Introduction
Liver transplantation (LT) is currently the most
viable and effective treatment option for patients with
hepatocellular carcinoma (HCC) except for surgical resection, thus
it may be used for patients where resection is not an option
(1). Although the implementation of
the Milan and University of California at San Francisco (UCSF)
criteria has improved post-LT survival rates, patients who do not
fit the UCSF criteria currently undergo LT worldwide (2,3). LT for
HCC has an associated high recurrence rate, particularly for
patients with advanced disease (10–60%), in addition, the overall
survival (OS) and disease-free survival (DFS) rates for post-LT
patients are also higher (LT, OS vs. DFS, 70–98% vs. 27–35%),
compared with those who undergo liver resection (LR OS vs. DFS,
60–72% vs. 18–37%) (3–7). Furthermore, no consensus on the most
suitable prophylaxis and treatment for recurrent HCC following LT
is currently available. Although sorafenib has demonstrated
efficacy for tumor recurrence (1,2,8,9), the
results are unsatisfactory, with a challenging adverse event
profile (10,11). Sorafenib also exhibits less favorable
results in Asian patients compared with European patients (9,10).
FK506 is an immunosuppressor that works by
inhibiting calmodulin (12). It is
currently used in LT patients, and is extensively used in post-LT
patients with benign liver disease. However, long-term application
of FK506 increases the risk of infection and de novo tumors,
and particularly contributes to tumor relapse in post-LT patients
with HCC (13–15). Until the introduction of sirolimus
(SRL) being used with organ transplantations, there were no
replacement drugs to overcome the effects of long-term use of FK506
(15,16). Accumulating evidence supports the
clinical safety and efficacy of immunosuppressant selection, and
replacing FK506 therapy with SRL following LT for patients with HCC
(13,15–17).
Additionally, SRL offers a dual function, as it acts as an
efficacious immunosuppressor and an antineoplastic agent (13,18,19), and
therefore, may effectively reduce graft rejection and reduce the
high relapse risk associated with long-term application of
FK506.
When combined with SRL, huaier granules (PS-T), a
type of traditional Chinese medicine (TCM), have been demonstrated
to inhibit tumor growth, reduce vascular endothelial growth factor
expression, and improve survival rates in patients with HCC
(8,18–22). As an
immunomodulator, thymalfasin (also known as Zadaxin) promotes
maturation and differentiation of T cells and NK cells by affecting
tumor-cell-related antigen expression and differentiation, and
increasing tumor immunogenicity; it has been demonstrated to be
safe for post-LT patients with HCC, with no increased risk of
rejection (23,24). Although monotherapy with SRL led to
rather effective results, no relevant research has been published
on combining SRL, PS-T and thymalfasin. Although the application of
SRL and huaier, thymalfasin and huaier, or single therapy, has been
studied (16–18,20), their
effects are limited in patients with LT, and there were fewer
studies regarding patients following LT (16,20).
Therefore, to improve survival and decrease recurrence rates, the
effectiveness of a combined therapy based on SRL, thymalfasin and
PS-T for LT patients with advanced HCC was investigated. In
consideration of the aforementioned background information, tumor
recurrence rates following LT at the Organ Transplant Institute of
the Chinese PLA 309th Hospital (Beijing, China) were
retrospectively analyzed to provide a potentially efficacious
treatment for these patients.
Materials and methods
Patients and groups
Clinicopathological data were collected from the
China Liver Transplant Registry database regarding 36 HCC patients
who did not fit the UCSF Criteria, and who had undergone LT at the
Organ Transplant Institute of the Chinese PLA 309th Hospital
between January 2008 and January 2014, including Model for
End-Stage Liver Disease (MELD) scores, Child-Pugh scores, liver
function tests [alanine aminotransferase (ALT), bilirubin (BIL),
alkaline phosphatase (ALP), γ-glutamyltransferase (γ-GGT)] and
serum α-fetoprotein (AFP) levels (Table
I).
 | Table I.Comparison of general data between
the treatment and control groups. |
Table I.
Comparison of general data between
the treatment and control groups.
|
Characteristics | Treatment group
(n=18) | Control group
(n=18) | P-value |
|---|
| Sex |
|
| 0.603a |
|
Male | 17 | 15 |
|
|
Female | 1 | 3 |
|
| Age (years) | 53.94±7.40 | 49.72±7.00 | 0.087a |
| MELD |
|
| 0.533c |
|
0–10 | 12 | 10 |
|
|
10–20 | 6 | 7 |
|
|
>20 | 0 | 1 |
|
| Child-Pugh |
|
| 0.531c |
| A
(5–6) | 11 | 9 |
|
| B
(7–9) | 7 | 8 |
|
| C
(≥10) | 0 | 1 |
|
| AFP preoperative
(ng/ml) |
|
| 0.489b |
|
400–1,000 | 8 | 5 |
|
|
>1,000 | 10 | 13 |
|
| HBV |
|
| 0.614b |
|
Positive | 16 | 17 |
|
|
Negative | 2 | 1 |
|
| ALT (ng/ml) |
|
| 0.738b |
|
<40 | 9 | 7 |
|
|
≥40 | 9 | 11 |
|
| Bilirubin
(ng/ml) |
|
| 0.519b |
|
<34 | 8 | 6 |
|
|
≥34 | 10 | 12 |
|
| Cr (ng/ml) |
|
| 0.282b |
|
<90 | 15 | 12 |
|
|
≥90 | 3 | 6 |
|
| White protein
(g/l) |
|
| 0.176b |
|
≤35 | 8 | 13 |
|
|
>35 | 10 | 5 |
|
Patients were divided into two groups: The group
treated with SRL, Zadaxin and PS-T (SRL+; n=18; 17 men and 1 woman;
median age, 53.94 years); and the control group (n=18; 15 men and 3
women; median age, 49.72 years). The present study was approved by
the Ethics Committee of Human Experimentation of the PLA 309th
Hospital (Beijing, China). Written informed consent was obtained
from all patients in accordance with the Declaration of Helsinki of
the World Medical Association.
Inclusion and exclusion criteria
The inclusion criteria were as follows: i) Patients
in whom liver malignancy was detected by at least an enhanced
computed tomography (CT) scan or magnetic resonance imaging scan
and abdominal ultrasound (MRI/US) and postoperative tissue biopsies
had been pathologically confirmed to be HCC; ii) whose AFP levels
were ≥1,000 ng/ml, or maintained at 400–1,000 ng/ml for 4 weeks;
iii) were 30–65 years old; iv) had no distant metastases (e.g.
lung, bone); and v) had undergone LT at the Organ Transplant
Institute of the Chinese PLA 309th Hospital. The following
exclusion criteria were maintained: i) Patients in whom
preoperative CT or MRI/US indicated current malignant liver tumors
and postoperative tissue biopsies had been pathologically confirmed
to be cholangiocarcinoma; ii) who were >65 years or <30
years; iii) with distant metastases (e.g. lung, bone); iv) were
confirmed to have hepatitis C virus (HCV) infection or alcoholic
liver disease (ALD); or v) had concurrent severe cardiopulmonary
disease or the inability to tolerate surgery.
Intra- and post-operative
examining
Preoperative demographical, clinical (MELD and
Child-Pugh scores) and laboratory data were recorded for these
patients. Patients underwent plain/enhanced CT scan or MRI/US
within 1 week before the surgery. Pathology diagnoses were
considered definitive for HCC. Pathological data were considered as
the standard for tumor characteristics. Microvascular invasion and
tumor differentiation were also assessed by pathology.
Blood cell tests, including blood and liver function
tests, is part of the routine work-up for patients with HCC who
undergo LT. The Fork head box P3 (FoxP3)+ Treg and
cluster of differentiation (CD)8+ T cell percentages was
calculated by flow cytometry. Tumor markers were recorded prior to
and following surgery. In addition, hepatitis B virus (HBV)
infection was quantitatively detected prior to LT and HBV-DNA
copies were assessed for HBV+ patients.
Intra- and post-operative
treatment
Basiliximab (20 mg) (Simulect; Novartis
International AG, Basel, Switzerland) and methylprednisolone (40
mg) (Pfizer, Inc., New York, NY, USA) were used as pre- and
intra-operative induction regimens. Small doses of
methylprednisolone were given post-operatively (20 mg on day 1, 10
mg on day 2 and 5 mg on day 3, once a day, for the first 3 days
following LT for both groups) and withdrawn 1 week following LT. In
addition, an immunosuppression regimen, including tacrolimus
(FK506; Astellas Ireland Co., Ltd., Dublin, Ireland), with or
without mycophenolate mofetil (Roche Applied Science, Madison, WI,
USA), was initially administered based according to individual
differences; FK506 was gradually withdrawn and completely replaced
with sirolimus (Wyeth Pharmaceuticals Co. Ltd., Philadelphia, PA,
USA) a month after LT. Patients were then switched to SRL combined
with Zadaxin and PS-T (SRL+) following stabilization.
