Introduction
Lung cancer has the highest mortality rates of all
cancer types, and non-small cell lung cancer (NSCLC) accounts for
85% all mortality cases globally (1).
The metastasis of NSCLC cells is rapid, the disease is usually at
an advanced stage by the time it is diagnosed and the effectiveness
of current treatments is limited, resulting in a low 5-year
survival rate and a poor prognosis (2). Therefore, in order to increase the
survival rate of patients with NSCLC, in-depth studies on the
pathogenesis of NSCLC focusing on inhibiting the malignant
proliferation and metastasis of the tumor are required. Long
non-coding RNAs (lncRNAs) are non-coding RNAs with a length of
>200 nucleotides, and are a novel research area that has emerged
in previous years (3). Studies have
revealed that lncRNAs are involved in a number of biological
regulation processes, such as cell differentiation, proliferation
and immune response (3,4). A number of studies have revealed that
lncRNAs are abnormally expressed in intestinal cancer (5), ovarian cancer (6), gastric cancer (7) and numerous other cancer types. The
abnormal expression of lncRNA may be associated with the occurrence
and metastasis of cancer (8).
Evidence has demonstrated that certain lncRNAs are associated with
the occurrence and progression of lung cancer (9). The lncRNA colon cancer-associated
transcript 2 (CCAT2) is located on chromosome 8 (10), and is involved in the occurrence and
progression of breast cancer, gastric cancer, colon cancer and lung
cancer (9,10). LncRNA CCAT2 is a novel lncRNA, first
identified to have abnormal expression in the tissues of colon
cancer, and may promote the growth and metastasis of tumor tissues
and induce chromosomal instability (11). The Wnt/β-catenin pathway is a classic
Wnt signaling pathway and β-catenin protein is its main member.
This pathway is able to activate the transcriptional activity of
its target gene through the nuclear translocation of β-catenin
(9–11). Previous studies have revealed that the
abnormal activation of the Wnt/β-catenin signaling pathway served a
role in the process of cell carcinogenesis, tumorigenesis and tumor
invasion (12), and the abnormalities
of the classic Wnt/β-catenin signaling pathway were associated with
the occurrence of a variety of malignant tumor types (13,14).
Previous studies have revealed that the lncRNA CCAT2 enhances
Wnt/β-catenin pathway activity, affecting glioma (15), oral squamous cell carcinoma (16), breast cancer (17), renal cell carcinoma (18) and other tumor types. However, there
are few studies on the effect of CCAT2 and the Wnt/β-catenin
signaling pathway in NSCLC. The present study aims to study the
association between CCAT2 and the Wnt/β-catenin pathway in NSCLC
and investigate its association with clinical features, in order to
provide insight for the early diagnosis and molecular targeted
therapy of NSCLC.
Materials and methods
Patient grouping
Patients were divided into two groups for each
characteristic, according to the median age (≥56 and <56 years),
sex, smoking history, tumor size (>3 and ≤3 cm),
Tumor-Node-Metastasis (TNM) staging (16) and the presence or absence of lymph
node metastasis. Samples were divided into a high expression group
and a low expression group according to the cut-off point of 2.58
times the CCAT2 expression level in normal tissues, with 2.58
included within the high expression group, including 15 cases in
the high expression group and 21 cases in the low expression
group.
Instruments and reagents
The lung cancer NCI-H1975 cell line was purchased
from the American Type Culture Collection cell bank (Manassas, VA,
USA). RPMI-1640 medium and fetal bovine serum (FBS) were purchased
from Gibco; Thermo Fisher Scientific, Inc. (Waltham, MA, USA). The
Cell Counting Kit-8 (CCK-8) was purchased from Dojindo Molecular
Technologies, Inc. (Kumamoto, Japan). The cell apoptosis kit was
purchased from Beyotime Institute of Biotechnology (Haimen, China).
The RNA extraction reagent TRIzol was obtained from Invitrogen;
Thermo Fisher Scientific, Inc. The reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) kit
was obtained from Takara Biotechnology Co., Ltd. (Dalian, China).
The gel imaging system and ViiA7 type Real-Time PCR instrument were
purchased from Applied Biosystems (Thermo Fisher Scientific, Inc.).
The total protein extraction kit was purchased from BestBio
(Shanghai, China). The Coomassie Brilliant Blue Protein assay kit
was purchased from Shanghai Majorbio Pharmaceutical Technology Co.,
Ltd. (Shanghai, China). SDS-PAGE, PBS with 0.1% Tween-20 (PBST)
solution, vertical electrophoresis apparatus and GIS-2020D gel
image analysis system were purchased from Sigma-Aldrich; Merck KGaA
(Darmstadt, Germany). β-actin antibody (cat no. MS123A1) was
purchased from Abcam (Cambridge, MA, USA). CCAT2 small interfering
RNA (siRNA) and negative control (NC) siRNA were purchased from
Guangzhou RiboBio Co., Ltd. (Guangzhou, China).
Lipofectamine® 2000 was purchased from Invitrogen;
Thermo Fisher Scientific, Inc.
Patients
A total of 36 cases of cancerous tissue resection
specimens were collected from patients with lung cancer who
attended in the First Affiliated Hospital of Bengbu Medical College
(Bengbu, China) between January 2013 and December 2015. Each
specimen included tumor tissue and normal para-carcinoma tissue (2
cm from the edge of the tumor). None of the patients had received
radiotherapy, chemotherapy or other treatments prior to surgery.
