Introduction
MicroRNAs (miRNAs/miRs) are a novel class of
non-coding RNAs that post-transcriptionally regulate gene
expression via translational inhibition or direct degradation of
the target RNAs (1). More than 30% of
all mRNAs are predicted to be targeted by miRNAs (2). miRNAs can negatively or positively
regulate not only the physiological processes, but also the
critical pathways of carcinogenesis, including cellular
proliferation, colony formation, apoptosis and migration (3).
Gastric cancer is one of the most prevalent human
cancer types, and is particularly common in the Far East region,
including China (4), and is the
second-leading cause of global cancer-associated mortality. For the
past few decades, surgical resection with chemoradiation has been
used to improve the 1- and 5-year survival rates (5,6). However,
the majority of gastric cancer cases are not diagnosed until they
reach advanced or metastatic stages, which markedly decreases the
1- and 5-year survival rates, owing to the increased
chemoresistance of metastatic disease (7). Although novel chemotherapeutic agents
have been used clinically, the median survival time remains <1
year (8). Thus, further research is
required to investigate the mechanisms of chemoresistance
reversal.
Recent studies have revealed data concerning the
involvement of miRNAs in carcinogenesis and increases in
chemoresistance. Among these miRNAs, miR-96 has recently been
demonstrated to be involved in the invasive and metastatic
potential of several types of carcinoma, including hepatocellular
carcinoma (9), breast cancer
(10), lung cancer (11), pancreatic cancer (12) and bladder cancer (13). One previous report has indicated that
miR-96 has a notable role in the induction of chemoresistance
(14). In non-small cell lung cancer
cells, miR-96 was found to be a critical inducer of cisplatin
chemoresistance (14). In breast
cancer, miR-96 was markedly upregulated in breast cancer cells and
tissues following chemotherapy (15).
The wide involvement of miR-96 promoted a focus on the association
of this miRNA with chemoresistance in gastric cancer.
In the current study, the expression of miR-96 in
gastric cancer cells following chemotherapeutic treatment was
quantified at different doses and time points to assess its
expression pattern under different conditions. It was determined
that miR-96 expression was likely to be promoted by
chemotherapeutic treatment and thus decreases the expression level
of forkhead box protein O1 (FOXO1) by targeting FOXO1 miRNA
directly, which is consistent with one previous report
demonstrating that miR-96 post-transcriptionally regulates FOXO1
(16). This reduced the expression of
cyclin-dependent kinase inhibitor 1A (CDK1A, also known as p21),
without disturbing that of tumor protein P53 (hereafter p53).
Materials and methods
Cell culture
The human gastric carcinoma SGC7901 cell line (Type
Culture Collection of the Chinese Academy of Sciences, Shanghai,
China) was used in the present study. Cells were cultured in
RPMI-1640 medium (Life Technologies; Thermo Fisher Scientific Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS;
Life Technologies; Thermo Fisher Scientific Inc.) at 37°C in an
incubator with 5% CO2.
miRNA mimics and short hairpin RNA
(shRNA) cell transfection
miRNA mimics and modified antisense oligonucleotide
(antago)-miRNA, with scrambled miRNA and scrambled antago-miRNA as
negative controls respectively, were synthesized and purchased
(Guangzhou RiboBio Co., Ltd., Guangzhou, China). Plasmids coding
for shRNA targeting human FOXO1 and scrambled shRNA as negative
control were purchased from Guangzhou RiboBio Co., Ltd. SGC7901
cells were seeded into six-well plates with a starting cell number
of 1×105, without serum and antibiotics. miRNA mimics or
antago-miRNA were transfected into cells at a concentration of 50
nmol/l using Lipofectamine RNAiMAX (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. For
shRNA plasmid transfection, 0.8 µg plasmid for each well was
transfected into SGC7901 cells using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. After 6 h incubation, the medium were
replaced with fresh medium containing 10% FBS. A total of 48 h
later, all cells were harvested for further analysis.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
For quantitative measurement of target microRNAs,
TaqMan microRNA assay kit containing specific primers; miR-96
forward, 5′-TTTGGCACTAGCACAT-3′ and reverse, 5′-GAGCAGGCTGGAGAA-3′;
Let-7a forward, 5′-TGAGGTAGTAGGTTGTGTGGTT-3′ and reverse,
5′-GCTGTCAACGATACGCTACCTA-3′; miR-9 forward,
5′-GGTCCTGGATCCCATCTTTT-3′ and reverse, 5′-GCGCAGTGTATGGGGTTATT-3′;
β-actin forward, 5′-CATGTACGTTGCTATCCAGGC-3′ and reverse,
5′-CTCCTTAATGTCACGCACGAT-3′ (Applied Biosystems; Thermo Fisher
Scientific, Inc.) were used for selected miRNAs, including miR-96,
Let-7a and miR-9, in accordance with the manufacturer's protocol.
Let-7a and miR-9 were used as negative controls, as their
expression levels are constant during chemotherapy (17). β-actin was used as reference. In
brief, total RNA was isolated from the target cells using a mirVana
miRNA Isolation kit (Ambion; Thermo Fisher Scientific, Inc.). A
total of 100 ng total RNA was used as template in each reaction
with miRNA-specific RT primers. The reaction was incubated for 30
min at 37°C. Next, the cDNA was used as a template following the
qPCR instructions. Thermocycling conditions were as follows: 20 sec
at 95°C (enzyme activation), and 40 cycles of 5 sec at 95°C
(denaturation) and 30 sec at 60°C (annealing and extension). For
data analysis, the 2−ΔΔCq method was performed (18).
