cells is unclear.
In the present study, the expression of KIAA1456 in
lung cancer tissue and adjacent tissue were compared, the
correlations of KIAA1456 with patient characteristics and
clinicopathological stages were analyzed, and the effects of
KIAA1456 on lung cancer cell proliferation, migration and invasion
were explored preliminarily.
Materials and methods
Lung cancer tissue samples
Human tissue samples (90 pairs of lung cancer and
adjacent tissues) were obtained from patients treated in the
Pneumology Department, Thoracic Surgery Department and Oncology
Department of the First People's Hospital of Xuzhou (Xuzhou, China)
from June 2008 to July 2011. These patients were diagnosed
according to the American Association for Thoracic Surgery
guidelines for lung cancer screening (8), had an average age of 68.26±13.75 years,
and included 56 males and 34 females. Postoperative pathological
and clinical data were collected from the Department of Pathology
and hospital records, and the patients were followed up by the
hospital and laboratories. Informed consent of patients was
obtained, and the study was approved by the Ethics Review Committee
of the First People's Hospital of Xuzhou (Jiangsu, China).
Cells and reagents
293T cells and human lung cancer cell lines (A549
and GLC-15) were purchased from the Shanghai Institute of
Biochemistry and Cell Biology, Shanghai Institutes for Biological
Sciences, Chinese Academy of Sciences (Shanghai, China) and
cultured in Dulbecco's modified Eagle's medium (DMEM) containing
10% calf serum in a 5% CO2 incubator at 37°C. Mouse
anti-human KIAA1456, neural cadherin (N-cadherin), cyclin D1 and
epithelial cadherin (E-cadherin) immunoglobulin G (IgG), internal
control antibody mouse anti-human glyceraldehyde 3-phosphate
dehydrogenase (GAPDH) IgG, and secondary rabbit anti-mouse
IgG-horseradish peroxidase (HRP)- and biotin-labeled rabbit
anti-mouse IgG were purchased from Abcam (Cambridge, MA, USA). The
remaining reagents were purchased from Beijing Zhongshan Golden
Bridge Biotechnology Co., Ltd. (Beijing, China).
Immunohistochemistry
Lung cancer tissue and adjacent tissue were
collected during surgical procedures from lung cancer patients. The
tissues were cut into sections, followed by routine dewaxing and
hydration. Then, sections were added with 0.01 mmol/l citrate
buffer for antigen retrieval, treated with 3%
H2O2 for 20 min, blocked with 10% sheep serum
at room temperature for 20 min, and added with mouse anti-human
KIAA1456 (1:1,000) for incubation overnight at 4°C. After that, the
sections were incubated with a secondary antibody, namely,
biotin-labeled rabbit anti-mouse IgG (1:2500), added with
diaminobenzidine (DAB) substrate for color development,
counterstained with hematoxylin, mounted with neutral resin, dried,
and then observed under a microscope (BX-42, Olympus Corporation,
Tokyo, Japan).