The thymalfasin (Zadaxin) (SciClone Pharmaceuticals,
Inc., Foster City, CA, USA) was administrated as a 16-mg
subcutaneous injection/day for 10 days, and then twice a week; the
PS-T (Qidong Gaitianli Pharmaceutical Co., Ltd., Qidong, China) was
administrated orally thrice a day (20 g/day) for ≥5 year later.
For the HBV+ patients, anti-viral drugs,
including lamivudine or entecavir, and injection of hepatitis B
immune globulin were regularly administered following LT to prevent
hepatitis B recurrence.
Of the 36 patients whose advanced HCC was treated
with LT, 18 were treated with SRL+ and 18 were treated with an
FK506-based regimen.
Follow up
The patients underwent follow-up procedures
immediately following LT. All patients were required to be
evaluated monthly during for the first 12 months post-LT,
(including plain/enhanced CT scans, abdominal ultrasound,
FoxP3+ Treg and CD8+ T cell percentages and
serum AFP levels), then every 3 months during the second year, then
every 3–6 months or when necessary in the following years. For this
study, each follow-up session also included physical examinations,
and the collection of data regarding rejection, survival time,
tumor recurrence sites, routine blood and liver function tests, SRL
concentration and HBV surface antibody titers.
If patients exhibited suspicious liver, lung or
brain lesions, as indicated by imaging and blood testing, follow-up
intervals were reduced to once a month. The date of tumor
recurrence was recorded as the time the FoxP3+ Treg
level began to elevate, once tumor recurrence was verified. Except
for the bone metastasis and progression of growth, patients with
other recurrent lesions were treated by γ-radiation therapy or
radiofrequency ablation (RFA)/resection (lung), RFA or
transcatheter hepatic arterial chemo-embolization (liver) or
resection (brain). Adverse events were also evaluated at each visit
for the SRL+ group. The follow-up deadline was July 31, 2017.
Flow cytometric analysis
Analysis of the Foxp3+Treg,
CD3+CD4+, and CD3+CD8+T
cell populations was performed for patients prior to and following
LT at different time points. The analysis of Foxp3+Treg
was performed using anti-CD4, anti-CD25 and anti-FoxP3 antibodies
(pre-diluted and contained in the Multitest IMK kit from
eBioscience; Thermo Fisher Scientific, Inc., Waltham, MA, USA), and
the analysis of the lymphocyte subpopulation was performed using
the Multitest IMK kit (BD Biosciences, USA) according to the
manufacturer's protocol. All the samples were detected and analyzed
with BD FACSCanto clinical software (BD Biosciences). The specific
testing steps as follows:
FoxP3+Treg detection was conducted with
the following procedure: i) A total of 4 µl anti-CD4 and 4 µl
anti-CD25 antibodies were added and incubated for 15 min at room
temperature, avoiding light; ii) 1 ml mixture containing
Fixation/permeabilization concentrate (250 µl) and
Fixation/permeabilization diluent (750 µl) (contained in the
Foxp3/Transcription Factor Staining Buffer Set kit; catalog no.
00-5523-00; eBioscience; Thermo Fisher Scientific, Inc.) were mixed
in a 1:3 ratio and added and incubated for 30 min at room
temperature according to the manufacturer's protocol, avoiding
light; iii) 1 ml 1X permeabilization buffer (eBioscience; Thermo
Fisher Scientific, Inc.) was added and incubated for 20 min at room
temperature, in the dark iv) then, centrifugation was performed at
435 × g/min for 5 min at room temperature and the supernatant was
removed; v) 2 µl anti-FoxP3 antibodies were added and incubated for
60 min at room temperature, avoiding light; vi) 1 ml 1X PBS was
added and centrifuged at 435 × g/min for 5 min at room temperature;
and vii), the supernatant was removed and detection was conducted
with a Flow Cytometer. CD3/CD4/CD8 T cell detection was then
conducted with the followed procedure: i) A total of 5 µl BD
multitest CD3/CD8/CD45/CD4 antibodies and 5 µl BD multitest
CD3/CD16+CD56/CD45/CD19 antibodies (BD Biosciences) were added and
incubated for 15 min at room temperature, avoiding light; ii) 1 ml
1X Lysing solution (BD Biosciences) was added and incubated for 30
min at room temperature, avoiding light; iii) then, centrifugation
was performed at 435 × g/min for 5 min at room temperature and the
supernatant was removed; iv) 1 ml 1X PBS was added and centrifuged
at 435 × g/min for 5 min at room temperature; v) the supernatant
was removed detection was conducted with a Flow Cytometer.
Statistical analysis
Statistical analysis was performed using SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA). Data are presented as the
mean ± standard deviation. The two groups were compared using the
unpaired Student's t-test, Fisher's exact test or the Chi-square
test. For multiple group comparisons using a two-way analysis of
variance followed by Tukey's post hoc test. For non-normal
distribution data the Wilcoxon rank-sum test was used. Patient and
graft survival were calculated using Kaplan-Meier curves and the
log-rank test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
Following the exclusion of patients with HCV
infection or ALD, or who met the UCSF criteria, 36 patients were
included in the present study. Among these patients, 91.7% (33/36)
were HBV+, with 44.5% (16/36) in the treatment group and
47.2% (17/36) in the control group. The SRL+ and control groups did
not significantly differ in sex or age, or in baseline MELD or
Child-Pugh scores, preoperative AFP or liver function (Table I).
All the patients had pathologically diagnosed HCC
following their LT, based on their liver specimens. The
tumor-associated indices [tumor type (single or multiple), size,
vascular invasion and pathological pattern] of the two groups are
presented in Table II. All patients
underwent LT for advanced liver cancer at the Organ Transplant
Institute of the Chinese PLA 309th Hospital and were discharged
following recovery. Patients were followed up directly following
their surgeries; none dropped out during the follow-up time. Median
follow-up time for the SRL+ group was 60 months (range, 47–84
months) excluding 1 case of mortality at 23 months; whereas for the
control group, the median period was 15.5 months (range, 9–24
months) until the mortality of all patients.
 | Table II.Comparison of clinicopathological
characteristics of two groups (n=36). |
Table II.
Comparison of clinicopathological
characteristics of two groups (n=36).
| Variables | Combined group
(n=18) | Control group
(n=18) | P-value |
|---|
| Tumor size, mm
(SD)c | 92 (38) | 72 (36) | 0.057a |
| Tumor type, n
(%) |
|
|
|
|
Single | 4 (22) | 10 (56) | 0.086b |
|
Multiple | 14 (78) | 8 (44) |
|
| Tumor pathology, n
(%) |
|
|
|
|
HCC | 9 (50) | 7 (39) | 0.738b |
| HCC
with cirrhosis | 9 (50) | 11 (61) |
|
| Macroscopic
vascular invasion, n (%) | 8 (44) | 9 (50) | 0.753b |
Liver function and rejection
The indices ALT, TBIL, DBIL, ALP and γ-GGT are used
to evaluate liver function and rejection. Whether preoperative
liver function was abnormal or not, all the aforementioned indices
typically decrease to normal levels postoperatively and remain in
near-normal ranges unless rejection occurs (Table III). The SRL+ group did not vary
from this pattern. The two groups did not significantly differ in
rejection rates (SRL+, 2/18; controls, 4/18; P>0.05). All the
rejection cases were treated following adjustment of
immunosuppressant dosages and glucocorticoid pulse therapy. No
evident rejection reactions were caused by Zadaxin or SRL-based
therapy throughout the follow-up period.
 | Table III.Comparison of liver function in
different time points following sirolimus-based therapy. |
Table III.
