Introduction
Smokeless tobacco (ST) has been used over centuries
by large numbers of the global population. ST is a type of tobacco
that is consumed orally or nasally, without burning the product
(1,2).
ST products include moist snuff (finely ground or shredded tobacco
applied to the gum or tongue), dry snuff (powdered tobacco inhaled
nasally) and chewing or sucking tobacco (3). The consumption of ST products has been
popular in several Asian countries including India and Pakistan,
and in Africa, northern Europe and the United States of America
(1,4).
The negative health consequences of ST remain controversial,
although there is increasing evidence demonstrating that >30
compounds within ST exhibit cancer-inducing activities, including
tobacco-specific nitrosamines (TSNAs), polycyclic aromatic
hydrocarbons and formaldehyde (5,6). Previous
studies have demonstrated that the chemical components in ST were
associated with an increased risk of a number of types of cancer,
including oral, or oropharyngeal and esophageal cancer (7–10).
N-nitrosonornicotine and
4-methyl-N-nitrosamino-1-(3-pyridyl)-1-butanoneare2principal human
carcinogens of TSNAs (11,12). These compounds were identified in the
urine of ST users, and studies involving rats demonstrated that
they may induce tumorigenesis in the pancreas, esophagus and oral
cavity (13–15).
ST consumption has been identified to generate free
radicals and result in increased oxidative stress, which destroys
the homeostasis between pro- and antioxidants (16). Cellular DNA is damaged by reactive
oxygen metabolites (ROMs), including superoxide anions,
malondialdehyde and nitric oxide, and therefore an imbalance
between pro- and antioxidants is directly associated with
carcinogenesis (16,17). The ability to eliminate ROMs using the
antioxidant enzyme system is critically important for smokers
(18). Active smokers and tobacco
users have been demonstrated to exhibit the lowest levels of
protective antioxidants in the general population, including
superoxide dismutase (SOD), glutathione peroxidase and catalase
(CAT) (19,20).
ST extracts (STE) are not only absorbed locally, but
may also enter the systemic circulation (21,22).
Evidence has demonstrated the effects of STE in several biological
processes, including inflammation, antioxidant defense and cell
apoptosis (23,24). Block et al (20) revealed the toxicity of chronic STE
exposure for 184 days in male and female rats, where the animals
experienced decreased body weight and a moderate toxic effect on a
number of organs including the stomach, liver, kidneys, esophagus
and lungs. The exact mechanisms of STE in human diseases remain
unknown.
In order to evaluate the toxicity of STE on oral
mucous fibroblasts, the present study first identified human oral
mucosa fibroblasts (hOMF) cells by detecting cytokeratin
expression. The effects of STE on the rates of proliferation and
apoptosis of hOMF cells were then investigated by a series of
experiments, including MTT assays, reverse transcription
quantitative polymerase chain reaction (RT-qPCR) and western blot
analysis. The present study analyzed the effect of the STE on the
oxidative status of hOMF cells by measuring the production of
reactive oxygen species (ROS), MDA, SOD and CAT following of
exposure of the cells to a range of concentrations of STE (0, 200,
400 and 800 µg/ml). It was identified that the tumor protein 53
(p53) and nuclear factor kappa light chain enhancer of activated B
cells (NF-κB)-transcription factor p65 (p65) signaling pathways
were involved in the effects of STE on cell proliferation and
apoptosis.
Materials and methods
Cell culture and treatment
The hOMF cells and human normal fibroblast (NF)
cells were purchased from Ai Yan Shanghai Biological Technology
Co., Ltd (Shanghai, China). The cells were cultured in Dulbecco's
modified Eagle's medium (DMEM; Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) with 10% fetal bovine serum
(FBS; Invitrogen; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin and 100 µg/ml streptomycin at 37°C in 95% humidity
incubator with 5% CO2. After culturing, the cellular
morphology of the hOMF cells was observed and images were captured
using fluorescence microscope (×200 magnification; Olympus
Corporation, Tokyo, Japan) in four randomly selected fields of
view.
The hOMF cells were digested, centrifuged in 500 × g
at 37°C for 5 min and seeded into plastic culture dishes (35 mm)
with 5.0×105 cells/well for 48 h. The cells were then
treated with STE (0, 200, 400 and 800 µg/ml) for 24, 48 and 72 h at
37°C. The treated cells were then used for the subsequent
experiments.
RT-qPCR assay
Total RNA was isolated with TRIzol®
reagent (Life Technologies; Thermo Fisher Scientific, Inc.), in
accordance with the manufacturer's protocol. The total RNA was
reverse transcribed into the first-strand cDNAs via a RevertAid
First Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.),
in accordance with the manufacturer's protocol. The thermocycler
conditions were as follows: 85°C for 15 min, 4°C for 10 min. The
mRNA expression levels of the GAPDH and the target genes were
evaluated with a qPCR assay using a SYBR GREEN PCR Master Mix
(Applied Biosystems; Thermo Fisher Scientific, Inc.) and an ABI
7500 Real-time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). The thermocycling conditions were as follows:
95°C for 10 min, (denaturation: 95°C for 30 sec; annealing: 55°C
for 30 sec; elongation: 72°C for 25 sec) for 30 cycles, extending:
72°C for 30 sec. The data was analyzed using the 2−ΔΔCq
method (25). The relative mRNA
expression levels were normalized to GAPDH. The primers for the
target genes and GAPDH are summarized in Table I.
 | Table I.Primer sequences for reverse
transcription quantitative polymerase chain reaction analysis. |
Table I.
Primer sequences for reverse
transcription quantitative polymerase chain reaction analysis.
