Introduction
Lung cancer is the leading cause of
cancer-associated mortality. It was estimated that 155,870
mortalities due to lung cancer occurred in the United States in
2017, which accounted for more than a quarter (26%) of all cancer
mortalities (1). Patients with
non-small cell lung cancer (NSCLC) approximately account for 80% of
lung cancer cases (2). Surgical
resection is the best treatment modality for localized lung cancer.
However, 79% of lung cancer cases are diagnosed at an advanced
stage. The cure rate of patients with advanced lung cancer using
conventional chemotherapy is low (3,4).
Tumor-associated genetic alterations have essential
roles in the tumorigenesis and progression of lung cancer (5). Extensive study has been focused on
finding oncogenes for the early diagnosis or effective therapy for
lung cancer. Several drugs that target molecular signaling pathways
have been applied to lung cancer treatment, particularly for
advanced lung cancer. For example, erlotinib, gefitinib and
afatinib worked efficiently for treating lung cancer cases with
epidermal growth factor receptor mutations (6–8).
Crizotinib is an anaplastic lymphoma kinase inhibitor for treating
lung cancer cases with ROS1 translocations (9). However, the molecular pathogenesis of
lung cancer has not been fully elucidated. Therefore, finding novel
key genes that are associated with tumor progression and prognosis
to further understand the molecular mechanisms of lung cancer is
necessary.
Replication factor C (RFC), also known as activator
1, was originally purified from the extraction of HeLa cells at the
end of 1980s (10). RFC was reported
to be a necessary factor for DNA replication of simian virus 40
in vitro (11). One large
subunit (RFC1/RFC140) and four small subunits (RFC2/RFC37,
RFC3/RFC36, RFC4/RFC40 and RFC5/RFC38) make up the RFC complex and
they were commonly found in numerous eukaryotes (12,13). RFC
functions as a clamp loader, which has a crucial role in loading
proliferating cell nuclear antigen (PCNA) onto primed DNA to
elongate the DNA chain (14,15).
Several RFC proteins have been found to be involved
in promoting cell proliferation in multiple carcinomas (16–20). Among
them, RFC5 subunit has been demonstrated to interact with
chromosome transmission fidelity protein 18 homolog (CTF18), This
type of interaction not only have a role in sister chromatid
cohesion but also stabilize the genome (21,22). Peng
et al (23) reported that RFC5
mediated resistance to temozolomide in glioma cells, which was
independent of methylguanine-DNA methyltransferase, and its
expression was positively regulated by forkhead box M1 (23). The upregulation of the RFC5 gene in
multidrug-resistant leukemia cells compared with parental HL-60
cell indicated that RFC5 might participate in drug resistance
(24). However, the molecular
mechanisms underlying lung cancer and the prognostic value of RFC5
remain to be reported.
The present study aimed to identify whether RFC5 has
a key role in promoting lung cancer progression and to investigate
its biological function. Furthermore, the prognostic implication
was also evaluated by using bioinformatic approaches. Our results
showed that RFC5 might serve as an independent predictor for
prognosis and a potential therapeutic target for lung cancer.
Materials and methods
Utilization of expression profile
datasets
All lung cancer microarray data with large sample
sizes were downloaded from the National Center for Biotechnology
Information (National Institutes of Health, Bethesda, MD, USA) Gene
Expression Omnibus database (GEO; http://www.ncbi.nlm.nih.gov/geo/). The relative
expression levels of RFC5 and clinical characteristics as well as
follow-up information were extracted for statistical analysis. The
probes corresponding to RFC5 were ‘203210_at’ and ‘203209_at’ with
which the expression of RFC5 was traced. The basic features of the
database were summarized in Table I.
In addition, ONCOMINE was used to compare RFC5 expression levels in
multiple datasets between cancer specimens and normal specimens.
Cluster analysis of RFC5 expression between lung cancer
histological subtypes and normal lung tissues was further performed
across 6 analyses. The thresholds, two-fold change, P<0.001 and
the top 10% gene rank, were used to reduce the false discovery rate
(FDR). The ONCOMINE data are available from https://www.oncomine.org.
 | Table I.Basic information of the 10 Gene
Expression Omnibus datasets. |
Table I.
Basic information of the 10 Gene
Expression Omnibus datasets.
| Cancer type | Accession no. | Number of samples
(tumor/normal) | P-value |
|---|
| Breast
carcinoma | GSE10780 | 42/143 | <0.0001 |
| Esophageal
carcinoma | GSE23400 | 53/53 | <0.0001 |
| Lung carcinoma | GSE30219 | 293/14 | <0.0001 |
| Gastric cancer | GSE13861 | 71/19 | <0.0001 |
| Acute lymphoblastic
leukemia | GSE26713 | 117/7 | 0.1439 |
| Colorectal
cancer | GSE32323 | 17/17 | 0.0022 |
| Bladder cancer | GSE3167 | 41/9 | 0.0134 |
| Melanoma | GSE3189 | 45/25 | 0.1813 |
| Cervical
cancer | GSE14407 | 33/24 | <0.0001 |
| Nasopharyngeal
carcinoma | GSE12452 | 31/10 | <0.0001 |
Tissue samples and RNA extraction
A total of 26 lung cancer and matched adjacent lung
cancer tissues were collected from patients with primary lung
cancer surgical resection at the Renmin Hospital of Wuhan
University (Hubei, China) from May to July 2017. None of the
patients received chemotherapy or radiotherapy prior to operation.
The present study was approved by the Ethics Committee of Renmin
Hospital of Wuhan University. Written informed consents were
obtained from all patients prior to enrollment in the study and
anonymity was guaranteed. All samples were immediately cut into
pieces and stored in liquid nitrogen. Total RNA was extracted from
tissue using TRIzol and quantified by NanoDrop 2000 (Thermo Fisher
Scientific, Inc., Waltham, MA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Reverse transcription was performed using 2 µg total
RNA with the RevertAid RT Reverse Transcription kit (Thermo Fisher
Scientific, Inc.). According to the manufacturer's protocol, qPCR
was performed using the QuantStudio 6 Flex Real-Time PCR system
(Thermo Fisher Scientific, Inc.) with SYBR Premix Ex Taq™ II
(Takara Bio, Inc., Otsu, Japan). The cycling parameters were 50°C
for 30 min, 94.5°C for 15 min, then 40 cycles of 96°C for 30 sec
and 59.7°C for 1 min. The sequences of the primers are as follows:
RFC5 forward, 5′-GAAGCAGACGCCATGACTCAG-3′ and reverse,
5′-GACCGAACCGAAACCTCGT-3′; β-actin forward,
5′-GAAGAGCTACGAGCTGCCTGA-3′ and reverse,
5′-CAGACAGCACTGTGTTGGCG-3′. The relative expression levels of RFC5
were quantified using RT-qPCR and the 2−ΔΔCq method in
triplicate and normalized to β-actin (25).