The fresh tissue specimens in vitro were placed in a −80°C
refrigerator for preservation subsequent to liquid nitrogen
freezing at −4°C for 30 min. All lung cancer cases were
pathologically confirmed with NSCLC. Relevant clinical data of the
patients were collected, including 28 male and 8 female cases, with
a mean age of 54.91±6.48 years (range, 43–65 years). Patients
provided written informed consents prior to the study, which was
ethically approved by the ethics committee of the First Affiliated
Hospital of Bengbu Medical College.
Cell lines and cell culture
NCI-H1975 cells were cultured in RPMI-1640 medium
containing, 100 U/ml penicillin, 10% FBS at 37°C, 20% O2
and 5% CO2.
si-CCAT2 transfection
The sequence of si-CCAT2 was
5′-GUGCAACUCUGCAAUUUAAUU-3′ and the control sequence of NC siRNA
was: 5′-AATGGACAACTGGTCGTGGAC-3′. NCI-H1975 cells were cultured at
a density of 1,000 cells/well in a 96-well plate and transfected
with 1.0 µl siRNA for 24 h using Lipofectamine 2000 according to
the manufacturer's protocol; they were divided into a transfection
group and an untransfected control group (control). Subsequent
experiments were performed 24–72 h following transfection.
RT-qPCR
RNA of cancerous tissues, para-carcinoma tissues and
NCI-H1975 cells was extracted using TRIzol reagent. The RNA purity
and content were detected using a nucleic acid protein analyzer
(DU640; Beckman Coulter, Inc., Brea, CA, USA). The RNA integrity
was identified using 1% agarose gel electrophoresis. A total of 1
µg RNA was reverse transcribed to obtain cDNAs using the RT-qPCR
kit (Takara Biotechnology Co., Ltd.) according to the
manufacturer's protocol. Next, the RT-qPCR reaction system was
prepared as follows: 5 µl 2X SYBR-Green Mixture, 0.5 µl cDNA, 0.5
µl Primer (10 µM) and 4 µl ddH2 (Takara Bio, Inc., Otsu, Japan).
The thermocycling conditions were as follows: 95°C pre-denaturation
for 10 min, 95°C denaturation for 15 sec and annealing extension
for 60 sec at 60°C and extension for 15 sec at 72°C for a total of
40 PCR cycles performed on a ViiA7 type fluorescence qPCR
apparatus. Three parallel samples were established for each
experiment and β-actin was used as the reference gene. RT-qPCR was
conducted as previously described (15). The primer sequence used were: CCAT2,
forward, 5′-GTTGTTGGGAGCTACATTGTCTGC-3′, and reverse,
5′-GTGTCGTGAACTCGGCAATTC-3′; and β-actin, forward,
5′-GAACCCTAAGGCCAAC-3′, and reverse, 5′-TGTCACGCACGATTTCC-3′.
CCK-8 assay for cell
proliferation
Cells (5×106) in the transfection, NC and
control groups following digestion and counting were inoculated
into 96-well plates, and cultured at 37°C in a 5% O2 and
5% CO2 atmosphere. In the transfection group,
experiments were performed 24, 48 and 72 h following transfection,
5 repeated wells were used at each time point and a blank control
group treated with PBS was established. Subsequently, the cells
were washed 3 times with PBS and 100 µl CCK-8 mixture (CCK-8
reagent; Qiagen GmbH, Hilden, Germany) was added to each well,
incubated at 37°C for 2 h, and then the absorbance value was
measured a wavelength of 450 nm using a microplate reader. Cell
proliferation was calculated according to the absorbance value.
Transwell assay
Cells were digested to adjust the concentration to
2×105/ml in serum-free Dulbecco's modified Eagle's
medium (DMEM; Sigma-Aldrich; Merck KGaA). A total of 200 ml cell
suspension was transferred to the upper chamber (8 mm pore size)
and placed in a 24-well plate, and 500 ml complete DMEM culture
medium containing 10% FBS was added to the lower chamber. The cells
were then incubated at 37°C for 24 h in an incubator, prior to the
chamber being removed and the cells on the upper surface being
removed using a sterile cotton swab. The cells were subsequently
fixed with 5% methanol at 4°C overnight and stained at 37°C using
crystal violet for 10 min. The number of membrane-penetrating cells
under 5 high magnification fields was counted under a light
microscope (×400), to evaluate the invasion ability of the
transfection group.
Western blot analysis
Cytoplasmic proteins and nucleoproteins were
separated and purified by radioimmunoprecipitation assay lysate
(Thermo Fisher Scientific, Inc.), the proteins were quantified
using the Coomassie Brilliant Blue Protein assay kit, and then
boiling at 100°C for 5 min to prepare the 10% SDS-PAGE gel
(Sigma-Aldrich; Merck KGaA). The protein samples (10 µl/lane) were
loaded for electrophoresis, prior to being transferred to an
electrophoresis tank for nitrocellulose membrane transfer by the
addition of transfer buffer containing 25 ml Tris, 0.193 M glycine
and 20% methanol (Sigma-Aldrich, Merck KGaA). The membranes were
blocked with PBST containing 5% skimmed milk for 2 h at room
temperature and washed three times using PBST, followed by
incubation with primary antibodies (cat. no. A21422; dilution:
1:2,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) overnight
at 4°C. Membranes were washed twice using PBST for 30 min, and the
horseradish peroxidase (HRP)-labeled secondary antibody (cat. no.
