Introduction
Gastric linitis plastica (GLP), also known as
‘leather bottle stomach’ or Borrmann type IV gastric cancer, is
believed to be a kind of diffuse infiltrative gastric cancer
(1,2).
In general, since tumor cells migrate throughout the submucosa
without severely affecting the mucosal lining of the stomach, it is
difficult to detect cancer cells by gastrointestinal series or
conventional endoscopic biopsy at an early stage (3–5). On one
hand, at the time of detection, these walls of GLP are occasionally
accompanied by peritoneal dissemination, considerable lymph node
metastasis and direct invasion into the surrounding organs, which
results in a poor prognosis (6,7). On the
other hand, a number of diseases may present with the same
thickened gastric wall as GLP including malignant tumors (lymphoma)
as well as benign diseases (Ménétrier's gastritis, amyloidosis, and
lymphoid hyperplasia); the therapeutic management of these diseases
is clearly different (8). In
addition, the incidence of GLP is increasing gradually at present
(9). Therefore, it is very important
to make a definitive diagnosis promptly and accurately.
Endoscopic ultrasound (EUS) is a reliable
non-surgical technique for diagnosis and staging of
gastrointestinal malignancies. Although some lesions have
distinctive EUS characteristics, using these diagnostic criteria
alone to distinguish other diseases from GLP is inadequate
(6). Consequently, tissue sampling is
necessary to establish a conclusive diagnosis. Endoscopic
ultrasound-guided fine-needle aspiration (EUS-FNA) biopsy has
evolved to become a leading method to confirm the diagnosis of the
pre-therapeutic evaluation in patients suspected of submucosal
tumors of the upper gastrointestinal tract (10,11).
However, its importance for the diagnosis of GLP has been reported
only by description of isolated cases (12), no systematic study has been
reported.
In the present study, we retrospectively
investigated the safety and efficacy of EUS-FNA for the diagnosis
of GLP with negative malignant endoscopy biopsies.
Patients and methods
Patients
Between January 2010 and January 2017, 46
consecutive patients who were suspected of GLP underwent EUS-FNA at
the Endoscopy Centers in Renmin Hospital of Wuhan University. All
patients had undergone ordinary endoscopic biopsies 2–8 times (4
times on average), and their pathology showed negative results. We
extracted and analyzed their medical data. The patient group was
composed of 20 males and 26 females, aged 26–72 years old with a
mean age of 47±10.3 years.
The study was approved by the Institutional Review
Board of Renmin Hospital of Wuhan University (Wuhan, China) and
Xiangyang First People's Hospital of Hubei University of Medicine
(Xiangyang, China). Written informed consent was obtained from all
patients.
Equipment
EUS was performed to determine the status (size,
shape, location, edge and echo intensity, surrounding organs or
lymph node metastasis) of the lesion by using a conventional linear
array EUS endoscope (Pentax EG-3270UK; Pentax, Tokyo, Japan) and
ultrasonic mainframe (Hitachi Preirus; Hitachi, Ltd., Tokyo,
Japan). Patients were hospitalized for the procedure and were
surveyed for complications till discharge from the Hospital.
EUS-FNA procedure
After the target lesion was detected on an EUS view,
EUS-FNA was performed by an endoscopic expert who used a disposable
19-gauge needle (EchoTip Ultra; Cook Medical, Inc., Winston-Salem,
NC, USA), as previously reported (13–15).
Briefly, color Doppler was used to prevent insertion of the needle
into the vessels. To select the appropriate safety path for EUS-FNA
4–8 times, 10 ml tissue was gained by negative pressure suction.
The needle was retracted after stopping the negative pressure. The
tissue in the needle was extracted.
The needle was advanced and moved back and forth
10–20 times while suction was applied. The lock of the syringe was
then closed and the needle removed.
If the collected specimen was a shaped tissue strip,
it was immediately placed in 10% formalin for histologic
examination, and the remaining extract was injected onto a dried
glass for cytological examination by an on-site cytopathologist.
