Introduction
Gastric cancer is rampant in many countries around
the world (1). According to Globocan
2012, gastric cancers rank as the 4th most common malignancy,
accounting for more than 6.8% of adult malignancies; and the third
leading cause of cancer death worldwide, accounting for more than
8.8% of adult malignancie; especially in China, gastric cancer
causing approximately 325,000 deaths per year (2). It's well known that prognosis of gastric
cancer remains poor due to the lack of effective therapies in
patients with advanced cancer (2,3).
Immunotherapy has become one of the most promising
treatments in gastric cancer, especially immunotherapy targeting
the immune checkpoints programmed cell death protein-1 (PD-1) and
programmed death-ligand-1 (PD-L1) (4). PD-1, a cluster of differentiation (CD)28
family member, is an immunosuppressive receptor expressed on T
cells, B cells, monocytes, natural killer cells (NKs) and many
tumor-infiltrating lymphocytes (TILs) (5,6). Previous
studies have shown that high-PD-1/CD4 ratio was associated with
poor prognosis in NSCLC patients (7);
high proportion of PD-1+CD4+ T cells in the
peripheral blood cells of PDAC patients was correlated to
chemotherapy resistance (8); high
surface expression of PD-1+CD8+ T cells
confered worse relapse-free and overall survival (OS) rates in
patients with colorectal cancer (9);
an overall low PD-1 and a concurrent high CD3+ T cells
expression was found in high-risk prostate cancer tissue (10). All these results indicated that the
surface expression of PD-1 on T-cells was tightly associated with
the prognosis of cancer patients.
Recent studies have confirmed the expression of PD-1
and its relationship with prognosis in gastric cancer patients
(11). In this study, we clarify the
significance of PD-1 expression on peripheral blood T-cell subsets,
then explore the relationships between PD-1 and the
clinicopathological features and prognosis of patients with
metastatic gastric cancer.
Patients and methods
Patients
A total of 100 outpatients with metastatic gastric
cancer met the inclusion criteria from the Zhengzhou University
Affiliated Cancer Hospital (Zhengzhou, China) were enrolled in the
study between May 2015 and May 2016. The inclusion criteria were
listed as follows: i) The diagnosis of metastatic gastric cancer
was confirmed by endoscopic biopsy and imageological examination;
ii) the disease was classified as pathologic M1 with any T and N
stage based on the standard of the WHO Classification of Tumors of
the Digestive System, 2010 edition (12,13); iii)
2 ml peripheral blood was drawn before the first line therapy; iv)
patients had completed 4 courses of first line chemotherapy as
documented in the medical record; and v) complete follow-up data
was available. This study was approved by the ethics committee at
the Zhengzhou University Affiliated Cancer Hospital, and informed
consent form was signed by every patient. The clinical
characteristics of these patients were detailed in Table I.
 | Table I.Association between PD-1 expression
on peripheral blood T-cell subsets and clinicopathological factors
in patients with metastatic gastric cancer. |
Table I.
Association between PD-1 expression
on peripheral blood T-cell subsets and clinicopathological factors
in patients with metastatic gastric cancer.
|
|
|
PD-1+/CD3+ |
| PD-1+/
CD3+CD4+ |
| PD-1+/
CD3+CD8+ |
|
|---|
|
|
|
|
|
|
|
|
|
|---|
|
Characteristics | Case (n=100) | High | Low | P-value | High | Low | P-value | High | Low | P-value |
|---|
| Total | 100 | 42 | 58 | 0.766 | 38 | 62 | 0.574 | 44 | 56 | 0.151 |
|
Male | 65 | 28 | 37 |
| 26 | 39 |
| 32 | 33 |
|
|
Female | 35 | 14 | 21 |
| 12 | 23 |
| 12 | 23 |
|
| Age (years) |
|
|
| 0.623 |
|
| 0.84 |
|
| 0.824 |
|
≥60 | 33 | 15 | 18 |
| 13 | 20 |
| 14 | 19 |
|
|
<60 | 67 | 27 | 40 |
| 25 | 42 |
| 30 | 37 |
|
| KPS score |
|
|
| 0.101 |
|
| 0.096 |
|
| 0.642 |
|
≥80 | 75 | 35 | 40 |
| 32 | 43 |
| 34 | 41 |
|
|
<80 | 25 | 7 | 18 |
| 6 | 19 |
| 10 | 15 |
|
|
Differentiation |
|
|
| 0.06 |
|
| 0.324 |
|
| 0.967 |
|
Well | 18 | 4 | 14 |
| 5 | 13 |
| 8 | 10 |
|
|
Poor | 82 | 38 | 44 |
| 33 | 49 |
| 36 | 46 |
|
| Number of
metastatic organs |
|
|
| 0.463 |
|
| 0.552 |
|
| 0.422 |
|
>2 | 41 | 19 | 22 |
| 17 | 24 |
| 20 | 21 |
|
| ≤2 | 59 | 23 | 36 |
| 21 | 38 |
| 24 | 35 |
|
| Liver
metastasis |
|
|
| 0.654 |
|
| 0.709 |
|
| 0.627 |
|
Present | 45 | 20 | 25 |
| 18 | 27 |
| 21 | 24 |
|
|
Absent | 55 | 22 | 33 |
| 20 | 35 |
| 23 | 32 |
|
| Peritoneal
metastasis |
|
|
| 0.609 |
|
| 0.638 |
|
| 0.594 |
|
Present | 47 | 21 | 26 |
| 19 | 28 |
| 22 | 25 |
|
|
Absent | 53 | 21 | 32 |
| 19 | 34 |
| 22 | 31 |
|
Treatment and response
Oxaliplatin (130 mg/m2 d1, every 3 weeks)
combined with Capecitabine (1,000 mg bid d1-14, every 3 weeks) was
undergone as the first line chemothrapy. Therapeutic efficacy was
evaluated every 2 cylcles of chemotherapy. The follow-up was
performed until July 30, 2017. progression-free survival (PFS) was
calculated from the date of first treatment to the date of
progression or death/censoring. OS was calculated from the date of
first treatment to the date of death or censoring. The therapy
regimen was replaced when the patient emerged progressive
disease.