Gene set enrichment analysis
(GSEA)
GSEA was performed using a Java GSEA desktop
application that was downloaded from http://www.broad.mit.edu/gsea/. The GSE3141 dataset
was analyzed with the GMT file (c5.all.v5.1) gene set, to obtain
biological processes enriched by RFC5. The samples were divided
into a high RFC5 expression group (top 50%) and a low RFC5
expression group (bottom 50%). A total of four files including
expression datasets, gene sets, phenotype labels and chip platforms
were loaded for running GSEA according to the manufacturer's
specifications. Significant gene sets were confirmed with nominal
P-value <0.05 and FDR <0.25.
Kaplan-Meier plotter database
analysis
Kaplan-Meier plotter (www.kmplot.com) was used to assess the prognostic
significance of RFC5 expression in lung cancer. The database
includes gene expression data and clinicopathological features of
lung (26), breast (27), ovarian (28) and gastric cancer (29). In order to evaluate the prognostic
value of RFC5, the patient samples were divided into two groups,
high expression and low expression, on the basis of the median
expression of the RFC5 (high expression, top 50%; low expression,
bottom 50%). The Kaplan-Meier survival plots were obtained by
entering the survival time [overall survival (OS), first
progression (FP) and post-progression survival (PPS)] of patients
with NSCLC, respectively. The P-values of the log-rank test and
hazard ratio (HR) with 95% confidence intervals (CIs) were also
calculated.
Statistical analysis
Statistical analyses were performed with GraphPad
Prism (version 5.0; GraphPad Software, Inc., La Jolla, CA, USA) and
SPSS (version 19.0; SPSS, Inc., Chicago, IL, USA). A paired t-test
was used to compare the differences between the expression level of
RFC5 in tumor and adjacent normal tissues. An unpaired t-test was
used for unpaired comparisons. The comparisons between the
experimental groups and the healthy group were performed using
one-way ANOVA followed by Dunnett's multiple comparisons test. The
P-values were derived from ranked data as variance was unequal
among groups (Bartlett's test, P<0.05), and the values were
adjusted by Bonferroni's test. The associations between RFC5
expression and clinicopathological parameters were analyzed using
the χ2 test. Cox regression was used for univariate and
multivariate analysis to determine the independent factors that
have a significant effect on patient survival. The HR and 95% CIs
of the prognostic factors were calculated. P<0.05 was considered
to indicate a statistically significant difference.
Results
RFC5 is upregulated in multiple types
of cancer
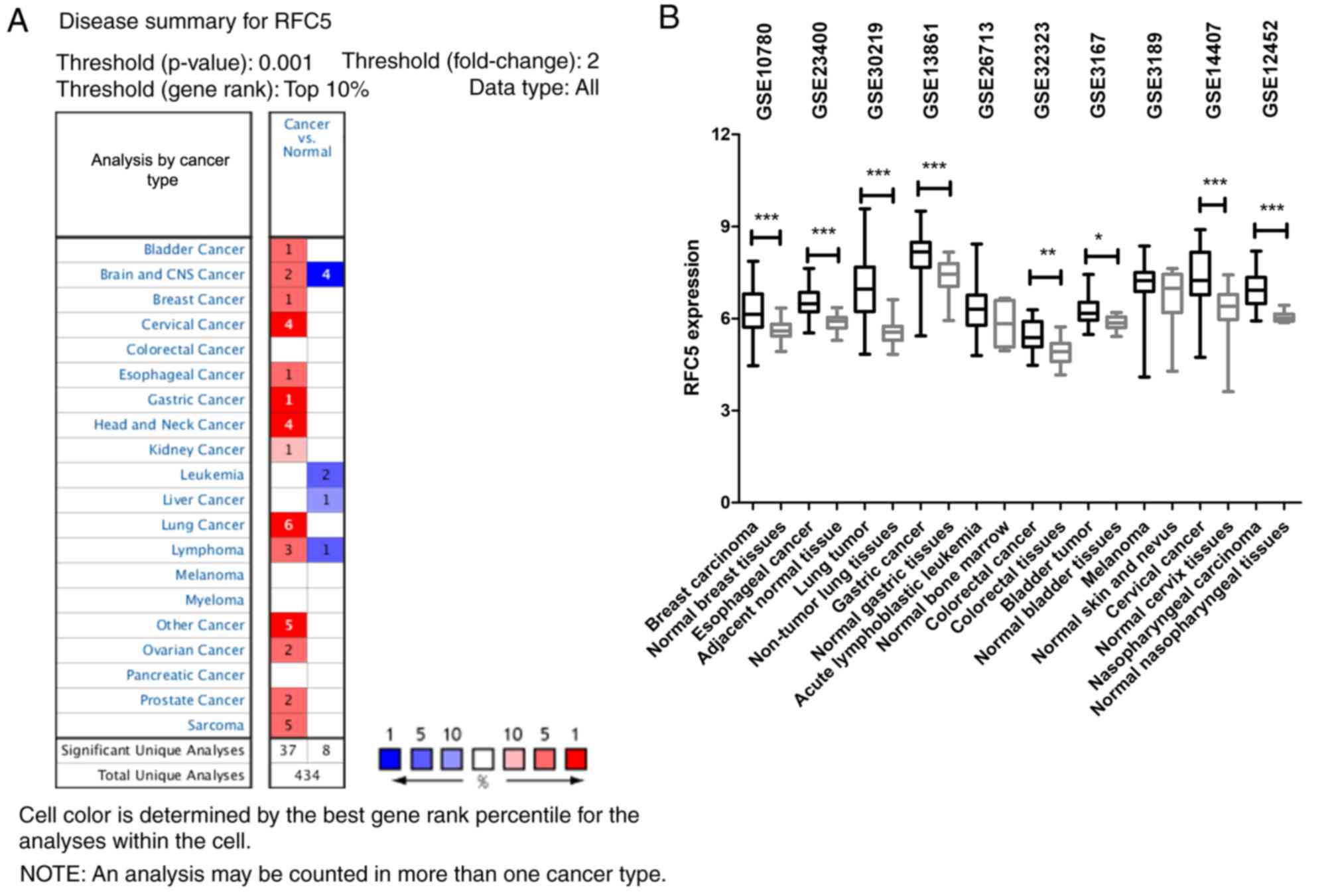
The ONCOMINE database was used to compare the levels
of RFC5 expression in cancer and normal samples. The results
indicated that RFC5 was overexpressed in multiple cancer types
(Fig. 1A). Among 20 common types of
cancer, the upregulation of RFC5 was identified in 14 cancer types.
