Introduction
Immune cells exist in many types of tissues to
protect host cells from inflammatory factors (1,2). It is now
clear that the infiltration of various inflammatory factors and
disease progression may cause cancer. For example, chronic
inflammation caused by Helicobacter pylori and hepatitis
virus and other microbial infections may induce the occurrence of
gastric and liver cancer (3,4). In addition, non-infectious chronic
inflammation is also closely correlated with the development of
tumors (5). Inflammation is caused by
the secretion of a variety of cytokines by innate immune cells, and
the infiltration of cytokines is associated with poor prognosis of
cancer patients (6). Some studies
suggest that these inflammatory factors may be directly involved in
tumor development and metastasis by inducing angiogenesis and
tissue remodeling (7).
As a member of the proinflammatory cytokine family
receptor, interleukin-23 receptor (IL-23R) is formed by p19 and p40
subunits. IL-23 can induce the production of Th1 cells and then
initiate Th17 cell cascade to produce IL-6 and TGF-β1 and activate
memory T cells (8). IL-23R is highly
expressed in tumor tissue to induce local inflammation and promote
the development of tumors (9). IL-17
plays a key role in inflammation and autoimmune diseases, including
inflammatory bowel disease, multiple sclerosis and rheumatoid
arthritis (10). Contradictory
findings also exist and some studies found that IL-17 supported
tumor growth (11). In contrast,
other studies have shown that IL-17 can promote T cell-mediated
tumor rejection (12). Study found
that IL-23 could induce IL-17 production by CD4 T cells (13). In addition, blocking the IL-23/IL-17
axis significantly inhibited the development of inflammatory bowel
disease in animal models. These studies suggest that IL-23/IL-17
axis may be a new therapeutic target for the treatment of chronic
inflammatory diseases (14).
Although the roles of IL-23R and IL-17 have been
extensively studied in autoimmunity, studies on their roles in
cancer, especially urinary bladder carcinoma (UBC), are still
relatively insufficient. In this study, levels of IL-23R and IL-17
in tumor tissue of patients with UBC and the effects on the
prognosis of patients were investigated. Our study provided
theoretical basis for clinical diagnosis and treatment of UBC.
Patients and methods
Clinical data
In the present study, 30 patients with UBC were
included. The patients included 14 females and 16 males, with an
average age of 58.3 years. All specimens were diagnosed by
pathological examinations. According to the classification criteria
established by the World Health Organization (1973), clinical stage
of each tumor was determined using histological grading, and there
were 5 patients (16.7%) in grade I, 9 in grade II (30%), and 16 in
grade III (53.3%). Based on radiographic and pathologic findings,
TNM staging was performed using the criteria established by
American Joint Committee on Cancer (AJCC). Tumor tissues and
adjacent healthy tissues were collected during surgical resection.
IL-23R and IL-17 mRNA expression level in tumor tissue of 30 UBC
patients were detected by RT-PCR. According to the median
expression levels of IL-23R and IL-17 mRNA, patients were divided
into IL-23R and IL-17 high expression and low expression groups,
respectively. Follow-up study was performed for ~30 months (median
length of 23 months) by phone or during patients' visit to
out-patient department. After surgery, disease progression was
observed in 10 patients, therefore, transurethral surgery was done,
and intravesical instillation was performed for 8 weeks. Systemic
chemotherapy was performed using methotrexate, cisplatin,
doxorubicin and vinblastine. This study was approved by the Ethics
Committee of Zhengzhou Central Hospital Affiliated to Zhengzhou
University (Zhengzhou, China) and all participants in the study
signed an informed consent.