EdU staining
An EdU detection kit (Guangzhou RiboBio Co., Ltd.)
was used according to the manufacturer's protocol. A total of
1×106 SGC7901 cells were incubated with 50 µM EdU
labeling medium (provided with the kit) at 37°C for 2 h. Following
immobilization, staining with Apollo® 567 solution
(provided with the kit) for 30 min at 37°C and Hoechst 33342
solution (provided with the kit) for 30 min at 37°C, cells were
imaged under a ×71 (U-RFL-T) fluorescence microscope (Olympus
Corporation, Tokyo, Japana).
Western blot analysis
Cells were lysed using Cell Lysis buffer (Beyotime
Institute of Biotechnology, Shanghai, China) that contained 1 mM
phenylmethylsulfonyl fluoride and 1× protease inhibitors cocktail
(Roche Applied Science, Penzberg, Germany). Following
quantification using a bicinchoninic acid assay kit (Sigma-Aldrich,
Merck KGaA, Darmstadt, Germany), a total of 20 µg protein were
subjected to 10% SDS-PAGE on 10% polyacrylamide gel. The resolved
proteins were transferred onto a polyvinylidene difluoride membrane
(EMD Millipore, Billerica, MA, USA), which was then blocked with 5%
non-fat dried milk in TBS-Tween-20 buffer (0.1%) for 1 h at room
temperature prior to incubation overnight at 4°C with primary
antibodies. Rabbit polyclonal antibodies against human FOXO1 (cat
no. ab39670), p21 (cat no. ab129520), p53 (cat no. ab131442) and
β-actin (cat no. ab8227) were obtained from Abcam (Cambridge, UK)
at a dilution of 1:2,000. The membrane was then washed three times
with TBS containing 0.05% Tween-20 prior to incubation for 1 h at
room temperature with horseradish peroxidase-conjugated goat
anti-rabbit antibodies IgG H&L (Abcam; cat no. ab6721) at a
dilution of 1:5,000. Immune complexes were detected with
chemiluminescence reagents (EMD Millipore, MA, USA).
Chromatin immunoprecipitation
(ChIP)
The ChIP kit (Upstate Biotechnology, Inc., Lake
Placid, NY, USA) was used to conduct the ChIP assay. A total of 1
µg/ml cisplatin with or without 10 µM pifithrin-α (PFT-α) was added
to regular medium for 24 h incubation. Then, 2×106 cells
were fixed with 1% formaldehyde for 15 min at room temperature and
quenched by adding glycine at final concentration of 125 mM. Cell
pellets were resuspended in SDS lysis buffer (Guangzhou RiboBio
Co., Ltd.) for a further 10 min incubation at room temperature. The
sheared DNA using sonication was diluted 10-fold in ChIP dilution
buffer (Guangzhou RiboBio Co., Ltd.) and incubated with the FOXO1
antibody (Abcam; cat no. 39670) at final concentration of 100 ng/µl
(1:1,000), 4°C with rocking for 12 h. Following the elution and
reverse-crosslinking step, the eluent was used for qPCR using the
SYBR® Premix Ex TaqTM kit (Takara Biotechnology Co.,
Ltd., Dalian, China) with the following primers: p21 promoter
region forward, 5′-CCTTTCTATCAGCCCCAGAGGATACC-3′ and reverse,
5′-GGGACGTCCTTAATTATCTGGGGTC-3′; DHFR 3′ untranslated region
forward, 5′-CTGATGTCCAGGAGGAGAAAGG-3′ and reverse,
5′-AGCCCGACAATGTCAAGGACTG-3′; β-actin forward,
5′-CATGTACGTTGCTATCCAGGC-3′ and reverse,
5′-CTCCTTAATGTCACGCACGAT-3′, β-actin was used as reference.
Thermocycling conditions were as follows: 20 sec at 95°C (enzyme
activation), followed by 40 cycles of 5 sec at 95°C (denaturation)
and 30 sec at 60°C (annealing and extension). The data was analyzed
using the 2−ΔΔCq method (18).
Cell death rate assay
Cell death caused by chemotherapeutic treatment (1
µg/ml cisplatin or 1 µmol/l doxorubicin co-incubation for 24 h) was
tested for lytic activities using a carboxyfluorescein succinimidyl
ester (CFSE)/propidium iodide (PI) labeling assay. Prior to
chemotreatment, cells were labeled with 5 µM CFSE (Sigma-Aldrich;
Merck KGaA) for 10 min at 37°C in PBS. This process was halted by
changing the supernatant for fresh medium containing chemoagent as
indicated (1 µg/ml cisplatin or 1 µmol/l doxorubicin). Following
chemotherapeutic treatment, cells were incubated with 5 µg/ml PI
(Sigma-Aldrich; Merck KGaA) at 37°C for 10 min. To assess the cell
death rate of SGC7901 cells following 1 µg/ml cisplatin or 1 µmol/l
doxorubicin treatment after 24 h, all cells were pre-stained with
CFSE. After 24 h, all cells were incubated with 5 µg/ml PI at 37°C
for 10 min, and dead cells were positively stained.