Cell smear was obtained from all of the 46 patients and complete
tissue strips were obtained in 10 cases. The number of needle
passages depended on the cytological diagnosis of the expert. If a
certain amount of eligible cells was found, the puncture was
completed. After the operation, all patients were fasted and given
treatment of fluid replacement and acid suppression.
Statistical analysis
SPSS 17.0 (SPSS, Inc., Chicago, IL, USA) software
was applied for data analysis. Measurement data are presented as
mean ± standard deviation; Chi-square (χ2) test was
applied to compare the enumeration data and rate between the two
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
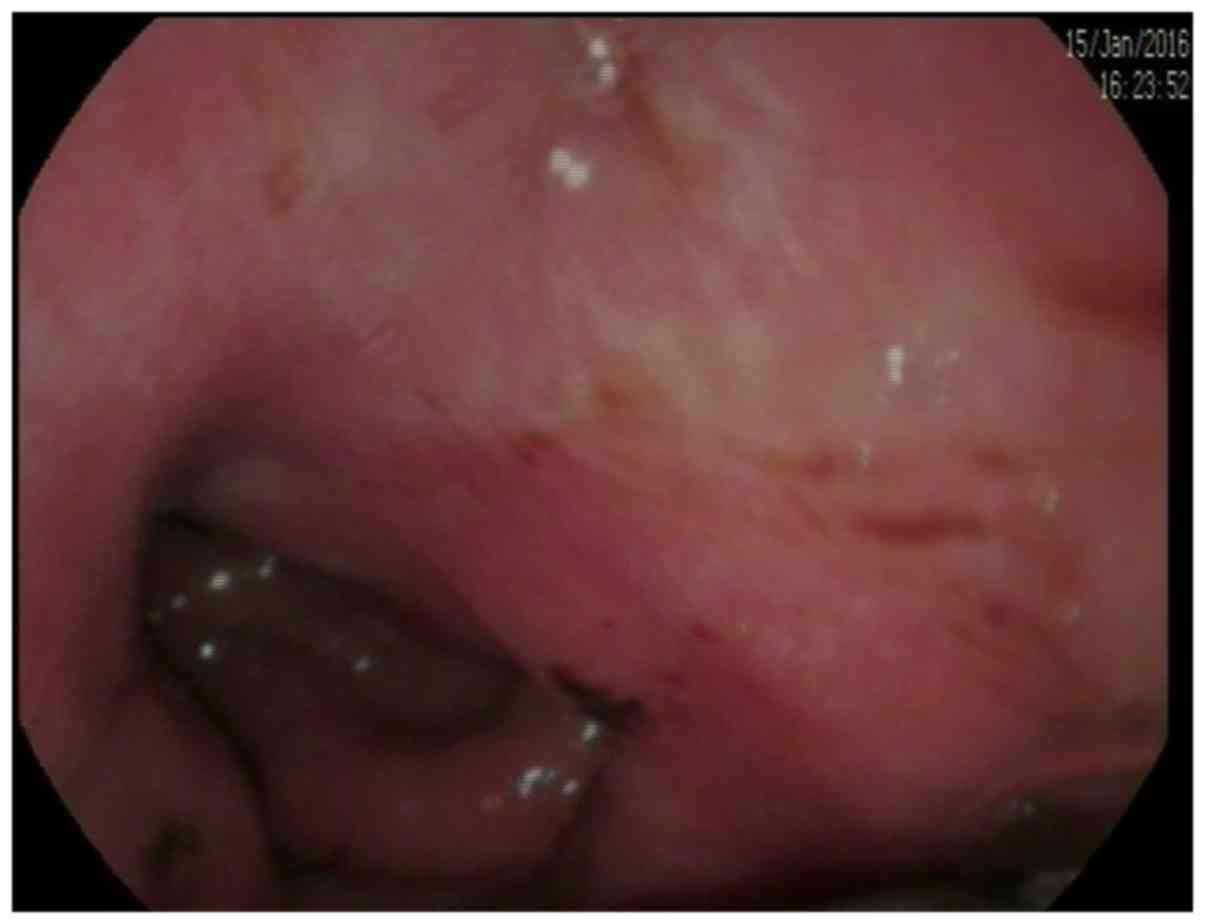
In the 46 cases, 40 cases were diagnosed as GLP by
EUS-FNA or operation. The lesions of 12 cases were situated in
total stomach, 16 cases in gastric body, 8 cases in gastric antrum,