Preparation of peripheral blood
mononuclear cells (PBMCs)
Approximately 40 ml of peripheral blood was drawn
from the patients on the day before surgery and on the day before
the first line therapy. A Ficoll-Paque (Pharmacia, Uppsala, Sweden)
density gradient was used to centrifuge blood samples.
Flow cytometric analysis
PBMC (1×105) were suspended in PBS
supplemented with 20% human AB serum and incubated on ice with
appropriate dilution of antibodies for 30 min. All antibodies used
in this study including anti-CD3-PE-Cy7, anti-CD4-PerCP-Cy5-5,
anti-CD8-APC, anti-CD279 (PD-1)-FITC, were purchased from BioLegend
(San Diego, CA, USA). APC mouse IgG1(j) (clone MOPC-21), PE-Cy7
mouse IgG2b(j) (clone MPC-11), PerCP-Cy5-5 mouse IgG1(j) (clone
MOPC-21) and FITC mouse IgG2b(j) (clone 27–35; all BioLegend), were
used as isotype controls. Additionally, FMO plus isotype controls
were used to help us gate the negative and positive populations of
CD3, CD4, CD8 and PD-1 (14).
Briefly, when we run flowcytometry, we stained all antibodies to
observe the expression of CD3, CD4, CD8 and PD-1, however, we also
stained CD3, CD4, CD8 and FITC mouse IgG2b as FMO plus isotype
control of PD-1 expression. The similar method was used to
distinguish the negative and positive populations of CD3, CD4, and
CD8. The staining cells were analyzed on BD FACS Canto II with FACS
Diva software (BD Biosciences, Franklin Lakes, NJ, USA).
Statistical analysis
All statistical analyses were performed using SPSS
24.0 (IBM Corp., Armonk, NY USA). Difference between two groups was
analyzed by student t-test, and difference between multiple groups
was analyzed by one way ananlysis of variance with Tukey's post hoc
test. Kaplan-Meier curve with the log rank test was used in
survival analysis. Prognostic factors were examined by univariate