A66585; dilution, 1:10,000; Santa Cruz Biotechnology, Inc.)
prepared using PBST containing 2.5% skimmed milk was added for
incubation at 37°C for 60 min. Once membranes were washed 3 times
with PBST, the proteins were evenly applied to the nitrocellulose
membrane, exposed in the dark room, the films were developed for 5
min in the 3,3′-Diaminobenzidine developing solution
(Sigma-Aldrich; Merck KGaA). Subsequent to washing with the
developing solution, the membranes were placed in the PBST fixing
solution (Sigma-Aldrich; Merck KGaA) at 37°C for 5 min, then dried
following washing. The optical density of the β-catenin protein,
β-actin protein and Histone3 protein bands in the si-CCAT2 group,
β-catenin inhibitor FH535 (37°C for 30 min; Sigma-Aldrich, Merck
KGaA) (15 µmol/l) group and si-CCAT2 + FH535 group was visualized
by Supersignal West Femto HRP sensitive chemiluminescent substrate
(Sigma-Aldrich, Merck KGaA) and analyzed using the GIS-2020D gel
image analysis system (Ningbo Shuangjia Instrument Co., Ltd.,
Hangzhou, China), and the ratio of the optical density of β-catenin
protein bands to that of β-actin bands or Histone3 bands was used
as the expression intensity of β-catenin protein.
TOP/FOP luciferase assay
Lung cancer NCI-H1975 cells at the logarithmic phase
were inoculated in a 96-well cell culture plate. Lung cancer
NCI-H1975 cells (1×106) were transfected with si-CCAT2
using Lipofectamine 2000 according to the manufacturer's protocol.
A total of 24 h later, cells were transfected with TOP/FOP
luciferase plasmids at 37°C for 30 min, according to the
manufacturer's protocols of Lipofectamine 2000 detection kit
(Invitrogen; Thermo Fisher Scientific, Inc.). The experiment was
repeated three times. The data are expressed as the ratio of
TOP/FOP. At 48 h after transfection, the TOP/FOP luciferase assay
was performed.
Statistical analysis
Data statistical analysis was performed using SPSS
19.0 statistical software (IBM Corp., Armonk, NY, USA). Statistical
data with a normal distribution are expressed as the mean ±
standard deviation. The comparison between CCAT2 expression in lung
cancer tissues and adjacent tissues was performed using a paired
Student's t-test. Analysis of the association between CCAT2
expression levels and the clinical characteristics of patients was
performed by χ2 test. Comparisons of measured data
between two groups with two independent samples were performed
using a paired Student's t-test. Comparisons of measured data
between multiple groups were performed using one-way analysis of
variance followed by Dunnett's test for comparisons between
specific groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
mRNA expression levels of CCAT2 in
lung cancer tissues and normal para-carcinoma tissues
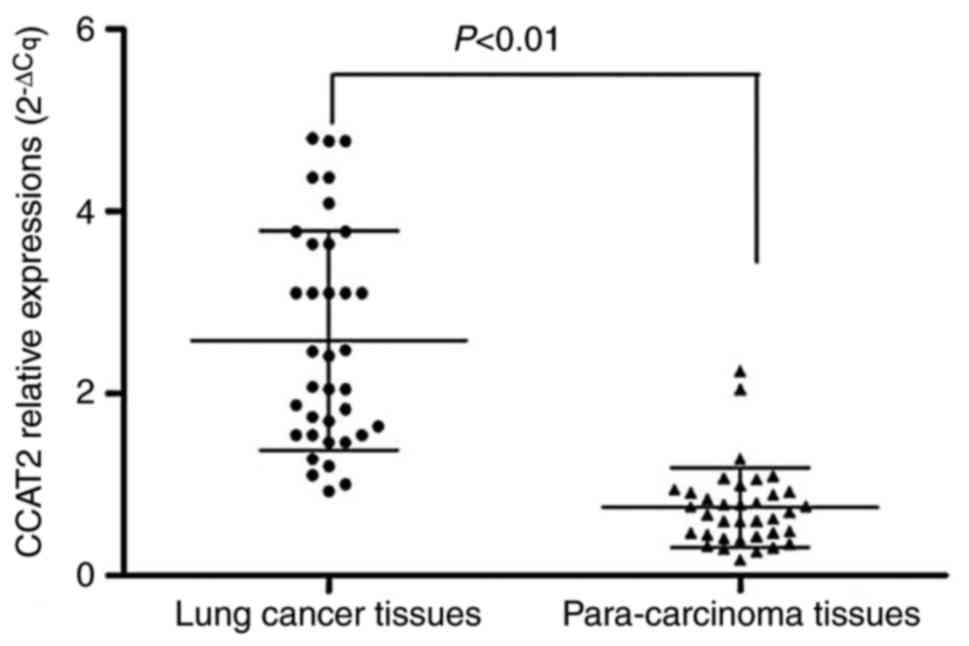
The mRNA expression levels of CCAT2 in lung cancer
tissues and normal para-carcinoma tissues in 36 patients with lung
cancer were detected using RT-qPCR. Results revealed that the
expression levels of CCAT2 in lung cancer tissues were
significantly higher compared with those in the normal
para-carcinoma tissues (t=8.580, P<0.01), as presented in
Fig. 1.