4 cases in cardia and fundus of stomach. The lesion site had been
replaced by a thick gastric wall. The maximum full thickness of the
stomach wall ranged from 7.4 to 22 mm, with an average thickness of
15.7±5.8 mm (Fig. 1). Thirty cases
had homogeneous hypoechoic changes, 8 cases had inhomogeneous
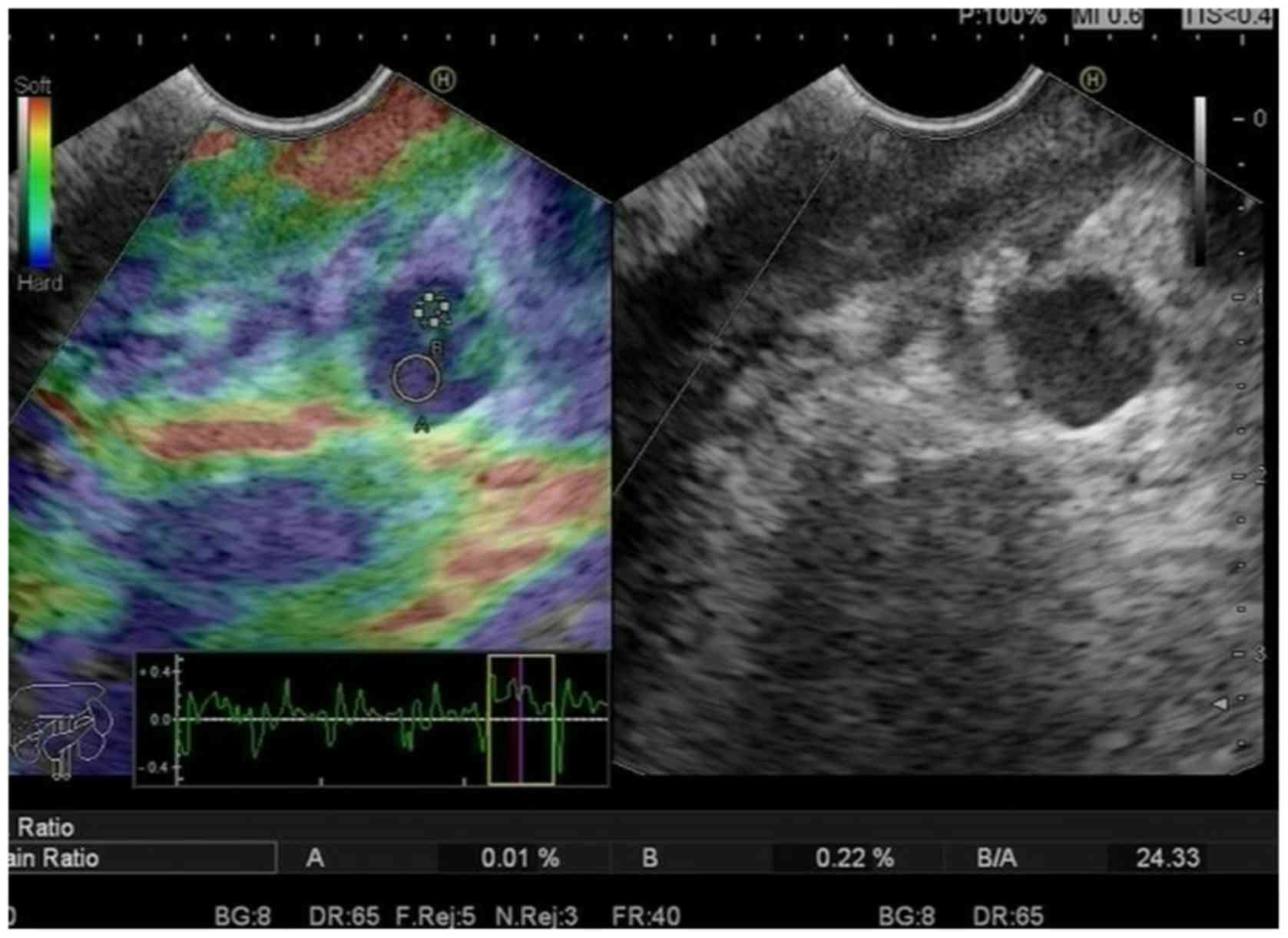
hypoechoic changes, and 2 cases had medium echo. Elastography is a
type of virtual biopsy that attempts to assess differences in
elasticity between normal and tumor tissue. In the present study
blue dominated in all the lesions. On elastographic images, soft
tissues are shown in red and hard tissues in blue. The elastic
strain rate (SR) ranged from 44 to 81, with an average value of
61±18.7. There were 24 cases with lymph node metastasis among the
40 patients (Fig. 2). Three cases had
ascites, and 4 cases had both lymph node metastasis and
ascites.