and multivariate analysis on the basis of Cox proportional hazards
model were used for. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of PD-1 on peripheral blood
lymphocytes
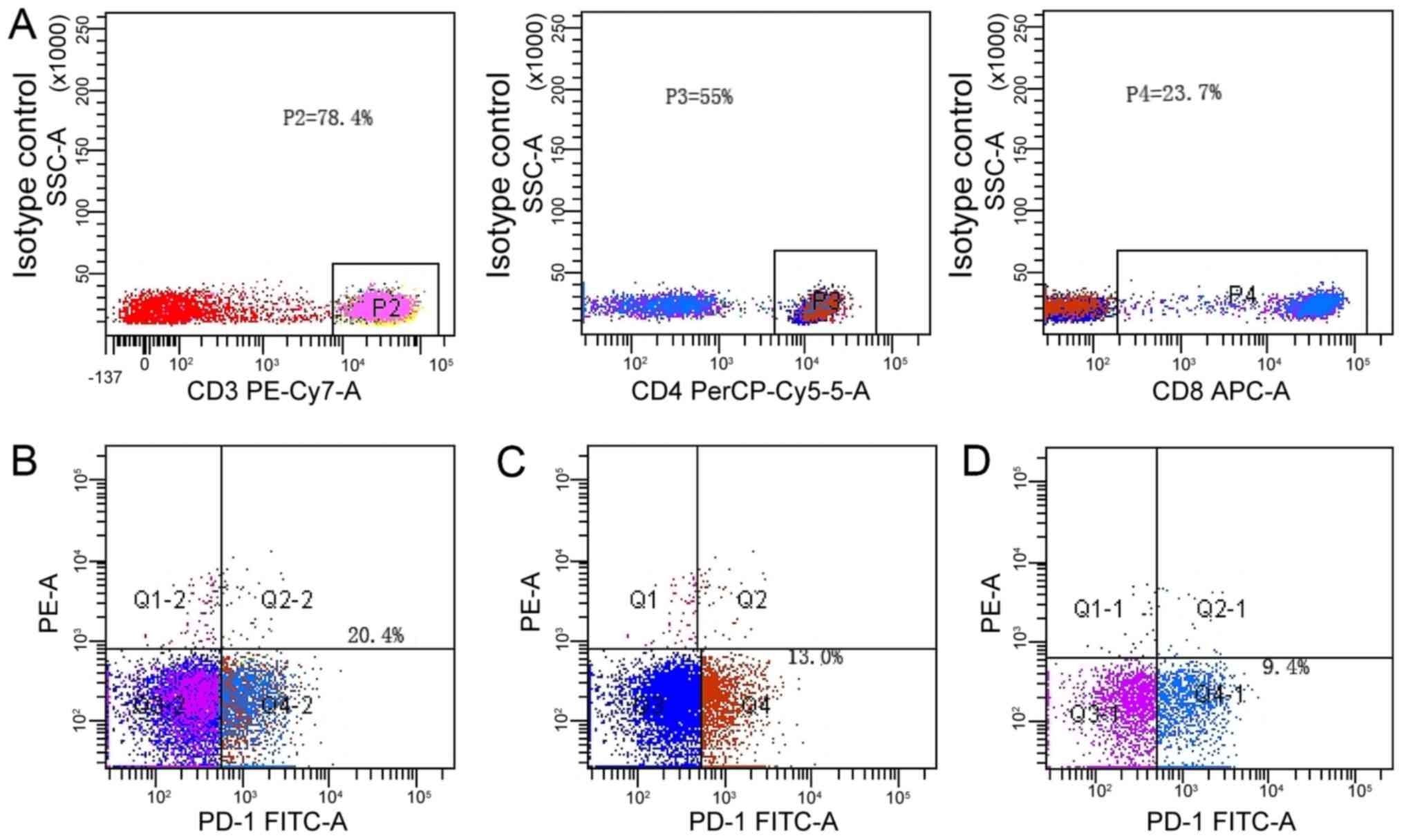
We detected the surface expression of PD-1 on
lymphocytes from the PBMCs of patients with metastatic gastric
cancer, and focused on the expression of PD-1 on CD3+,
CD3+CD4+ and CD3+CD8+
T-cell subsets. The representative staining patterns of PD-1 on
these subsets were shown in Fig. 1.
For further analysis, we defined that lymphocytes with
PD-1+/CD3+ >15%,
PD-1+/CD3+CD4+ >10%, or
PD-1+/CD3+CD8+ >5% showed high
surface PD-1 expression on T-cell subsets by using isotype-matched
control, on the contrary PD-1 expression level is low. The percent
of CD3+, CD3+CD4+ and
CD3+CD8+ T-cells with high surface PD-1
expression were 20.4, 13.0 and 9.4%, respectively.
Relationship between PD-1 expression
on peripheral blood T-cell subsets and clinicopathological factors
in metastatic gastric cancer
The correlation between PD-1 expression on
peripheral blood T-cell subsets and clinicopathological factors of
patients with metastatic gastric cancer was analyzed. However, no
correlation was found between PD-1 expression on peripheral blood
T-cell subsets and clinicopathological factors of patients with
metastatic gastric cancer (all P>0.05) (Table I).
Relationship between PD-1 expression
on peripheral blood T-cell subsets and OS in metastatic gastric
cancer
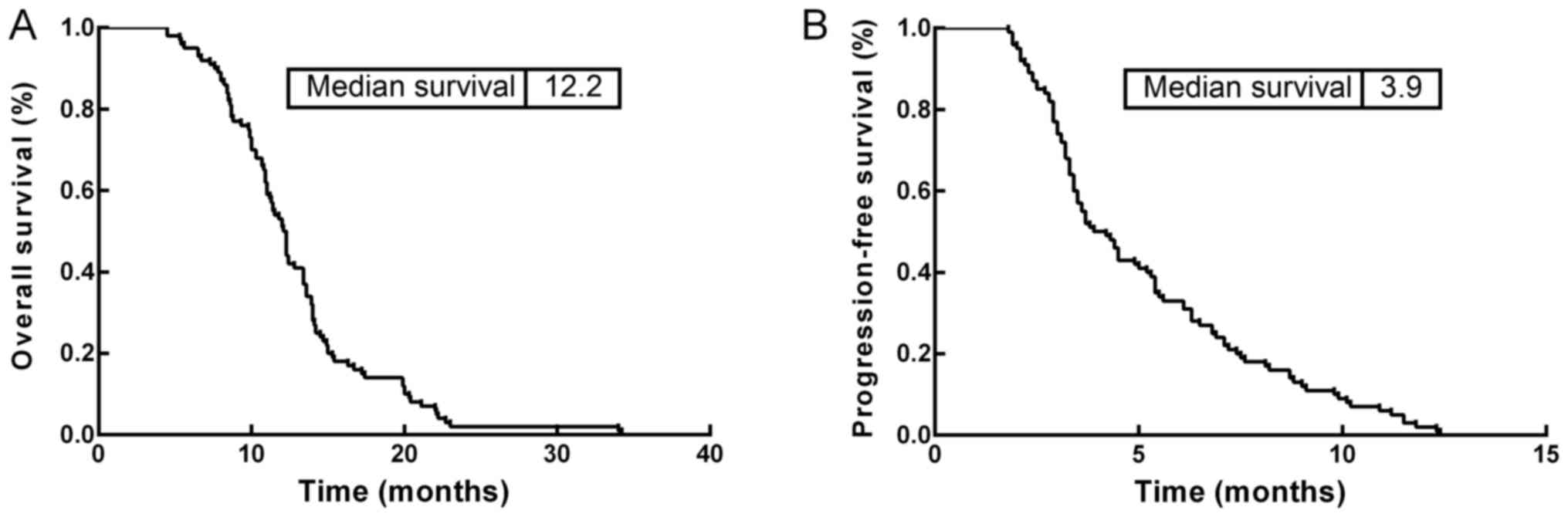
The correlation of PD-1 expression on peripheral
blood T-cell subsets with the prognosis of patients with metastatic
gastric cancer was analyzed. The OS and PFS rate of the 100
patients with metastatic gastric cancer were 12.2 and 3.9 months,
respectively (Fig. 2). The 1-year and
2-year OS rates were 53.0 and 2%, respectively.