| Gene | Primer
sequences |
|---|
| GAPDH | Forward:
5′-TATGATGATATCAAGAGGGTAGT-3′ |
|
| Reverse:
5′-TGTATCCAAACTCATTGTCATAC-3′ |
| p21 | Forward:
5′-CTGGTGACTCTCAGGGTCGAA-3′ |
|
| Reverse:
5′-GGATTAGGGCTTCCTCTTGGA-3′ |
| Cyclin D1 | Forward:
5′-GCTGCTCCTGGTGAACAAGC-3 |
|
| Reverse:
5′-TTGCGTCTCAGCTCAGGGAC-3 |
| Bax | Forward:
5′-CAGCTCTGAGCAGATCATGAAGACA-3′ |
|
| Reverse:
5′-GCCCATCTTCTTCCAGATGGTGAGC-3′ |
| Bcl-2 | Forward:
5′-ACTTGTGGCCCAGATAGGCACCCAG-3′ |
|
| Reverse:
5′-CGACTTCGCCGAGATGTCCAGCCAG-3′ |
| p53 | Forward:
5′-CAGCGTGATGATGGTAAGGA-3′ |
|
| Reverse:
5′-GCGTTGCTCTGATGGTGA-3′ |
| NF-κB p65 | Forward:
5′-ACGATCTGTTTCCCCTCATCT-3′ |
|
| Reverse:
5′-TGCTTCTCTCCCCAGGAATA-3′ |
| Nrf2 | Forward:
5′-TACTCCCAGGTTGCCCACA-3′ |
|
| Reverse:
5′-CATCTACAAACGGGAATGTCTGC-3′ |
| HO-1 | Forward:
5′-CACGCATATACCCGCTACCT-3′ |
|
| Reverse:
5′-AAGGCGGTCTTAGCCTCTTC-3′ |
| NQO1 | Forward:
5′-CATTCTGAAAGGCTGGTTTGA-3′ |
|
| Reverse:
5′-CTAGCTTTGATCTGGTTGTCAG-3′ |
Western blot analysis
The hOMF cells were lysed with a radio
immunoprecipitation assay (RIPA) buffer (Thermo Fisher Scientific,
Inc.) supplemented with a Protease Inhibitor Cocktail (Thermo
Fisher Scientific, Inc.). The protein concentrations were
quantified with a BCA protein assay kit (Qcbio Science Technologies
Co., Ltd., Shanghai, China). The proteins (30 µg) were separated
with polyacrylamide gel electrophoresis. The proteins were then
transferred on to polyvinylidene fluoride membranes (EMD Millipore,
Billerica, MA, USA), and TBST buffer containing 5% skim milk was
used to block the membranes at room temperature for 90 min. The
primary antibodies were incubated with the membranes overnight at
4°C, followed by incubation with a horseradish
peroxidase-conjugated secondary antibody (anti-mouse; cat. no.,
SC-2005; anti-rabbit, cat. no., SC-2004; dilution: 1:700, Santa
Cruz Biotechnology, Inc., Dallas, TX, USA) at room temperature for
60 min and then incubation with a chemiluminescence substrate
(Amersham; GE Healthcare, Chicago, IL, USA) at room temperature for
2 min. The results were analyzed with an ECL system (Amersham; GE
Healthcare). Images were captured by the LAS-3000 imaging system
(FUJIFILM, Tokyo, Japan) and Multi Gauge Version 2.0 software
(FUJIFILM). The primary antibodies used were as follows: Anti-GAPDH
(cat. no., SC-47724, dilution, 1:2,000, Santa Cruz Biotechnology),
anti-cyclin-dependent kinase inhibitor 1 (p21; dilution, 1:500,
Abcam, Cambridge, MA, USA; cat. no., ab54562); anti-cyclin D1
(dilution, 1:1,000, Abcam; cat.no, ab134175); anti-B cell-lymphoma
2 (Bcl-2)-associated X protein (Bax; dilution, 1:1,000; Abcam;
cat.no. ab32503); anti-Bcl-2 (dilution, 1:1,000; Abcam; cat. no.,
ab32124); anti-p53 (dilution, 1:100; Abcam; cat. no., ab28);
anti-phosphorylated (p)-p53 (dilution, 1:500; Cell Signaling
Technology, Inc., Danvers, MA, USA; cat. no., 9284); anti-NF-κB p65
(dilution, 1: 1,000, Abcam, cat. no., ab76026); anti-Nuclear factor
(erythroid-derived 2)-like 2 (Nrf2; dilution, 1:1,000; Abcam; cat.
no., ab76026); anti-Hemeoxygenase 1 (HO-1; dilution, 1:1,000;
Abcam; cat. no., ab13248); and anti-NAD(P)H quinone dehydrogenase 1
(NQO1; dilution, 1:1,000; Abcam; cat. no., ab28947).
Cell viability assay
The treated hOMF cells were seeded in 96-well plates
with 100 µl DMEM medium (10% FBS) at a density of 2×103
cells/well. They were cultured at 5% CO2 for 48 h at
37°C. A total of 20 µl MTT solution (5 mg/ml) was added into each
well. The cells were then incubated for 4 h at 37°C. A total of 200
µl dimethyl sulfoxide was then added into each well for 10 min at
room temperature, which dissolved the formazan product. The
absorbance was measured at 490 nm with an Elx800 Reader (Bio-Tek
Instruments Inc., Winooski, VT, USA).
Immunofluorescence assay
The treated hOMF cells (1×106 cells/ml)
were fixed with 3.7% paraformaldehyde in PBS at room temperature
for 20 min. Triton X-100 (0.2%) solutions in PBS were used for
permeabilizing cells at room temperature for 10 min. 5% BSA
(Bovogen Biologicals, Keilor East, VIC, Australia) was used for
blocking cells at room temperature for 20 min. The cells were then
incubated with cytokeratin antibody (cat. no. ab9377, dilution:
1:100; Abcam) overnight at 4°C. The goat-anti-rabbit-Alexa
594-conjugated secondary antibody (cat. no. R37117, dilution:
1:6,000; Life Technologies; Thermo Fisher Scientific, Inc.) was
then incubated with the treated coverslips for 1 h at room
temperature. The treated coverslips were then incubated with DAPI
(Life Technologies; Thermo Fisher Scientific, Inc.) for 10 min at
room temperature. The images were obtained with a fluorescence
microscope (×400 magnification), in four fields of view.
Flow cytometric analysis
A cell cycle analysis was performed with propidium
iodide (PI; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
staining, as described previously (26,27). The
cells were collected and fixed in 70% (v/v) ethanol (cat. no.
5054.2; Carl Roth GmbH & Co., KG, Karlsruhe, Germany) for 15
min at room temperature, then washed with PBS and suspended in a PI
staining solution (50 mg/l; Biolegend, Inc., San Diego, CA, USA;
cat. no. 421301) supplemented with 0.1% Triton X-100 and
Ribonuclease A (0.25 mg/ml; Sigma-Aldrich; Merck KGaA). The cells
were incubated at 37°C for 30 min and then the cell fluorescence
was measured with a FACS Calibur flow cytometer (BD Biosciences,
San Jose, CA, USA). The cell cycle analysis was performed with
ModFit LT 2.0 software (Verity SoftwareHouse, Topsham, ME,
USA).
The cell apoptosis assay was performed with an
Annexin V-fluorescein isothiocyanate (FITC)/PI apoptosis detection
kit (BestBio, Co., Shanghai, China) as described previously
(28). The treated hOMF cells
(1×106 cells/ml) were re-suspended with a1X binding
buffer (100 µl), and then double-stained with 20 µg/ml Annexin
V-FITC and 50 µg/ml PI for 15 min at room temperature. Cell
apoptosis levels were then detected by flow cytometry with version
5.1 Cell Quest Pro software (BD FACSCalibur, San Jose, CA,
USA).