Of the 26 patients who underwent EUS in the lower
stomach wall whose 1–5 layer structure disappeared, the average
stomach wall thickness was 16.6±2.1 mm. Among these, 24 patients
were with gastric lesions, such as ascites or lymph nodes. Of the
13 patients with merged 1st-to-3rd layer structure and thickened
4th layer, the average thickness of gastric wall was 13.1±2.9 mm,
and in patients with gastric lesions (6/14), the gastric wall
thickness and the incidence of gastric lesions in the former (6
patients) were significantly higher.
In the 46 patients, there were 38 cases who were
diagnosed clearly by EUS-FNA, and the positive rate of aspiration
diagnosis was 82.6%. Pathological findings showed that among the 38
lesions, 26 were adenocarcinoma, 8 were signet-ring cell carcinoma,
and 4 were lymphoma. The final operations were performed in 16
patients, and the postoperative pathological findings were
consistent with EUS-FNA in 14 cases. The diagnostic accuracy of
EUS-FNA was 87.5% (14/16). However, another 2 cases had
indeterminate diagnoses by EUS-FNA, while the pathological findings
after operation were poorly differentiated adenocarcinoma and
undifferentiated adenocarcinoma, respectively. In 16 cases, the
findings of EUS were compared with postoperative assessments of T
and N staging. The diagnostic accuracy of EUS was 80% for T2
staging and 66.7% for T3 staging. Twelve of 16 GLPs were staged
correctly and the overall diagnostic accuracy of the T stage was
75% (Table I). The diagnostic
accuracy of EUS was 66.7% for N0 staging and 80% for N+
staging. The overall diagnostic accuracy of N staging was 75%
(Table II). Lymph nodes with sharp
borders and hypoechoic structures, and >10 mm in size, were
considered as malignant. Stage N0 denotes no sign of metastasis.
N+ denotes metastases in perigastric lymph nodes
(15). According to the clinical and
imaging follow-up, the diagnoses of patients without surgical
resection were consistent with the EUS-FNA findings.
 | Table I.Accuracy of EUS preoperative T staging
in 16 patients with linitis plastica. |
Table I.
Accuracy of EUS preoperative T staging
in 16 patients with linitis plastica.
|
|
| Pathologic stage |
|
|---|
|
|
|
|
|
|---|
| EUS stage | n | T2 | T3 | T4 | Accuracy of EUS
(%) |
|---|
| T2 | 10 | 8 | 2 | 0 | 80 |
| T3 | 6 | 0 | 4 | 2 | 66.7 |
| T4 | 0 | 0 | 0 | 0 |
|
| Total | 16 | 8 | 6 | 2 | 75 |
 | Table II.Accuracy of EUS preoperative N staging
in 16 patients with linitis plastica. |
Table II.