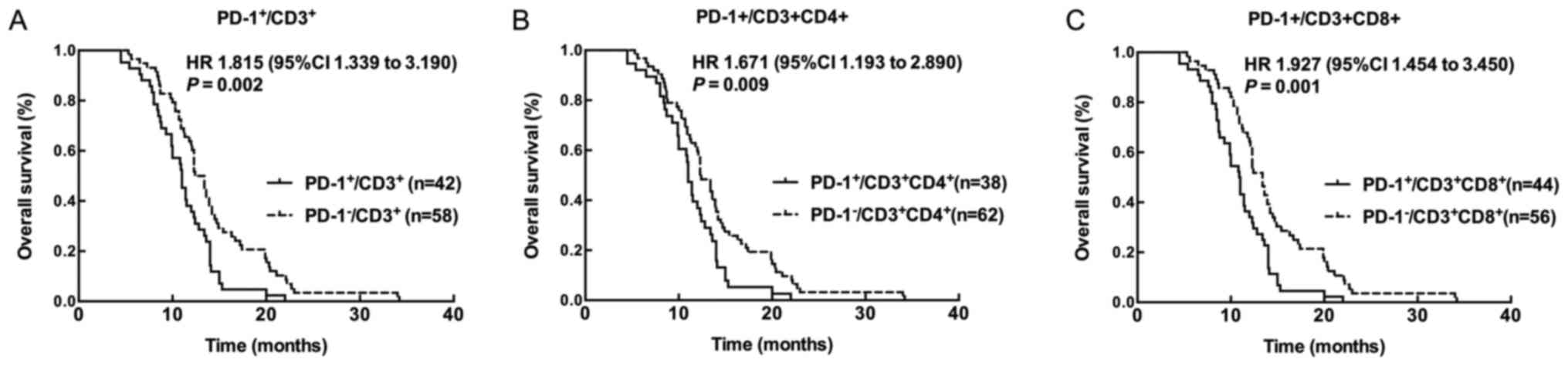
Kaplan-Meier survival analysis revealed that
patients with high PD-1+/CD3+,
PD-1+/CD3+CD4+, and
PD-1+/CD3+CD8+ level showed worse
prognosis compared with patients with low
PD-1+/CD3+,
PD-1+/CD3+CD4+, and
PD-1+/CD3+CD8+ level (all
P<0.05) (Fig. 3). Univariate
analysis was performed to estimate the clinical significances of
various parameters that may affect survival of patients with
metastatic gastric cancer. As shown in Table II, Karnofsky performance score (KPS)
scores [hazard ratio (HR); 2.059; P=0.043], tumor differentiation
(HR, 2.167; P=0.031), number of metastatic organs (HR, 3.041;
P=0.032), liver metastasis (HR, 3.234; P=0.047), peritoneal
metastasis (HR, 2.567; P=0.038), high
PD-1+/CD3+ level (HR, 2.066; P=0.005), high
PD-1+/CD3+CD4+ level (HR, 1.857;
P=0.028), and high PD-1+/CD3+CD8+
level (HR, 1.796; P=0.023) were all predictive factors for
prognosis of patients with metastatic gastric cancer. Then
multivariate analysis based on Cox's proportional hazards model was
performed to determine the independent prognostic factors for
patients with metastatic gastric cancer in our cohort. The results
indicated that high PD-1+/CD3+ (HR, 2.145;
P=0.015), high PD-1+/CD3+CD4+ (HR,
1.866; P=0.034), and high
PD-1+/CD3+CD8+ (HR, 1.817;
P=0.033) level were independent risk factors for predicting the
survival time of metastatic gastric cancer (Table III).
 | Table II.Univariate analysis of
clinicopathological factors for overall survival in gastric
cancer. |
Table II.