Cell death rate analysis using flow
cytometry
Cells were diluted into 1×106 cells/ml,
fixed with ice-chilled 75% ethanol in 1× PBS overnight at 4°C and
rinsed three times with PBS (5 min each). The cells were analyzed
with a flow cytometer following staining with 1 ml PI (including
RNase) for 30 min at room temperature. The excitation wavelength
488 nm and the wavelength of emitted light was >630 nm. The
software Cell Quest and Modfit LF (version 3.0; BD Biosciences,
Franklin Lakes, NJ, USA) were used to assay the cell death rate of
10,000 cells.
Cell Counting Kit-8 (CCK-8) assay
Target cells were trypsinized, resuspended and
seeded into 96-well plate, each well with 5,000 cells. Cell
viability was evaluated using Cell Counting kit-8 (Beyotime
Institute of Biotechnology) according to the protocol of the
manufacture at daily intervals on days 1–5 after seeding. Following
treatment with CCK-8 at 37°C for 1 h, the absorbance was measured
at 450 nm using a Multiskan spectrum microplate reader (Thermo
Fisher Scientific, Inc.).
Statistical analysis
The data were expressed as the mean ± standard
deviation of three independent experiments. Statistical analyses
were performed using IBM SPSS version 20 (IBM Corp., Armonk, NY,
USA). Statistical comparisons were performed using unpaired
Student's t-test for two-group comparisons of means. One-way
analysis of variance with Dunnett's post hoc was used for
comparisons of three or more groups. In all cases, P<0.05 was
considered to indicate a statistically significant difference.
Results
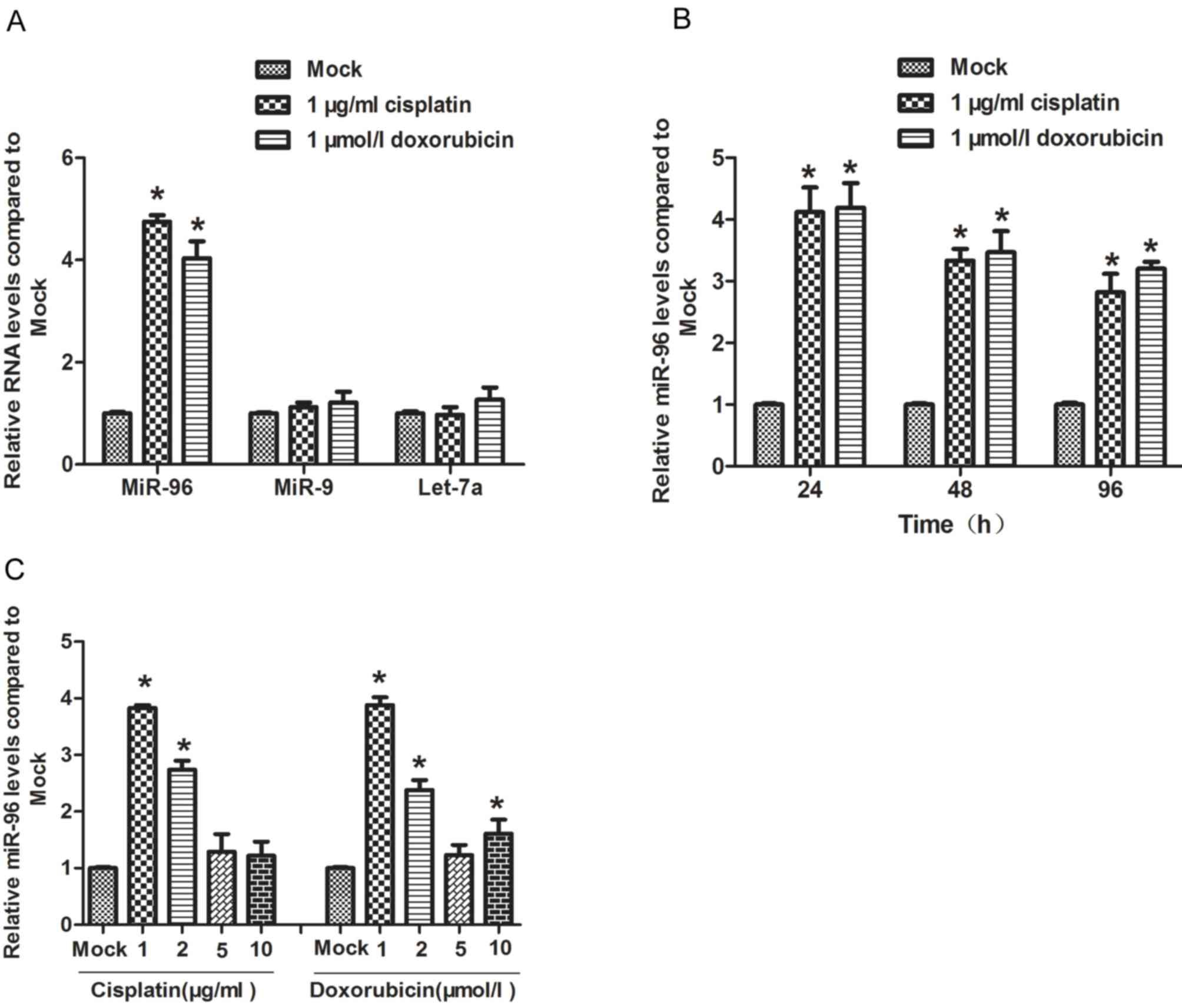
Low dose of chemotherapeutic agents
treatment induces expression of miR-96 in gastric cancer cells
The gastric cancer SGC7901 cell line is frequently
resistant to chemotherapeutic agents, including cisplatin and
doxorubicin (19). Here, SGC7901 was
treated with 1 µg/ml cisplatin or 1 µmol/l doxorubicin for 24 h,
and the expression level of miR-96 was detected by RT-qPCR. The
results of this analysis revealed that treatment with
chemotherapeutic agents significantly induced the expression of
miR-96, but not Let-7a or miR-9 (Fig.