Flow cytometry for ROS expression
ROS levels were detected with the membrane-permeable
fluorescent probes 2′,7′-dichlorofluorescin diacetate (DCFH-DA), as
described previously (29). The
treated hOMF cells (1×106 cells/ml) were incubated with
2.5 mmol/l DCFH-DA at 37°C for 25 min. The cells were then washed
with PBS, digested with 0.25% trypsin (Sigma-Aldrich; Merck KGaA),
treated with DCFH-DA at 37°C for 25 min, and finally detected by
flow cytometry with version 5.1 Cell Quest Pro software. The
fluorescence intensity was measured, and the average was calculated
using at least 3 replicates.
SOD, MDA and CAT expression
levels
SOD assay kit (cat, no. A001-3), MDA assay kit (cat,
no. A003-1) and CAT assay kit (cat, no. A007-2) were purchased from
Nanjing Jiancheng Bioengineering Institute (Nanjing, China). SOD,
MDA and CAT activities (30,31) were based on a colorimetric method and
used in accordance with the manufacturer's protocol, as described
previously (30–32). The colorimetric reactions were
determined at 450 and 405 nm using UV-5800H spectrophotometer
(Metash Instruments, Shanghai, China).
Statistical analysis
Statistical analyses were conducted with SPSS 19.0
software (SPSS, Inc., Chicago, IL, USA). The data are expressed as
the mean ± standard deviation. A one-way analysis of variance and
Dunnett's post-hoc tests were performed to compare the differences
between each group. P<0.05 was considered to indicate a
statistically significant difference.
Results
Morphology and cytokeratin of hOMF
cells are identified
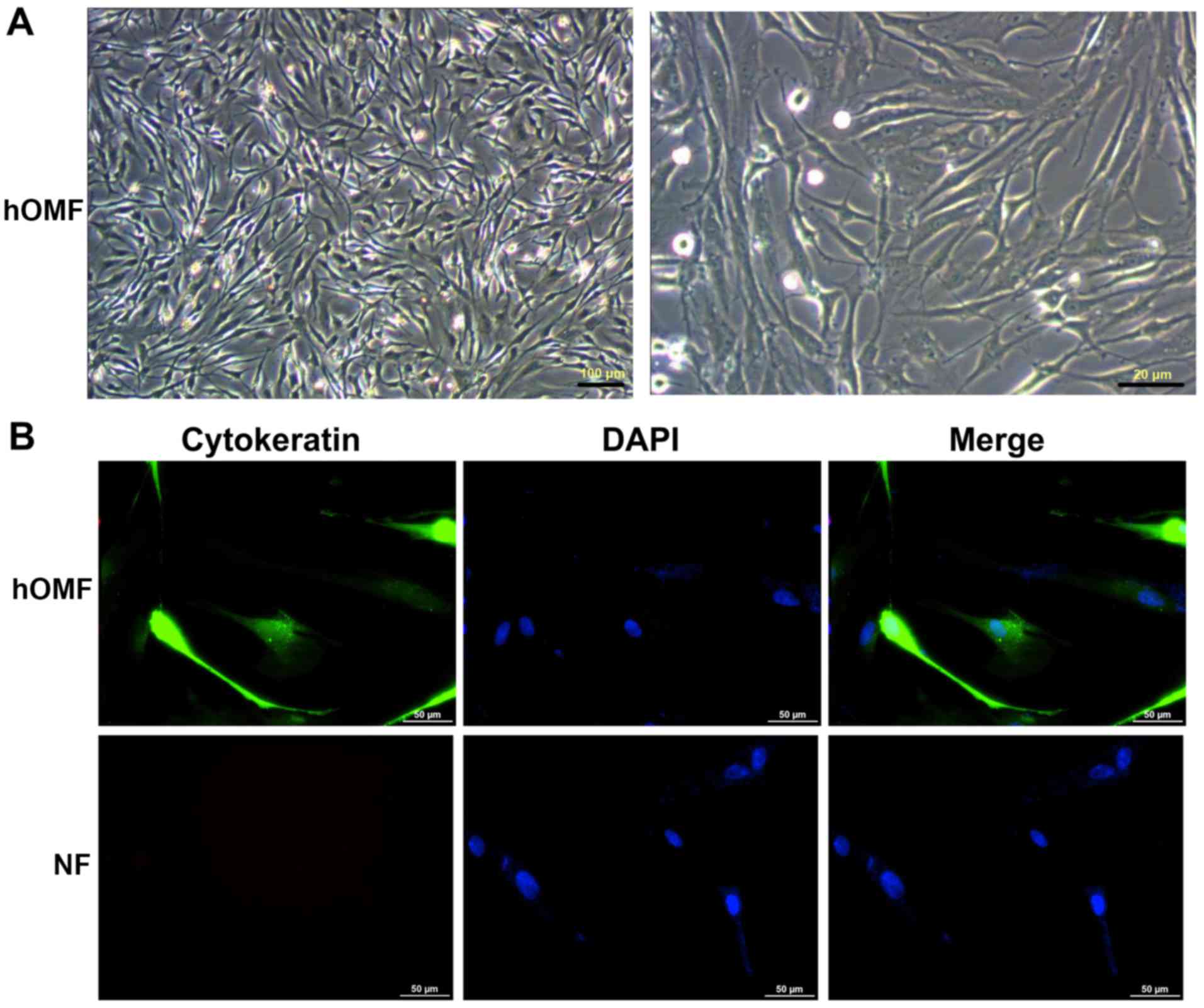
The results indicated that the hOMF cells exhibited
a typical morphology (Fig. 1A). The
cytokeratin level was detected with immunofluorescence staining in
the hOMF and NF cells. It was identified that cytokeratin was
expressed in the hOMF cells, but not in the NF cells (Fig. 1B).