Association between CCAT2 mRNA
expression levels and the clinicopathological features of
patients
The association between the CCAT2 expression level
and clinicopathological features was investigated by Fisher's
exact test. As presented in Table
I, the expression level of CCAT2 in samples with a >3 cm
tumor tissue size and lymph node metastasis was significantly
increased compared with samples with a ≤3 cm tumor tissue size and
no lymph node metastasis, respectively (P<0.05). The expression
level of CCAT2 was not significantly associated with age, sex,
smoking history or TNM staging (P>0.05).
 | Table I.Association between CCAT2 expression
and patient clinicopathological features. |
Table I.
Association between CCAT2 expression
and patient clinicopathological features.
|
|
| Relative expression
level of CCAT2 |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
feature | No. cases | Low | High | P-value |
|---|
| Age, years |
|
|
| 0.311 |
| ≥56 | 19 | 13 | 6 |
|
|
<56 | 17 | 8 | 9 |
|
| Sex |
|
|
| 0.236 |
| Male | 28 | 18 | 10 |
|
|
Female | 8 | 3 | 5 |
|
| Smoker |
|
|
| 0.219 |
| Yes | 29 | 17 | 12 |
|
| No | 7 | 2 | 5 |
|
| Tumor size, cm |
|
|
| 0.039 |
| ≥3 | 22 | 7 | 15 |
|
|
<3 | 14 | 10 | 4 |
|
| Tumor-Node-Mode
stage |
|
|
| 0.071 |
| I and
II | 12 | 8 | 4 |
|
| III and
IV | 24 | 7 | 17 |
|
| Lymph node
metastasis |
|
|
| 0.006 |
|
Yes | 20 | 4 | 16 |
|
| No | 16 | 11 | 5 |
|
Expression levels of CCAT2 in lung
cancer NCI-H1975 cells following transfection with si-CCAT2
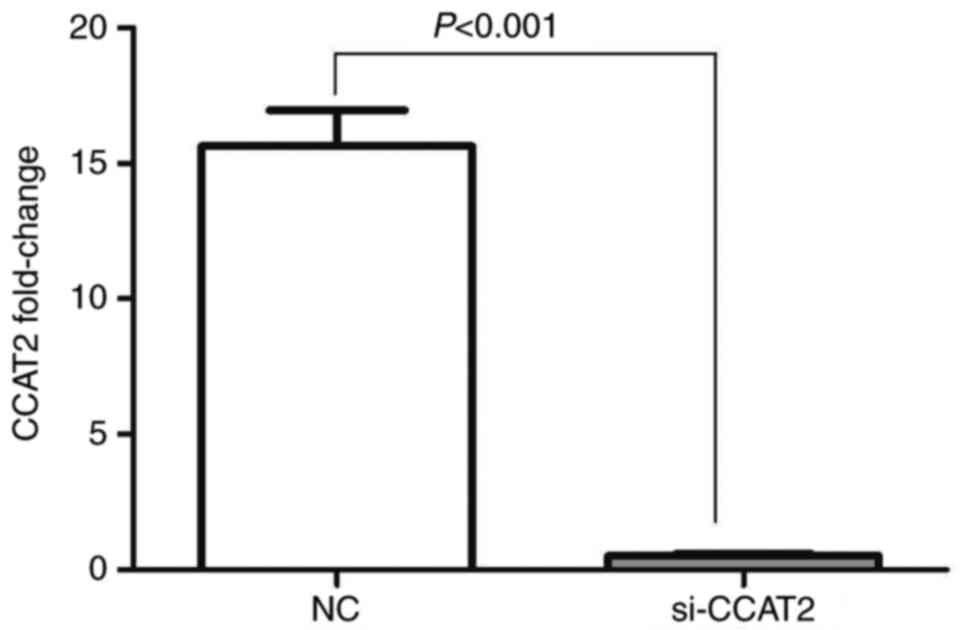
NCI-H1975 lung cancer cells were transfected with
siRNA to inhibit the expression of CCAT2. RT-qPCR results revealed
that following the transfection of si-CCAT2, the mRNA expression
levels of CCAT2 in lung cancer NCI-H1975 cells were significantly
inhibited (t=19.98, P<0.001), as presented in Fig. 2.
Effect on proliferation and apoptosis
of lung cancer NCI-H1975 cells following CCAT2 silencing
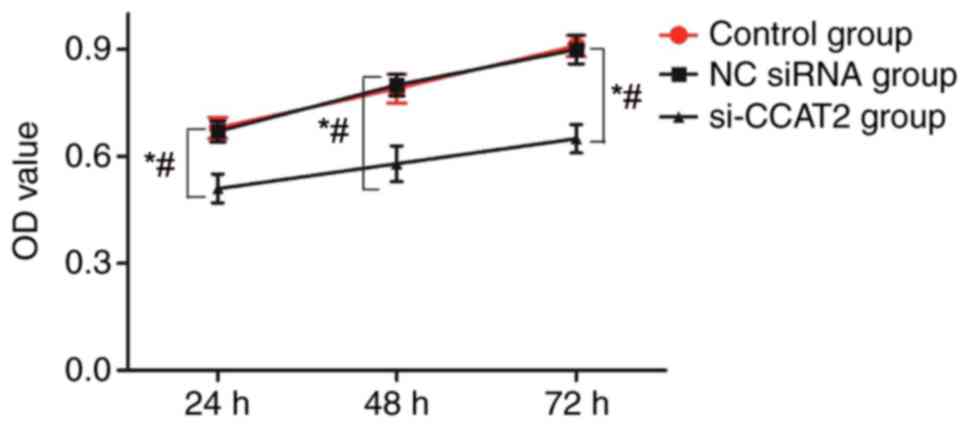
A CCK-8 assay was performed to detect the effect on
the proliferation of lung cancer NCI-H1975 cells subsequent to
CCAT2 silencing, and the optical density values are presented in
Fig. 3. At 24–72 h after
transfection, the cell proliferation rate in the si-CCAT2
transfection group was significantly lower than that of the NC
siRNA group and the control group (P<0.05). There was no
significant difference between the cell proliferation rate of the
NC siRNA group and that of the control group (P>0.05).