RT-qPCR
Total RNA was extracted using RNA extraction kit
(Qiagen China Co., Ltd., Shanghai, China) according to the
manufacturer's instructions, and 1 µg of total RNA was reversely
transcribed into cDNA. RT-qPCR was performed using the
SYBR® Premix Ex Taq™ II kit (Takara Biotechnology Co.,
Ltd., Dalian, China). PCR conditions: 95°C for 1 min, followed by
40 cycles of 95°C for 10 sec and 55°C for 40 sec. Ct values were
processed using 2−ΔCt method (15). With GAPDH as endogenous control,
relative expression level of each gene was calculated using the
following formula: 2−ΔCt [ΔCt = Cq (target gene) - Cq
(GAPDH)]. Primer sequences are as follows:
5′-CTATGCGGTACTCATATCGCAG-3′ (forward) and
5′-GGTTGTATCAATGAATTCC-3′ (reverse) for IL-17;
5′-CTTTGAGGAGTTCATATTGTA-3′ (forward) and
5′-AAATTAGCCAGATTGTGGTCT-3′ (reverse) for IL-23R;
5′-ATTGATGGATGCTAFGAGTATT-3′ (forward) and
5′-AGTCTTCTGGGTGGCAGTGAT-3′ (reverse) for GAPDH. This experiment
was repeated three times.
ELISA to measure serum levels of
IL-23R and IL-17
Whole blood (25 ml) was collected from UBC patients
and healthy subjects before operation, and the blood was
centrifuged at 1,000 × g for 1 h at room temperature to collect
serum. Standard sample was diluted with a ratio of 1:50 to draw
standard curve. ELISA kit was provided by R&D Systems, Inc.,
(Minneapolis, MN, USA). Protein levels of IL-17 (no. P5326; New
York, USA) and IL-23R (no. P43432) in serum samples were measured
according to the instructions. This experiment was repeated three
times to obtain the average value.
Statistical analysis
Results were analyzed using GraphPad Prism software
(version 5.01; GraphPad Software, Inc., San Diego, Chile). The data
are presented as mean ± standard deviation. Differences between the
two groups were compared using independent sample t-test.
Chi-square test was used to analyze the relationship between IL-23R
and IL-17 protein levels and clinical parameters of UBC patients.
Kaplan-Meier method was used to plot the patient's survival curve
and survival curves were compared using log-rank test. The
relationship between the expression of IL-23R, IL-17 and the
clinical index of UBC was analyzed by COX risk ratio model. Roc
curve was used for detecting the diagnostic efficiency of IL-23,
IL-17 and combined detection. P<0.05 was considered to indicate
a statistically significant difference.
Results
Expression levels of IL-23R and IL-17
detected by RT-PCR
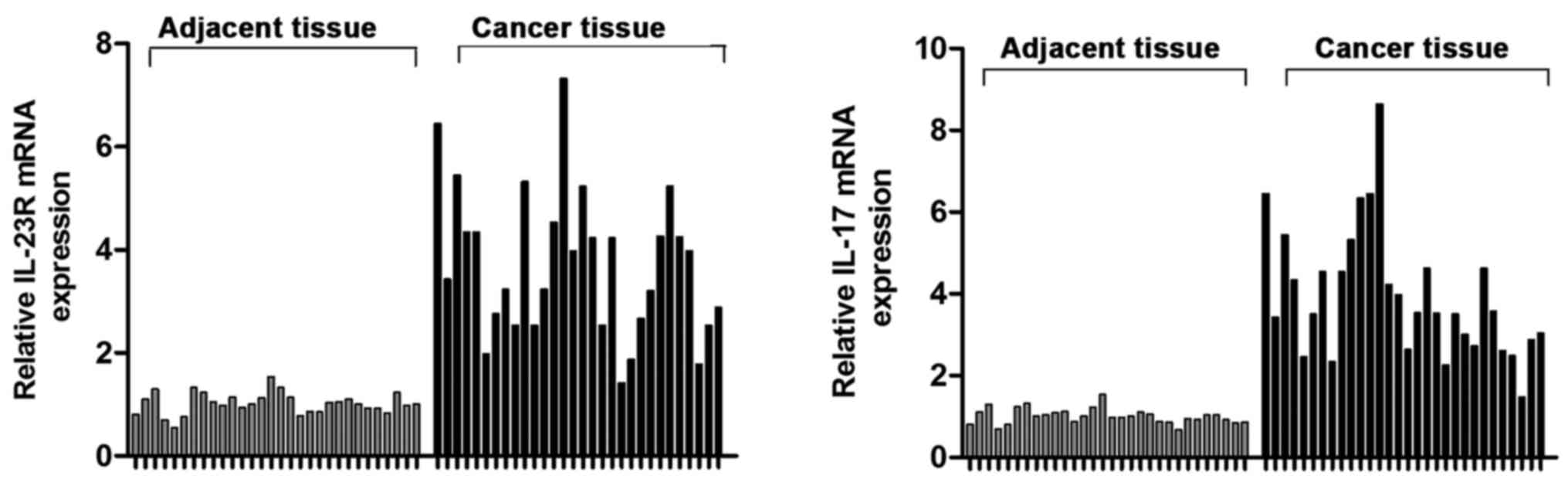
Expression levels of IL-23R and IL-17 mRNAs in tumor
tissues and adjacent tissues of 30 UBC patients were detected by
RT-PCR. Results showed that IL-23R and IL-17 mRNA levels in tumor
tissues were 3.26 and 2.65 times higher than those in adjacent
tissues (Fig. 1), respectively
(P<0.05).