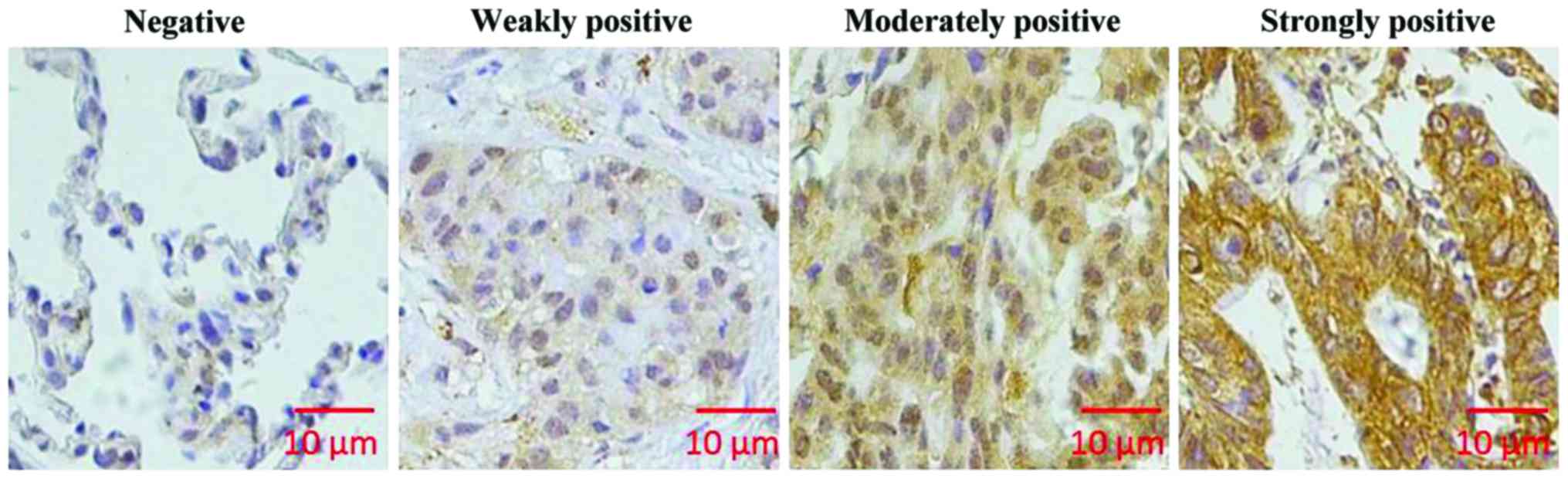
To determine immunohistochemical results, cells
stained in sepia were considered positive cells. ImageJ software
(National Institutes of Health, Bethesda, MD, USA) was used to
analyze staining intensity. The scoring for staining intensity was:
0 point (negative), 1 point (weakly positive), 2 points (moderately
positive), and 3 points (strongly positive). Scoring for percentage
of stained cells was: 1 point (1–24%), 2 points (25–49%), 3 points
(50–74%), and 4 points (75–100%). After multiplying the above two
scores, the following scores 0, 1, 2, 3, 4, 6, 8, 9 and 12 were
obtained and further scored was: 0 point, negative; 1–4 points,
weakly positive; 6–8 points, moderately positive; 9–12 points,
strongly positive. A score <6 points was defined as a low
expression of KIAA1456, and a score ≥6 points was defined as a high
expression of KIAA1456.
Lentivirus infection
The recombinant lentiviral vector plasmid pLP was
co-transfected with 293T cells via the Lipofectamine 2000 reagent
and incubated at 37°C for 48 h. The supernatant of the cell medium
was collected to obtain lentivirus carrying KIAA1456 gene or empty
vector lentivirus solutions. A549 or GLC-15 cells
(5×103) were inoculated into a 96-well plate and
cultured overnight at 37°C until the rate of cell fusion was
50–60%. The lentivirus pLP carrying KIAA1456 gene [multiplicity of
infection (MOI) =4] was added and incubated at 37°C for 24 h. The
next day, the medium containing virus was discarded and replaced by
DMEM + 10% calf serum medium, followed by 48 h of continuous
cultivation. The lentivirus pLP carrying no inserted genes was also
used to transfect A549 and GLC-15 cells, used as negative
controls.
Western blotting
After 72 h of lentiviral infection of A549 or GLC-15
cells, cells were collected, and added with Tween-20 cell lysis
buffer for cell lysis. Then, the supernatant was collected and
subjected to sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE). After that, protein was transferred
onto a polyvinylidene fluoride (PVDF) membrane, followed by
blocking with 1% bovine serum albumin (BSA) for 1 h at room
temperature. Then, protein was added with mouse polyclonal KIAA1456
antibody (1:500; cat. no. ab68919), N-cadherin (1:500; cat. no.