Accuracy of EUS preoperative N staging
in 16 patients with linitis plastica.
|
|
| Pathologic stage |
|
|---|
|
|
|
|
|
|---|
| EUS stage | n | N0 | N+ | Accuracy of EUS
(%) |
|---|
| N0 | 6 | 4 | 2 | 66.7 |
| N+ | 10 | 2 | 8 | 80 |
| Total | 16 | 6 | 10 | 75 |
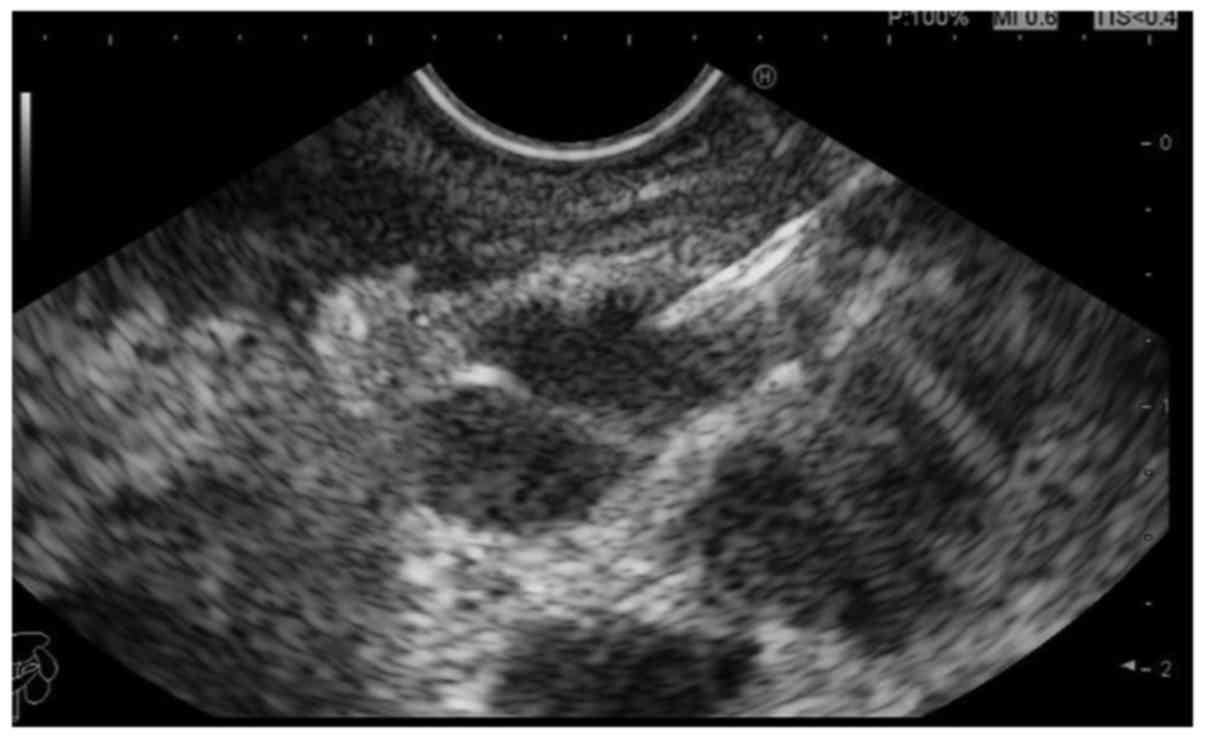
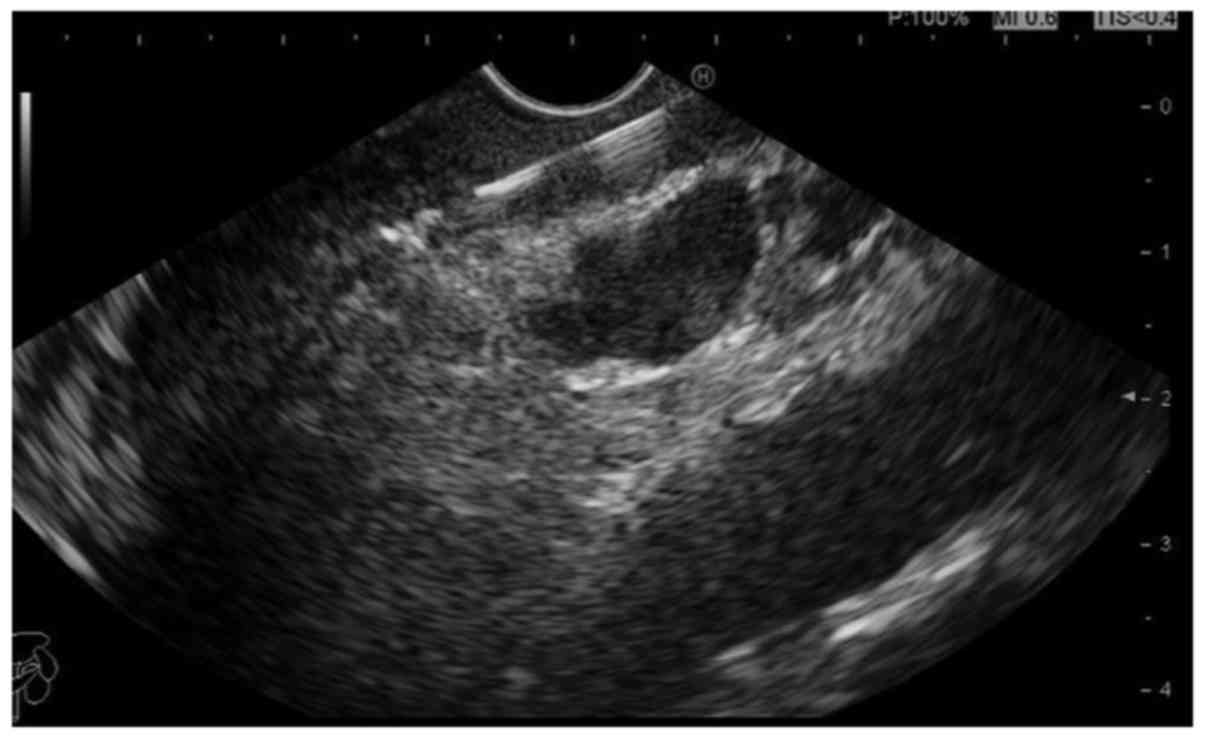
There were 24 cases with lymph node metastasis, the
abnormal stomach walls (gastric lesions) and lymph nodes of these
patients were punctured, respectively, by EUS-FNA. The diagnostic
accuracy in different puncture sites was compared. The results
showed that there were 18 cases with accurate diagnoses by
puncturing lymph nodes (the positive rate of diagnosis was 75%).
However, of the 24 patients, only 13 cases had positive and
accurate diagnoses by puncturing gastric walls (the diagnostic
accuracy was only 54.2%). Accordingly, the diagnostic accuracy by
puncturing lymph nodes was higher than the thick stomach walls
(Figs. 3 and 4), and this difference was statistically
significant (P=0.033, <0.05) (Table
III).
 | Table III.The positive rates of diagnosis in
different puncture sites. |
Table III.
The positive rates of diagnosis in
different puncture sites.
|
| Pathological
findings of aspiration |
|
|---|
|
|
|
|
|---|
| Puncture sites | Positive | Negative | Diagnostic accuracy
of aspiration (%) |
|---|
| Metastatic lymph
node | 18 | 6 | 75 |
| Stomach walls
(gastric lesions) | 13 | 11 | 54.2 |
According to the follow-up (the follow-up interval
after EUS-FNA ranged from 1 week to 1 month), none of the 46
patients required any procedures related to adverse events.
Discussion
GLP has a unique pathological growth pattern. The
tumor tissue originates from the submucosa and infiltrates around
the gastric wall, resulting in reactive fibrosis (16). The positive rate for superficial
biopsies in GLP patients is low. Kim et al (17) showed that the missed rate of ordinary
biopsies in diagnosing Borrmann type IV gastric cancer was as high
as 55.9%. Therefore, misdiagnosed and missed diagnosis are common
in conventional endoscopic biopsy and that not only affects the
treatment and prognosis of the disease, but also increases the
patient's pain and psychological, and financial burden.