The GEO database was searched to analyze the differences in RFC5
expression levels in various tumors. As indicated in Fig. 1B, RFC5 was upregulated in breast
carcinoma (P<0.0001), esophageal cancer (P<0.0001), lung
tumor (P<0.0001), gastric cancer (P<0.0001), colorectal
cancer (P=0.0022), bladder tumor (P=0.0134), cervical cancer
(P<0.0001) and nasopharyngeal carcinoma (P<0.0001) tissues
compared with normal tissues. These data indicated that RFC5 was
upregulated in a variety of tumor types, which suggested it might
be relevant to the oncogenesis of these tumors.
RFC5 is significantly overexpressed in
lung cancer
The publicly available GEO datasets were used to
analyze the expression levels of RFC5 mRNA in cancer and normal
tissues. Compared with normal tissues, the expression levels of
RFC5 were significantly higher in lung tumor (Fig. 2A), NSCLC (Fig. 2B), paired tumor (Fig. 2C and D), lung adenocarcinoma (ADC),
squamous cell carcinoma (SCC) and large cell carcinoma (LCC)
tissues (Fig. 2E). Furthermore, RFC5
was uniformly upregulated in six analyses in ONCOMINE. The level of
RFC5 expression was confirmed in 26 paired cancerous and adjacent
normal tissues from patients with lung cancer by RT-qPCR.
Consistent with the findings of previous bioinformatic analyses,
RFC5 was significantly overexpressed in lung cancer tissue compared
with adjacent tissues.
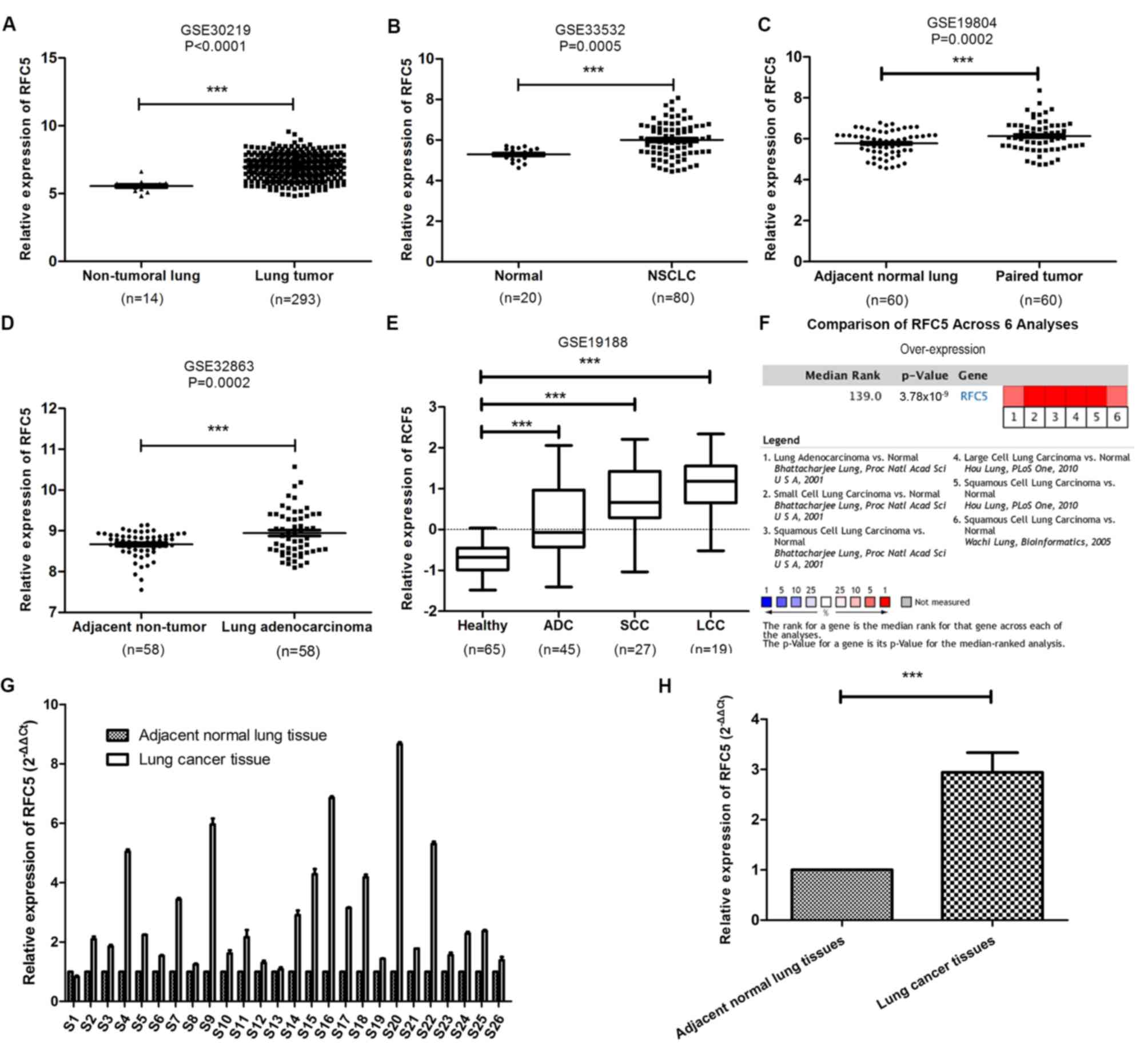 | Figure 2.Identification of RFC5 as an
overexpressed gene in lung cancer. (A-D) Comparison of the level of
RFC5 mRNA expression between cancerous and normal tissue from the
datasets (A) GSE30219, (B) GSE33532, (C) GSE19804 (paired samples)
and (D) GSE32863 (paired samples). ***P<0.001, paired t-test and
unpaired t-test. (E) Expression level of RFC5 in ADC, SCC and LCC
in the GSE19188 dataset. ***P<0.001, one-way ANOVA and Dunnett's
test. (F) Cluster analysis of RFC5 in different data sets of lung
cancer subtypes. Red, significant overexpression. The P-value was
calculated using the meta-analysis. (G) Expression levels of RFC5
mRNA in 26-paired lung cancer were assessed by RT-qPCR. (H)
Comparison of the expression levels of RFC5 in lung cancer tissues
and adjacent tissues. ***P<0.001; RFC5, replication factor C
subunit 5; ADC, adenocarcinoma; LCC, large cell carcinoma; SCC,
squamous cell carcinoma. |
RFC5 expression is associated with the
clinicopathological characteristics of lung cancer
In order to elucidate the association between RFC5
expression and the clinicopathological characteristics of lung
cancer, the GSE30219 dataset was analyzed by χ2 test.
RFC5 expression was associated with sex (P=0.0300), T stage
(P<0.0001), N stage (P<0.0001) and relapse (P=0.0010).