Treatment with STE inhibits cell
viability
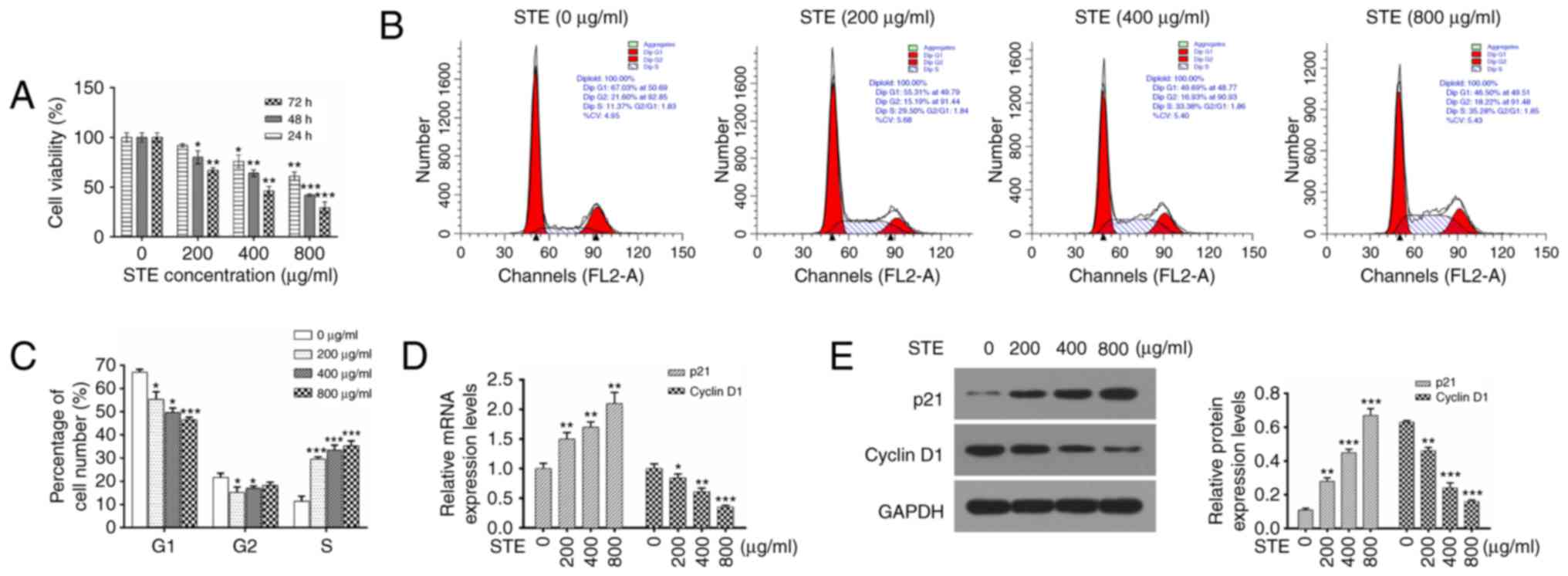
The effects of STE on the proliferation of hOMF
cells were determined by culturing the hOMF cells with specified
concentrations of STE (0, 200, 400 or 800 µg/ml) for 24, 48 and 72
h. MTT assays was performed to analyze the viability rate. It was
identified that the STE inhibited the viability HOMF cells in dose-
and a time-dependent manners (Fig.
2A).
Treatment with STE induces cycle
progression of hOMF cells
The cell cycle distribution was additionally
analyzed via flow cytometry with PI staining. The results revealed
that the average percentages of cells in G1 phase were 67.03,
55.31, 49.69 and 46.50% at 0, 200, 400 and 800 µg/ml STE
respectively. The average percentages of cells in the S phase were
11.37, 29.50, 33.38 and 35.28% in the hOMF cells that were treated
with 0, 200, 400 and 800 µg/ml STE, (Fig.
2B). In comparison with the control group (STE, 0 µg/ml), STE
treatment decreased the percentage of cells in the G1 phase, and
increased the percentage of cells in the S phase at 200, 400 and
800 µg/ml STE (Fig. 2C). The results
indicated that the G1-S cell cycle progression was induced by the
STE in the hOMF cells. The mRNA and the protein expression levels
of p21 and cyclin D1 were evaluated by RT-qPCR and western blot
analysis. Fig. 2D indicates that STE
treatment upregulated p21 expression and downregulated cyclin D1
expression in a concentration-dependent manner. It was also
identified that the protein expression levels of p21 were increased
and the protein expression levels of cyclin D1 were decreased in
the STE treatment group (STE, 0 µg/ml; Fig. 2E).
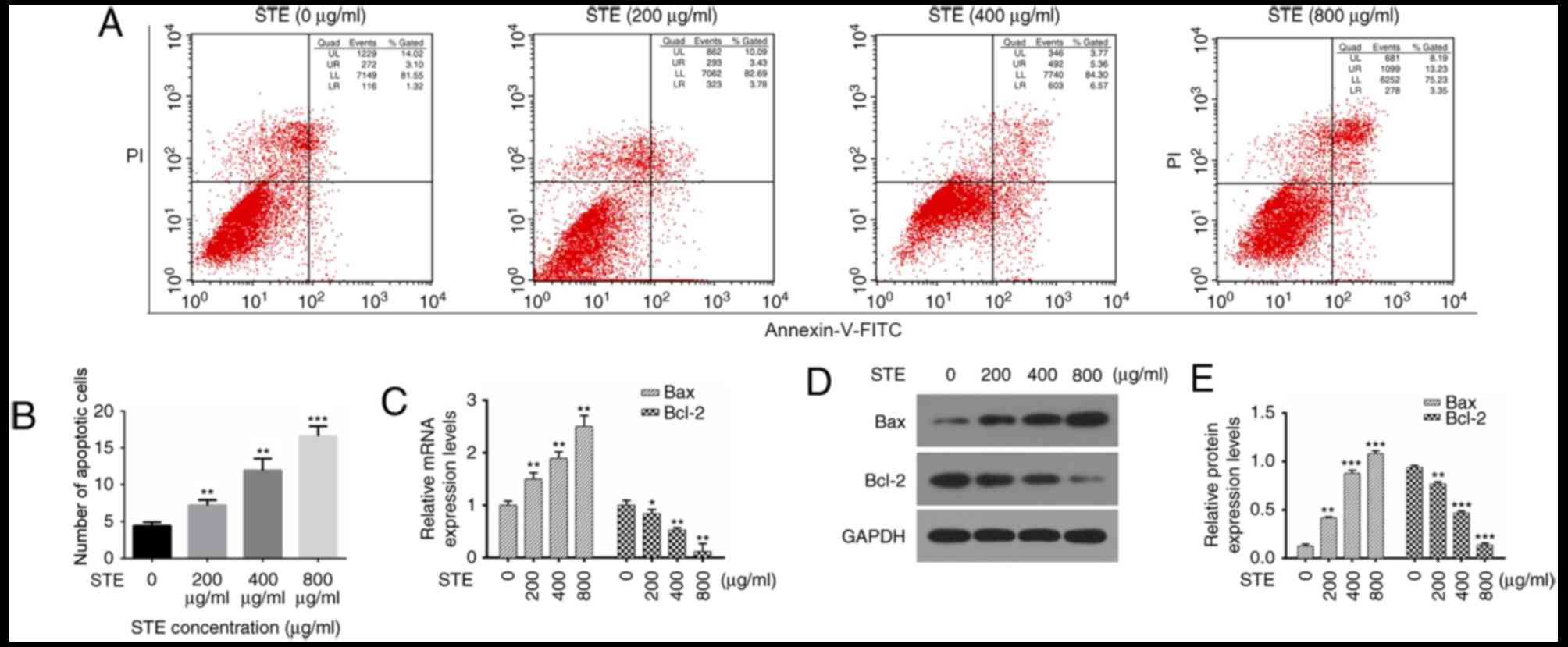
Treatment with STE promotes the
apoptosis ability of hOMF cells
The present study analyzed the effects of the STE on
apoptosis via flow cytometry (Fig.