Serum levels of IL-23R and IL-17
detected by ELISA
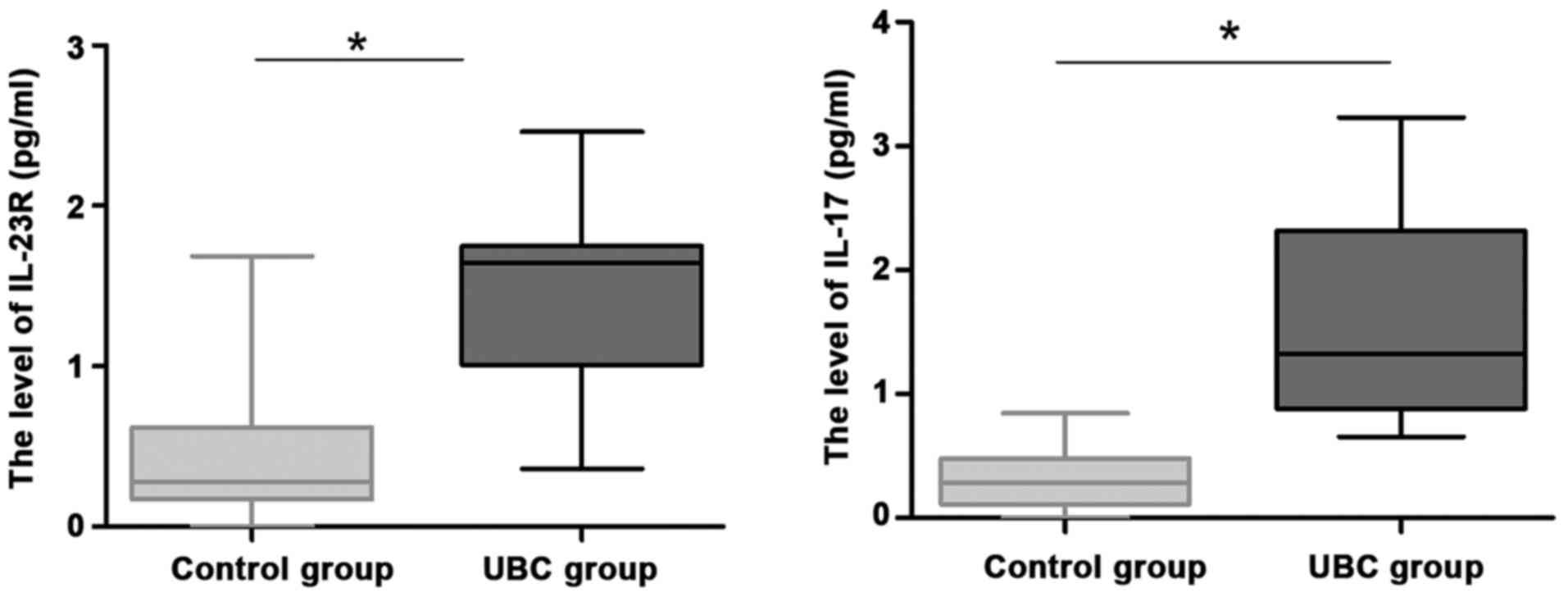
To further explore the role of IL-23R and IL-17 in
UBC, we recruited 30 healthy volunteers as controls. Serum levels
of IL-23R and IL-17 protein in patients were detected by ELISA.