However, there were no significant associations between RFC5
expression with age (P=0.5580) and M stage (P=0.2820; Table II). These results demonstrated that
RFC5 expression was associated with the progression of lung
cancer.
 | Table II.Associations between RFC5 expression
and clinicopathological characteristics of patients with lung
cancer in the GSE30219 dataset. |
Table II.
Associations between RFC5 expression
and clinicopathological characteristics of patients with lung
cancer in the GSE30219 dataset.
|
|
| RFC5
expression |
|
|
|---|
|
|
|
|
|
|
|---|
|
Characteristics | N | High
expression | Low expression | χ2 | P-value |
|---|
| Age |
|
|
| 0.342 | 0.5580 |
|
≤62.0 | 146 | 71 | 75 |
|
|
|
>62.0 | 146 | 76 | 70 |
|
|
| Sex |
|
|
| 4.711 | 0.0300 |
|
Female | 43 | 15 | 28 |
|
|
|
Male | 250 | 132 | 118 |
|
|
| T stage |
|
|
| 29.997 | <0.0001 |
| T1 | 166 | 61 | 105 |
|
|
| T2 | 69 | 42 | 27 |
|
|
|
T3-4 | 52 | 40 | 12 |
|
|
| N stage |
|
|
| 37.451 | <0.0001 |
|
Positive | 93 | 71 | 22 |
|
|
|
Negative | 198 | 75 | 123 |
|
|
| Metastasis |
|
|
| 2.000 | 0.2820a |
|
Yes | 8 | 6 | 2 |
|
|
| No | 282 | 140 | 142 |
|
|
| Relapse |
|
|
| 11.757 | 0.0010 |
| No | 164 | 65 | 99 |
|
|
|
Yes | 114 | 69 | 45 |
|
|
High expression of RFC5 indicates a
poor prognosis
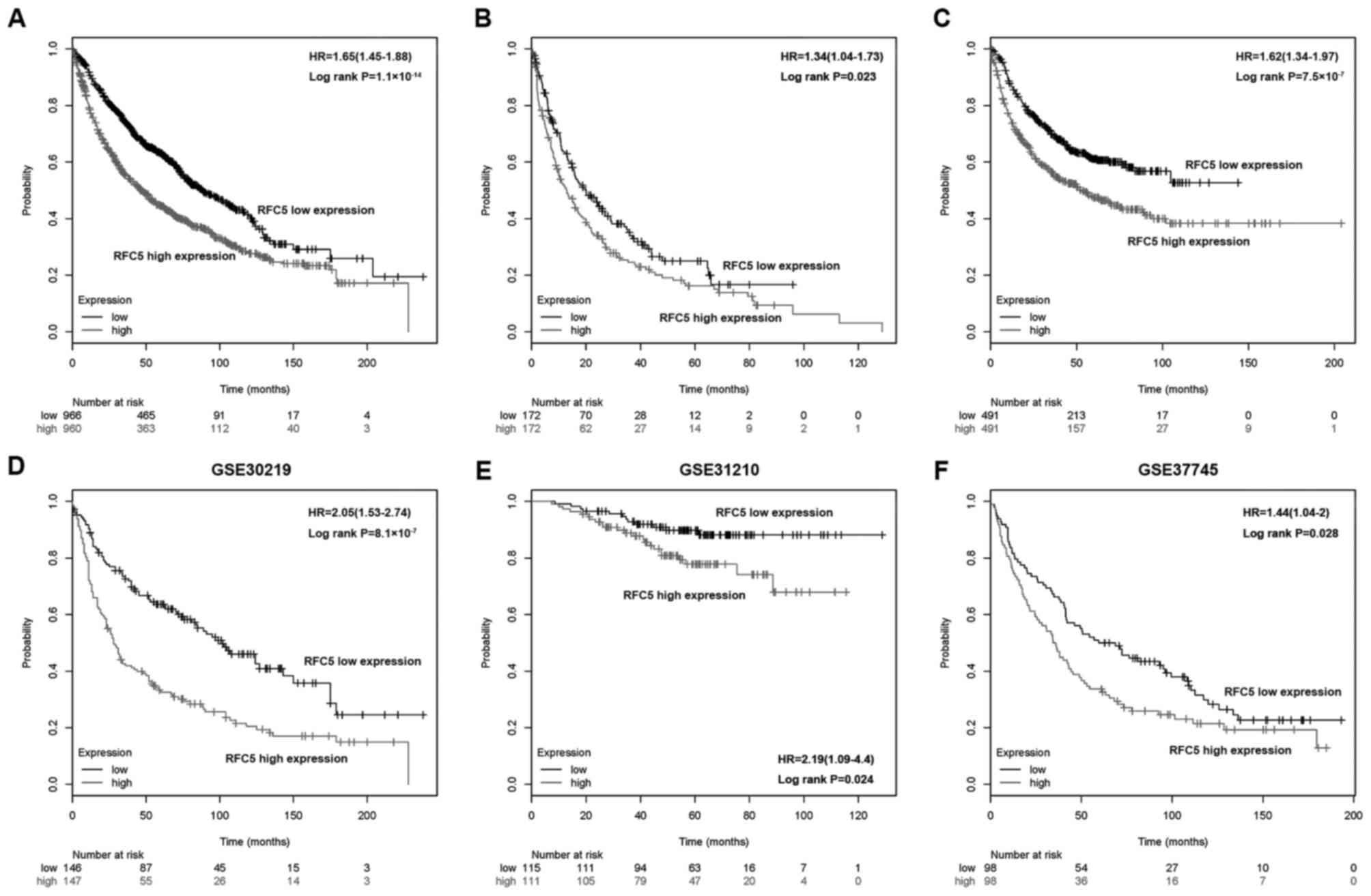
To further investigate the association between RFC5
expression and the outcomes of patients with lung cancer,
Kaplan-Meier analysis was applied to assess the OS, FP and PPS in
the high RFC5 expression and low RFC5 expression groups. As shown
in Fig. 3A-C, a high expression of
RFC5 corresponded to a poorer OS (HR, 1.65; 95% CI, 1.45–1.88;
P=1.1×10−14), FP (HR, 1.34; 95% CI, 1.04–1.73; P=0.023)
and PPS (HR, 1.62; 95% CI, 1.34–1.97; P=7.5×10−7),
respectively. Kaplan-Meier analysis was performed in three GEO
datasets (GSE30219, GSE31210 and GSE37745, respectively), which
consistently indicated that a low RFC5 expression was associated
with improved OS (Fig. 3D-F).