Univariate analysis of
clinicopathological factors for overall survival in gastric
cancer.
| Parameters | Hazard ratio | 95% confidence
interval | P-value |
|---|
| Age (≥60 vs. <60
years) | 1.885 | 1.024–3.895 | 0.067 |
| Sex (male vs.
female) | 1.603 | 0.876–4.563 | 0.168 |
| KPS score (≥8 vs.
<80) | 2.059 | 1.556–6.345 | 0.043 |
| Differentiation
(well vs. poor) | 2.167 | 1.543–4.324 | 0.031 |
| Number of
metastatic organs (<2 vs. ≤2) | 3.041 | 2.123–7.342 | 0.032 |
| Liver metastasis
(present vs. absent) | 3.234 | 2.246–9.213 | 0.047 |
| Peritoneal
metastasis (present vs. absent) | 2.567 | 1.526–5.563 | 0.038 |
|
PD-1+/CD3+ (high vs.
low) | 2.066 | 1.311–3.257 | 0.005 |
|
PD-1+/CD3+CD4+
(high vs. low) | 1.857 | 1.171–2.946 | 0.028 |
|
PD-1+/CD3+CD8+
(high vs. low) | 1.796 | 1.159–2.784 | 0.023 |
 | Table III.Multivariate analysis of
clinicopathological factors for disease special survival in gastric
cancer. |
Table III.
Multivariate analysis of
clinicopathological factors for disease special survival in gastric
cancer.
| Parameters | Hazard ratio | 95% confidence
interval | P-value |
|---|
| KPS score (≥8 vs.
<80) | 1.767 | 1.543–6.324 | 0.131 |
| Differentiation
(well vs. poor) | 1.159 | 0.756–4.345 | 0.243 |
| Number of
metastatic organs (>2 vs. ≤2) | 2.041 | 1.123–7.342 | 0.126 |
| Liver metastasis
(present vs. absent) | 2.289 | 2.239–11.213 | 0.147 |
| Peritoneal
metastasis (present vs. absent) | 2.136 | 1.238–8.175 | 0.264 |
|
PD-1+/CD3+ (high vs.
low) | 2.145 | 1.325–5.232 | 0.015 |
|
PD-1+/CD3+CD4+
(high vs. low) | 1.866 | 1.273–3.243 | 0.034 |
|
PD-1+/CD3+CD8+
(high vs. low) | 1.817 | 1.099–3.675 | 0.033 |
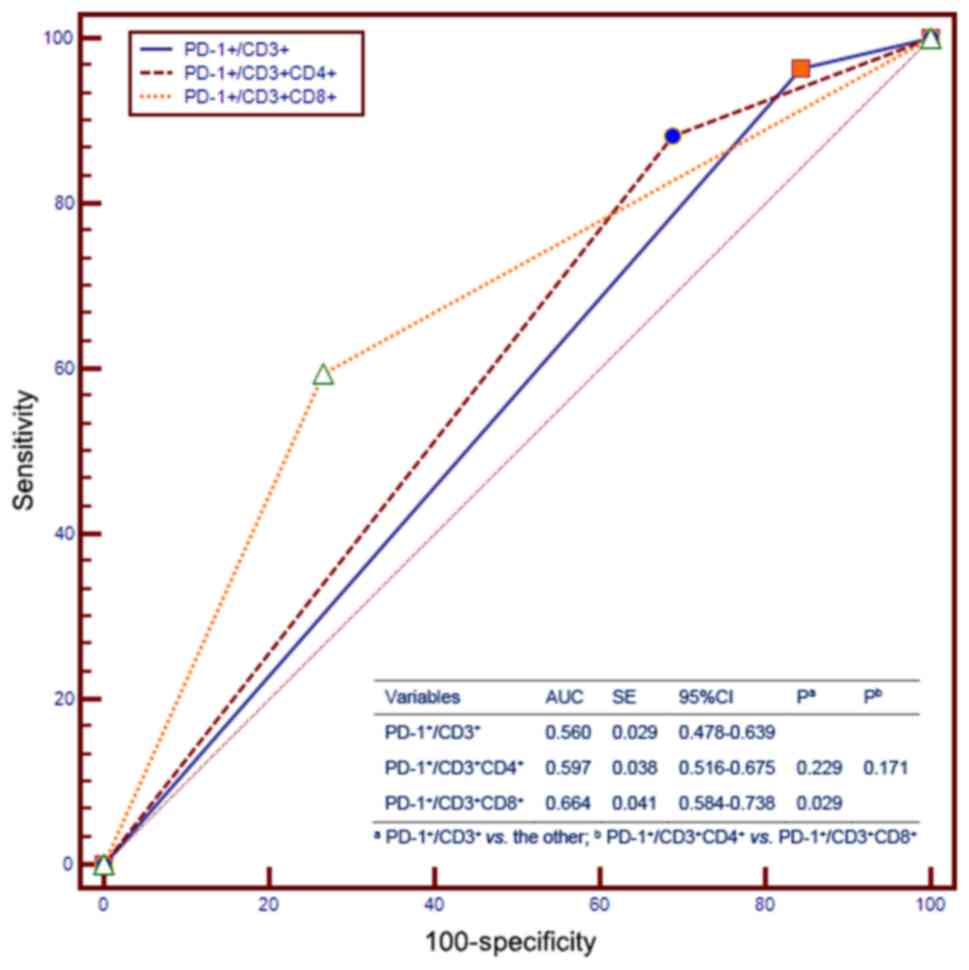
Furthermore, we compared the prognostic value of
PD-1 expression on peripheral blood T-cell subsets for OS with that
of other independent prognostic factors using receiver operating
characteristics (ROC) curves, which showed that the
PD-1+/CD3+ expression was not different from
PD-1+/CD3+CD4+ with regards to OS,
and the PD-1+/CD3+CD4+ expression
was not different from
PD-1+/CD3+CD8+ with regards to OS
(Fig. 4). Interestingly, when we
compared PD-1+/CD3+ with
PD-1+/CD3+CD8+, the
PD-1+/CD3+CD8+ had a better
prognostic value than PD-1+/CD3+ (Fig. 4).