Comparison of liver function in
different time points following sirolimus-based therapy.
| Variables | 1 year | 2 years | 3 years | F | P-value |
|---|
| ALT (0–40 U/l) | 15.97±6.79 | 14.74±9.69 | 14.62±5.35 | 0.116 | 0.891 |
| TBIL (0–21
µmol/l) | 13.84±6.48 | 13.91±3.96 | 16.58±2.67 | 0.541 | 0.587 |
| DBIL (0–6.8
µmol/l) | 3.18±1.89 | 4.20±2.18 | 4.06±1.49 | 1.077 | 0.353 |
| ALP (40–150
U/l) | 87.57±32.66 | 88.18±23.99 | 110.8±9.83 | 1.442 | 0.252 |
| γ-GGT (8–58
U/l) | 42.79±23.79 | 35.63±14.92 | 50.40±14.02 | 0.988 | 0.384 |
Imaging detection
All patients underwent regular ultrasonography and
CT scans (lung and abdomen), no remarkable changes were observed in
the bloodstream, bile duct or solid tissue of the liver and lung,
except in cases of recurrence. Among the recurrent patients,
compared with the control group, the metastasis and relapse foci in
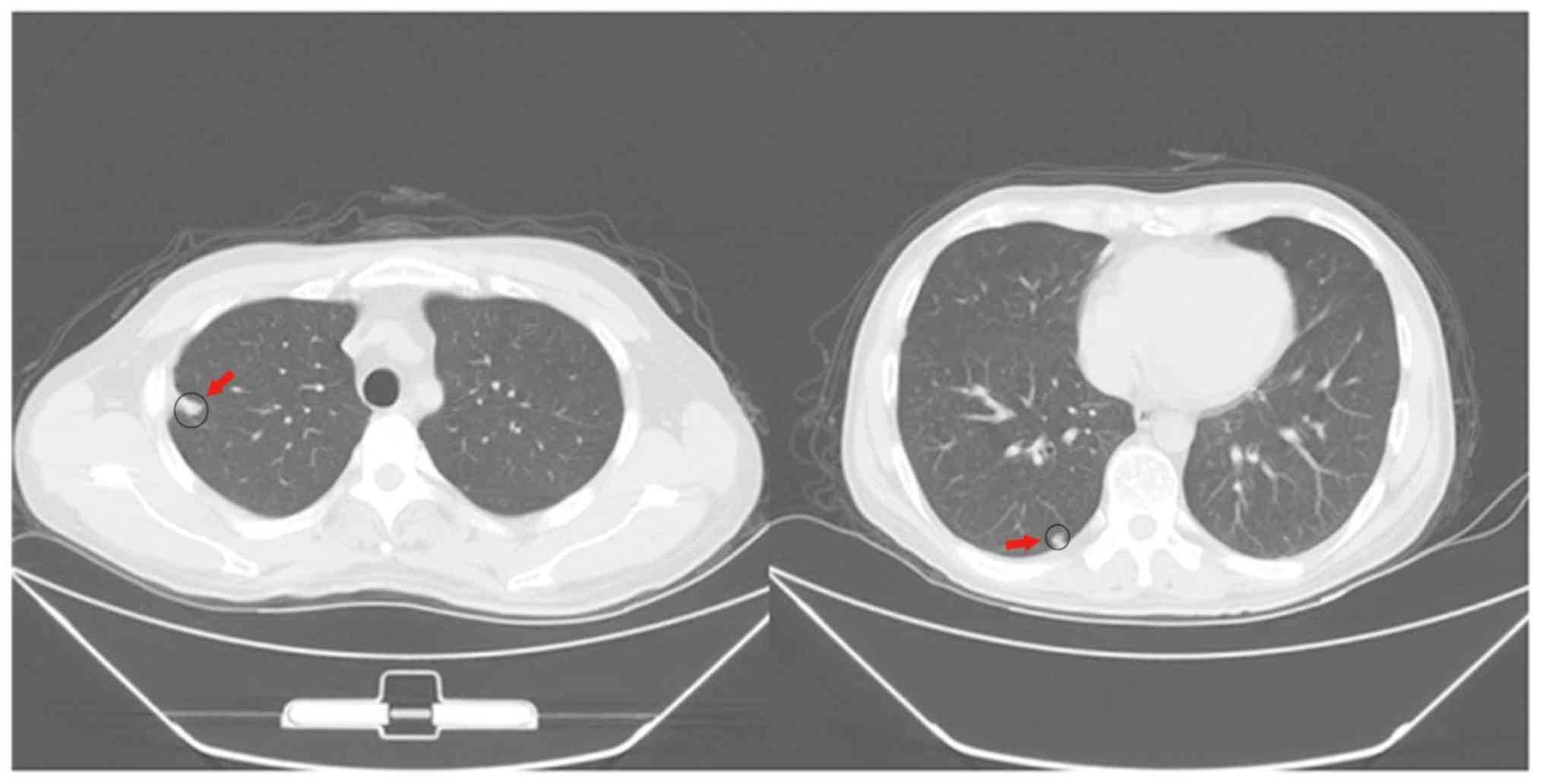
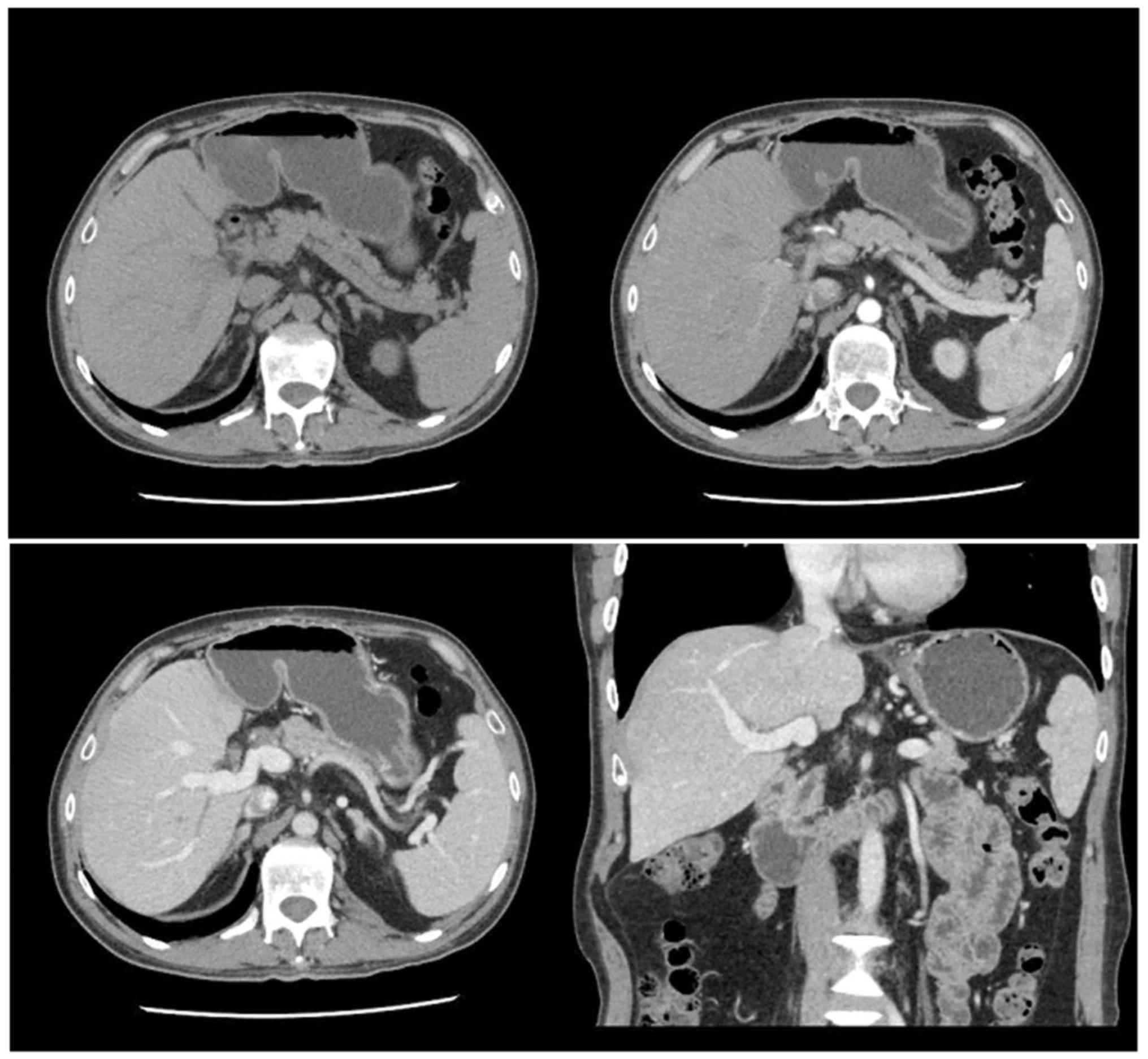
lung were significantly reduced in the SRL+ group (Fig. 1). In addition, no metastasis and
relapse liver foci were detected for the lung relapse patients of
the SRL+ group (Fig. 2).