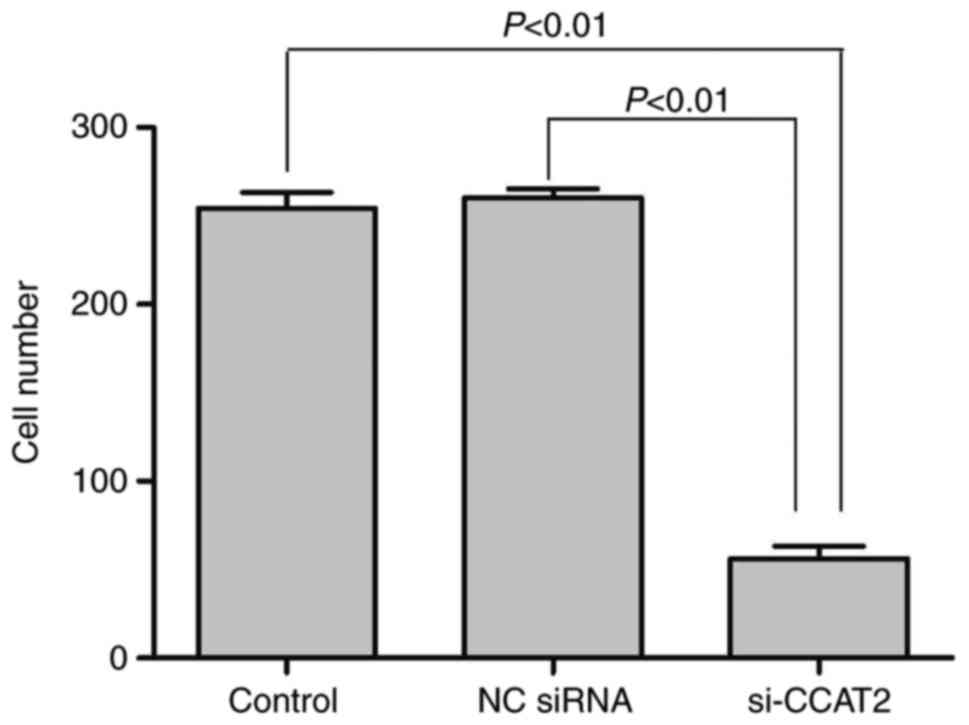
A Transwell assay was performed to detect the effect
on the invasion ability of lung cancer NCI-H1975 cells subsequent
to CCAT2 silencing. The number of cells that passed through the
chamber is presented in Fig. 4.
Results revealed that the cell invasion ability in the si-CCAT2
group was significantly lower than that in the NC siRNA group
(t=39.50, P<0.01) and the control group (t=29.86,
P<0.01).
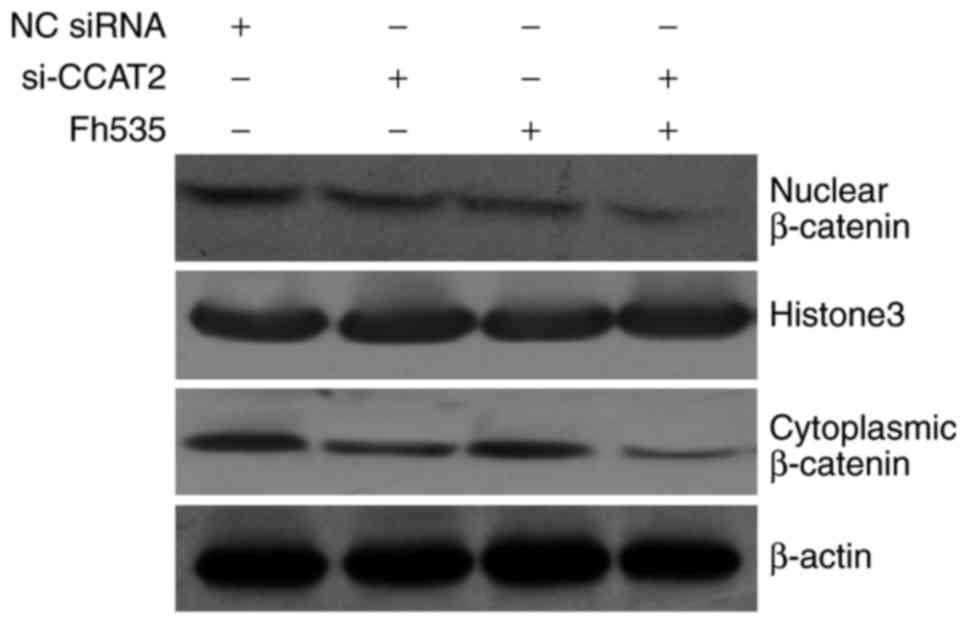
Effect of si-CCAT2 on Wnt pathway
β-catenin proteins
FH535 was used as an inhibitor of Wnt pathway
β-catenin proteins. The effect of CCAT2 silencing on the protein
expression of β-catenin protein in the nucleus and cytoplasm of
lung cancer NCI-H1975 cells and the effect of CCAT2 on the entry of
β-catenin into the nucleus were detected using western blot
analysis, and the results are presented in Fig. 5. The expression of β-catenin proteins
in the cell nucleus and cytoplasm were decreased in the si-CCAT2
group compared with that in the NC siRNA group; in the FH535 group,
the protein expression of β-catenin was decreased in the cell
nucleus and maintained in the cell cytoplasm compared with the NC
siRNA group; and in the si-CCAT2 + FH535 group, the protein
expression of β-catenin in the cell nucleus and cytoplasm was
markedly decreased compared with that in the NC siRNA group.