Levels of IL-23R and IL-17 in serum of UBC patients were
significantly higher than those in control group (Fig. 2; P<0.05).
Relationship between IL-23R and IL-17
expression and clinicopathological features
Chi-square test was used to analyze the relationship
between IL-23R and IL-17 protein levels and clinical parameters of
UBC patients. As shown in Table I,
expression levels of IL-23R and IL-17 were not associated with sex,
age, tumor size and pathological grade (P>0.05), but was
significantly associated with clinical stage and lymph node
metastasis (P<0.05).
 | Table I.Relationship between IL-23R and IL-17
expression and clinicopathological features of UBC patients using
Chi-square test. |
Table I.
Relationship between IL-23R and IL-17
expression and clinicopathological features of UBC patients using
Chi-square test.
|
|
| IL-23R |
|
| IL-17 |
|
|---|
| Clinicopathological
features | No. | Low (11) | High (19) | P-value | No. | Low (10) | High (20) | P-value |
|---|
| Sex |
| Male | 16 | 5 | 11 | 0.073 | 17 | 6 | 11 | 0.846 |
|
Female | 14 | 6 | 8 |
| 13 | 4 | 9 |
|
| Age (years) |
| ≤58 | 15 | 7 | 8 | 0.242 | 11 | 5 | 6 | 0.319 |
|
>58 | 15 | 4 | 11 |
| 19 | 5 | 14 |
|
| Tumor size (cm) |
| ≤3 | 18 | 6 | 12 | 0.054 | 16 | 3 | 13 | 0.544 |
|
>3 | 12 | 5 | 7 |
| 14 | 17 | 7 |
|
| PG |
| I | 5 | 3 | 2 | 0.054 | 4 | 1 | 3 | 0.075 |
| II | 9 | 6 | 3 |
| 10 | 6 | 4 |
|
| III | 16 | 2 | 14 |
| 16 | 3 | 13 |
|
| CS |
|
T0-T1 | 17 | 9 | 8 | 0.034 | 18 | 6 | 12 | 0.043 |
|
T2-T4 | 13 | 2 | 11 |
| 12 | 4 | 8 |
|
| LM |
| No | 12 | 6 | 6 | 0.035 | 14 | 4 | 10 | 0.046 |
| Yes | 18 | 5 | 13 |
| 16 | 6 | 10 |
|
Cox hazard model analysis
Relationship between IL-23R and IL-17 expression and
clinical parameters of UBC patients was analyzed using Cox hazard
model analysis. As shown in Table
II, sex, age, tumor size and pathological grade were not
independent prognostic risk factors for UBC (P>0.05), so they
have no significantly clinical correction with the prognosis of
UBC. While clinical stage, lymph node metastasis, the levels of
IL-23R and IL-17 were independent prognostic risk factor for UBC
(P<0.05), which were closely related to poor prognosis of
patients with UBC.
 | Table II.Results of Cox hazard model
analysis. |
Table II.
Results of Cox hazard model
analysis.