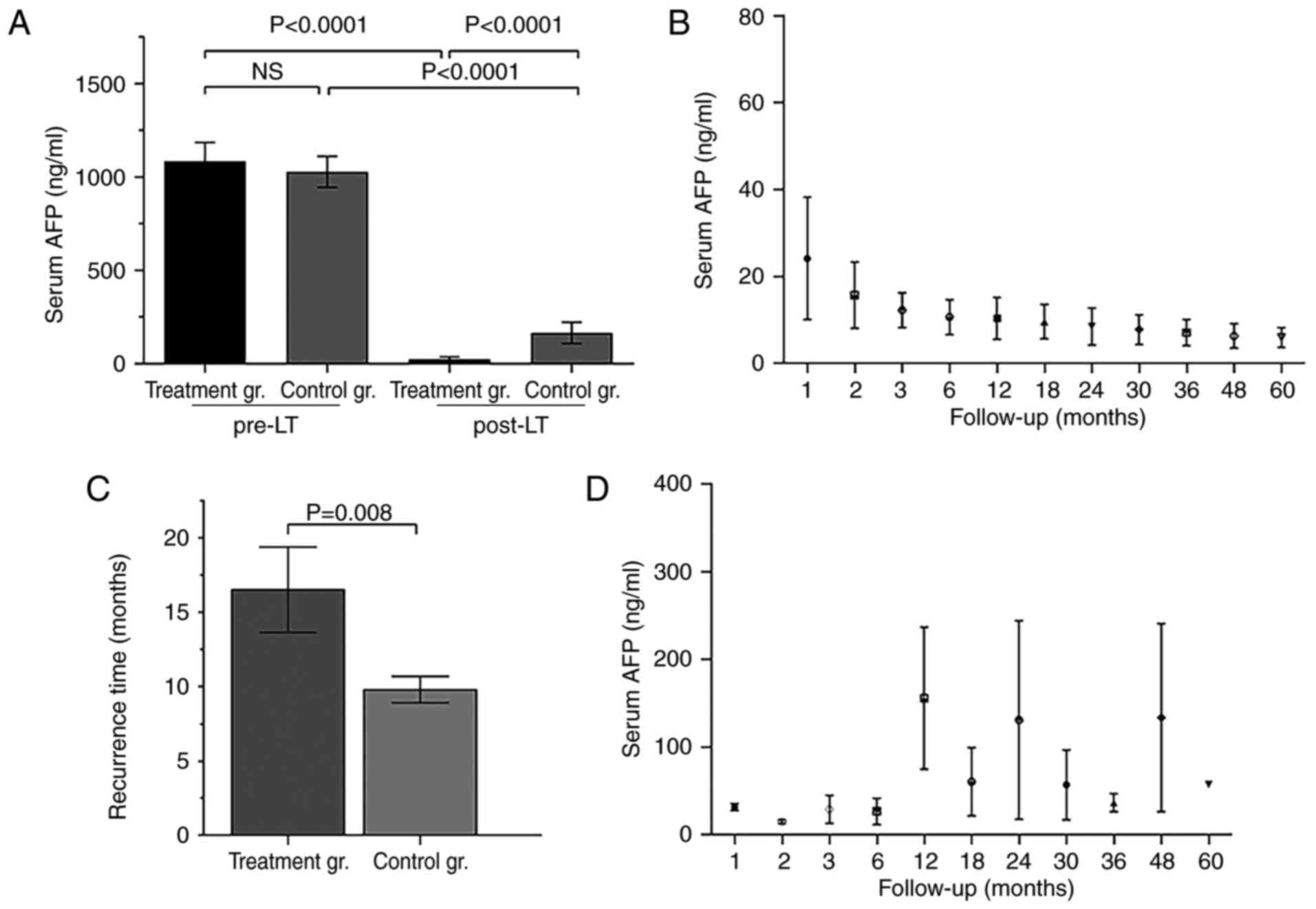
AFP levels pre- and post- LT
The positive rate of liver cancer-specificity tumor
marker, AFP, is >70% among patients with liver cancer.
Preoperative AFP levels of all 36 patients were positive, and did
not significantly differ between the SRL+ and control groups
(Fig. 3A), but did significantly
differ at 1 year post-LT (Fig. 3A).
Post-LT AFP titers in the SRL+ group dropped and were maintained at
normal levels for a long time, although for the recurrent patients,
the AFP titers may remain at low levels (<400 ng/ml) or decline
to normal for some time (Fig. 3B and
C). Furthermore, the tumor recurrence time of the SRL+ group
was significantly longer, compared with the control group (Fig. 3D).
T-cell-associated indices
In addition to AFP, Tregs, particularly
Foxp3+ Tregs, are negatively correlated with prognosis
in patients with HCC, and have been used as an independent
predictor of tumor recurrence (20).
The percentage of Treg (Treg%) and Foxp3+ Treg
(Foxp3+ Treg%) among lymphocytes, and the ratios for
CD8+T/CD3+ T cells and for FoxP3+
Treg/CD8+ T cells at 1, 3 and 5 years after LT were used
to evaluate anti-cancer effects and predict survival benefits.
Preoperative Treg%, Foxp3+ Treg% and FoxP3+
Treg/CD8+ T cell ratios were significantly higher at 1,
3 and 5 years compared with immediately following treatment (all
P<0.05; Fig. 4; Table IV). Furthermore, the association of
CD8+T/CD3+T preoperatively and
postoperatively at different time points was indirect (Table IV). Compared with the preoperative
result, as the level of Foxp3+ Treg% gradually reduced,
the level of CD8+T/CD3+T increased gradually.
This indicated a direct association between the reduced level of
FoxP3+ Treg and the reduced T cell inhibition.
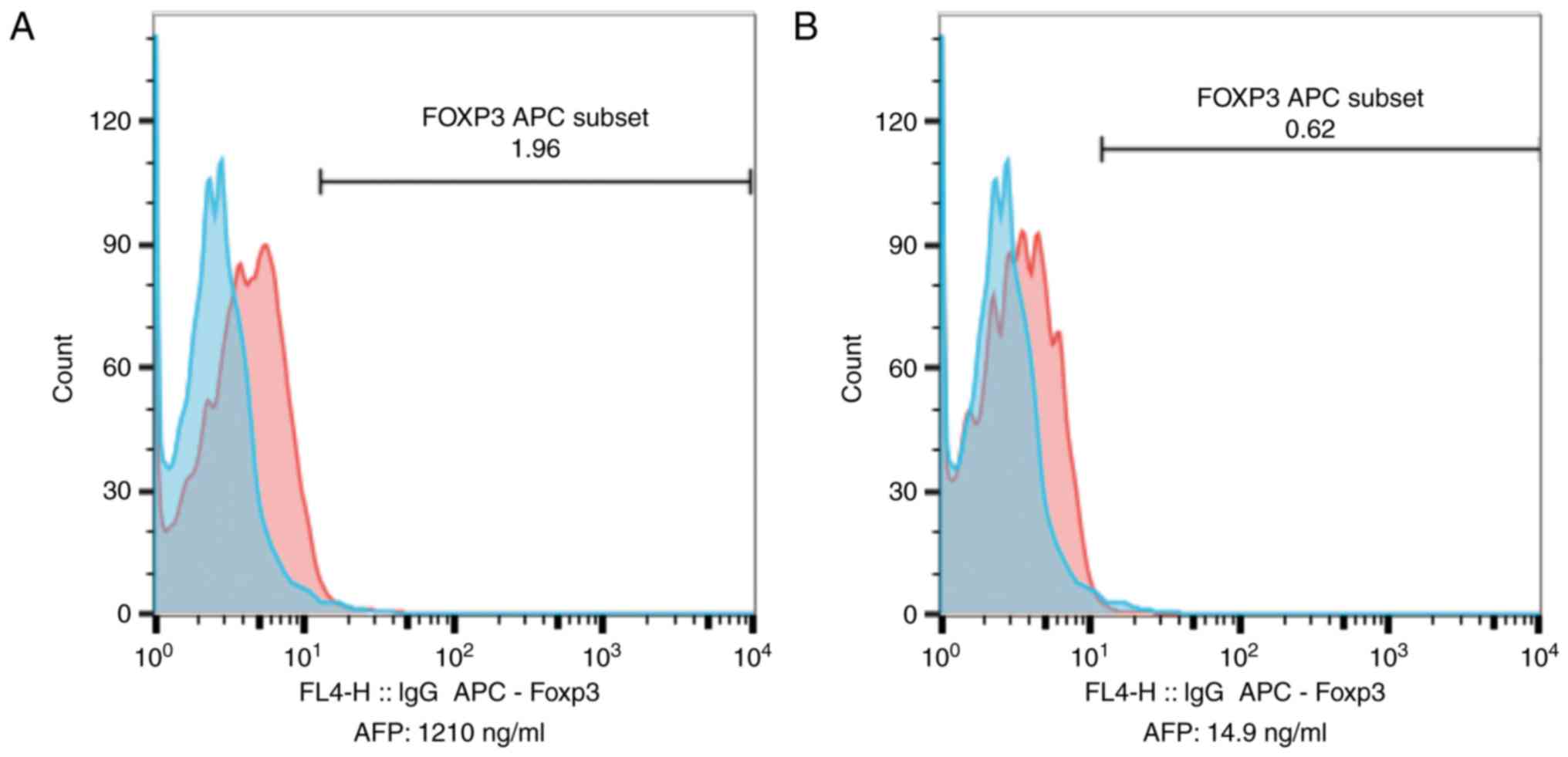
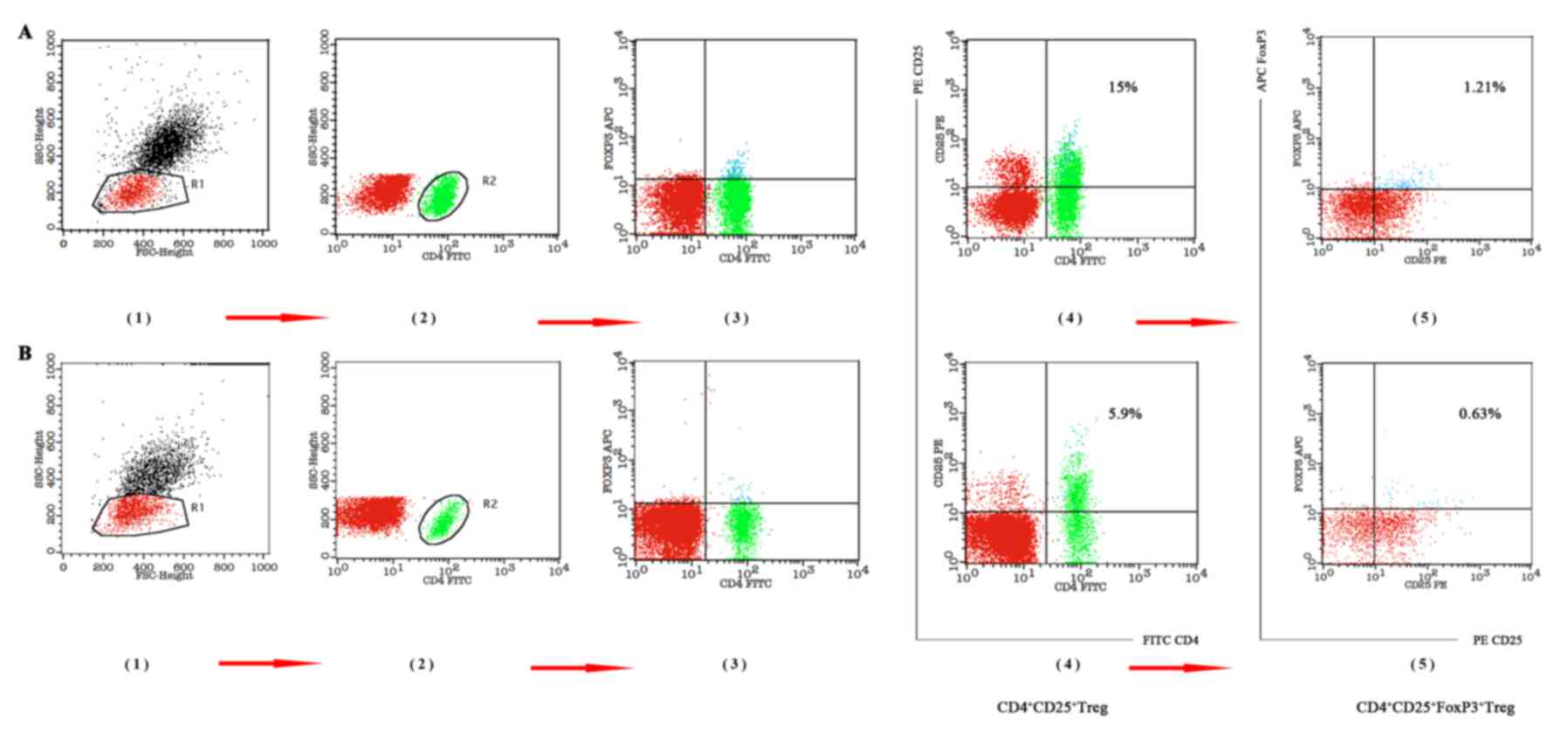 | Figure 4.Changes in Treg and Foxp3+ Treg
percentages, pre-LT and post-LT. (A) Pre-LT Treg and
Foxp3+ Treg percentages of lymphocytes (Treg, 15%;
Foxp3+ Treg, 1.21%). (B) Percentages of Treg and
Foxp3+ Treg in lymphocytes at 1 year post-LT (Treg,
5.9%; Foxp3+ Treg, 0.63%). FoxP3, Forkhead box P3; LT,
liver transplantation; FITC, fluorescein isothiocyanate; PE,
phycoerythrin; CD, cluster of differentiation; SSC, side scatter
height; FSC, forward scatter height; APC, allophycocyanin. |
 | Table IV.Changes in T cell-associated tumor
recurrence index in treatment group at different time points. |
Table IV.