ab98952), cyclin D1 (1:500; cat. no. ab134175) and E-cadherin
(1:500; cat. no. ab1416) as well as secondary goat anti-rabbit
(HRP) IgG antibody (1:2,000; cat. no. ab6721) and secondary rabbit
anti-mouse (HRP) IgG antibody (1:2,000; cat. no. ab6728) for 1 h of
incubation at room temperature. All the antibodies were purchased
from Abcam. After that, secondary rabbit anti-mouse (HRP) IgG
antibody (1:2,000; cat. no. ab6728) was added for 1 h of incubation
at room temperature, followed by membrane washing with
phosphate-buffered saline (PBS) three times and color development
with DAB substrate. GAPDH was used as an internal reference. An
Odyssey chemiluminescence instrument was utilized to record the
colorimetric results. ImageJ software was used for gray analysis to
detect the expression level of the protein of interest.
Statistical analysis
Data were processed using Statistical Product and
Service Solutions (SPSS) 17.0 statistical software (SPSS, Inc.,
Chicago, IL, USA). Measurement data are expressed as mean ±
standard deviation, repeated 3 times for each group, and Student's
t-test was employed for comparisons among groups. Enumeration data
were expressed as cases or percentage, using χ2 test for
comparison among groups. The correlations of KIAA1456 expression in
lung cancer tissue with the clinicopathological parameters were
analyzed via the χ2 test. Survival rate analysis was
performed by using Kaplan-Meier survival curves. Threshold of
significance was α=0.05, and p<0.05 indicated that the
difference was statistically significant.
Results
KIAA1456 expression in lung cancer
tissue and adjacent tissue
Immunohistochemical analysis was used to analyze the
expression of KIAA1456 in lung cancer tissue and adjacent tissue.
As shown in Fig. 1, KIAA1456 was
mainly expressed in cytoplasm in lung cancer tissue. In 90 cases of
lung cancer tissue, the number of tissues with high expression of
KIAA1456 accounted for 51.1%, while in adjacent tissue, that
accounted for 70%. KIAA1456 expression in lung cancer tissue was
distinctly lower than that in adjacent tissue (p<0.05; Table I).
 | Table I.Expression of KIAA1456 in lung cancer
tissue and adjacent tissue. |
Table I.
Expression of KIAA1456 in lung cancer
tissue and adjacent tissue.
|
|
| KIAA1456
staining |
|---|
|
|
|
|
|---|
| Tissues | n | Low expression (n,
%) | High expression (n,
%) |
|---|
| Lung cancer | 90 | 44
(48.9) | 46
(51.1) |
| Adjacent | 90 | 27 (30) | 63 (70) |
| χ2 |
| 6.722 | 5.761 |
| P-value |
| 0.026 | 0.035 |
Correlation of KIAA1456 expression in
lung cancer tissue with clinicopathological parameters
The expression of KIAA1456 in lung cancer tissue had
no significant correlation with sex, age, tumor size or
histological type of the patient (p>0.05), but were
significantly correlated with the clinicopathological features of
the lung cancer patient, for example, pathological tumor (pT) stage
(p<0.01), pathological node (pN) stage (p<0.01),
tumor-node-metastasis (TNM) stage (p<0.01) and pathological
stage (p<0.01; Table II).
 | Table II.Correlation analyses of KIAA1456
expression in lung cancer tissue with clinicopathological
parameters. |
Table II.
Correlation analyses of KIAA1456
expression in lung cancer tissue with clinicopathological
parameters.