Discussion
Recently, immunotherapy has become a promising
treatment for many types of cancers, including gastric cancer,
which has given rise to a growing interest in cancer immunotherapy
(15). Many clinical trials have
demonstrated that anti-PD-1 therapy was a successful treatment for
malignancies, including NSCLC, metastatic melanoma, renal cell
carcinoma, Hodgkin's lymphoma, and metastatic gastric cancer
(16–18). It has been indicated that PD-1 and
PD-L1 expression were independent prognosis factors in gastric
cancer by immunohistochemistry (IHC) analysis (19–22). Since
it is difficult to get fresh specimens from patients to determine
the expression of PD-1 or PD-L1 expression by IHC, no uniform
standard was defined for PD-L1 positivity up to now. Eto et
al defined PD-L1 positivity by staining of the tumor-cell in
four grade: 6–25, 26–50 or 51–75 and 76–100% of cells (12,23–29); Ti
Wen and co-worker defined staining pattern of PD-L1 as positive if
<5 or ≥5% in tumor cells (13);
whereas Wenfeng Fang assessed the expression of PD-L1 by
semi-quantitative H-score and defined cases with greater than 10%
PD-L1 expression on tumor cells were considered positive (26). Therefore, consistency detection of
PD-1 and PD-L1 expression by IHC is limited due to the difference
of antibody qualities from different manufacturers, the
interpretative subjectivity, and the different standard on
positivity thresholds.
For the development of new treatment modalities, the
co-development of novel convenient detection methods of biomarkers
is important. Here, we detected PD-1 expression in PBMC from
patients with metastatic gastric cancer using flow cytometry
analysis, which was an antibody-independent assay for PD-1 protein
detection of circulation. To analysis the role of the expression
level of PD-1 on peripheral blood T-cell subsets in
clinicopathological factors and prognosis of patients with
metastatic gastric cancer, we defined that lymphocytes with
PD-1+/CD3+ >15%,
PD-1+/CD3+CD4+ >10%, or
PD-1+/CD3+CD8+ >5% showed high
PD-1 expression level on T-cell subsets, on the contrary PD-1
expression level is low. However, our data indicated that there was
no significant difference on the expression level of PD-1 on
peripheral blood T-cell subsets between patients with high
PD-1+/CD3+,
PD-1+/CD3+CD4+,
PD-1+/CD3+CD8+ expression and
patients with low PD-1+/CD3+,
PD-1+/CD3+CD4+,
PD-1+/CD3+CD8+ expression.
In addition, the expression of PD-1 showed no
correlation with clinicopathological factors of patients with
metastatic gastric cancer. Then, we analyzed the correlation
between PD-1+/CD3+,
PD-1+/CD3+CD4+,
PD-1+/CD3+CD8+ expression and PFS,
the data demonstrated that patients with high
PD-1+/CD3+,
PD-1+/CD3+CD4+,
PD-1+/CD3+CD8+ level showed worse
OS. Further multivariate analysis using Cox's proportional hazards
model indicated that high PD-1+/CD3+,
PD-1+/CD3+CD4+, and
PD-1+/CD3+CD8+ were independent
risk factors for predicting the survival of metastatic gastric
cancer.
Furthermore, ROC analysis suggested that
PD-1+/CD3+CD4+ expression has a
similar survival predictive ability as PD-1+/CD3 and
PD-1+/CD3+CD8+, while
PD-1+/CD3+CD8+ higher predictive
ability than PD-1+/CD3, implying that identification of
PD-1+/CD3+CD8+ in patients might
be a more straightforward procedure.