Changes in T cell-associated tumor
recurrence index in treatment group at different time points.
| Variables | n=36 | Foxp3+
Treg %a | F-value |
CD8+T/CD3+Ta | F-value | FoxP3+
Treg/CD8+Ta | F-value |
|---|
| Pre-LT | 18 | 1.23±0.35 | 62.11 | 0.31±0.07 | 9.16 | 0.07±0.02 | 83.89 |
| Post-LT (1
year) | 18 | 0.38±0.16 |
| 0.47±0.15 |
| 0.02±0.01 |
|
| Post-LT (3
year) | 17 | 0.36±0.14 |
| 0.43±0.08 |
| 0.012±0.005 |
|
| Post-LT (5
year) | 10 | 0.29±0.15 |
| 0.47±0.06 |
| 0.014±0.004 |
|
Higher FoxP3 titers in relapsed patients gradually
decreased to levels observed in non-relapsed patients following
γ-radiation and SRL-based therapy (Fig.
5).
Tumor recurrence and treatment
After 12 months, 4 patients in the SRL+ group had
suffered tumor recurrences; of whom 2 had pulmonary metastases, but
no liver recurrence or other metastases. Another patient was
identified to have pulmonary metastases at 18 months after LT. The
patient obtained survival benefits with tumor silencing without
progression following γ-radiation therapy; unfortunately, the
patient was diagnosed with brain metastases 50 months after LT, but
survived well after resection of the metastases (Table V).
 | Table V.Treatment and survival status of the
patients with tumor recurrence. |
Table V.
Treatment and survival status of the
patients with tumor recurrence.
| Case | Recurrence
time/months | Recurrence
site/number | Treatment
method | Therapeutic
effect | Prognosis |
|---|
| 1 | 12 | Lung/single | γ-radiation | Cured | Survived |
| 2 | 12 | Lung/two | γ-radiation | Remission | Succumbed at 23
months after LT |
| 3 | 18 | Lung/two | γ-radiation | Tumor silence, no
progress | Survived |
|
| 50 | Brain/single | Surgical
resection | Cured |
|
| 4 | 24 | Lung/single | γ-radiation | Cured | Survived |
Notably, although 1 patient with multiple
recurrences succumbed from tumor complications, the others achieved
a good quality of life and survived well after γ-radiation therapy
when the data were collected (Table
V). The higher AFP and FoxP3 levels in relapsed patients also
declined to near-normal levels, similar to those of non-relapsed
patients (Fig. 5). By contrast, 10
control patients (10/18) had tumor recurrences during the first 3
to 12 months post-LT, and the other 8 had recurrences at 12 to 15
months; even following positive γ-radiation therapy and
chemotherapy, all the patients in the control group succumbed
within 24 months from multiple tumor metastases and resulting
complications.