1A). Next, the effect of the two chemotherapeutic agents was
assessed on miR-96 expression level at different time points (24,
48 and 96 h). It was observed that, compared with untreated cells,
the miR-96 expression level at time points 24, 48 and 96 h
following treatment with chemotherapeutics were all higher, and
miR-96 was expressed at a significantly higher level at 24 h
(Fig. 1B). To determine the dose of
chemotherapeutic agents that efficiently induced the expression of
miR-96, the cells was treated with 1, 2, 5 or 10 µg/ml cisplatin,
or 1, 2, 5 or 10 µmol/l doxorubicin respectively for 24 h. A dose
of 1 µg/ml cisplatin and 1 µmol/l doxorubicin induced miR-96
expression most efficiently, with a higher dose of either agent
exhibiting no stronger an effect on miR-96 expression (Fig. 1C), indicating that the dose required
to induce miR-96 expression is comparatively low.
Induction of miR-96
post-transcriptionally represses FOXO1 expression and decreases the
transcription of p21
Identified as the direct target of miR-96, FOXO1
protein level is potentially regulated by miR-96 induced by
chemotreatment (16). This promoted
to identify whether miR-96 regulates FOXO1 expression in SGC7901
cells. Synthesized miR-96 mimics (miR-96), scrambled miR-96 mimics
(scrambled), shRNA target to FOXO1 mRNA (shFOXO1), and scrambled
shFOXO1 were introduced into SGC7901 for 48 h. Western blot
analysis was performed to detect FOXO1, GAPDH and β-actin protein
levels. The results confirmed that miR-96 mimics exhibited similar
effect on repressing FOXO1 protein level as treatment with shFOXO1
(Fig. 2A). Following treatment with
cisplatin or doxorubicin, the FOXO1 protein level decreased
significantly compared with the Mock group (Fig. 2B; right panel), whereas FOXO1 mRNA
exhibited no observed changes (Fig.
2B; left panel). FOXO1 is reported to transcriptionally
activate p21 in a p53-independent manner (20), with the results of the present study