3A), and the results indicated that the apoptotic indexes of
the control (0 µg/ml) and STE treatment groups (200, 400 and 800
µg/ml) were 4.43, 7.21, 8.93 and 16.58%, respectively (Fig. 3B). The expression levels of the
apoptosis-associated genes (Bax and Bcl-2) were then measured with
RT-qPCR and western blot analysis. The results indicated that Bax
expression levels were increased and the Bcl-2 levels were
decreased in the hOMF cells treated with STE in a
concentration-dependent manner (Fig. 3C
and D).
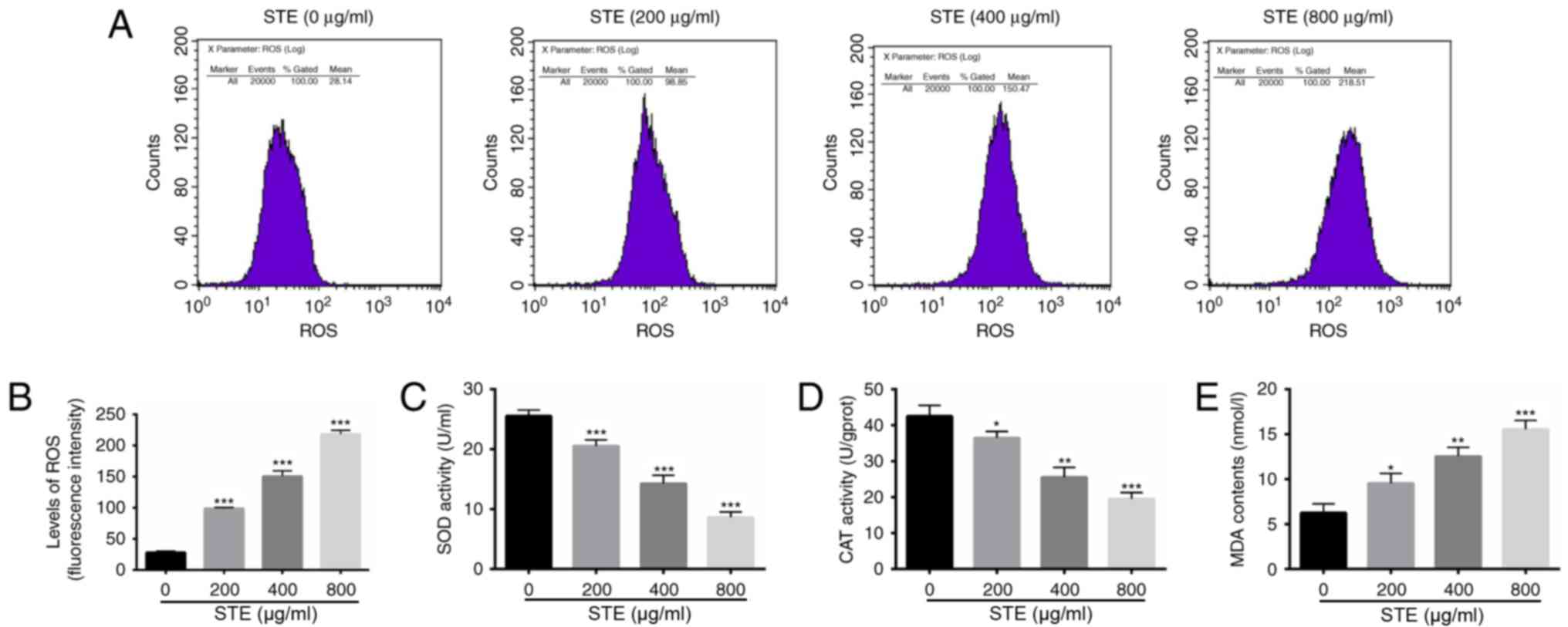
STE increases the concentrations of
ROS and MDA and decreases the concentrations of SOD and CAT
There have been several previous studies
demonstrating that ROS serves a significant role in apoptosis
induction under physiological and pathological conditions (33,34). In
the present study, the concentration of ROS was analyzed with a
flow cytometer with DCFH-DA fluorescent probe in the hOMF cells
treated with STE (0, 200, 400 and 800 µg/ml) for 48 h. The results
indicated significant differences in the ROS levels among the STE
treatment and the control (STE, 0 µg/ml) groups (Fig. 4A). The concentration of ROS was
significantly increased in the STE treatment groups (Fig. 4B). The results indicated that the SOD
(Fig. 4C) and the CAT (Fig. 4D) activity levels were significantly
decreased compared with 0 µg/ml. The MDA activity level was
significantly increased in the STE treatment group, compared with 0
µg/ml (Fig. 4E).
STE upregulates p-p53, NF-κB p65,
Nrf2, HO-1, and NQO1 expression levels in the hOMF cells
The regulatory effects of STE treatment were
investigated by detecting the expression levels of p53, NF-κB p65,
Nrf2, HO-1, and NQO1 via RT-qPCR and western blot analysis in hOMF
cells treated with the STE (0, 200, 400 and 800 µg/ml) for 48 h. As
demonstrated in Fig. 5A, the
expression level of p-p53 was increased in the STE treatment group
compared with 0 µg/ml. The results also indicated that STE
treatment increased the expression level of NF-κB p65 in the hOMF
cells in a dose-dependent manner (Fig.
5B). The Nrf2, HO-1 and NQO1 expression levels were upregulated
in the STE treatment group compared with 0 µg/ml (Fig. 5C).