Furthermore, univariate and multivariate Cox regression analyses
were performed to investigate the independent factors that affect
patient survival. As shown in Table
III, the high RFC5 expression group exhibited a significantly
increased risk of OS (HR, 2.027; 95% CI, 1.498–2.742, P<0.0001)
compared with the low RFC5 expression group. The characteristics
that were significant in the univariate analyses were then
incorporated into the multivariate analyses. Age (HR, 2.014; 95%
CI, 1.478–2.744, P<0.0001), T stage (HR, 1.363; 95% CI,
1.106–1.678, P<0.0040) and RFC5 expression (HR, 1.557; 95% CI,
1.137–2.132, P<0.0060) were indicated to be significant risk
factors in multivariate analysis and were determined as independent
prognostic factors of OS. These results also indicated that RFC5
expression (HR, 1.736; 95% CI, 1.143–2.636, P <0.0100); T stage
(HR, 1.461; 95% CI, 1.137–1.878, P<0.0030) and N stage (HR,
1.461; 95% CI, 1.016–2.100, P<0.0410) were independent
prognostic factors for disease-free survival (Table IV).
 | Table III.Univariate and Multivariate analysis
of the effect of covariates on overall survival for patients with
lung cancer in the GSE30219 dataset. |
Table III.
Univariate and Multivariate analysis
of the effect of covariates on overall survival for patients with
lung cancer in the GSE30219 dataset.
| A, Univariate
analysis |
|---|
|
|---|
| Variables | HR (95% CI) | P-value |
|---|
| Age |
|
|
| ≤62 vs.
>62 years | 2.101
(1.544–2.858) | <0.0001 |
| Sex |
|
|
| Female
vs. male | 1.789
(1.098–2.916) |
0.0200 |
| T stage |
|
|
| T1 vs.
T2 vs. T3 vs. T4 | 1.589
(1.368–1.845) | <0.0001 |
| N stage |
|
|
| N0 vs.
N1 | 1.770
(1.431–2.188) | <0.0001 |
| M stage |
|
|
| M0 vs.
M1 | 2.456
(0.908–6.644) |
0.0770 |
| RFC5
expression |
|
|
| High
vs. low | 2.027
(1.498–2.742) | <0.0001 |
|
| B, Multivariate
analysis |
|
|
Variables | HR (95%
CI) | P-value |
|
| Age |
|
|
| ≤62 vs.
>62 years | 2.014
(1.478–2.744) | <0.0001 |
| Sex |
|
|
| Female
vs. male | 1.515
(0.926–2.478) |
0.0980 |
| T stage |
|
|
| T1 vs.
T2 vs. T3 vs. T4 | 1.363
(1.106–1.678) |
0.0040 |
| N stage |
|
|
| N0 vs.
N1 | 1.259
(0.935–1.696) |
0.1280 |
| M stage |
|
|
| M0 vs.
M1 | – | – |
| RFC5
expression |
|
|
| High
vs. low | 1.557
(1.137–2.132) |
0.0060 |
 | Table IV.Univariate and multivariate analysis
of the effect of covariates on disease-free survival for patients
with lung cancer in the GSE30219 dataset. |
Table IV.
Univariate and multivariate analysis
of the effect of covariates on disease-free survival for patients
with lung cancer in the GSE30219 dataset.
| A, Univariate
analysis |
|---|
|
|---|
| Variables | HR (95% CI) | P-value |
|---|
| Age |
|
|
| ≤62 vs.
>62 years | 1.332
(0.902–1.968) |
0.1490 |
| Sex |
|
|
| Female
vs. male | 1.301
(0.740–2.286) |
0.3610 |
| T stage |
|
|
| T1 vs.
T2 vs. T3 vs. T4 | 1.852
(1.541–2.226) | <0.0001 |
| N stage |
|
|
| N0 vs.
N1 | 2.309
(1.786–2.986) | <0.0001 |
| M stage |
|
|
| M0 vs.
M1 | 3.705
(1.359–10.095) |
0.0100 |
| RFC5
expression |
|
|
| High
vs. low | 2.327
(1.563–3.464) | <0.0001 |
|
| B, Multivariate
analysis |
|
|
Variables | HR (95%
CI) | P-value |
|
| Age |
|
|
| ≤62 vs.
>62 years | – | – |
| Sex |
|
|
| Female
vs. male | – | – |
| T stage |
|
|
| T1 vs.
T2 vs. T3 vs. T4 | 1.461
(1.137–1.878) | 0.0030 |
| N stage |
|
|
| N0 vs.
N1 | 1.461
(1.016–2.100) | 0.0410 |
| M stage |
|
|
| M0 vs.
M1 | 2.640
(0.960–7.265) | 0.0600 |
| RFC5
expression |
|
|
| High
vs. low | 1.736
(1.143–2.636) | 0.0100 |
RFC5 enhances the proliferation of
tumor cells in lung cancer
GSEA was used to analyze the biological processes of
RFC5 in lung cancer. The GSE3141 dataset was analyzed with GMT file
C5 (GO gene set). The first 20 relevant biological processes that
met P-value <0.05 and false discovery rates <0.25 are shown
in Table V. The results showed that
several gene sets that are associated with cell cycle and DNA
damage were enriched in the RFC5 overexpression group, which
suggested the RFC5 is involved in the proliferation of lung cancer
cells (Fig. 4).