| Items | Regression
coefficient (B) | SE | Wald test | Degrees of
freedom | P-value | RR | 95.0% confidence
interval |
|---|
| Sex | 1.073 | 0.263 | 1.342 | 1 | 0.302 | 0.872 | 0.543–1.378 |
| Age | 0.873 | 0.211 | 0.892 | 1 | 0.236 | 1.237 | 0.915–1.528 |
| Tumor size | 0.462 | 0.376 | 1.773 | 1 | 0.092 | 1.152 | 0.842–1.352 |
| PG | 1.0832 | 0.353 | 2.421 | 2 | 0.517 | 1.037 | 0.884–1.379 |
| CS | 0.542 | 0.426 | 8.342 | 1 | 0.044 | 2.603 | 1.741–2.905 |
| LM | 0.782 | 0.284 | 10.261 | 1 | 0.009 | 2.154 | 2.035–2.553 |
| IL-23R level | 0.882 | 0.118 | 7.231 | 1 | 0.011 | 3.021 | 2.648–3.527 |
| IL-17 level | 1.245 | 0.205 | 11.528 | 1 | 0.006 | 2.604 | 2.184–3.017 |
Correlation between IL-23R and IL-17
protein expression and prognosis of UBC patients
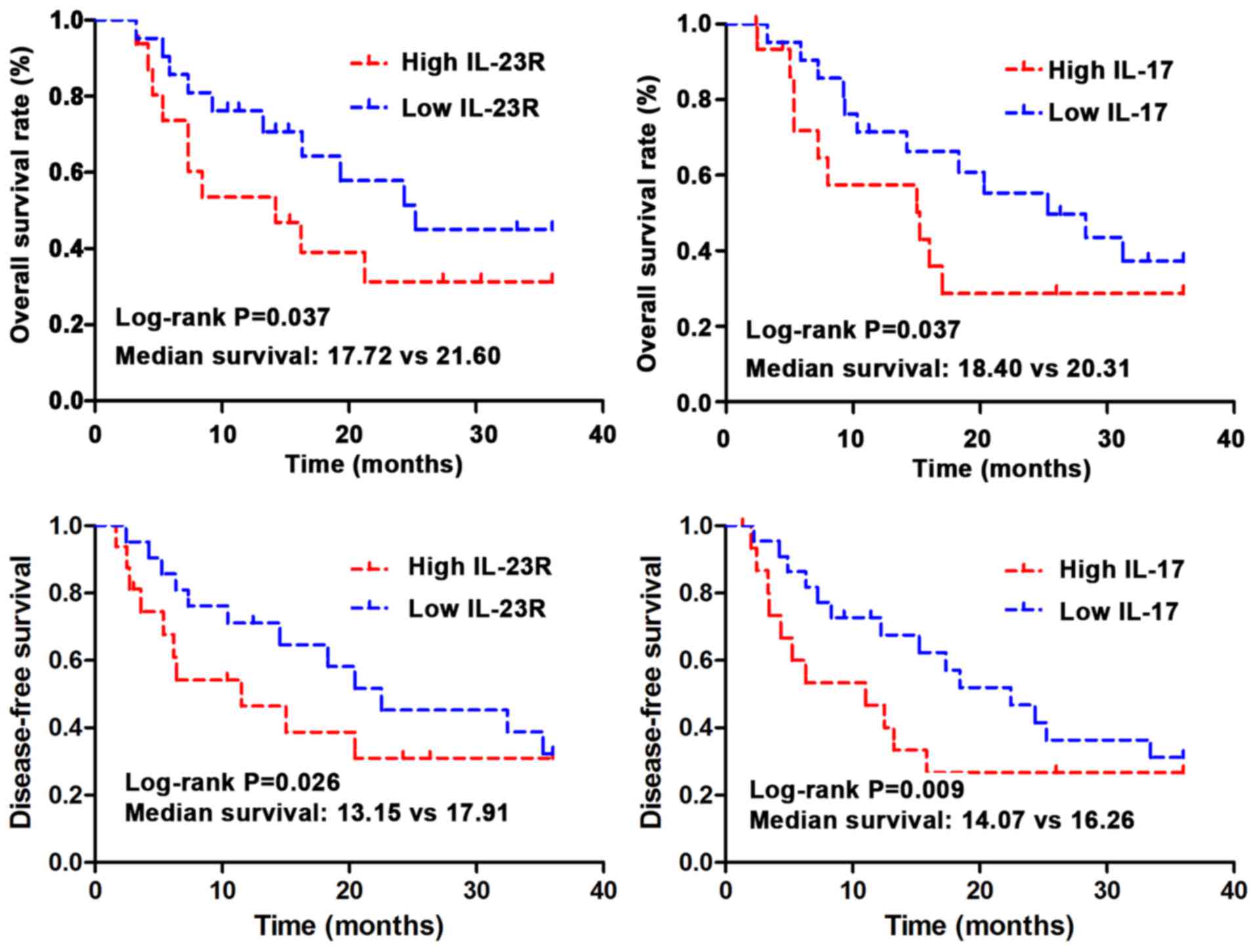
Kaplan-Meier method was used to investigate the
correlations between expression of IL-23R and IL-17 and the overall
survival (OS) time and disease-free survival (DFS). The data were
analyzed using log-rank test. As shown in Fig. 3, OS and DFS is significantly shorter
in IL-23R and IL-17 high expression group than those in low
expression group (P<0.05). Median OS was 20.31 months in IL-17
low expression group, and 18.40 months in IL-17 high expression
group (log-rank test, P=0.037). Median OS was 21.60 months in
IL-23R low expression group, and 17.72 months in IL-23R high
expression group (log-rank test, P=0.037). Median DFS was 16.26
months in IL-17 low expression group, and 14.07 months in IL-17
high expression group (log-rank test, P=0.009). Median DFS was
17.91 months in IL-23R low expression group, and 13.15 months in
IL-23R high expression group (log-rank test, P=0.026).