revealing that chemotreatment repressed p21 mRNA and protein
expression levels without disturbing the p53 expression level
(Fig. 2B). PFT-α, which specifically
inhibits transcription of p53, was pre-incubated with SGC7901 cells
treated with 1 µg/ml cisplatin. As expected, inhibition of p53
exerted no additional effect on p21 mRNA expression level (Fig. 2C). To determine whether FOXO1 was
involved in the regulation of p21 under treatment with
chemotherapeutics, the binding of FOXO1 to p21 promoter region was
assessed by ChIP. As expected, the binding of FOXO1 to p21 promoter
decreased following treatment with chemotherapeutics (Fig. 2D).
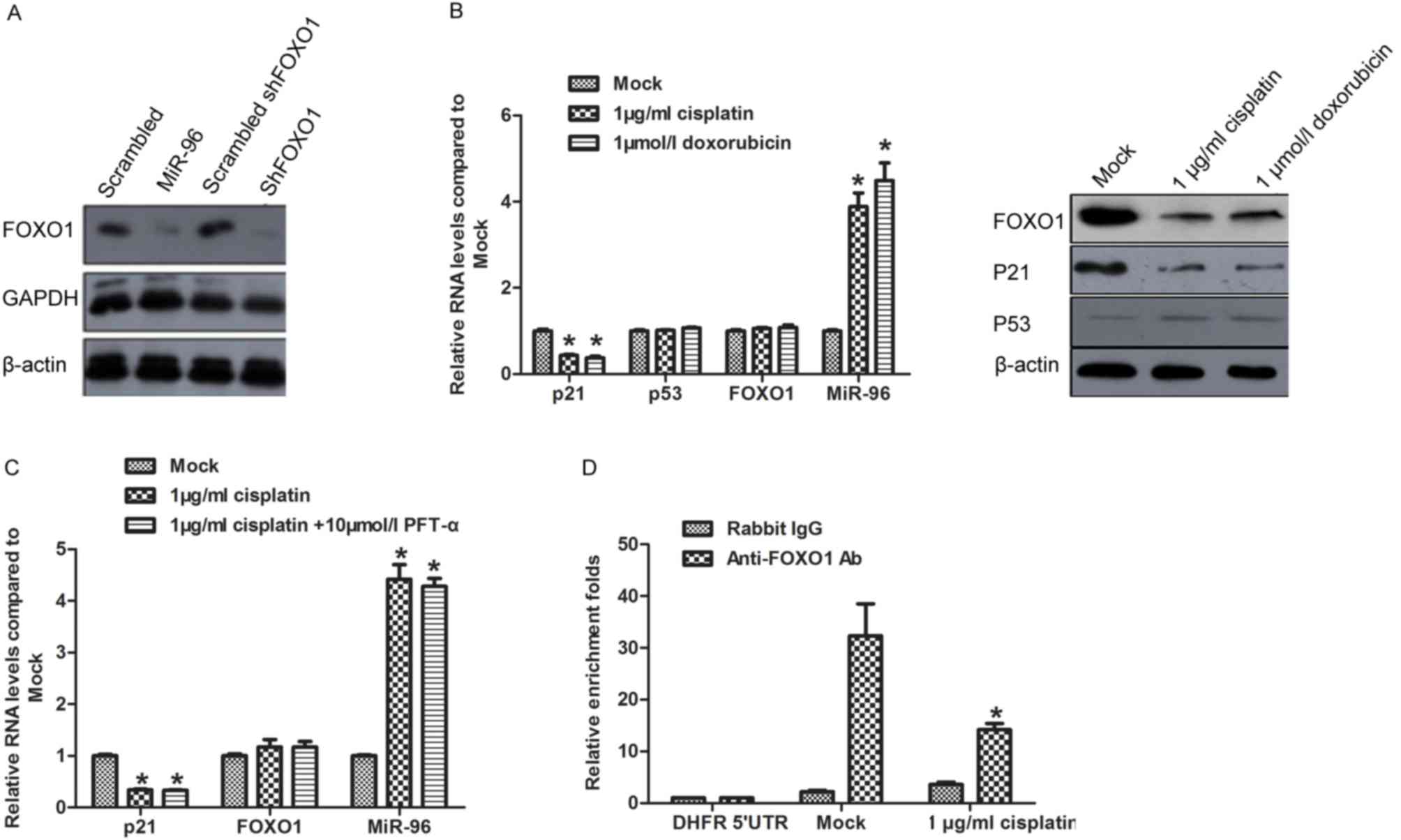 | Figure 2.miR-96 expression
post-transcriptionally regulates FOXO1 and thus modulates the
expression of p21. (A) Protein levels of FOXO1 in SGC7901 cells
transfected with miR-96 mimics or scrambled mimic negative
controls. (B) To confirm that the downregulation of FOXO1 was not
caused by chemotherapeutic treatment, the mRNA or protein level was
detected following chemotherapeutic treatment. (C) p21 expression
level following chemotherapeutic treatment. (D) ChIP assay,
revealing the binding activity of FOXO1 to the p21 promoter region.
*P<0.05, compared with mock. miR-96, microRNA-96; FOXO1,
forkhead box protein O1; ChIP, chromatin immunoprecipitation;
shRNA, short hairpin RNA; Ab, antibody; UTR, untranslated region;
p21, cyclin-dependent kinase inhibitor 1A; p53, tumor protein P53;
PFT-α, Pifithrin-α. |
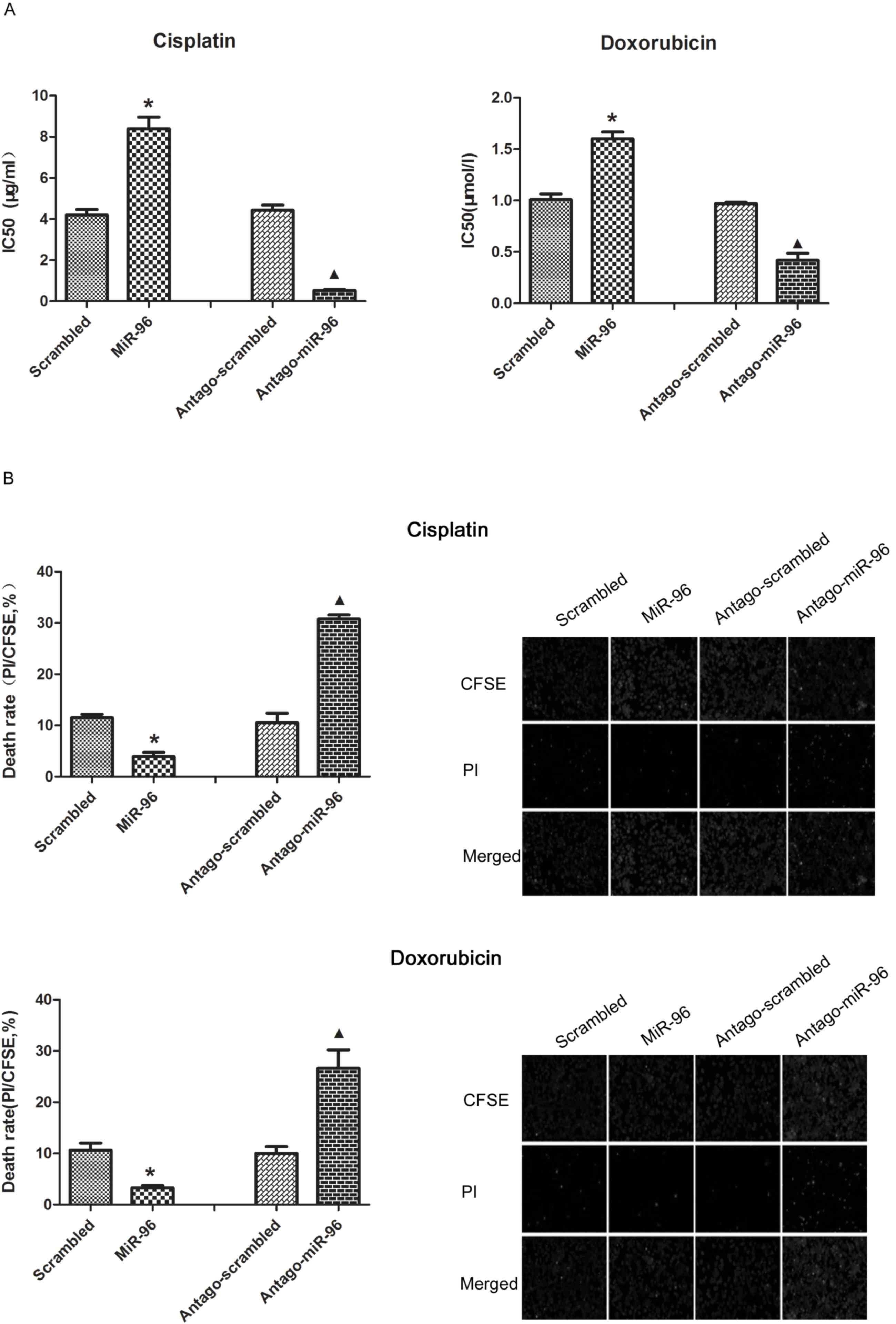
Overexpression of miR-96 induces the
chemoresistance and decreased the death rate following
chemotherapeutic treatment
As expected, overexpression of miR-96 in SGC7901
increased the half-maximal inhibitory concentration
(IC50) of cisplatin and doxorubicin. The repression of
miR-96 upon treatment with antago-miR-96 significantly increased
the chemosensitivity of SGC7901 (Fig.