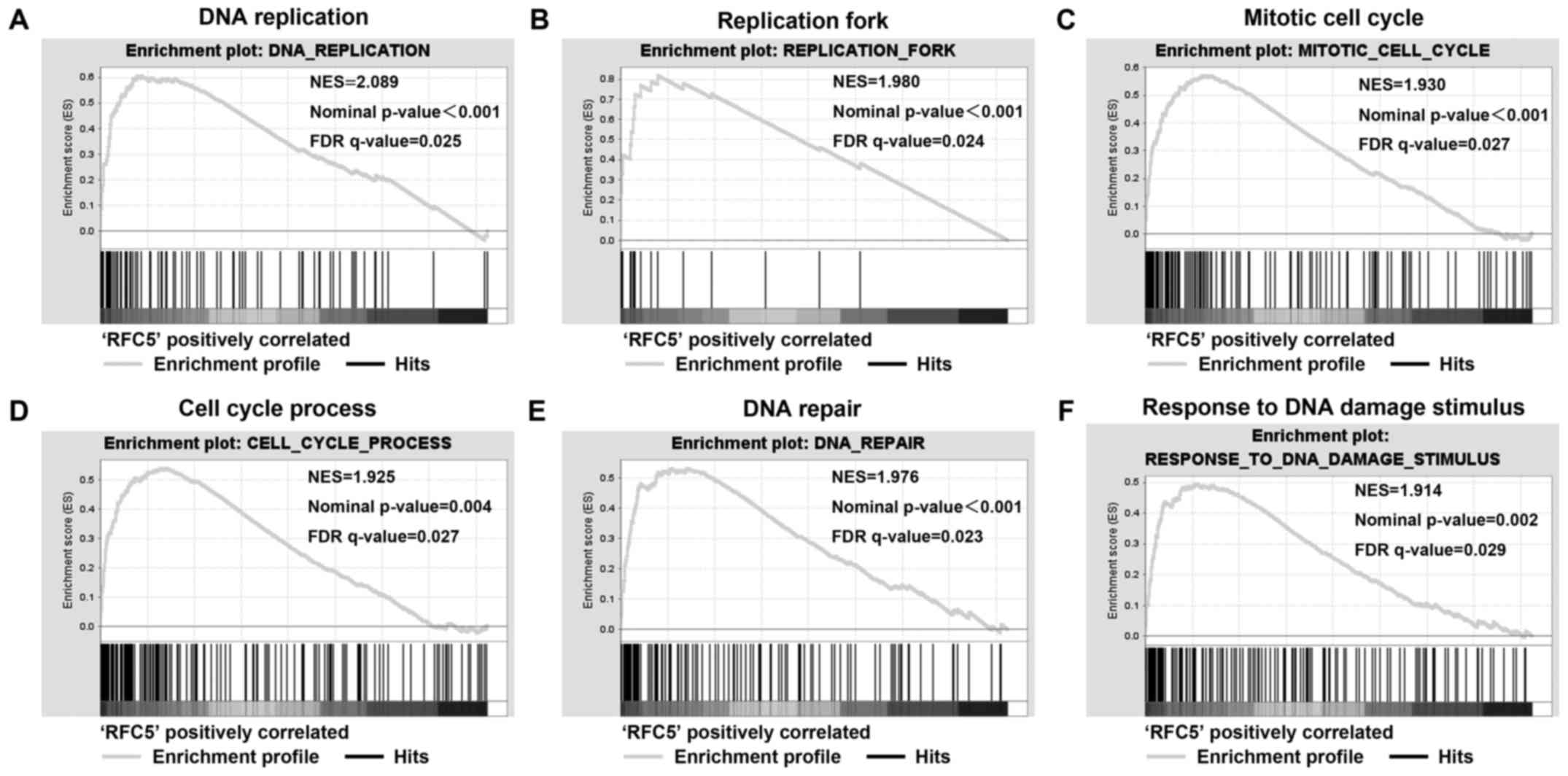 | Figure 4.RFC5 enriched cell cycle and DNA
damage process in lung cancer. Gene set enrichment analysis was
performed by GSEA using the GSE3141 dataset. The gene profile of
high RFC5 expression groups (top 50%) and low RFC5 expression
groups (bottom 50%) were loaded to the GSEA software, and the
‘c5.all.v5.1’ gene set was selected to process the analysis. (A)
RFC5 high expression enriched DNA replication, (B) replication
fork, (C) mitotic cell cycle, (D) cell cycle process, (E) DNA
repair, (F) response to DNA damage stimulus signature genes. GSEA,
gene set enrichment analysis; NES, normal enrichment score; NOM
P-value, normal P-value; FDR q-value, false discovery rate
q-value. |
 | Table V.Enrichment of biological processes in
the RFC5 high expression group. |
Table V.
Enrichment of biological processes in
the RFC5 high expression group.
| No. | GS details | Size | ES | NES | NOM P-value | FDR q-value |
|---|
| 1 | DNA
replication | 88 | 0.607 | 2.085 | 0.002 | 0.037 |
| 2 | Chromosomal
part | 88 | 0.667 | 2.083 | <0.001 | 0.020 |
| 3 | DNA-dependent DNA
replication | 47 | 0.672 | 2.072 | 0.002 | 0.015 |
| 4 | Chromosome | 114 | 0.632 | 2.071 | <0.001 | 0.012 |
| 5 | DNA metabolic
process | 232 | 0.518 | 2.026 | <0.001 | 0.018 |
| 6 | Double-strand break
repair | 22 | 0.739 | 2.013 | 0.002 | 0.019 |
| 7 | Nuclear part | 487 | 0.527 | 1.988 | <0.001 | 0.024 |
| 8 | Nuclear
membrane | 47 | 0.651 | 1.985 | <0.001 | 0.022 |
| 9 | Nuclear pore | 28 | 0.761 | 1.982 | <0.001 | 0.020 |
| 10 | Nuclear membrane
part | 39 | 0.688 | 1.958 | <0.001 | 0.026 |
| 11 | Nuclear
envelope | 67 | 0.607 | 1.951 | <0.001 | 0.026 |
| 12 | Organelle
lumen | 391 | 0.512 | 1.940 | <0.001 | 0.027 |
| 13 | Membrane-enclosed
lumen | 391 | 0.512 | 1.940 | <0.001 | 0.025 |
| 14 | Mitotic cell
cycle | 136 | 0.571 | 1.922 | 0.006 | 0.029 |
| 15 | DNA repair | 117 | 0.533 | 1.918 | <0.001 | 0.029 |
| 16 | Envelope | 158 | 0.587 | 1.912 | <0.001 | 0.030 |
| 17 | Organelle
envelope | 158 | 0.587 | 1.912 | <0.001 | 0.028 |
| 18 | Translation factor
activity nucleic acid binding | 29 | 0.696 | 1.908 | <0.001 | 0.029 |
| 19 | RNA splicing | 66 | 0.651 | 1.907 | <0.001 | 0.027 |
| 20 | Cell cycle
process | 172 | 0.539 | 1.905 | 0.006 | 0.027 |
Discussion
There is no doubt that DNA replication is a crucial
process during cell proliferation and enables the ability to
proliferate indefinitely, which is a hallmark of tumor cells
(5). A number of genes that are
associated with DNA replication are deregulated in cancer cells.
Therefore, the present study focused on RFC, which is involved in
DNA replication and damage repair as well as cell division and
proliferation (30–34). In general, RFC is a structurally
specific DNA-binding protein that preferentially binds to the 3′
end of the template primer. A study has indicated that RFC
catalyzed the formation of a cyclic structure of PCNA around the
primers in an ATP-dependent manner (35). Munshi et al (36) observed that cyclin-dependent kinases
reduced the stability of RFC, inactivating it in the S phase to
regulate DNA replication (36).
However despite being an important component of the RFC, the role
of RFC5 in lung cancer remains unknown. Therefore, the present
study investigated the expression patterns, potential biological
functions and the prognostic value of RFC5 in lung cancer.
Bioinformatics is a multidisciplinary research
field, and bioinformatic analysis is particularly used for
developing methods and software tools to identify candidate genes.
Such understanding allows the efficient elucidation of the genetic
basis of diseases (37). In the
present study, the ONCOMINE database and GEO datasets were applied
to analyze RFC5 expression in lung cancer. The results suggested
that RFC5 was highly expressed in lung cancer samples compared with
normal samples. The expression levels of RFC5 in 26 paired samples
were confirmed by RT-qPCR, and consistent results were
attained.