Cumulative studies revealed that PD-L1 was widely
expressed in human malignancies and was a component of the
immunosuppressive microenvironment (24). A problems that cannot be neglected is
that the heterogeneity of fresh specimens and peripheral blood. It
was not clear the status of PD-1 expression on peripheral blood
accurately reflect the status of PD-1 expression in tumor
microenvironment. However, tumor-derived DNA and RNA can be
released and circulated in the peripheral circulation of cancer
patients, which operationally allowing for non-invasive gene
expression profiling by body fluid analysis. Indeed, there is a
wealth of information indicating a correlation between
tumor-associated changes in genomic, epigenetic, or transcriptional
patterns and alterations in the levels of cell-free circulating
nucleic acids (cfCNAs) (30,31). Thus, detected PD-L1 in peripheral
blood may after all be accepted as a kind of practical methods for
prostraction of disease progression. In the other hands, although
we did not determined the expression of PD-L1 and PD-L2, two
ligands for PD-1, on peripheral blood T-cell subsets in the present
study, it has been already demonstrated that PD-L1 expression in
gastric cancer cells was significantly correlated with worse
prognosis in gastric cancer patients (32). As to PD-L2, which deliver
co-inhibitory signal by binding to PD-1, it is very interesting to
determine PD-L2 expression on peripheral blood T-cell subsets to
see its role on immune evasion in gastric cancer patients.
In conclusion, in our cohort, PD-1 expression on
peripheral blood T-cell subsets is an independent prognostic factor
in metastatic gastric cancer, suggest that PD-1 expression in
peripheral blood T-cell subsets may potential be a novel prognostic
marker for gastric cancer patients at advanced stage.
Acknowledgements
The authors would like to thank Dr Weiquan Lu
(Department of Cancer Prevention, Henan Cancer Hospital, Changsha,
China) for his assistance with statistical analysis.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JC had full access to all of the data in the study
and takes responsibility for the integrity of the data and the
accuracy of the data analysis. BS performed the clinical analysis,
including patient selection, tissue procurement, histology, patient
case collection and biostatistics. QL and XM performed the data
analysis and interpretation of results. BS and QG performed the
experiments and statistical analysis. JC and LL planned and
supervised the project, performed data analysis and wrote the
manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee at Zhengzhou University Affiliated Cancer Hospital
(Henan, China) and written informed consent was obtained from each
patient.
Consent for publication
Written informed consent was obtained from all
patients for the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hamamoto Y: Complications in advanced or
recurrent gastric cancer patients with peritoneal metastasis during
and after palliative systemic chemotherapy. Mol Clin Oncol.
3:539–542. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN, 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Price TJ, Shapiro JD, Segelov E, Karapetis
CS, Pavlakis N, Van Cutsem E, Shah MA, Kang YK and Tebbutt NC:
Management of advanced gastric cancer. Expert Rev Gastroenterol
Hepatol. 6:199–209. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Topalian SL, Taube JM, Anders RA and
Pardoll DM: Mechanism-driven biomarkers to guide immune checkpoint
blockade in cancer therapy. Nat Rev Cancer. 16:275–287. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Freeman GJ, Long AJ, Iwai Y, Bourque K,
Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne
MC, et al: Engagement of the PD-1 immunoinhibitory receptor by a
novel B7 family member leads to negative regulation of lymphocyte
activation. J Exp Med. 192:1027–1034. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Latchman Y, Wood CR, Chernova T, Chaudhary
D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, et al:
PD-L2 is a second ligand for PD-1 and inhibits T cell activation.
Nat Immunol. 2:261–268. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zheng H, Liu X, Zhang J, Rice SJ, Wagman
M, Kong Y, Zhu L, Zhu J, Joshi M and Belani CP: Expression of PD-1
on CD4+ T cells in peripheral blood associates with poor clinical
outcome in non-small cell lung cancer. Oncotarget. 7:56233–56240.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Komura T, Sakai Y, Harada K, Kawaguchi K,
Takabatake H, Kitagawa H, Wada T, Honda M, Ohta T, Nakanuma Y and
Kaneko S: Inflammatory features of pancreatic cancer highlighted by
monocytes/macrophages and CD4+ T cells with clinical impact. Cancer
Sci. 106:672–686. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shibutani M, Maeda K, Nagahara H, Fukuoka
T, Nakao S, Matsutani S, Hirakawa K and Ohira M: The prognostic
significance of the tumor-infiltrating programmed cell
death-1+ to CD8+ lymphocyte ratio in patients
with colorectal cancer. Anticancer Res. 37:4165–4172.
2017.PubMed/NCBI
|
|
11
|
Baas W, Gershburg S, Dynda D, Delfino K,
Robinson K, Nie D, Yearley JH and Alanee S: Immune characterization
of the programmed death receptor pathway in high risk prostate
cancer. Clin Genitourin Cancer. 15:577–281. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yuan J, Zhang J, Zhu Y, Li N, Tian T, Li
Y, Li Y, Li Z, Lai Y, Gao J and Shen L: Programmed death-ligand-1
expression in advanced gastric cancer detected with RNA in situ
hybridization and its clinical significance. Oncotarget.