3A). The results of CFSE/PI revealed that SGC7901 cells
transfected with exogenous miR-96 exhibited fewer PI-positive cells
and SGC7901 cells transfected with antago-miR-96 exhibited much
more PI positive cells by compared with their control group
(Fig. 3B, right). The results of the
flow cytometry assay confirmed that overexpression of miR-96 is
critical for decreasing the cell death rate of SGC7901 cells
(Fig. 3B, left).
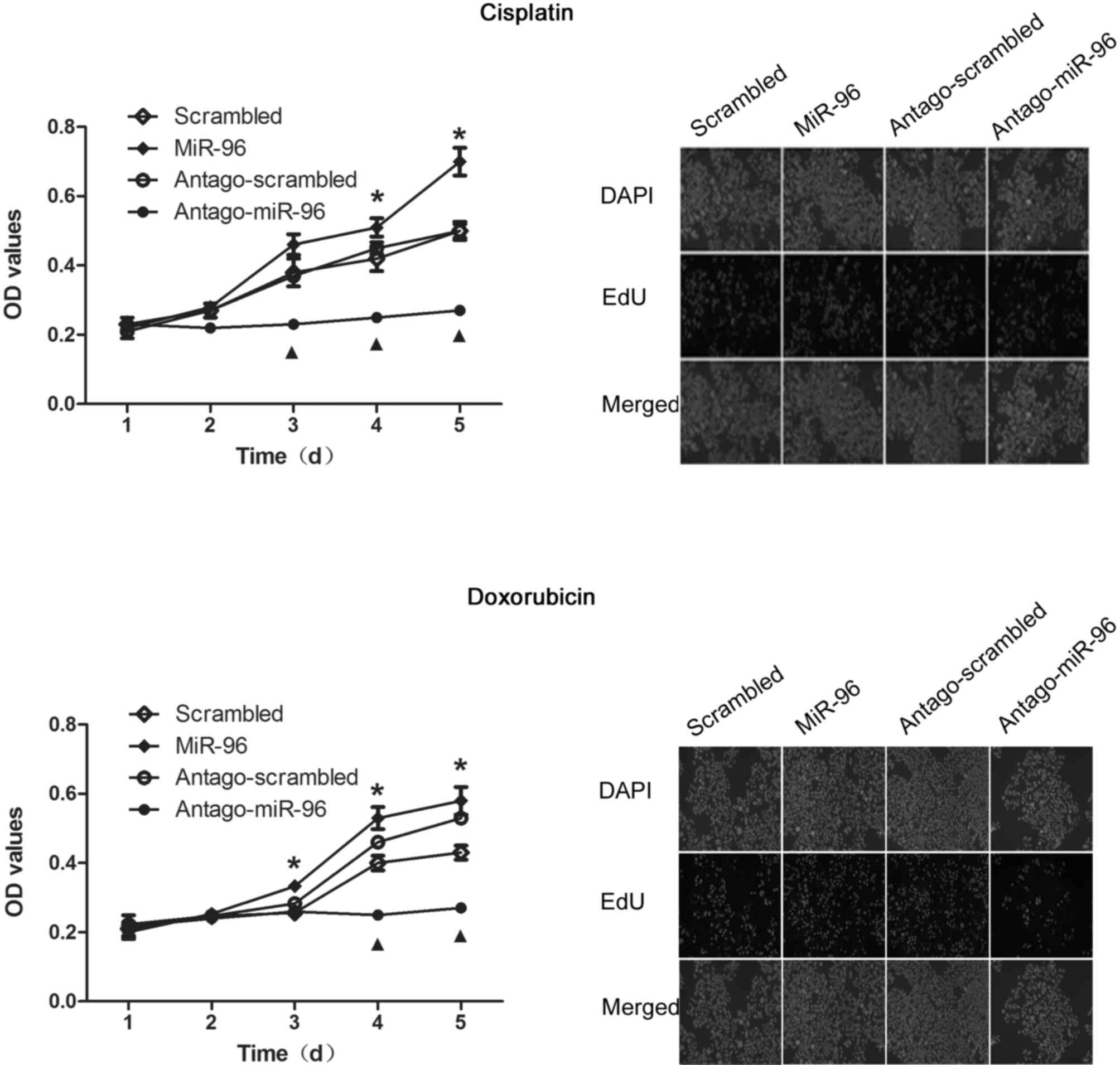
miR-96 promotes proliferation of
SGC7901
According to previous results, overexpression of
miR-96 possibly affects cell proliferation by regulating p21
(21). The results of the CCK-8 assay
revealed that, miR-96 overexpression promoted the proliferation of
SGC7901, and conversely, treatment with antago-miR-96 significantly
inhibited proliferation (Fig. 4, left
panel). Similar results were obtained from the EdU staining assay,
which was further performed for confirming this observation
(Fig. 4, right panel).