ROC curve to analyze the diagnostic
values of IL-23R and IL-17 protein levels for UBC
ROC curve was applied to analyze the diagnostic
values of IL-23R and IL-17 protein levels for UBC. As shown with
ROC curve (Table III) serum IL-23R
and IL-17 protein levels can be used to accurately and effectively
diagnose UBC (P<0.05). Serum IL-23R and IL-17 protein levels can
be applied in the clinical stage and the forecast of lymph node
metastasis of UBC (P<0.05), and in the clinical stage, area
under ROC curve of serum IL-17 protein level was larger than that
of serum IL-23R protein level, so serum IL-17 protein level was
more significantly significant in the clinical stage of UBC.
However, area under ROC curve of serum IL-23R protein level in the
forecast of lymph node metastasis was larger than that of serum
IL-17 protein level, so serum IL-23R protein level was more
obviously significant in the forecast of lymph node metastasis of
UBC.
 | Table III.Effect of IL-23R and IL-17 protein
levels on parameters of clinical staging and lymph node prediction
in UBC patients. |
Table III.
Effect of IL-23R and IL-17 protein
levels on parameters of clinical staging and lymph node prediction
in UBC patients.
| Items | Proteins | Area under ROC
curve | Area standard
error | P-value | 95% confidence
interval |
|---|
| CS | IL-23R | 0.639 | 0.055 | 0.011 | 0.009–0.032 |
|
| IL-17 | 0.740 | 0.043 | 0.008 | 0.003–0.683 |
| LM | IL-23R | 0.836 | 0.084 | 0.028 | 0.016–0.053 |
|
| IL-17 | 0.694 | 0.105 | 0.013 | 0.007–0.063 |
Analysis of diagnostic efficiency of
IL-23R combined with IL-17 for UBC
The diagnostic sensitivity and specificity of serum
IL-23R + IL-17 for UBC patients were significantly higher than
those of IL-23R or IL-17 alone (Table
IV).
 | Table IV.Diagnostic efficiency of IL-23R
combined with IL-17 for UBC. |
Table IV.
Diagnostic efficiency of IL-23R
combined with IL-17 for UBC.
| Detection
index | Specificity
(%) | Sensitivity
(%) | Positive predictive
value | Negative predictive
value |
|---|
| IL-23R | 75.3 | 38.3 | 75.4 | 38.5 |
| IL-17 | 83.8 | 53.7 | 80.4 | 48.3 |
| IL-23R + IL-17 | 89.4a,b | 62.5a,b | 87.9 | 45.7 |
Discussion
Inflammation is considered to be an important factor
in the progression of tumors. Studies have shown that
proinflammatory cytokines can promote the proliferation of
different tumor cells and tumor-related white blood cells, so as to
play an important role in inhibiting tumor regeneration (16). But at the same time proinflammatory
cytokines have also been proven to contribute to tumor growth and
proliferation. For example, IL-1 and IL-6 can induce tumor cell
proliferation and prolong tumor cell survival (17). IL-17 is mainly produced and secreted
by activated CD4 T cells and CD8 T cells. In addition to
proinflammatory reactions, IL-17 can also regulate the formation of
tight junctions of cells (18).
IL-17-positive cells have been observed in prostate and
hepatocellular carcinoma. IL-17 plays a role in promoting tumor
regeneration and increasing the invasive ability of tumors in
cervical cancer (19). In addition,
IL-17 is also highly expressed in ovarian cancer and colorectal
cancer to play a role in promoting tumor angiogenesis (20). Bagheri et al reported that
IL-17 had important functions in T cell-mediated angiogenesis
(21). Infiltration of
proinflammatory cytokines is considered to be the growth promoter
of UBC. Recently, IL-17 has been shown to increase the production
of active metalloproteinase-9, so as to promote angiogenesis and
tumor growth (22). Therefore, IL-17
may also have important functions in UBC.