|
|
| KIAA1456
staining |
|
|
|---|
|
|
|
|
|
|
|---|
| Clinicopathological
parameters | n | Low expression (n,
%) | High expression (n,
%) | P-valuea | χ2 |
|---|
| Sex |
|
|
| 0.118 | 3.271 |
| Male | 56 | 29 (51.8) | 27 (48.2) |
|
|
|
Female | 34 | 16 (47.1) | 18 (52.9) |
|
|
| Age (years) |
|
|
| 0.154 | 2.147 |
| ≤60 | 53 | 23 (43.4) | 30 (56.6) |
|
|
|
>60 | 37 | 21 (56.8) | 16 (43.2) |
|
|
| Max. tumor
diameter |
|
|
| 0.125 | 4.158 |
| ≤3
cm | 42 | 19 (45.2) | 23 (54.8) |
|
|
| >3
cm | 48 | 27 (56.2) | 21 (43.8) |
|
|
| Histology |
|
|
| 0.424 | 3.297 |
|
Adenocarcinoma | 68 | 32 (47.1) | 36 (52.9) |
|
|
| Squamous
cell carcinoma | 16 | 8 (50) | 8 (50) |
|
|
|
Others | 6 | 4 (66.7) | 2 (33.3) |
|
|
| pT stage |
|
|
| <0.01 | 6.713 |
| pT1 | 27 | 7 (25.9) | 20 (74.1) |
|
|
| pT2 | 40 | 19 (47.5) | 21 (52.5) |
|
|
|
pT3-pT4 | 23 | 18 (78.3) | 5 (21.7) |
|
|
| pN stage |
|
|
| <0.01 | 7.291 |
| pN0 | 45 | 17 (37.8) | 28 (62.2) |
|
|
| pN1 | 17 | 7 (41.2) | 10 (58.8) |
|
|
|
pN2-pN3 | 28 | 20 (71.4) | 8 (28.6) |
|
|
| TNM stage |
|
|
| <0.01 | 6.246 |
| I | 33 | 8 (24.2) | 25 (75.8) |
|
|
|
II–III | 57 | 32 (56.1) | 25 (43.9) |
|
|
| Pathological
stage |
|
|
| <0.01 | 6.349 |
|
G1-G2 | 61 | 23 (37.7) | 38 (62.3) |
|
|
| G3 | 29 | 21 (72.4) | 8 (27.6) |
|
|
Analysis on the relationship between
KIAA1456 expression and prognostic survival in patients
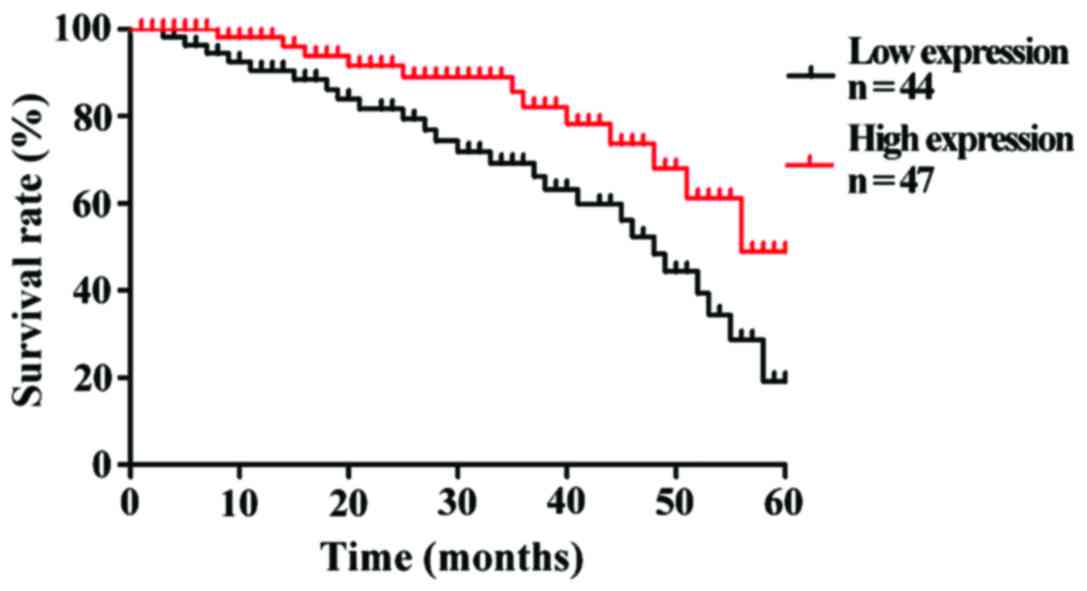
To understand the correlation of KIAA1456 expression
level with the prognostic survival in patients, Kaplan-Meier
survival curves were used to analyze the 5-year survival rates of
90 patients with lung cancer. The results revealed that the overall
5-year survival rate of patients with a high expression of KIAA1456
after operation was obviously higher than that of patients with a
low expression of KIAA1456 (p<0.05). The low expression of
KIAA1456 was associated with poor postoperative outcomes of
patients (Fig. 2).