Discussion
Chemotherapy is the most widely used therapeutic
strategy for cancer treatment besides surgery (21). However, drug resistance, particularly
multidrug resistance, greatly limits its clinical use. Changes in
miRNA expression levels have been found to contribute to multiple
cancer-associated processes, including initiation, progression and
chemoresistance (22). The
association between the expression level of miRNAs and cancer has
indicated that changes to miRNA expression profiles may be
associated with responses to the treatment of patients with
chemotherapeutic agents. Therefore, further research on the miRNAs
whose expression is altered by chemotherapy is required.
The present study revealed that miR-96 expression
was positively induced by chemotherapeutic treatment at certain
doses, which post-transcriptionally repressed the expression of
FOXO1 and thus led to the inhibition of p21 transcription, which is
a FOXO1-target gene in gastric cancer cells. These results
indicated that miR-96 could function as a promoter of
chemoresistance by promoting cell proliferation in gastric cancer
cells. To the best of our knowledge, the present study is the first
to investigate the association between miR-96 and chemotherapy in
gastric cancer cells. In the previous reports, miR-96 has a
critical role in various types of tumor. Yu et al (12) revealed that in pancreatic cancer
cells, miR-96 repressed expression of the oncogene KRAS by directly
targeting its mRNA, thus functioning as a tumor-suppressor miRNA
and causing a decrease in proliferation, invasion, migration and
tumor growth. Vishwamitra et al (23) revealed that the heterogeneous
expression of miR-96 in lymphoma kinase-expressing cancer cells
decreased the proliferation, colony formation, and migration of
cells via a posttranscriptional regulatory mechanism. Contrarily,
miR-96 has also been shown to function as a tumor-promoting miRNA
by increasing the proliferation, migration, and invasion of various
different types of cancer cell, including breast cancer (24). In hepatocellular carcinoma cells
(HCC), induction of miR-96 expression promotes the invasion of HCC
cells, indicating that miR-96 may be a therapeutic target for
inhibiting HCC metastasis (25).
FOXO1, reported to be a downstream mediator of
CPT-triggered apoptosis (26,27), is phosphorylated by CDK1 and protein
kinase B (AKT), and thus be activated as a transcriptional
regulator on its target gene, p21 (20). FOXO1 is transcriptionally regulated by
several mechanisms, aforementioned, including AKT signaling. The
present study confirmed that FOXO1 mRNA was bound by miR-96 in
gastric cancer cells. Consequently, the specific binding of miR-96
to FOXO1 mRNA markedly downregulated the protein level and caused
the alteration of phenotype in SGC7901.
In summary, the characterization of miR-96
identified that it was a novel inducer of cell proliferation,
migration, invasion and tumor formation following chemotherapeutic
treatment of gastric cancer cells. The oncogenic function of miR-96
is in part explained by its inhibition of FOXO1. The results of the
present study indicate that functional FOXO1 could be considered as
a biomarker for processing chemotherapy and miR-96 could be
considered as a novel target for reversing chemoresistance in
gastric cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CL designed the study and was a major contributor in
writing and revising the manuscript. MX performed the cell culture,
analyzed and interpreted the data. ZZ performed the reverse
transcription-quantitative polymerase chain reaction and other
molecular biology experiments and revised the manuscript. JC and LZ
made substantial contribution to designing this study and revised
the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to publish
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lagos-Quintana M, Rauhut R, Lendeckel W
and Tuschl T: Identification of novel genes coding for small
expressed RNAs. Science. 294:853–858. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Filipowicz W, Bhattacharyya SN and
Sonenberg N: Mechanisms of post-transcriptional regulation by
microRNAs: Are the answers in sight? Nat Rev Genet. 9:102–114.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Esquela-Kerscher A and Slack FJ:
Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer.
6:259–269. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu CW, Hsiung CA, Lo SS, Hsieh MC, Chen
JH, Li AF, Lui WY and Whang-Peng J: Nodal dissection for patients
with gastric cancer: A randomised controlled trial. Lancet Oncol.