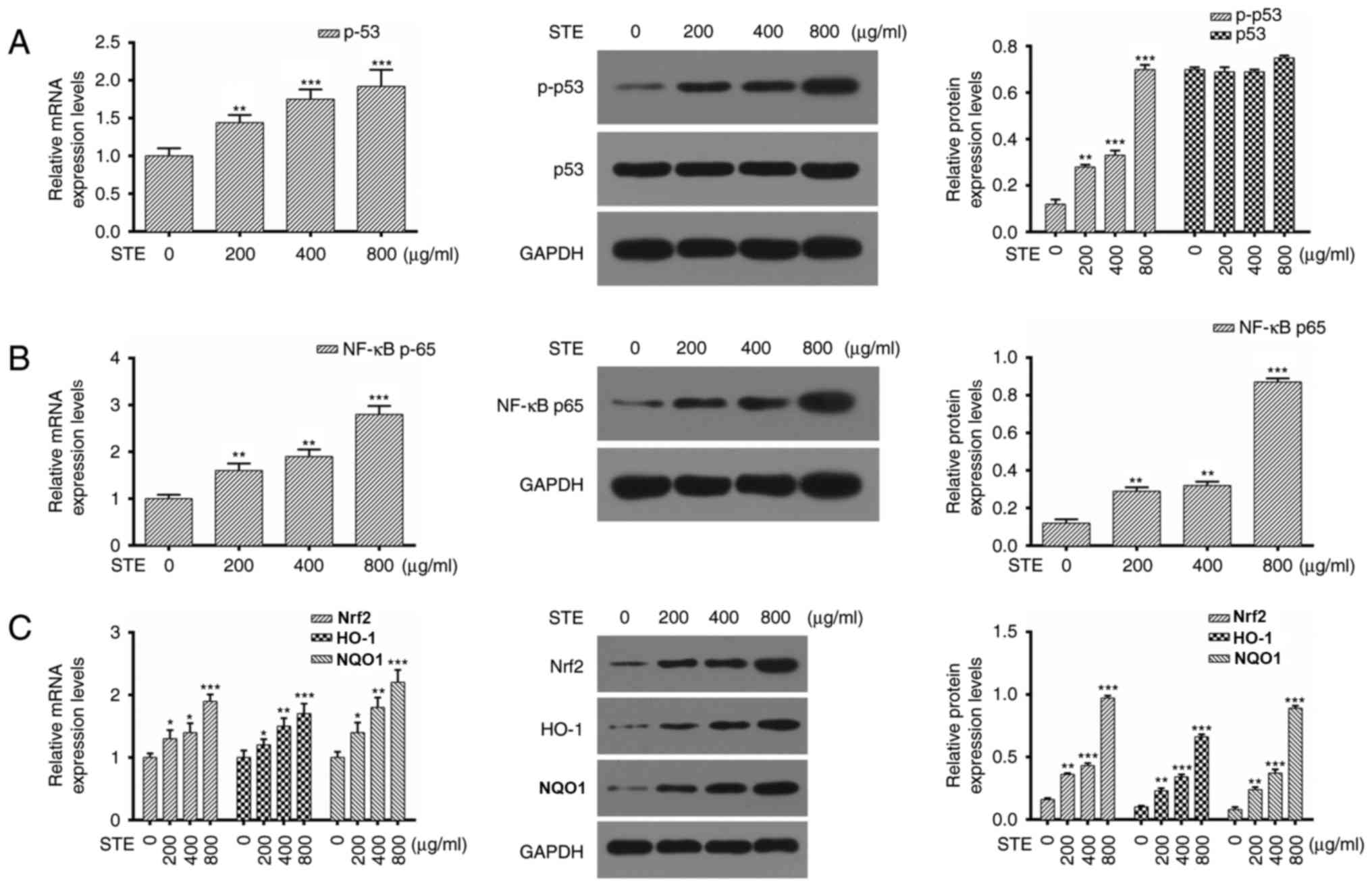 | Figure 5.STE upregulates p-p53, NF-κB p65,
Nrf2, HO-1 and NQO1 expression levels in hOMF cells. hOMF cells
were treated with STE (0, 200, 400 and 800 µg/ml) for 48 h
respectively. (A) p53 expression was detected by RT-qPCR and
western blot analysis and GAPDH was used as an internal reference
(**P<0.01 and ***P<0.001 vs. 0 µg/ml). (B) RT-qPCR and
western blot analysis of NF-κB p65 in hOMF cells (**P<0.01 and
***P<0.001 vs. 0 µg/ml). (C) Levels of Nrf2, HO-1 and NQO1 were
measured by RT-qPCR and western blot analysis (*P<0.05,
**P<0.01 and ***P<0.001 vs. 0 µg/ml). hOMF, human oral mucosa
fibroblasts; STE, smokeless tobacco extracts; p53, tumor protein
53; p, phosphorylated p53; NF-κB, nuclear factor kappa light chain
enhancer of activated B cells, p65, transcription factor p65; Nrf2,
Nuclear factor (erythroid-derived 2)-like 2; HO-1, Hemeoxygenase 1;
NQO1, NAD(P)H quinone dehydrogenase 1; RT-qPCR, reverse
transcription quantitative polymerase chain reaction. |
Discussion
The use of tobacco remains a major public health
concern in a number of countries, due to its established
associations with a number of diseases and nicotine addiction
(35,36). The nicotine within tobacco is the
primary psychoactive substance, and the major source of alkaloids
(37,38). Previous studies have identified that
tobacco contains a variety of toxic substances, including
polycyclic aromatic hydrocarbons, nitrosamines, nicotine,
formaldehyde and hydrogen (39). The
use of general tobacco may result in a variety of oral diseases,
including oral inflammation, oral injury and Snuff dipper's lesions
(40,41). Previous studies also have indicated
that smokeless tobacco causes other diseases, including peripheral
vascular disease, cardiovascular disease, hypertension and
increased fetal morbidity and mortality (42). In the present study, the effect of STE
on hOMF cells over a range of concentrations and time intervals was
explored, in order to identify the potential pathogenic mechanism
of STE.
Previous studies have indicated that p21 was present
in several biological progresses and served as a negative regulator
of cell proliferation (43). An
association between poor prognosis and an accumulation of nuclear
p21 in oral squamous cell carcinoma (OSCC) was demonstrated
(44). A number of studies also
indicated that STE affected the cell proliferation and cell cycle
in OSCC (45,46). STE promoted NF-κB expression and the
expression levels of cell cycle-associated proteins p53 and p21 in
premalignant lesions in the oral cavity (47,48). The
results of the present study indicated that STE significantly
inhibited cell proliferation and induced G1-S cell cycle
progression in the hOMF cells. It was also identified that STE
increased p21 expression and decreased cyclin D1 expression in the
hOMF cells.
Apoptosis is a gene-controlled cell-independent
death process, which is an important mechanism for the maintenance
of a stable internal environment. Apoptosis serves an important
role of normal biological function (49,50).
Previous studies have indicated that STE may lead to the apoptosis
of multiple cell types, including hamster cheek pouch cells,
Epstein-Barr virus-infected B cells and oral keratinocytes
(51–54). Bcl-2 and Bax proteins are the 2
primary members of apoptosis pathway (55). Bcl-2 serves an anti-apoptotic role,
and the Bax protein is similar to the Bcl-2 in structure, but
serves an antagonistic role to that of Bcl-2 (56). The molecular mechanisms of STE-induced
apoptosis are not fully understood in hOMF cells. The present study
identified that the percentage of apoptotic cells was significantly
increased subsequent to treatment of the hOMF cells with STE in a
dose dependent manner. In addition, Bax expression levels were
increased and Bcl-2 levels were decreased in the hOMF cells treated
with STE in a dose-dependent manner. The present study indicated
that the ROS were chemically reactive molecules containing oxygen,
which may induce apoptosis. The results of the present study also
revealed that STE increased the concentrations of ROS, and that ROS
was closely associated with MDA, SOD and CAT.
NF-κB is a nuclear transcription factor that
regulates the expression of various genes that are critical for the
regulation of apoptosis, inflammation, viral replication,
tumorigenesis and various autoimmune diseases (57). Nrf2 is a transcription factor
activated by oxidative stress that binds to the antioxidant
response element (ARE) (58).