To date, conventional surgery and chemotherapy
remain the main treatment modalities for treating lung cancer
(38). However, numerous lung cancer
cases are diagnosed at an advanced stage. When treating patients
with advanced lung cancer using conventional surgical resection and
chemotherapy, the 5-year relative survival rate is only 4%
(3). Therefore, it is important to
find a novel target molecule that can be used to diagnose and used
for therapy. The prognostic value of RFC5 was rarely investigated
in previous studies. In the present study, RFC5 was identified to
be a potential independent prognostic factor for patients with lung
cancer. A higher expression of RFC5 was significantly associated
with higher T stage, more advanced regional lymph node metastasis
and a higher probability of relapse. These findings suggested a
potential role of RFC5 in the progression of lung cancer, which
might contribute to the development of accurate diagnosis and
personalized treatment strategies.
A number of studies revealed the association between
RFC5 overexpression and DNA replication. The GSEA provides an
improved understanding of the biological functional enrichment in
the high RFC5 expression groups. The gene sets that were most
significant were associated with DNA replication or DNA damage,
which suggested that RFC5 might promote the proliferation of lung
cancer cells. A study of other RFC subunits found that the
interaction between RFC2, RFC3 and c-myc promoted cell division
(39). Furthermore, cDNA microarray
analysis and meta-analysis confirmed that the RFC4 mRNA was
abnormally high in a variety of malignant tumors, including ADC,
cervical cancer, head and neck squamous cell carcinoma and
colorectal cancer (18,40–43). Chae
et al (17) reported that RFC3
might promote cell proliferation, and its promoter directly binds
with CAMP responsive element binding protein in acute myeloid
leukemia. The copy number of RFC3 also reported to be increased in
other types of cancer, and knocking out RFC3 was demonstrated to
inhibit the growth of tumor cells. As the five RFC subunits possess
similar conserved regions of ATP/GTP-binding proteins, each of the
subunit has a remarkable degree of similarity (13); which further indicated that they might
be involved in similar biological processes.
In conclusion, this is the first study to
systematically demonstrate that RFC5 is an oncogene, which is
closely associated with the prognosis of lung cancer. RFC5 was
significantly overexpressed in lung cancer tissues compared with
normal tissues. Furthermore, a high expression level of RFC5 was
associated with poor clinicopathological characteristics and
proliferation of tumor cells. In summary, RFC5 might serve as a
prognostic biomarker and novel therapeutic target for lung
cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grants no. 81501352, 81270607
and 81541027).
Availability of data and materials
The GEO datasets analyzed during the current study
are available from the National Center for Biotechnology
Information (National Institutes of Health, Bethesda, MD, USA)
(http://www.ncbi.nlm.nih.gov/geo/). The
online database Kaplan-Meier Plotter are available from http://kmplot.com/analysis/index.php?p=service&cancer=lung.
The ONCOMINE data are available from https://www.oncomine.org.
Authors' contributions
MW and TX conducted the experiments and performed
the statistical analysis. YW and QY interpreted the experimental
results, wrote and revised the manuscript. SX and QY collected
clinical samples and provided critical advice. JX and QZ conceived
and designed the study. All authors contributed to this manuscript.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Renmin Hospital of Wuhan University (Wuhan, China).
Written informed consents were obtained from all patients prior to
enrollment in the study, and anonymity was ensured.
Patient consent for publication
Written informed consent were obtained from all
examined patients for the publication of their data.
Competing interests
The authors declare that they have no conflicts of
interest.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA A Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar
|
|
2
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torre LA, Siegel RL and Jemal A: Lung
Cancer Statistics. Adv Exp Med Biol. 893:1–19. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sung WJ, Kim H and Park KK: The biological
role of epithelial-mesenchymal transition in lung cancer (Review).
Oncol Rep. 36:1199–1206. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kato T, Yoshioka H, Okamoto I, Yokoyama A,
Hida T, Seto T, Kiura K, Massey D, Seki Y and Yamamoto N: Afatinib
versus cisplatin plus pemetrexed in Japanese patients with advanced
non-small cell lung cancer harboring activating EGFR mutations:
Subgroup analysis of LUX-Lung 3. Cancer Sci. 106:1202–1211. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hoshi H, Hiyama G, Ishikawa K, Inageda K,
Fujimoto J, Wakamatsu A, Togashi T, Kawamura Y, Takahashi N, Higa
A, et al: Construction of a novel cell-based assay for the
evaluation of anti-EGFR drug efficacy against EGFR mutation. Oncol
Rep. 37:66–76. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li K, Yang M, Liang N and Li S:
Determining EGFR-TKI sensitivity of G719X and other uncommon EGFR
mutations in non-small cell lung cancer: Perplexity and solution
(Review). Oncol Rep. 37:1347–1358. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Juan O and Popat S: Crizotinib for ROS1
patients: One small step in biomarker testing, one giant leap for
advanced NSCLC patients. Lung Cancer. 104:131–133. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen M, Pan ZQ and Hurwitz J: Studies of
the cloned 37-kDa subunit of activator 1 (replication factor C) of
HeLa cells. Proc Natl Acad Sci USA. 89:5211–5215. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Virshup DM and Kelly TJ: Purification of
replication protein C, a cellular protein involved in the initial
stages of simian virus 40 DNA replication in vitro. Proc Natl Acad
Sci USA. 86:3584–3588. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Uhlmann F, Cai J, Flores-Rozas H, Dean FB,
Finkelstein J, O'Donnell M and Hurwitz J: In vitro reconstitution
of human replication factor C from its five subunits. Proc Natl
Acad Sci USA. 93:6521–6526. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cullmann G, Fien K, Kobayashi R and
Stillman B: Characterization of the five replication factor C genes
of Saccharomyces cerevisiae. Mol Cell Biol. 15:4661–4671. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Majka J and Burgers PM: The PCNA-RFC
families of DNA clamps and clamp loaders. Prog Nucleic Acid Res Mol
Biol. 78:227–260. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang G, Gibbs E, Kelman Z, O'Donnell M
and Hurwitz J: Studies on the interactions between human
replication factor C and human proliferating cell nuclear antigen.
Proc Natl Acad Sci USA. 96:1869–1874. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Niu G, Wang D, Pei Y and Sun L: Systematic
identification of key genes and pathways in the development of
invasive cervical cancer. Gene. 618:28–41. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chae HD, Mitton B, Lacayo NJ and Sakamoto
KM: Replication factor C3 is a CREB target gene that regulates cell
cycle progression through the modulation of chromatin loading of
PCNA. Leukemia. 29:1379–1389. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xiang J, Fang L, Luo Y, Yang Z, Liao Y,
Cui J, Huang M, Yang Z, Huang Y, Fan X, et al: Levels of human
replication factor C4, a clamp loader, correlate with tumor
progression and predict the prognosis for colorectal cancer. J
Transl Med. 12:3202014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lockwood WW, Thu KL, Lin L, Pikor LA,
Chari R, Lam WL and Beer DG: Integrative genomics identified RFC3
as an amplified candidate oncogene in esophageal adenocarcinoma.