7:309–315. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sharma MR and Schilsky RL: GI cancers in
2010: New standards and a predictive biomarker for adjuvant
therapy. Nat Rev Clin Oncol. 8:70–72. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Smyth EC and Cunningham D: Gastric cancer
in 2012: Defining treatment standards and novel insights into
disease biology. Nat Rev Clin Oncol. 10:73–74. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dassen AE, Lemmens VE, van de Poll-Franse
LV, Creemers GJ, Brenninkmeijer SJ, Lips DJ, Vd Wurff AA, Bosscha K
and Coebergh JW: Trends in incidence, treatment and survival of
gastric adenocarcinoma between 1990 and 2007: A population-based
study in the Netherlands. Eur J Cancer. 46:1101–1110. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
GASTRIC (Global Advanced/Adjuvant Stomach
Tumor Research International Collaboration) Group, . Oba K,
Paoletti X, Bang YJ, Bleiberg H, Burzykowski T, Fuse N, Michiels S,
Morita S, Ohashi Y, et al: Role of chemotherapy for
advanced/recurrent gastric cancer: An individual-patient-data
meta-analysis. Eur J Cancer. 49:1565–1577. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang TH, Yeh CT, Ho JY, Ng KF and Chen TC:
OncomiR miR-96 and miR-182 promote cell proliferation and invasion
through targeting ephrinA5 in hepatocellular carcinoma. Mol
Carcinog. 55:366–375. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang J, Kong X, Li J, Luo Q, Li X, Shen
L, Chen L and Fang L: miR-96 promotes tumor proliferation and
invasion by targeting RECK in breast cancer. Oncol Rep.
31:1357–1363. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guo H, Li Q, Li W, Zheng T, Zhao S and Liu
Z: MiR-96 downregulates RECK to promote growth and motility of
non-small cell lung cancer cells. Mol Cell Biochem. 390:155–160.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu S, Lu Z, Liu C, Meng Y, Ma Y, Zhao W,
Liu J, Yu J and Chen J: miRNA-96 suppresses KRAS and functions as a
tumor suppressor gene in pancreatic cancer. Cancer Res.
70:6015–6025. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Y, Luo H, Li Y, Chen T, Wu S and Yang
L: hsa-miR-96 up-regulates MAP4K1 and IRS1 and may function as a
promising diagnostic marker in human bladder urothelial carcinomas.
Mol Med Rep. 5:260–265. 2012.PubMed/NCBI
|
|
14
|
Wu L, Pu X, Wang Q, Cao J, Xu F, Xu LI and
Li K: miR-96 induces cisplatin chemoresistance in non-small cell
lung cancer cells by downregulating SAMD9. Oncol Lett. 11:945–952.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin H, Dai T, Xiong H, Zhao X, Chen X, Yu
C, Li J, Wang X and Song L: Unregulated miR-96 induces cell
proliferation in human breast cancer by downregulating
transcriptional factor FOXO3a. PLoS One. 5:e157972010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Haflidadóttir BS, Larne O, Martin M,
Persson M, Edsjö A, Bjartell A and Ceder Y: Upregulation of miR-96
enhances cellular proliferation of prostate cancer cells through
FOXO1. PLoS One. 8:e724002013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bazzoni F, Rossato M, Fabbri M, Gaudiosi
D, Mirolo M, Mori L, Tamassia N, Mantovani A, Cassatella MA and
Locati M: Induction and regulatory function of miR-9 in human
monocytes and neutrophils exposed to proinflammatory signals. Proc
Natl Acad Sci USA. 106:5282–5287. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xie XQ, Zhao QH, Wang H and Gu KS:
Dysregulation of mRNA profile in cisplatin-resistant gastric cancer
cell line SGC7901. World J Gastroenterol. 23:1189–1202. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Machida S, Spangenburg EE and Booth FW:
Forkhead transcription factor FoxO1 transduces insulin-like growth
factor's signal to p27Kip1 in primary skeletal muscle satellite
cells. J Cell Physiol. 196:523–531. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu Z, Liu K, Wang Y, Xu Z, Meng J and Gu
S: Upregulation of microRNA-96 and its oncogenic functions by
targeting CDKN1A in bladder cancer. Cancer Cell Int. 15:1072015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang XL, Shi HJ, Wang JP, Tang HS, Wu YB,
Fang ZY, Cui SZ and Wang LT: MicroRNA-218 is upregulated in gastric
cancer after cytoreductive surgery and hyperthermic intraperitoneal
chemotherapy and increases chemosensitivity to cisplatin. World J
Gastroenterol. 20:11347–11355. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vishwamitra D, Li Y, Wilson D, Manshouri
R, Curry CV, Shi B, Tang XM, Sheehan AM, Wistuba II, Shi P and Amin
HM: MicroRNA 96 is a post-transcriptional suppressor of anaplastic
lymphoma kinase expression. Am J Pathol. 180:1772–1780. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hong Y, Liang H, Uzair-Ur-Rehman, Wang Y,
Zhang W, Zhou Y, Chen S, Yu M, Cui S, Liu M, et al: miR-96 promotes
cell proliferation, migration and invasion by targeting PTPN9 in
breast cancer. Sci Rep. 6:374212016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen RX, Xia YH, Xue TC and Ye SL:
Suppression of microRNA-96 expression inhibits the invasion of
hepatocellular carcinoma cells. Mol Med Rep. 5:800–804.
2012.PubMed/NCBI
|
|
26
|
Han S and Wei W: Camptothecin induces
apoptosis of human retinoblastoma cells via activation of FOXO1.
Curr Eye Res. 36:71–77. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang H, Regan KM, Lou Z, Chen J and
Tindall DJ: CDK2-dependent phosphorylation of FOXO1 as an apoptotic
response to DNA damage. Science. 314:294–297. 2006. View Article : Google Scholar : PubMed/NCBI
|