Survival benefits
In addition to the recurrent patients, other
patients of the SRL+ group survived well past-transplantation till
the deadline of data collection. The respective 1-year OS and DFS
rates were 100 and 88.9% for the SRL+ group, and 77.8 and 22.2% in
the control group. Whereas none of the control patients lived >2
years post-LT, the OS rates at 3 and 5 years 94.5 and 77.8%,
respectively in the SRL+ group. In addition, the DFS rates at 3 and
5 years were 55.6 and 50.0% in the SRL+ group, respectively. The
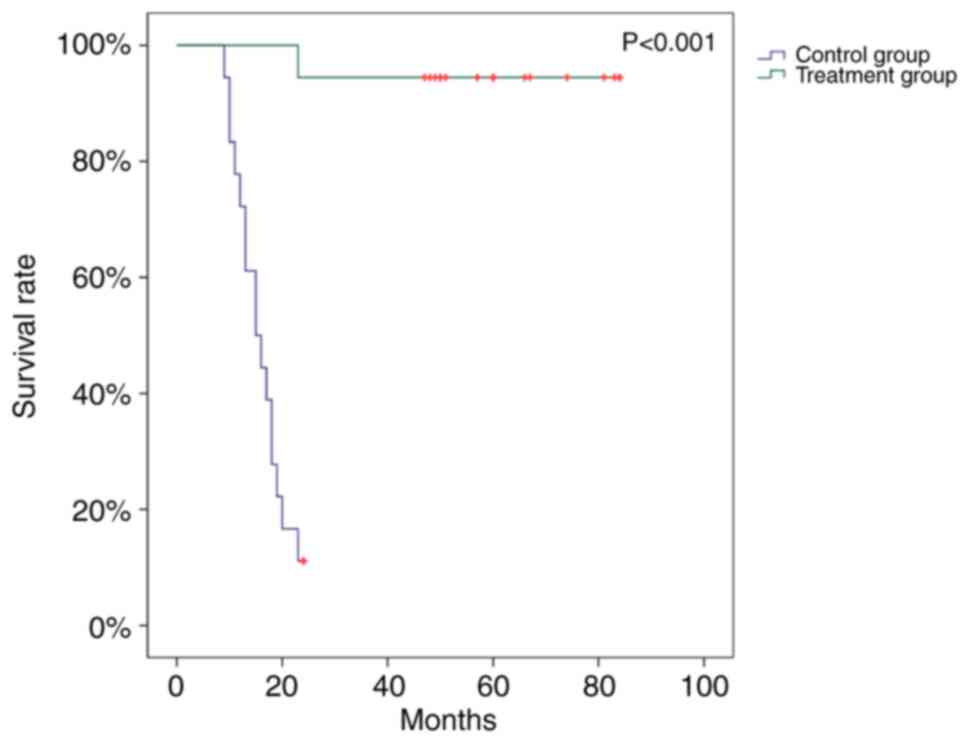
survival curves demonstrated that the SRL+ group had a
significantly longer survival time (P<0.001) compared with the
control group, although the groups did not significantly differ in
baseline values that influence patient survival, including age and
rejection rate (Fig. 6).
Discussion
LT is the currently only effective treatment for
advanced liver cancer, and may improve patient prognosis and
quality of life. However, patients who undergo LT have a high risk
of tumor recurrence due to long-term use of FK506; whereby
subsequent administration of immunosuppressive drugs may accelerate
the risk of mortality (1–3,25).
Although current treatments for post-LT recurrence in patients with
advanced HCC has limited efficacy (2,5,8–11), the
recent ‘SiLIVER’ study demonstrated that SRL may act as an
efficacious immunosuppressive drug and an antineoplastic agent for
LT patients, with favorable clinical safety and efficacy comparable
to that of FK506 (13). This trial
indicated that switching to SRL from FK506 following LT
significantly improved the survival rates for patients with HCC in
the first 3 to 5 years, particularly among low-risk patients,
including those who meet the Milan criterion (13). It also provided the first high-level
evidence for selecting SRL-based immunosuppression in LT recipients
with HCC (13). Similarly, this
retrospective analysis of the combined application of SRL, Zadaxin
and PS-T has revealed promising results with long-term survival and
low relapse rates.
Despite the high recurrence rate of HCC following LT
(10–60%), OS and DFS rates are hopeful, at 70–98% and 60–72%,
respectively, following LT (3–4,7,13,16,26). The
present study demonstrated that the SRL-based therapy improved
post0LT survival and decreased recurrence rates compared with the
control group (OS, 1-year: 100%, 3-year: 94.4%, 5-year: 77.8%; DFS,
1-year: 88.9%, 3-year: 55.6%, 5-year: 50.0%). Furthermore, the
tumor recurrence rate in the present study was 11.1 and 22.2% for 1
and 3 years, respectively, and there were no new relapse cases
beyond 3 years.
Although single applications of SRL are reportedly
effective in preventing post-transplantation recurrence (13,16), they
were only 10–30% effective for patients who met the Milan criteria
(8,13). Excessive SRL doses also have serious
side effects, including myelosuppression, thrombocytopenia and
interstitial pneumonia, which are not observed with FK506 treatment
(8,18). In the present study, it was indicated
that the therapeutic concentration of SRL does not rely on drug
dosage, but primarily on liver and renal function, rejection status
and anti-tumor effect. Therefore, it is suggested that serum SRL
levels should be maintained at ≤10 ng/ml to avoid severe adverse
reactions. Furthermore, as FK506 and SRL doses are collocated
regularly, FK506 should be gradually withdrawn, starting during the
second month after LT.
Research on anticancer TCM for solid malignant
tumors is increasing and yielding promising findings. Trametes
robiniophila Murr. has been used for >1,600 years in TCM
practice as a medicinal fungus for the treatment of inflammation
and cancer in China (22,27), and the TCM PS-T has been used as an
adjuvant drug for chemoradiotherapy, with good clinical efficacy
against liver, lung, gastric and breast cancer (22,27–29). PS-T
has been revealed to be a multitarget drug with proteoglycans that
improve immune functions, particularly those of T effector cells
and natural killer cells, and kill tumor cells (29,30). Thus,
it was considered that PS-T alone or in combination with other
drugs may improve the quality of life and prolong the survival time
of patients with HCC treated with resection and LT.
In vivo research on PS-T in combination with
SRL has confirmed that combined therapy inhibits the tumorigenesis
through activation of mechanistic target of rapamycin (mTOR)
signaling by PS-T, thus increasing the sensitivity of cells more
effectively compared with PS-T or SRL alone (29). In the present study, including PS-T in
post-LT treatment (taken continuously, 20 g orally, 3/day, started
as soon as possible following LT) exhibited survival benefits
compared with FK506-based therapy. Increasing numbers of studies on
the treatment of patients with HCC with SRL combined with PS-T
post-LT have been conducted in Chinese medical centers (31–33).
The effects of the mTOR signaling pathway on cell
growth, and of CD8+ T cells (CTLs) and FoxP3+
Tregs on antitumor activity are involved in immune tolerance
induction by SRL (34,35). Numerous studies have identified a
negative association between high FoxP3+ Tregs levels
and the prognosis of patients with HCC, as well as a positive
association with the risk of tumor relapse (36,37).
Furthermore, Zadaxin may improve cellular immune function of CTLs
and affect FoxP3+ Treg function. Certain centers have
attempted to use Zadaxin in organ transplantation, and have
indicated that it does not increase the risk of rejection even when
it was theoretically demonstrated to be infeasible (38–40).
Therefore, Zadaxin was used in this study to prevent tumor relapse.
It did not increase the rejection rate or cause other adverse
events. In the present study, the SRL-based therapy significantly
downregulated expression of FoxP3+ Treg and increased
CTL levels, to those comparable with pre-LT levels and relapse
cases. To improve the cellular immune function of patients with
HCC, early application of Zadaxin following LT is recommended as a
subcutaneous injection of 1.6-mg dose 1/day for 10 days, followed
by 2/week, with long-term maintenance.
Application of immunosuppressant produces a
systemically inhibitory effect, which may affect the immune system,
not just the CD4+ T lymphocytes (40). Foxp3 expression is the highest in
CD4+ T lymphocytes, but is also partially expressed in
CD8+ T, NK lymphocytes and other lymphocytes (38,41). Tumor
immunity for HCC and allograft tolerance for LT are associated with
FoxP3+ Tregs (34,42). However, allograft tolerance induction
requires high levels of FoxP3+ Tregs (35). Thus, FoxP3+ Tregs may
exhibit contradictory roles in immune tolerance induction (43,44) and
tumor recurrence prevention in patients with HCC following LT
(34,45). Additionally, FoxP3+ Tregs
directly and indirectly interact with CD4+ and
CD8+ T cells (43,44,46). CTLs
are important effector cells in antitumor reactions and graft
rejection. During the present study, it was observed that SRL-based
therapy decreased FoxP3+ Tregs among lymphocytes and the
FoxP3+ Treg/CD8+ T cell ratio, and increased
the CD8+/CD3+ T-cell ratio. It was also
demonstrated that these cell levels may supply sufficient
suppression strength and immune ability for surveillance of the
tumor cell relapse due to the lower and delayed tumor recurrence.