IL-23 and IL-23R subunits make up a new complex of
IL-12RB1 called IL-23R10. These two cytokines are mainly expressed
by activated dendritic cells and phagocytic cells. IL-23R is mainly
expressed in T cells, natural killer cells and natural killer T
cells (23). Recently, expression of
IL-23R was detected in macrophages and dendritic cells in human and
mouse tumor tissues. Studies also found that IL-23 induced the
expression of IL-17 and matrix metalloproteinase, while
IL-23R-deficient mice showed reduced tumor growth. In addition,
with the progress of tumor, the number of Th17 cells gradually
increased, and levels of IL-23R and its complex also gradually
increased (24).
Chronic inflammation plays pivotal roles in cancer
and autoimmune diseases. We hypothesized that IL-17 and IL-23 may
also play important roles in UBC. To verify this, RT-PCR and ELISA
were used to detect the expression of IL-23R in tumor tissue and
serum of patients with UBC. Results showed that levels of IL-23R
and IL-17 mRNAs were higher in tumor tissue than in adjacent
tissue. In addition, levels of IL-23R and IL-17 in serum of
patients with UBC were also higher than those in normal control
group. Correlation analysis suggested that IL-23R and IL-17 protein
levels were correlated with clinical stage and lymphatic
metastasis. Further Cox hazard model analysis showed that IL-23R
and IL-17 may be independent factors for UBC. In addition, protein
levels of IL-23R and IL-17 in serum of UBC patients can be used to
effectively predict clinical stage and lymphatic metastasis of UBC.
The sensitivity and specificity of combined diagnosis using both
IL-23R and IL-17 were higher than those of diagnosis using IL-23R
or IL-17 alone. In conclusion, we found that IL-23R and IL-17 were
upregulated in UBC tumor tissue, and the increased expression
levels were associated with poor prognosis. IL-23R and IL-17 may
serve as potential diagnostic and prognostic indicators for
UBC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL conceived and designed the study. JL and LW were
responsible for the collection and analysis of the patient data. TW
and JW interpreted the data and drafted the manuscript. JL and JW
revised the manuscript critically for important intellectual
content. All authors read and approved the final study.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Zhengzhou Central Hospital Affiliated to Zhengzhou University,
(Zhengzhou, China). Signed informed consents were obtained from the
patients or the guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pernot S, Terme M, Voron T, Colussi O,
Marcheteau E, Tartour E and Taieb J: Colorectal cancer and
immunity: What we know and perspectives. World J Gastroenterol.
20:3738–3750. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ikemoto T, Shimada M, Ishikawa D, Teraoku
H, Yoshikawa M, Yamada S, Takasu C, Saito Y, Morine Y and Imura S:
The clinical impact of CD4+ CD49b+ regulatory
T cells addition to FOXp3+ regulatory T cells in cancer
patients' immunity. J Am Coll Surg. 221:e222015. View Article : Google Scholar
|
|
3
|
Zhong Z, Sanchez-Lopez E and Karin M:
Autophagy, inflammation, and immunity: A troika governing cancer
and its treatment. Cell. 166:288–298. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chang WJ, Du Y, Zhao X, Ma LY and Cao GW:
Inflammation-related factors predicting prognosis of gastric
cancer. World J Gastroenterol. 20:4586–4596. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shalapour S and Karin M: Immunity,
inflammation, and cancer: An eternal fight between good and evil. J
Clin Invest. 125:3347–3355. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tran E, Turcotte S, Gros A, Robbins PF, Lu
YC, Dudley ME, Wunderlich JR, Somerville RP, Hogan K, Hinrichs CS,
et al: Cancer immunotherapy based on mutation-specific
CD4+ T cells in a patient with epithelial cancer.