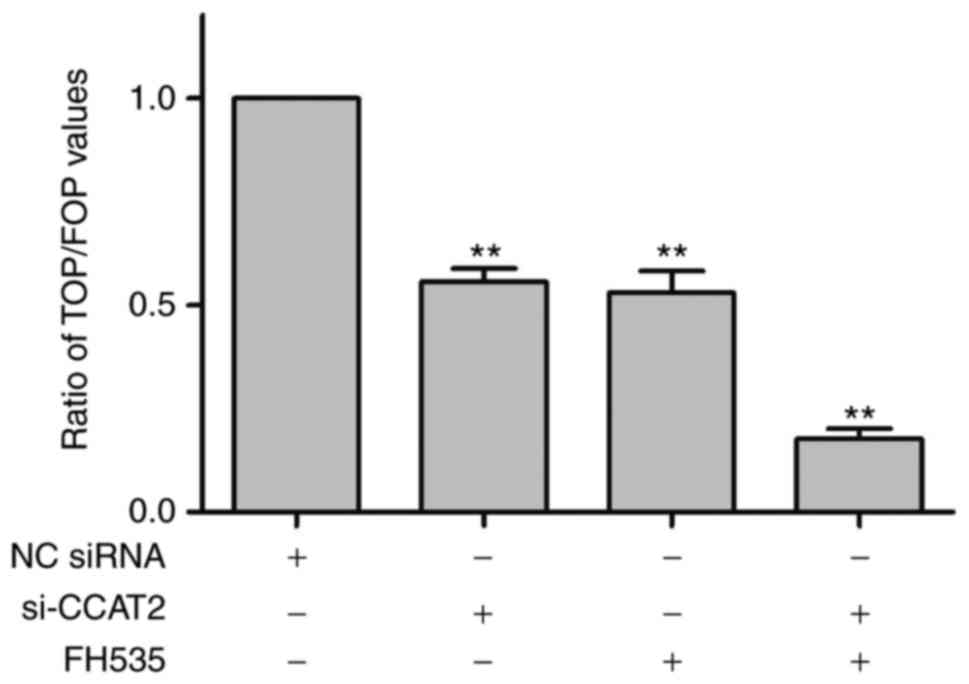
Detection of Wnt/-β-catenin signaling
pathway activity using a TOP/FOP luciferase ratio assay
In order to further verify the effect of si-CCAT2 on
the Wnt/-β-catenin signaling pathway, a TOP/FOP luciferase ratio
assay was performed to detect the activity of the Wnt/-β-catenin
signaling pathway. Results revealed that si-CCAT2 and FH535
significantly decreased the activity of the β-catenin signaling
pathway compared with the NC siRNA group (t=12.49, P<0.01 and
t=11.23, P<0.01, respectively). si-CCAT2 and FH535 combined
exerted a synergistic, significant inhibitory effect on the
activity of the β-catenin signaling pathway in lung cancer
NCI-H1975 cells compared with the NC siRNA group (t=23.56,
P<0.01), as presented in Fig.
6.
Discussion
A previous study concluded that lncRNAs are novel
tumor biomarkers that may provide a novel approach to the early
diagnosis and treatment of cancer (19). Qiu et al (20) reported that CCAT2 is an lncRNA
specifically expressed in lung adenocarcinoma, and that it promotes
tumor proliferation and invasion. CCAT2 combined with
carcinoembryonic antigen may be used to predict the potential for
lymph node metastasis in patients (20). In the present study, the expression
level of CCAT2 in the lung cancer tissues was higher compared with
that in the normal para-carcinoma tissues of 36 patients with lung
cancer, suggesting that CCAT2 may be associated with the occurrence
of lung cancer. In addition, Fisher's exact test revealed that the
high expression of CCAT2 was associated with lymph node metastasis,
consistent with the results reported by Qiu et al (20). Wang et al (21) collected the tumor samples and clinical
data of patients with gastric cancer and revealed that the patients
with a high expression of CCAT2 were at an increased risk of lymph
node metastasis and distant metastasis. In the present study, the
high expression of CCAT2 was associated with lymph node metastasis
and tumor size, suggesting that CCAT2 was highly expressed in lung
cancer. A previous study demonstrated that CCAT2 may promote the
invasion and proliferation of gastric cancer by regulating
E-cadherin and large tumor suppressor kinase 2, and that CCAT2
serves the biological role of an oncogene (22). Zhao et al (23) believed that CCAT2 may promote the
occurrence of NSCLC through the tumor suppressor gene
cyclin-dependent kinase inhibitor 1. In the present study, CCK-8
and Transwell assays revealed that CCAT2 may promote the
proliferation and metastasis of NSCLC, indicating that CCAT2 may
promote the occurrence and progression of NSCLC, which was
consistent with the results reported by Qiu et al (20) and Wang et al (21).