SRL-based therapy may also decrease serum AFP, which is consistent
with the Treg trends. We hypothesize that regulation of Tregs
through mTOR signaling may be the mechanism through which SRL-based
therapy prevents recurrence.
In the present study, the primary immunosuppressant
used post-transplantation was SRL. When SRL was combined with
Zadaxin and PS-T, the SRL-based therapy significantly prolonged OS
and DFS time and decreased post-LT tumor recurrence rate, whereby
22% (4/18) of the SRL+ group suffered recurrence, compared with
100% (18/18) in the control group. It was also suggested that
monitoring post-LT changes in FoxP3+ Treg and
CD8+ T cell levels along with serum AFP levels is a
reliable predictive index of tumor recurrence and long-term
survival in patients whose advanced HCC is treated with LT.
This clinical experiment was limited by its small
study cohort, short observation time and single-institution design.
Larger, multi-center studies are required to verify the conclusions
of the current study. In addition, animal experiments are required
to investigate the underlying mechanism of the combined treatment,
and to determine the relevant molecular and cellular changes.
The present study has demonstrated the safety and
efficacy of Zadaxin use in patients with HCC following LT. In
conclusion, early application of SRL-based therapy following LT in
advanced HCC may improve quality of life and delay tumor recurrence
without increasing the rejection rate. Therefore, this clinical
study performed at the Organ Transplant Institute of the Chinese
PLA 309th Hospital suggests a novel regimen to prevent tumor
recurrence in patients treated with LT for advanced HCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of China (grant no. 81771717).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ, LCP, YGZ and GSD designed the study. LZ, LCP,
YGZ, XQF, XZS, LLS and SZY collected the data. LZ, JYS, WC, DHZ and
ZDZ analyzed the data and LZ, LCP, DHZ and LLS drew the diagrams.
LZ, LCP and GSD drafted the manuscript. GSD, DHZ and ZJL made
critical revisions and approved the paper.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Human Experimentation of the Chinese PLA 309th
Hospital (Beijing, China). Written informed consent was obtained
from all patients in accordance with the Declaration of Helsinki of
the World Medical Association.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AFP
|
α-fetoprotein
|
|
ALD
|
alcoholic liver disease
|
|
ALP
|
alkaline phosphatase
|
|
ALT
|
alanine aminotransferase
|
|
CT
|
enhanced computed tomography
|
|
DBIL
|
direct bilirubin
|
|
DFS
|
disease-free survival
|
|
FK506
|
tacrolimus
|
|
HBIG
|
human hepatitis B immunoglobulin
|
|
HBV
|
hepatitis B virus
|
|
HCC
|
hepatocellular carcinoma
|
|
HCV
|
hepatitis C virus
|
|
LT
|
liver transplantation
|
|
MRI/US
|
magnetic resonance imaging scan and
abdominal ultrasound
|
|
mTOR
|
mechanistic target of rapamycin
|
|
OS
|
overall survival
|
|
PS-T
|
huaier granules
|
|
RFA
|
radiofrequency ablation
|
|
SRL
|
sirolimus
|
|
SRL+
|
sirolimus with huaier granules and
thymalfasin
|
|
Thymalfasin
|
Zadaxin
|
|
TBIL
|
total bilirubin
|
|
TCM
|
traditional Chinese medicine
|
|
UCSF
|
University of California at San
Francisco
|
|
γ-GGT
|
γ-glutamyl-transpeptidase
|
References
|
1
|
Yan J, Tan C, Gu F, Jiang J, Xu M, Huang
X, Dai Z, Wang Z, Fan J and Zhou J: Sorafenib delays recurrence and
metastasis after liver transplantation in a rat model of
hepatocellular carcinoma with high expression of phosphorylated
extracellular signal-regulated kinase. Liver Transpl. 19:507–520.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Roh YN, Kwon David CH, Song S, Shin M, Kim
Man J, Kim S, Joh JW and Lee SK: The prognosis and treatment
outcomes of patients with recurrent hepatocellular carcinoma after
liver transplantation. Clin Transplant. 28:141–148. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alsina AE: Liver transplantation for
hepatocellular carcinoma. Cancer Control. 17:83–86. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Baccarani U, Isola M, Adani GL, Benzoni E,
Avellini C, Lorenzin D, Bresadola F, Uzzau A, Risaliti A, Beltrami
AP, et al: Superiority of transplantation versus resection for the
treatment of small hepatocellular carcinoma. Transpl Int.
21:247–254. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim HR, Cheon SH, Rha SY, Lee S, Han KH,
Chon CY, Lee JD, Sung JS and Chung HC: Treatment of recurrent
hepatocellular carcinoma after liver transplantation. Asia Pac J
Clin Oncol. 7:258–269. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee KK, Kim DG, Moon IS, Lee MD and Park
JH: Liver transplantation versus liver resection for the treatment
of hepatocellular carcinoma. J Surg Oncol. 101:47–53. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shah SA, Cleary SP, Wei AC, Yang I, Taylor
BR, Hemming AW, Langer B, Grant DR, Greig PD and Gallinger S:
Recurrence after liver resection for hepatocellular carcinoma: Risk
factors, treatment, and outcomes. Surgery. 141:330–339. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gomez-Martin C, Bustamante J, Castroagudin
JF, Salcedo M, Garralda E, Testillano M, Herrero I, Matilla A and
Sangro B: Efficacy and safety of sorafenib in combination with
mammalian target of rapamycin inhibitors for recurrent
hepatocellular carcinoma after liver transplantation. Liver
Transpl. 18:45–52. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tan WF, Qiu ZQ, Yu Y, Ran RZ, Yi B, Lau
WY, Liu C, Qiu YH, Feng FL, Wang JH, et al: Sorafenib extends the
survival time of patients with multiple recurrences of
hepatocellular carcinoma after liver transplantation. Acta
Pharmacol Sin. 31:1643–1648. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Staufer K, Fischer L, Seegers B,
Vettorazzi E, Nashan B and Sterneck M: High toxicity of sorafenib
for recurrent hepatocellular carcinoma after liver transplantation.
Transpl Int. 25:1158–1164. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zavaglia C, Airoldi A, Mancuso A, Vangeli
M, Viganò R, Cordone G, Gentiluomo M and Belli LS: Adverse events
affect sorafenib efficacy in patients with recurrent hepatocellular
carcinoma after liver transplantation: Experience at a single
center and review of the literature. Eur J Gastroenterol Hepatol.
25:180–186. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mueller AR, Platz KP, Bechstein WO,
Schattenfroh N, Stoltenburg-Didinger G, Blumhardt G, Christe W and
Neuhaus P: Neurotoxicity after orthotopic liver transplantation. A
comparison between cyclosporine and FK506. Transplantation.
58:155–170. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Geissler EK, Schnitzbauer AA, Zülke C,
Lamby PE, Proneth A, Duvoux C, Burra P, Jauch KW, Rentsch M, Ganten
TM, et al: Sirolimus use in liver transplant recipients with
hepatocellular carcinoma: A randomized, multicenter, Open-Label
phase 3 trial. Transplantation. 100:116–125. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Alessiani M, Cillo U, Fung JJ, Irish W,
Abu-Elmagd K, Jain A, Takaya S, Van Thiel D and Starzl TE: Adverse
effects of FK 506 overdosage after liver transplantation.
Transplant Proc. 25:628–634. 1993.PubMed/NCBI
|
|
15
|
Taniai N, Akimaru K, Ishikawa Y, Kanada T,
Kakinuma D, Mizuguchi Y, Mamada Y, Yoshida H and Tajiri T:
Hepatotoxicity caused by both tacrolimus and cyclosporine after
living donor liver transplantation. J Nippon Med Sch. 75:187–191.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang ZH, Li LX, Li P, Lv SC, Pan B and He
Q: Sirolimus in liver transplant recipients with hepatocellular
carcinoma: An updated meta-analysis. J Invest Surg. 1–10. 2018.