Clin Cancer Res. 18:1936–1946. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arai M, Kondoh N, Imazeki N, Hada A,
Hatsuse K, Matsubara O and Yamamoto M: The knockdown of endogenous
replication factor C4 decreases the growth and enhances the
chemosensitivity of hepatocellular carcinoma cells. Liver Int.
29:55–62. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Murakami T, Takano R, Takeo S, Taniguchi
R, Ogawa K, Ohashi E and Tsurimoto T: Stable interaction between
the human proliferating cell nuclear antigen loader complex
Ctf18-replication factor C (RFC) and DNA polymerase {epsilon} is
mediated by the cohesion-specific subunits, Ctf18, Dcc1 and Ctf8. J
Biol Chem. 285:34608–34615. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maradeo ME, Garg A and Skibbens RV: Rfc5p
regulates alternate RFC complex functions in sister chromatid
pairing reactions in budding yeast. Cell Cycle. 9:4370–4378. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Peng WX, Han X, Zhang CL, Ge L, Du FY, Jin
J and Gong AH: FoxM1-mediated RFC5 expression promotes temozolomide
resistance. Cell Biol Toxicol. 33:527–537. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu SM, Chen W and Wang J: Distinguishing
between cancer cell differentiation and resistance induced by
all-trans retinoic acid using transcriptional profiles and
functional pathway analysis. Sci Rep. 4:55772014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Klein D: Quantification using real-time
PCR technology: Applications and limitations. Trends Mol Med.
8:257–260. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gyorffy B, Surowiak P, Budczies J and
Lanczky A: Online survival analysis software to assess the
prognostic value of biomarkers using transcriptomic data in
non-small-cell lung cancer. PLoS One. 8:e822412013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gyorffy B, Lanczky A, Eklund AC, Denkert
C, Budczies J, Li Q and Szallasi Z: An online survival analysis
tool to rapidly assess the effect of 22,277 genes on breast cancer
prognosis using microarray data of 1,809 patients. Breast Cancer
Res Treat. 123:725–731. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gyorffy B, Lanczky A and Szallasi Z:
Implementing an online tool for genome-wide validation of
survival-associated biomarkers in ovarian-cancer using microarray
data from 1287 patients. Endocr Relat Cancer. 19:197–208. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Szasz AM, Lanczky A, Nagy A, Förster S,
Hark K, Green JE, Boussioutas A, Busuttil R, Szabó A and Győrffy B:
Cross-validation of survival associated biomarkers in gastric
cancer using transcriptomic data of 1,065 patients. Oncotarget.
7:49322–49333. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gorbea CM, Marchand P, Jiang W, Copeland
NG, Gilbert DJ, Jenkins NA and Bond JS: Cloning, expression and
chromosomal localization of the mouse meprin beta subunit. J Biol
Chem. 268:21035–21043. 1993.PubMed/NCBI
|
|
31
|
Shimada M, Okuzaki D, Tanaka S, Tougan T,
Tamai KK, Shimoda C and Nojima H: Replication factor C3 of
Schizosaccharomyces pombe, a small subunit of replication factor C
complex, plays a role in both replication and damage checkpoints.
Mol Bio Cell. 10:3991–4003. 1999. View Article : Google Scholar
|
|
32
|
Naiki T, Shimomura T, Kondo T, Matsumoto K
and Sugimoto K: Rfc5, in cooperation with rad24, controls DNA
damage checkpoints throughout the cell cycle in Saccharomyces
cerevisiae. Mol Cell Biol. 20:5888–5896. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim HS and Brill SJ: Rfc4 interacts with
Rpa1 and is required for both DNA replication and DNA damage
checkpoints in Saccharomyces cerevisiae. Mol Cell Biol.
21:3725–3737. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tomida J, Masuda Y, Hiroaki H, Ishikawa T,
Song I, Tsurimoto T, Tateishi S, Shiomi T, Kamei Y, Kim J, et al:
DNA damage-induced ubiquitylation of RFC2 subunit of replication
factor C complex. J Biol Chem. 283:9071–9079. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shiomi Y, Usukura J, Masamura Y, Takeyasu
K, Nakayama Y, Obuse C, Yoshikawa H and Tsurimoto T: ATP-dependent
structural change of the eukaryotic clamp-loader protein,
replication factor C. Proc Natl Acad Sci USA. 97:14127–14132. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Munshi A, Cannella D, Brickner H,
Salles-Passador I, Podust V, Fotedar R and Fotedar A: Cell
cycle-dependent phosphorylation of the large subunit of replication
factor C (RF-C) leads to its dissociation from the RF-C complex. J
Biol Chem. 278:48467–48473. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Qi S, Wang CH, Li CL, Wang P and Liu MH:
Candidate genes investigation for severe nonalcoholic fatty liver
disease based on bioinformatics analysis. Medicine (Baltimore).
96:e77432017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Arnold BN, Thomas DC, Rosen JE, Salazar
MC, Detterbeck FC, Blasberg JD, Boffa DJ and Kim AW: Effectiveness
of local therapy for stage I non-small-cell lung cancer in
nonagenarians. Surgery. 162:640–651. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Koch HB, Zhang R, Verdoodt B, Bailey A,
Zhang CD, Yates JR III, Menssen A and Hermeking H: Large-scale
identification of c-MYC-associated proteins using a combined
TAP/MudPIT approach. Cell cycle. 6:205–217. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Erdogan E, Klee EW, Thompson EA and Fields
AP: Meta-analysis of oncogenic protein kinase Ciota signaling in
lung adenocarcinoma. Clin Cancer Res. 15:1527–1533. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kang BY, You H, Bandyopadhyay S, Agrawal
N, Melchert RB, Basnakian AG, Liu Y and Hermonat PL: Cervical
cancer isolate PT3, super-permissive for adeno-associated virus
replication, over-expresses DNA polymerase delta, PCNA, RFC and
RPA. BMC Microbiol. 9:792009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Narayan G, Bourdon V, Chaganti S,
Arias-Pulido H, Nandula SV, Rao PH, Gissmann L, Dürst M, Schneider
A, Pothuri B, et al: Gene dosage alterations revealed by cDNA
microarray analysis in cervical cancer: Identification of candidate
amplified and overexpressed genes. Genes chromosomes cancer.
46:373–384. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Slebos RJ, Yi Y, Ely K, Carter J, Evjen A,
Zhang X, Shyr Y, Murphy BM, Cmelak AJ and Burkey BB: Gene
expression differences associated with human papillomavirus status
in head and neck squamous cell carcinoma. Clinical cancer research:
an official journal of the American Association for Cancer
Research. 12:701–709. 2006. View Article : Google Scholar : PubMed/NCBI
|