ARE-associated genes may participate in the maintenance of redox
homeostasis through the activation of proteins, including HO-1 and
NQO1 (58). In the present study, it
was identified that STE increased the expression level of NF-κB p65
in the hOMF cells in a dose-dependent manner, and the levels of
Nrf2, HO-1 and NQO1 expression were upregulated in the STE
treatment group.
In the present study, the potential roles that STE
serves in proliferation, cell cycle and apoptosis of hOMF cells
were identified. The results indicated that STE increased the rate
of cell cycle progression and apoptosis via cell cycle- and
apoptosis-associated proteins. STE increased the concentrations of
ROS and MDA, decreased the concentrations of SOD and CAT and
upregulatedp-p53, NF-κB, p65, Nrf2, HO-1 and NQO1 expression levels
in hOMF cells.
Acknowledgements
Not applicable.
Funding
Not funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LL and XZ designed the experimental scheme. YW
analyzed and interpreted data. LL was a major contributor in
writing the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Boffetta P, Hecht S, Gray N, Gupta P and
Straif K: Smokeless tobacco and cancer. Lancet Oncol. 9:667–675.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chagué F, Guenancia C, Gudjoncik A, Moreau
D, Cottin Y and Zeller M: Smokeless tobacco, sport and the heart.
Arch Cardiovasc Dis. 108:75–83. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
England LJ, Kim SY, Tomar SL, Ray CS,
Gupta PC, Eissenberg T, Cnattingius S, Bernert JT, Tita AT, Winn
DM, et al: Non-cigarette tobacco use among women and adverse
pregnancy outcomes. Acta Obstet Gynecol Scand. 89:454–464. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bates C, Fagerström K, Jarvis MJ, Kunze M,
McNeill A and Ramström L: European Union policy on smokeless
tobacco: A statement in favour of evidence based regulation for
public health. Tob Control. 12:360–367. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McAdam K, Kimpton H, Vas C, Rushforth D,
Porter A and Rodu B: The acrylamide content of smokeless tobacco
products. Chem Cent J. 9:562015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Janbaz KH, Qadir MI, Basser HT, Bokhari TH
and Ahmad B: Risk for oral cancer from smokeless tobacco. Contemp
Oncol (Pozn). 18:160–164. 2014.PubMed/NCBI
|
|
7
|
Lee CH, Lee JM, Wu DC, Hsu HK, Kao EL,
Huang HL, Wang TN, Huang MC and Wu MT: Independent and combined
effects of alcohol intake, tobacco smoking and betel quid chewing
on the risk of esophageal cancer in Taiwan. Int J Cancer.
113:475–482. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Znaor A, Brennan P, Gajalakshmi V, Mathew
A, Shanta V, Varghese C and Boffetta P: Independent and combined
effects of tobacco smoking, chewing and alcohol drinking on the
risk of oral, pharyngeal and esophageal cancers in Indian men. Int
J Cancer. 105:681–686. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brinton LA, Blot WJ, Becker JA, Winn DM,
Browder JP, Farmer JC Jr and Fraumeni JF Jr: A case-control study
of cancers of the nasal cavity and paranasal sinuses. Am J
Epidemiol. 119:896–906. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Johansson SL, Hirsch JM, Larsson PA, Saidi
J and Osterdahl BG: Snuff-induced carcinogenesis: Effect of snuff
in rats initiated with 4-nitroquinoline N-oxide. Cancer Res.
49:3063–3069. 1989.PubMed/NCBI
|
|
11
|
Lam E, Kelley E, Martin S and Buettner G:
Tobacco xenobiotics release nitric oxide. Tob Induc Dis. 1:207–211.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stich HF, Parida BB and Brunnemann KD:
Localized formation of micronuclei in the oral mucosa and
tobacco-specific nitrosamines in the saliva of ‘reverse’ smokers,
Khaini-tobacco chewers and gudakhu users. Int J Cancer. 50:172–176.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hecht SS, Rivenson A, Braley J, DiBello J,
Adams JD and Hoffmann D: Induction of oral cavity tumors in F344
rats by tobacco-specific nitrosamines and snuff. Cancer Res.
46:4162–4166. 1986.PubMed/NCBI
|
|
14
|
Rivenson A, Hoffmann D, Prokopczyk B, Amin
S and Hecht SS: Induction of lung and exocrine pancreas tumors in
F344 rats by tobacco-specific and Areca-derived N-nitrosamines.
Cancer Res. 48:6912–6917. 1988.PubMed/NCBI
|
|
15
|
Hecht SS: Biochemistry, biology, and
carcinogenicity of tobacco-specific N-nitrosamines. Chem Res
Toxicol. 11:559–603. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Patel BP, Rawal UM, Shah PM, Prajapati JA,
Rawal RM, Dave TK and Patel PS: Study of tobacco habits and
alterations in enzymatic antioxidant system in oral cancer.
Oncology. 68:511–519. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Naga Sirisha CV and Manohar RM: Study of
antioxidant enzymes superoxide dismutase and glutathione peroxidase
levels in tobacco chewers and smokers: A pilot study. J Cancer Res
Ther. 9:210–214. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sugiura T, Dohi Y, Takase H, Yamashita S,
Fujii S and Ohte N: Oxidative stress is closely associated with
increased arterial stiffness, especially in aged male smokers
without previous cardiovascular events: A cross-sectional study. J
Atheroscler Thromb. 24:1186–1198. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ermis B, Ors R, Yildirim A, Tastekin A,
Kardas F and Akcay F: Influence of smoking on maternal and neonatal
serum malondialdehyde, superoxide dismutase, and glutathione
peroxidase levels. Ann Clin Lab Sci. 34:405–409. 2004.PubMed/NCBI
|
|
20
|
Block G, Dietrich M, Norkus EP, Morrow JD,
Hudes M, Caan B and Packer L: Factors associated with oxidative
stress in human populations. Am J Epidemiol. 156:274–285. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Harris AC, Tally L, Schmidt CE, Muelken P,
Stepanov I, Saha S, Vogel RI and LeSage MG: Animal models to assess
the abuse liability of tobacco products: Effects of smokeless
tobacco extracts on intracranial self-stimulation. Drug Alcohol
Depend. 147:60–67. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu C, Zhang Z, Liu Y, Zong Y, Chen Y, Du
X, Chen J, Feng S, Hu J, Cui S and Lu G: Toxicity of smokeless
tobacco extract after 184-day repeated oral administration in rats.