Endoscopic ultrasonography has developed rapidly and
has dual functions of endoscope and ultrasound. It can clearly
display the structure of gastric wall and its relation with tumors
(18). Therefore, EUS greatly
improves the diagnostic rate of GLP (8). EUS has special sonographic signs for
gastric cancer, and correct diagnosis and staging can be achieved
in most patients. EUS can be used to observe the gastric wall and
extramural lesions. At the same time, according to the
characteristics of the EUS sonogram, it is possible to distinguish
whether the thickened gastric wall has a destructive lesion and
infer its properties. Therefore, there is an advantage in
diagnosing the leather stomach. By conventional endoscopy it is
difficult to distinguish primary gastric lymphoma, Ménétrier's
disease, and hypertrophic gastritis from leather stomach. Caletti
et al reported that the hypertrophic gastritis EUS showed
diffuse thickening of the 2nd and 3rd layers of the stomach wall,
but thickened lesions usually show hyperechoic changes (19). Some studies have found that the
lesions of primary gastric lymphoma under EUS are multifocal, and
the diffuse thickened layer 2 and 3 hypoechoic lesions pass through
the pylorus to the duodenum. Further comparison of the difference
between primary gastric lymphoma and leather stomach under EUS
revealed that the former tends to grow along the longitudinal axis
of the stomach, whereas the leather stomach grows along the
transverse axis of the stomach (20).
In this study, we summarized the characteristics of EUS sonograms
of 40 cases of leather stomach: i) the lesions were widely
distributed, with continuous diffuse infiltration around the
stomach wall as the main type, lesion area is beyond the abnormal
area under endoscopy, and the lesions were mainly located in the
stomach; ii) all layers of the stomach wall at the lesion were
thickened, 1st-3rd layers were most commonly thickened and
sometimes the 4th layer was also thickened. The mean thickness of
the stomach wall measured by ultrasound was 15.7±5.8 mm. The
thicker the stomach wall, the higher the incidence of gastric
lesions; iii) the lesions were mainly hypoechoic; iv) lesions tend
to grow along the transverse axis of the stomach; v) lesions are
hard tissue, elasticity ultrasound shows mainly blue signal,
average SR value was 61±18.7. The characteristics of these
ultrasound images are basically consistent with those reported by
Shan et al (21), and they are
consistent with the special biological characteristics of leather
stomach, which is helpful for the diagnosis of leather stomach.
In addition, the peripheral lymph node metastasis
rate of GLP is slightly higher. These sonographic features which
are consistent with the findings of Shan et al (21) and the special biological
characteristics of GLP (22) are
beneficial for the diagnosis of GLP. In addition, EUS can
accurately judge the TNM staging of GLP, and is of great value for
the resectability and prognosis (23). In the present study, compared with
postoperative staging of the 16 patients who underwent surgical
resection, EUS had a diagnostic accuracy of 75% for T staging and
75% for N staging. The accuracies are similar to those from the
previous studies of Cardoso et al (24). However, Park et al considered
that the level of experience and proficiency of an operating doctor
could directly affect the accuracy of staging (25). Therefore, with the increasing
diagnostic experience of endoscopic doctors, the accuracy of EUS
for T and N staging of GLP will be further improved.
Although EUS is a reliable imaging method for the
diagnosis of GLP, a clear diagnosis based only on the sonographic
features is inadequate (20). More
importantly, we need definitive diagnosis to guide the treatment
and prognosis of GLP. In recent years, with the improvement of
endoscopic diagnosis and treatment technology, a number of new
endoscopic biopsy techniques have emerged, such as jumbo biopsy,
endoscopic submucosal resection, endoscopic submucosal dissection
and the bite-on-bite technique (26–28).
Although jumbo biopsy and endoscopic submucosal resection may
increase the surface area of the tissue sample, they do not
significantly increase the depth (29), and there are procedural risks and
complications such as perforation and hemorrhage (30). In contrast to EUS-FNA, endoscopic
submucosal resection is more costly and GLP usually has a thickened
epithelium which may limit the use of bite-on-bite technique
(10,31). EUS-FNA biopsy can accept partial
submucosal lesions and surrounding metastatic tissue purposely,
which significantly improves the biopsy positive rate of GLP
(32). In this study, we assessed the
diagnostic yield of EUS-FNA for GLP that had received negative
results for malignancy via endoscopy biopsies. EUS-FNA provided a
definitive and confirmative diagnosis in 38 (82.6%) of the 46
patients. Pathology of these patients was mainly adenocarcinoma.