Effects of KIAA1456 on lung cancer
cell proliferation, migration and invasion
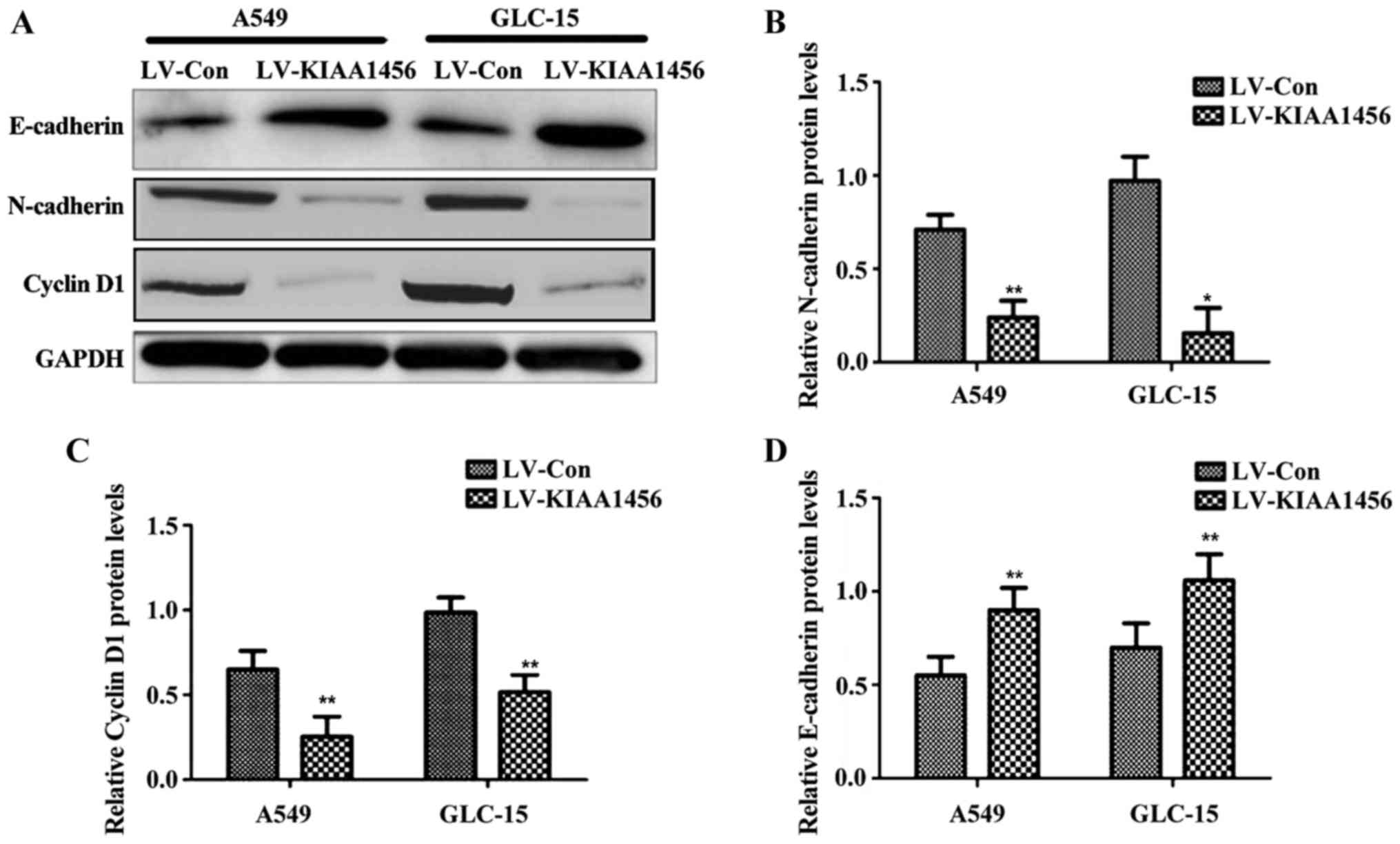
Following the overexpression of KIAA1456 in lung
cancer cell lines (A549 and GLC-15), the expression levels of
N-cadherin and cyclin D1 were significantly reduced compared with
those in control virus-infected cells (p<0.05), while the
expression level of E-cadherin was distinctly higher than that in
control virus-infected cells (p<0.05; Fig. 3).