Int J Environ Res Public Health. 13(pii): E2812016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Avti PK, Vaiphei K, Pathak CM and Khanduja
KL: Involvement of various molecular events in cellular injury
induced by smokeless tobacco. Chem Res Toxicol. 23:1163–1174. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Avti PK, Kumar S, Pathak CM, Vaiphei K and
Khanduja KL: Smokeless tobacco impairs the antioxidant defense in
liver, lung, and kidney of rats. Toxicol Sci. 89:547–553. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li CJ: Flow cytometry analysis of cell
cycle and specific cell synchronization with butyrate. Methods Mol
Biol. 1524:149–159. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Filby A, Day W, Purewal S and
Martinez-Martin N: The analysis of cell cycle, proliferation, and
asymmetric cell division by imaging flow cytometry. Methods Mol
Biol. 1389:71–95. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang W and Liang Z: Comparison between
annexin V-FITC/PI and Hoechst33342/PI double stainings in the
detection of apoptosis by flow cytometry. Xi Bao Yu Fen Zi Mian Yi
Xue Za Zhi. 30:1209–1212. 2014.(In Chinese). PubMed/NCBI
|
|
29
|
Shen Y, Guo W, Wang Z, Zhang Y, Zhong L
and Zhu Y: Protective effects of hydrogen sulfide in hypoxic human
umbilical vein endothelial cells: A possible mitochondria-dependent
pathway. Int J Mol Sci. 14:13093–13108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
McCord JM and Fridovich I: Superoxide
dismutase. An enzymic function for erythrocuprein (hemocuprein). J
Biol Chem. 244:6049–6055. 1969.PubMed/NCBI
|
|
31
|
Archbald RM: Enzymatic methods in amino
acid analysis. Ann N Y Acad Sci. 47:181–186. 1946. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cheng C, Yi J, Wang R, Cheng L, Wang Z and
Lu W: Protection of Spleen Tissue of γ-ray irradiated mice against
immunosuppressive and oxidative effects of radiation by adenosine
5′-monophosphate. Int J Mol Sci. 19(pii): E12732018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Simon HU, Haj-Yehia A and Levi-Schaffer F:
Role of reactive oxygen species (ROS) in apoptosis induction.
Apoptosis. 5:415–418. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yee C, Yang W and Hekimi S: The intrinsic
apoptosis pathway mediates the pro-longevity response to
mitochondrial ROS in C. elegans. Cell. 157:897–909. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Napierala M and Florek E: Historical
trends in prevalence of tobacco smoking among women. Przegl Lek.
72:155–157. 2015.(In Polish). PubMed/NCBI
|
|
36
|
Weintraub JM and Hamilton WL: Trends in
prevalence of current smoking, Massachusetts and states without
tobacco control programmes, 1990 to 1999. Tob Control. 11 Suppl
2:ii8–ii13. 2002.PubMed/NCBI
|
|
37
|
Corrigall WA: Nicotine self-administration
in animals as a dependence model. Nicotine Tob Res. 1:11–20. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rose JE and Corrigall WA: Nicotine
self-administration in animals and humans: Similarities and
differences. Psychopharmacology (Berl). 130:28–40. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schick SF, Farraro KF, Perrino C, Sleiman
M, van de Vossenberg G, Trinh MP, Hammond SK, Jenkins BM and Balmes
J: Thirdhand cigarette smoke in an experimental chamber: Evidence
of surface deposition of nicotine, nitrosamines and polycyclic
aromatic hydrocarbons and de novo formation of NNK. Tob Control.
23:152–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Furukawa S, Ueno M and Kawaguchi Y:
Influence of tobacco on dental and oral diseases. Nihon Rinsho.
71:459–463. 2013.(In Japanese). PubMed/NCBI
|
|
41
|
Didilescu A: Tobacco induced oral
diseases. Pneumologia. 49:300–303. 2000.(In Romanian). PubMed/NCBI
|
|
42
|
Critchley JA and Unal B: Health effects
associated with smokeless tobacco: A systematic review. Thorax.
58:435–443. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gartel AL, Serfas MS and Tyner AL:
p21-negative regulator of the cell cycle. Proc Soc Exp Biol Med.
213:138–149. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Huang KJ, Kuo CH, Chen SH, Lin CY and Lee
YR: Honokiol inhibits in vitro and in vivo growth of oral squamous
cell carcinoma through induction of apoptosis, cell cycle arrest
and autophagy. J Cell Mol Med. 22:1894–1908. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mishra R and Das BR: Activation of STAT
5-cyclin D1 pathway in chewing tobacco mediated oral squamous cell
carcinoma. Mol Biol Rep. 32:159–166. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mishra R and Das BR: Cyclin D1 expression
and its possible regulation in chewing tobacco mediated oral
squamous cell carcinoma progression. Arch Oral Biol. 54:917–923.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Rohatgi N, Kaur J, Srivastava A and Ralhan
R: Smokeless tobacco (khaini) extracts modulate gene expression in
epithelial cell culture from an oral hyperplasia. Oral Oncol.
41:806–820. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sawhney M, Rohatgi N, Kaur J, Shishodia S,
Sethi G, Gupta SD, Deo SV, Shukla NK, Aggarwal BB and Ralhan R:
Expression of NF-kappaB parallels COX-2 expression in oral
precancer and cancer: Association with smokeless tobacco. Int J
Cancer. 120:2545–2556. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jiang P and Yue Y: Human papillomavirus
oncoproteins and apoptosis (Review). Exp Ther Med. 7:3–7. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ni Nyoman AD and Lüder CG: Apoptosis-like
cell death pathways in the unicellular parasite Toxoplasma gondii
following treatment with apoptosis inducers and chemotherapeutic
agents: A proof-of-concept study. Apoptosis. 18:664–680. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bagchi M, Balmoori J, Bagchi D, Ray SD,
Kuszynski C and Stohs SJ: Smokeless tobacco, oxidative stress,
apoptosis, and antioxidants in human oral keratinocytes. Free Radic
Biol Med. 26:992–1000. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jenson HB, Baillargeon J, Heard P and
Moyer MP: Effects of smokeless tobacco and tumor promoters on cell
population growth and apoptosis of B lymphocytes infected with
epstein-barr virus types 1 and 2. Toxicol Appl Pharmacol.
160:171–182. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Mangipudy RS and Vishwanatha JK: Role of
nitric oxide in the induction of apoptosis by smokeless tobacco
extract. Mol Cell Biochem. 200:51–57. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Banerjee AG, Gopalakrishnan VK and
Vishwanatha JK: Inhibition of nitric oxide-induced apoptosis by
nicotine in oral epithelial cells. Mol Cell Biochem. 305:113–121.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kuwana T and Newmeyer DD: Bcl-2-family
proteins and the role of mitochondria in apoptosis. Curr Opin Cell
Biol. 15:691–699. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Breckenridge DG and Xue D: Regulation of
mitochondrial membrane permeabilization by BCL-2 family proteins
and caspases. Curr Opin Cell Biol. 16:647–652. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Panday A, Inda ME, Bagam P, Sahoo MK,
Osorio D and Batra S: Transcription factor NF-κB: An update on
intervention strategies. Arch Immunol Ther Exp (Warsz). 64:463–483.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Jaiswal AK: Nrf2 signaling in coordinated
activation of antioxidant gene expression. Free Radic Biol Med.
36:1199–1207. 2004. View Article : Google Scholar : PubMed/NCBI
|