7:39671–39679. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eto S, Yoshikawa K, Nishi M, Higashijima
J, Tokunaga T, Nakao T, Kashihara H, Takasu C, Iwata T and Shimada
M: Programmed cell death protein 1 expression is an independent
prognostic factor in gastric cancer after curative resection.
Gastric Cancer. 19:466–471. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vali B, Jones RB, Sakhdari A, Sheth PM,
Clayton K, Yue FY, Gyenes G, Wong D, Klein MB, Saeed S, et al:
HCV-specific T cells in HCV/HIV co-infection show elevated
frequencies of dual Tim-3/PD-1 expression that correlate with liver
disease progression. Eur J Immunol. 40:2493–2505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lauwers GY CF and Graham DY: Curado MP
tumours of the stomach. WHO classification of tumours of the
digestive system Bosman FT CF. Hruban RH and Theise ND: 48–59.
2010.
|
|
16
|
Aldarouish M and Wang C: Trends and
advances in tumor immunology and lung cancer immunotherapy. J Exp
Clin Cancer Res. 35:1572016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dougan M and Dranoff G: Immune therapy for
cancer. Annu Rev Immunol. 27:83–117. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sharon E, Streicher H, Goncalves P and
Chen HX: Immune checkpoint inhibitors in clinical trials. Chin J
Cancer. 33:434–444. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ansell SM, Lesokhin AM, Borrello I,
Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry
D, Freeman GJ, et al: PD-1 blockade with nivolumab in relapsed or
refractory Hodgkin's lymphoma. N Engl J Med. 372:311–319. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brahmer JR, Hammers H and Lipson EJ:
Nivolumab: Targeting PD-1 to bolster antitumor immunity. Future
Oncol. 11:1307–1326. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Muro K, Chung HC, Shankaran V, Geva R,
Catenacci D, Gupta S, Eder JP, Golan T, Le DT, Burtness B, et al:
Pembrolizumab for patients with PD-L1-positive advanced gastric
cancer (KEYNOTE-012): A multicentre, open-label, phase 1b trial.
Lancet Oncol. 17:717–726. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rizvi NA, Mazières J, Planchard D,
Stinchcombe TE, Dy GK, Antonia SJ, Horn L, Lena H, Minenza E,
Mennecier B, et al: Activity and safety of nivolumab, an anti-PD-1
immune checkpoint inhibitor, for patients with advanced, refractory
squamous non-small-cell lung cancer (CheckMate 063): A phase 2,
single-arm trial. Lancet Oncol. 16:257–265. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Böger C, Behrens HM, Mathiak M, Krüger S,
Kalthoff H and Röcken C: PD-L1 is an independent prognostic
predictor in gastric cancer of Western patients. Oncotarget.
7:24269–24283. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fang W, Chen Y, Sheng J, Zhou T, Zhang Y,
Zhan J, Liu L, Huang J, Peng P and Zhang L: Association between
PD-L1 expression on tumour-infiltrating lymphocytes and overall
survival in patients with gastric cancer. J Cancer. 8:1579–1585.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Saito R, Abe H, Kunita A, Yamashita H,
Seto Y and Fukayama M: Overexpression and gene amplification of
PD-L1 in cancer cells and PD-L1+ immune cells in Epstein-Barr
virus-associated gastric cancer: The prognostic implications. Mod
Pathol. 30:427–439. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wen T, Wang Z, Li Y, Li Z, Che X, Fan Y,
Wang S, Qu J, Yang X, Hou K, et al: A four-factor immunoscore
system that predicts clinical outcome for stage II/III gastric
cancer. Cancer Immunol Res. 5:524–534. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang L, Qiu M, Jin Y, Ji J, Li B, Wang X,
Yan S, Xu R and Yang D: Programmed cell death ligand 1 (PD-L1)
expression on gastric cancer and its relationship with
clinicopathologic factors. Int J Clin Exp Pathol. 8:11084–11091.
2015.PubMed/NCBI
|
|
28
|
Zhang M, Dong Y, Liu H, Wang Y, Zhao S,
Xuan Q, Wang Y and Zhang Q: The clinicopathological and prognostic
significance of PD-L1 expression in gastric cancer: A meta-analysis
of 10 studies with 1,901 patients. Sci Rep. 6:379332016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu Y, Cao D, Qu L, Cao X, Jia Z, Zhao T,
Wang Q and Jiang J: PD-1 and PD-L1 co-expression predicts favorable
prognosis in gastric cancer. Oncotarget. 8:64066–64082.
2017.PubMed/NCBI
|
|
30
|
Brown JA, Dorfman DM, Ma FR, Sullivan EL,
Munoz O, Wood CR, Greenfield EA and Freeman GJ: Blockade of
programmed death-1 ligands on dendritic cells enhances T cell
activation and cytokine production. J Immunol. 170:1257–1266. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dong H and Chen L: B7-H1 pathway and its
role in the evasion of tumor immunity. J Mol Med (Berl).
81:281–287. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schwarzenbach H, Hoon DS and Pantel K:
Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev
Cancer. 11:426–437. 2011. View Article : Google Scholar : PubMed/NCBI
|