The Wnt signaling pathway is a complex and
conservative signal transduction pathway, serving an important role
in embryonic development, regulating cell growth and
differentiation, as well as tumor occurrence, development and
metastasis (24). The activity of the
Wnt signaling pathway is regulated by a variety of proteins
(25). The abnormal activation of Wnt
signaling results in Wnt activating the disheveled protein,
transducing intracellular signals and β-catenin is inhibited by the
activated disheveled protein to form a complex with Axin-APC and
Wnt signaling pathway regulator-glycogen synthase kinase-3β
(26). Through combining with T-cell
factor/lymphoid enhancer factor, the transcription of downstream
target genes matrix metalloproteinase-7, MYC proto-oncogene, BHLH
transcription factor (c-Myc) is stimulated to induce cell
proliferation (26). The abnormal
activation of the Wnt signaling pathway is associated with cellular
malignant transformation and tumorigenesis (27). The core of the Wnt signaling pathway
is the accumulation of β-catenin in the cell, which results in the
transcription of specific target genes through its downstream
pathway (15). CCAT2 is a downstream
target site of the Wnt signaling pathway (9,10), which
is involved in the occurrence and progression of cancer (15,28). Ling
et al (10) reported that
CCAT2 may upregulate Myc, microRNA (miR)-17-5p and miR-20a via
transcription factor 7-like 2 in colon cancer tissues and that it
may enhance the activity of the Wnt signaling pathway. In the
present study, the expression of Wnt signaling pathway β-catenin
protein in the nucleus and cytoplasm of NCI-H1975 cells and the
activity of the Wnt/β-catenin signaling pathway was detected using
a TOP/FOP luciferase assay following CCAT2 silencing. The results
revealed that si-CCAT2 may reduce the expression of β-catenin in
the cytoplasm and nucleus of NCI-H1975 cells and inhibit the Wnt
signaling pathway, while FH535 may only inhibit the expression of
β-catenin in the nucleus. The combination of the two may further
reduce the expression of β-catenin in the nucleus of NCI-H1975
cells and may further inhibit the activity of the Wnt signaling
pathway. This suggests that CCAT2 activates the Wnt signaling
pathway by enhancing β-catenin stability and the entry of β-catenin
protein into the nucleus.
In the present study, it was revealed that CCAT2 may
be involved in the occurrence and progression of NSCLC through the
Wnt/β-catenin signaling pathway. To investigate the potential use
of CCAT2 in the early diagnosis and molecular targeted therapy of
patients with NSCLC, further in vivo experiments are
required.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Anhui Province (grant no. 1608085QH189) and
the National Natural Science Foundation of China (grant no.
81172213).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CZ is responsible for study conception and design.
CQ analyzed and interpreted the data. LZ was responsible for
clinical sampling collection and manuscript draft. YC was in charge
of cell experiment and manuscript revision.
Ethics approval and consent to
participate
All specimens were collected with the patient's
written informed consent and approved by the Ethics Committee of
the First Affiliated Hospital of Bengbu Medical College.
Patient consent for publication
Witten informed consent was obtained from the
patients for the publication of any associated data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu X and Cho WC: Precision medicine in
immune checkpoint blockade therapy for non-small cell lung cancer.
Clin Transl Med. 6:72017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kunz M, Wolf B, Schulze H, Atlan D, Walles
T, Walles H and Dandekar T: Non-coding RNAs in lung cancer:
Contribution of bioinformatics analysis to the development of
non-invasive diagnostic tools. Genes. 8:pii: E8. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Thean LF, Wong YH, Lo M, Loi C, Chew MH,
Tang CL and Cheah PY: Chromosome 19q13 disruption alters
expressions of CYP2A7, MIA and MIA-RAB4B lncRNA and contributes to
FAP-like phenotype in APC mutation-negative familial colorectal
cancer patients. PLoS One. 12:e1737722017. View Article : Google Scholar
|
|
4
|
Li LJ, Chai Y, Guo XJ, Chu SL and Zhang
LS: The effects of the long non-coding RNA MALAT-1 regulated
autophagy-related signaling pathway on chemotherapy resistance in
diffuse large B-cell lymphoma. Biomed Pharmacother. 89:939–948.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang LM, Wang P, Liu XM and Zhang YJ:
LncRNA SUMO1P3 drives colon cancer growth, metastasis and
angiogenesis. Am J Transl Res. 9:5461–5472. 2017.PubMed/NCBI
|
|
6
|
Li AH and Zhang HH: Overexpression of
lncRNA MNX1-AS1 is associated with poor clinical outcome in
epithelial ovarian cancer. Eur Rev Med Pharmacol Sci. 21:5618–5623.
2017.PubMed/NCBI
|
|
7
|
Huang Y, Zhang J, Hou L, Wang G, Liu H,
Zhang R, Chen X and Zhu J: LncRNA AK023391 promotes tumorigenesis
and invasion of gastric cancer through activation of the PI3K/Akt
signaling pathway. J Exp Clin Cancer Res. 36:1942017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang J, Qiu M, Xu Y, Li M, Dong G, Mao Q,
Yin R and Xu L: Long noncoding RNA CCAT2 correlates with smoking in
esophageal squamous cell carcinoma. Tumour Biol. 36:5523–5528.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tian FM, Meng FQ and Wang XB:
Overexpression of long-noncoding RNA ZFAS1 decreases survival in
human NSCLC patients. Eur Rev Med Pharmacol Sci. 20:5126–5131.