View Article : Google Scholar
|
|
17
|
Toso C, Merani S, Bigam DL, Shapiro AM and
Kneteman NM: Sirolimus-based immunosuppression is associated with
increased survival after liver transplantation for hepatocellular
carcinoma. Hepatology. 51:1237–1243. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guba M, von Breitenbuch P, Steinbauer M,
Koehl G, Flegel S, Hornung M, Bruns CJ, Zuelke C, Farkas S,
Anthuber M, et al: Rapamycin inhibits primary and metastatic tumor
growth by antiangiogenesis: Involvement of vascular endothelial
growth factor. Nat Med. 8:128–135. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shao H, Gao C, Tang H, Zhang H, Roberts
LR, Hylander BL, Repasky EA, Ma WW, Qiu J, Adjei AA, et al: Dual
targeting of mTORC1/C2 complexes enhances histone deacetylase
inhibitor-mediated anti-tumor efficacy in primary HCC cancer in
vitro and in vivo. J Hepatol. 56:176–183. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li C, Wu X, Zhang H, Yang G, Hao M, Sheng
S, Sun Y, Long J, Hu C, Sun X, et al: A Huaier polysaccharide
inhibits hepatocellular carcinoma growth and metastasis. Tumour
Biol. 36:1739–1745. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang X, Zhang N, Huo Q and Yang Q:
Anti-angiogenic and antitumor activities of Huaier aqueous extract.
Oncol Rep. 28:1167–1175. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang N, Kong X, Yan S, Yuan C and Yang Q:
Huaier aqueous extract inhibits proliferation of breast cancer
cells by inducing apoptosis. Cancer Sci. 101:2375–2383. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Garaci E, Pica F, Serafino A, Balestrieri
E, Matteucci C, Moroni G, Sorrentino R, Zonfrillo M, Pierimarchi P
and Sinibaldi-Vallebona P: Thymosin α1 and cancer: Action on immune
effector and tumor target cells. Ann N Y Acad Sci. 1269:26–33.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Serafino A, Pierimarchi P, Pica F,
Andreola F, Gaziano R, Moroni N, Zonfrillo M, Sinibaldi-Vallebona P
and Garaci E: Thymosin α1 as a stimulatory agent of innate
cell-mediated immune response. Ann N Y Acad Sci. 1270:13–20. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Alsina AE, Nakshabandi A, Makris AM and
Torres EA: Liver transplantation for hepatocellular carcinoma in
Puerto Ricans: Underutilization of a curative therapy. P R Health
Sci J. 33:170–176. 2014.PubMed/NCBI
|
|
26
|
Mathai AM, Kapadia MJ, Alexander J,
Kernochan LE, Swanson PE and Yeh MM: Role of Foxp3-positive
tumor-infiltrating lymphocytes in the histologic features and
clinical outcomes of hepatocellular carcinoma. Am J Surg Pathol.
36:980–986. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen L, Lu P, Lu Z and Dechun L:
Anticancer effect of PS-T on the experimental hepatocellular
carcinoma. Chin German J Clin Oncol. 3:55–59. 2004. View Article : Google Scholar
|
|
28
|
Wang CY, Bai XY and Wang CH: Traditional
Chinese medicine: A treasured natural resource of anticancer drug
research and development. Am J Chin Med. 42:543–559. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu Z, Yang A, Fan H, Wang Y, Zhao Y, Zha
X, Zhang H and Tu P: Huaier aqueous extract sensitizes cells to
rapamycin and cisplatin through activating mTOR signaling. J
Ethnopharmacol. 186:143–150. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun Y, Sun T, Wang F, Zhang J, Li C, Chen
X, Li Q and Sun S: A polysaccharide from the fungi of Huaier
exhibits anti-tumor potential and immunomodulatory effects.
Carbohydr Polym. 92:577–582. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou L, Su XJ, Pan LC, Du GS, Zheng YG,
Xiao L, Kong XR, Gao Y, Zhu ZD, Song JY, et al: Long-Time survival
experience of sirolimus in combination with thymosin alpha −1 and
huaier granule for prevention tumor-recurrence in liver
transplantation recipient of advanced intrahepatic
cholangiocarcinoma: A case report. Ausin J Cancer Clin.
1:10752017.
|
|
32
|
Lei JY, Yan LN, Zhu JQ and Wang WT:
Hepatocellular carcinoma patients may benefit from postoperative
huaier aqueous extract after liver transplantation. Transplant
Proc. 47:2920–2924. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou L, Du GS, Pan LC, Zheng YG, Liu ZJ,
Shi HD, Yang SZ, Shi XJ, Xuan M, Feng LK and Zhu ZD: Sirolimus
treatment for cirrhosis or hepatocellular carcinoma patients
accompanied by psoriasis after liver transplantation: A single
center experience. Oncol Lett. 14:7817–7824. 2017.PubMed/NCBI
|
|
34
|
Chen X, Du Y and Huang Z: CD4+CD25+ Treg
derived from hepatocellular carcinoma mice inhibits tumor immunity.
Immunol Lett. 148:83–89. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee WC, Wu TJ, Chou HS, Yu MC, Hsu PY, Hsu
HY and Wang CC: The impact of CD4+CD25+ T cells in the tumor
microenvironment of hepatocellular carcinoma. Surgery. 151:213–222.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li F, Guo Z, Lizée G, Yu H, Wang H and Si
T: Clinical prognostic value of CD4+CD25+FOXP3+regulatory T cells
in peripheral blood of Barcelona Clinic Liver Cancer (BCLC) stage B
hepatocellular carcinoma patients. Clin Chem Lab Med. 52:1357–1365.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ozgur HH, Ercetin AP, Eliyatkin N, Seren
A, Kupelioglu A, Ortac R, Diniz G and Aktas S: Regulatory T cells
and their prognostic value in hepatopancreatobiliary tumours.
Hepatogastroenterology. 61:1847–1851. 2014.PubMed/NCBI
|
|
38
|
Romani L, Bistoni F, Montagnoli C, Gaziano
R, Bozza S, Bonifazi P, Zelante T, Moretti S, Rasi G, Garaci E and
Puccetti P: Thymosin alpha1: An endogenous regulator of
inflammation, immunity, and tolerance. Ann N Y Acad Sci.
1112:326–338. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ji SM, Li LS, Sun QQ, Chen JS, Sha GZ and
Liu ZH: Immunoregulation of thymosin alpha 1 treatment of
cytomegalovirus infection accompanied with acute respiratory
distress syndrome after renal transplantation. Transplant Proc.
39:115–119. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wolf E, Milazzo S, Boehm K, Zwahlen M and
Horneber M: Thymic peptides for treatment of cancer patients.
Cochrane Database Syst Rev: CD003993. 2011. View Article : Google Scholar
|
|
41
|
Joosten SA, van Meijgaarden KE, Savage ND,
de Boer T, Triebel F, van der Wal A, de Heer E, Klein MR, Geluk A
and Ottenhoff TH: Identification of a human CD8+ regulatory T cell
subset that mediates suppression through the chemokine CC chemokine
ligand 4. Proc Natl Acad Sci USA. 104:8029–8034. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Huang Y, Liao H, Zhang Y, Yuan R, Wang F,
Gao Y, Wang P and Du Z: Prognostic value of Tumor-Infiltrating
FoxP3+ T cells in gastrointestinal cancers: A meta analysis. PLoS
One. 9:e943762014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pons JA, Revillanuin B, Ramírez P,
Baroja-Mazo A and Parrilla P: Development of immune tolerance in
liver transplantation. Gastroenterol Hepatol. 34:155–169. 2011.(In
Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Karczewski M, Karczewski J, Kostrzewa A,
Wiktorowicz K and Glyda M: The role of foxp3+ regulatory T cells in
kidney transplantation. Transpl Proc. 41:1527–1529. 2009.
View Article : Google Scholar
|
|
45
|
Shang B and Liu Y, Jiang SJ and Liu Y:
Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in
cancers: A systematic review and meta-analysis. Sci Rep.
5:151792015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Casares N, Arribillaga L, Sarobe P, Dotor
J, de Cerio Lopez-Diaz A, Melero I, Prieto J, Borrás-Cuesta F and
Lasarte JJ: CD4+/CD25+ regulatory cells inhibit activation of
tumor-primed CD4+ T cells with IFN-gamma-dependent antiangiogenic
activity, as well as long-lasting tumor immunity elicited by
peptide vaccination. J Immunol. 171:5931–5939. 2003. View Article : Google Scholar : PubMed/NCBI
|