Discussion
KIAA1456 is an enzyme catalyzing the swing of tRNA
bases, which plays an important role in making tRNA mature with
bioactivity. Prasad et al reported that KIAA1456 gene is a
potential tumor suppressor gene (9).
A study by Begley et al also indicated that KIAA1456 gene is
negatively correlated with tumor growth and has significantly
decreased expressions in bladder cancer, breast cancer, testicular
cancer, colon cancer and cervical cancer (2). There is no research study on
KIAA1456 gene in lung cancer. In this study, lung cancer
tissue and adjacent tissue were stained by immunohistochemistry to
observe whether the expression of KIAA1456 protein was changed in
lung cancer tissue. The results showed that the expression level of
KIAA1456 in lung cancer tissue was lower than that in adjacent
tissue. KIAA1456 expression level was not significantly correlated
with sex, age, tumor size and histological type of the patient, but
obviously associated with pT stage, pN stage, TNM stage and
pathological stage. The overall 5-year survival rate of patients
with high KIAA1456 expression after operation was significantly
higher than that of patients with low KIAA1456 expression. The low
expression of KIAA1456 was related to poor postoperative outcomes
of patients.
Cyclin D1, a member of cyclin family, binds
cyclin-dependent kinase-4 (CDK-4) proteins to accelerate
progression of phase G1-S of cell cycle, thereby promoting tumor
cell proliferation (10). Western
blot analysis in this study indicated that cyclin D1 protein was
clearly decreased when KIAA1456 gene was overexpressed in lung
cancer cells, suggesting that KIAA1456 can inhibit the
proliferation of lung cancer cells.
Lung cancer is a malignant tumor that has high
invasiveness and is easy to metastasize distantly.
Epithelial-mesenchymal transition (EMT) promotes the invasion and
metastasis of lung cancer cells, which is one of the major causes
of high mortality in lung cancer (11). E-cadherin and N-cadherin are the most
important markers in the process of EMT. The loss of E-cadherin can
lead to the loss of cell-cell junctions, and N-cadherin can
increase the motility and invasiveness of tumor cells (12). In this study, when KIAA1456 was
overexpressed in lung cancer cells, N-cadherin protein expression
was significantly decreased, while E-cadherin protein expression
was overtly increased, suggesting that KIAA1456 can reduce lung
cancer cell migration, invasion and metastasis.
In conclusion, KIAA1456 protein expression is low in
lung cancer tissue. The expression level of KIAA1456 is obviously
associated with the clinicopathological features and prognoses of
lung cancer. Overexpression of KIAA1456 inhibits the proliferation,
migration and invasion of lung cancer cells. KIAA1456 gene can
serve as a tumor suppressor gene, and an independent prognostic
factor in patients with lung cancer. Studies have shown that the
10-year survival rates of patients with early-stage lung cancer
will be up to 75% if lung cancer is diagnosed in the early stage
(13). KIAA1456 can act as a new
biological indicator for lung cancer detection, which is of great
significance for the early diagnosis and targeted therapy of lung
cancer.
Acknowledgements
Not applicable.