Science. 344:641–645. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen DS and Mellman I: Oncology meets
immunology: The cancer-immunity cycle. Immunity. 39:1–10. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yan J, Smyth MJ and Teng MWL: Interleukin
(IL)-12 and IL-23 and their conflicting roles in cancer. Cold
Spring Harb Perspect Biol. Jul 17–2017.(Epub ahead of print).
|
|
9
|
Stanilov N, Miteva L, Deliysky T, Jovchev
J and Stanilova S: Advanced colorectal cancer is associated with
enhanced IL-23 and IL-10 serum levels. Lab Med. 41:159–163. 2010.
View Article : Google Scholar
|
|
10
|
Coffelt SB, Kersten K, Doornebal CW,
Weiden J, Vrijland K, Hau CS, Verstegen NJM, Ciampricotti M,
Hawinkels LJAC, Jonkers J, et al: IL-17-producing γδ T cells and
neutrophils conspire to promote breast cancer metastasis. Nature.
522:345–348. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin Q, Xue L, Tian T, Zhang B, Guo L, Lin
G, Chen Z, Fan K and Gu X: Prognostic value of serum IL-17 and VEGF
levels in small cell lung cancer. Int J Biol Markers. 30:e359–e363.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fabre J, Giustiniani J, Garbar C,
Antonicelli F, Merrouche Y, Bensussan A, Bagot M and Al-Dacak R:
Targeting the tumor microenvironment: The protumor effects of IL-17
related to cancer type. Int J Mol Sci. 17:E14332016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Iwakura Y and Ishigame H: The IL-23/IL-17
axis in inflammation. J Clin Invest. 116:1218–1222. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liang SC, Tan XY, Luxenberg DP, Karim R,
Dunussi-Joannopoulos K, Collins M and Fouser LA: Interleukin
(IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively
enhance expression of antimicrobial peptides. J Exp Med.
203:2271–2279. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sica A, Allavena P and Mantovani A: Cancer
related inflammation: The macrophage connection. Cancer Lett.
267:204–215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Grivennikov S, Karin E, Terzic J, Mucida
D, Yu GY, Vallabhapurapu S, Scheller J, Rose-John S, Cheroutre H,
Eckmann L, et al: IL-6 and Stat3 are required for survival of
intestinal epithelial cells and development of colitis-associated
cancer. Cancer Cell. 15:103–113. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamaguchi Y, Fujio K, Shoda H, Okamoto A,
Tsuno NH, Takahashi K and Yamamoto K: IL-17B and IL-17C are
associated with TNF-alpha production and contribute to the
exacerbation of inflammatory arthritis. J Immunol. 179:7128–7136.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Punt S, Fleuren GJ, Kritikou E, Lubberts
E, Trimbos JB, Jordanova ES and Gorter A: Angels and demons: Th17
cells represent a beneficial response, while neutrophil IL-17 is
associated with poor prognosis in squamous cervical cancer.
OncoImmunology. 4:e9845392015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Straus DS: TNFα and IL-17 cooperatively
stimulate glucose metabolism and growth factor production in human
colorectal cancer cells. Mol Cancer. 12:782013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bagheri N, Azadegan-Dehkordi F, Shirzad H,
Rafieian-Kopaei M, Rahimian G and Razavi A: The biological
functions of IL-17 in different clinical expressions of
Helicobacter pylori-infection. Microb Pathog. 81:33–38.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li L and Boussiotis VA: The role of
IL-17-producing Foxp3+CD4+ T cells in
inflammatory bowel disease and colon cancer. Clin Immunol.
148:246–253. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Helbling M, Lukesch A, Haimovici A,
Karamitopoulou E, Berger MD, Hädrich M, Mallaev M, Schnüriger B,
Koelzer VH, Dawson H, et al: Investigation of IL-23 (p19, p40) and
IL-23R identifies nuclear expression of IL-23 p19 as a favorable
prognostic factor in colorectal cancer: A retrospective multicenter
study of 675 patients. Oncotarget. 5:4671–4682. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen G, Liang Y, Guan X, Chen H, Liu Q,
Lin B, Chen C, Huang M, Chen J, Wu W, et al: Circulating low IL-23:
IL-35 cytokine ratio promotes progression associated with poor
prognosisin breast cancer. Am J Transl Res. 8:2255–2264.
2016.PubMed/NCBI
|