2016.PubMed/NCBI
|
|
10
|
Ling H, Spizzo R, Atlasi Y, Nicoloso M,
Shimizu M, Redis RS, Nishida N, Gafà R, Song J, Guo Z, et al:
CCAT2, a novel noncoding RNA mapping to 8q24, underlies metastatic
progression and chromosomal instability in colon cancer. Genome
Res. 23:1446–1461. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kasagi Y, Oki E, Ando K, Ito S, Iguchi T,
Sugiyama M, Nakashima Y, Ohgaki K, Saeki H, Mimori K, et al: The
expression of CCAT2, a novel long noncoding RNA transcript, and
rs6983267 single-mucleotide polymorphism genotypes in colorectal
cancers. Oncology. 92:48–54. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lyu X, Li J, Yun X, Huang R, Deng X, Wang
Y, Chen Y and Xiao G: miR-181a-5p, an inducer of Wnt-signaling,
facilitates cell proliferation in acute lymphoblastic leukemia.
Oncol Rep. 37:1469–1476. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu XB, Zhang ZC, Han GS, Han JZ and Qiu
DP: Overexpression of miR-214 promotes the progression of human
osteosarcoma by regulating the Wnt/β-catenin signaling pathway. Mol
Med Rep. 15:1884–1892. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lecarpentier Y, Claes V, Vallée A and
Hébert JL: Interactions between PPAR gamma and the canonical
Wnt/beta-catenin pathway in type 2 diabetes and colon cancer. PPAR
Res. 2017:58790902017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo H, Hu G, Yang Q, Zhang P, Kuang W, Zhu
X and Wu L: Knockdown of long non-coding RNA CCAT2 suppressed
proliferation and migration of glioma cells. Oncotarget.
7:81806–81814. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma Y, Hu X, Shang C, Zhong M and Guo Y:
Silencing of long non-coding RNA CCAT2 depressed malignancy of oral
squamous cell carcinoma via Wnt/β-catenin pathway. Tumour Biol.
39:10104283177176702017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sarrafzadeh Sh, Geranpayeh L, Tasharrofi
B, Soudyab M, Nikpayam E, Iranpour M, Mirfakhraie R, Gharesouran J
and Ghafouri-Fard S and Ghafouri-Fard S: Expression study and
clinical correlations of MYC and CCAT2 in breast cancer patients.
Iran Biomed J. 21:303–311. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang JL, Liao Y, Qiu MX, Li J and An Y:
Long non-coding RNA CCAT2 promotes cell proliferation and invasion
through regulating Wnt/β-catenin signaling pathway in clear cell
renal cell carcinoma. Tumour Biol. 39:1010428317711312017.
View Article : Google Scholar
|
|
19
|
Meryet-Figuière M, Lambert B, Gauduchon P,
Vigneron N, Brotin E, Poulain L and Denoyelle C: An overview of
long non-coding RNAs in ovarian cancers. Oncotarget. 7:44719–44734.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qiu M, Xu Y, Yang X, Wang J, Hu J, Xu L
and Yin R: CCAT2 is a lung adenocarcinoma-specific long non-coding
RNA and promotes invasion of non-small cell lung cancer. Tumour
Biol. 35:5375–5380. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang CY, Hua L, Yao KH, Chen JT, Zhang JJ
and Hu JH: Long non-coding RNA CCAT2 is up-regulated in gastric
cancer and associated with poor prognosis. Int J Clin Exp Pathol.
8:779–785. 2015.PubMed/NCBI
|
|
22
|
Wang YJ, Liu JZ, Lv P, Dang Y, Gao JY and
Wang Y: Long non-coding RNA CCAT2 promotes gastric cancer
proliferation and invasion by regulating the E-cadherin and LATS2.
Am J Cancer Res. 6:2651–2660. 2016.PubMed/NCBI
|
|
23
|
Zhao Z, Wang J, Wang S, Chang H, Zhang T
and Qu J: LncRNA CCAT2 promotes tumorigenesis by over-expressed
Pokemon in non-small cell lung cancer. Biomed Pharmacother.
87:692–697. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sokol SY: Spatial and temporal aspects of
Wnt signaling and planar cell polarity during vertebrate embryonic
development. Semin Cell Dev Biol. 42:78–85. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang J, Jiang HY, Zhang LK, Xu WL, Qiao
YT, Zhu XG, Liu W, Zheng QQ and Hua ZC: C-FLIPL modulated
Wnt/β-catenin activation via association with TIP49 protein. J Biol
Chem. 292:2132–2142. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brautigam C, Raggioli A and Winter J: The
Wnt/β-catenin pathway regulates the expression of the miR-302
cluster in mouse ESCs and P19 cells. PLoS One. 8:e753152013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Picco G, Petti C, Centonze A, Torchiaro E,
Crisafulli G, Novara L, Acquaviva A, Bardelli A and Medico E: Loss
of AXIN1 drives acquired resistance to WNT pathway blockade in
colorectal cancer cells carrying RSPO3 fusions. EMBO Mol Med.
9:2932017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cai Y, He J and Zhang D: Long noncoding
RNA CCAT2 promotes breast tumor growth by regulating the Wnt
signaling pathway. Onco Targets Ther. 8:2657–2664. 2015.PubMed/NCBI
|