Funding
This study was supported by the Project of Xuzhou
Science and Technology Bureau (project no. KC16SH034), Medical Key
Subjects of Jiangsu Province (project no. ZDXKB2016007), Suzhou
Clinical Medicine Center (project no. Szzx201502), Key Technology
Application Research Project of Suzhou (project no. SS201630).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
SW and XL conceived and designed the experiments, SW
wrote the manuscript; JH, SW and YZ performed the experiments; CS
and XL analyzed the data of human tissue samples; TL and JY
contributed in collecting clinical tissue samples. All authors read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
Informed consent of patients was obtained, and the
study was approved by the Ethics Review Committee of the First
People's Hospital of Xuzhou (Xuzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Goodarzi H, Nguyen HCB, Zhang S, Dill BD,
Molina H and Tavazoie SF: Modulated expression of specific tRNAs
drives gene expression and cancer progression. Cell. 165:1416–1427.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Begley U, Sosa MS, Avivar-Valderas A,
Patil A, Endres L, Estrada Y, Chan CT, Su D, Dedon PC,
Aguirre-Ghiso JA, et al: A human tRNA methyltransferase 9-like
protein prevents tumour growth by regulating LIN9 and HIF1-α. EMBO
Mol Med. 5:366–383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Flanagan JM, Healey S, Young J, Whitehall
V, Trott DA, Newbold RF and Chenevix-Trench G: Mapping of a
candidate colorectal cancer tumor-suppressor gene to a 900-kilobase
region on the short arm of chromosome 8. Genes Chromosomes Cancer.
40:247–260. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shanmugam R, Aklujkar M, Schäfer M,
Reinhardt R, Nickel O, Reuter G, Lovley DR, Ehrenhofer-Murray A,
Nellen W, Ankri S, et al: The Dnmt2 RNA methyltransferase homolog
of Geobacter sulfurreducens specifically methylates
tRNA-Glu. Nucleic Acids Res. 42:6487–6496. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goodarzi H, Liu X, Nguyen HC, Zhang S,
Fish L and Tavazoie SF: Endogenous tRNA-derived fragments suppress
breast cancer progression via YBX1 displacement. Cell. 161:790–802.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abbott JA, Francklyn CS and Robey-Bond SM:
Transfer RNA and human disease. Front Genet. 5:1582014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Peng C, Zhang Z, Wu J, Lv Z, Tang J, Xie
H, Zhou L and Zheng S: A critical role for ZDHHC2 in metastasis and
recurrence in human hepatocellular carcinoma. BioMed Res Int.
2014:8327122014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jaklitsch MT, Jacobson FL, Austin JH,
Field JK, Jett JR, Keshavjee S, MacMahon H, Mulshine JL, Munden RF,
Salgia R, et al: The American Association for Thoracic Surgery
guidelines for lung cancer screening using low-dose computed
tomography scans for lung cancer survivors and other high-risk
groups. J Thorac Cardiovasc Surg. 144:33–38. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Prasad MA, Trybus TM, Wojno KJ and Macoska
JA: Homozygous and frequent deletion of proximal 8p sequences in
human prostate cancers: Identification of a potential tumor
suppressor gene site. Genes Chromosomes Cancer. 23:255–262. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bramanti V, Tomassoni D, Bronzi D, Grasso
S, Currò M, Avitabile M, Li Volsi G, Renis M, Ientile R, Amenta F,
et al: Alpha-lipoic acid modulates GFAP, vimentin, nestin, cyclin
D1 and MAP-kinase expression in astroglial cell cultures. Neurochem
Res. 35:2070–2077. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xiao D and He J: Epithelial mesenchymal
transition and lung cancer. J Thorac Dis. 2:154–159.
2010.PubMed/NCBI
|
|
12
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Henschke CI, Yankelevitz DF, Libby DM,
Pasmantier MW, Smith JP and Miettinen OS: International Early Lung
Cancer Action Program Investigators: Survival of patients with
stage I lung cancer detected on CT screening. N Engl J Med.
355:1763–1771. 2006. View Article : Google Scholar : PubMed/NCBI
|