Based on the systemic assessment, patients were given a definite
diagnosis and underwent individualized treatment. Finally, of the
16 patients who received surgery, the pathological results of 14
cases were the same by operation and EUS-FNA. The diagnostic
accuracy of EUS-FNA was 87.5%. Carter et al obtained similar
results (12). Futhermore, we
compared the positive rates of diagnosis in different puncture
sites, and the result showed that the positive rate of diagnosis in
lymph node was significantly higher than that of gastric lesions
(P<0.05). Nevertheless, some studies have shown that positive
rate of diagnosis is low by EUS-FNA, because the cytological
tissues obtained by EUS-FAN are small (31). In our study, the positive rate of
diagnosis for GLP by EUS-FNA is high, and we obtained ideal tissue
samples. The main reasons are: first and foremost, the thick wall
of GLP is fibrotic, while the lymph nodes around the stomach have
more cancerous tissues (1), so we
punctured the metastatic lymph nodes. Secondly, we had rapid
on-site evaluation by a cytopathologist during EUS-FNA, which
assisted us to judge whether the adequate samples were obtained or
not (33). Accordingly, on-site
pathology contributes to improved diagnostic accuracy and reduced
complications (34). Last but not
least, EUS-FNA has less risk of bleeding and perforation, thus, it
can be repeated several times (35).
Therefore, if we select the metastatic lymph nodes as far as
possible and have cytology experts present, the positive rate and
accuracy of GLP by EUS-FNA can be improved greatly.
As a limitation, this study was performed with a
small number of patients. Further studies on this method are needed
to clarify the indications and clinical outcomes.
In conclusion, EUS-FNA biopsies provided extremely
accurate pathological diagnoses and were associated with no major
complications or disease recurrence. The results of this small
sample size study suggested that EUS-FNA could obtain submucosal
lesions and puncturing lymph node tissue significantly improving
the diagnostic accuracy of GLP with negative endoscopy biopsies.
Moreover, EUS-FNA is an effective and safe diagnostic method for
GLP.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YY collected and analyzed the data. ST was
responsible for the EUS-FNA procedure. Both authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Institutional Review
Board of Renmin Hospital of Wuhan University (Wuhan, China) and
Xiangyang First People's Hospital of Hubei University of Medicine
(Xiangyang, China). Informed consents were signed by the
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Endo K, Sakurai M, Kusumoto E, Uehara H,
Yamaguchi S, Tsutsumi N and Ikejiri K: Biological significance of
localized Type IV scirrhous gastric cancer. Oncol Lett. 3:94–99.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yook JH, Oh ST and Kim BS:
Clinicopathological analysis of Borrmann type IV gastric cancer.
Cancer Res Treat. 37:87–91. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ikeguchi M, Miyake T, Matsunaga T,
Yamamoto M, Fukumoto Y, Yamada Y, Fukuda K, Saito H, Tatebe S and
Tsujitani S: Recent results of therapy for scirrhous gastric
cancer. Surg Today. 39:290–294. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sasaki T, Koizumi W, Tanabe S, Higuchi K,
Nakayama N and Saigenji K: TS-1 as first-line therapy for gastric
linitis plastica: Historical control study. Anticancer Drugs.
17:581–586. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kodera Y, Nakanishi H, Ito S, Mochizuki Y,
Yamamura Y, Fujiwara M, Hibi K, Ito K, Akiyama S, Tatematsu M, et
al: Detection of disseminated cancer cells in linitis plastica-type
gastric carcinoma. Jpn J Clin Oncol. 34:525–531. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jung K, Park MI, Kim SE and Park SJ:
Borrmann type 4 advanced gastric cancer: Focus on the development
of scirrhous gastric cancer. Clin Endosc. 49:336–345. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Agnes A, Estrella JS and Badgwell B: The
significance of a nineteenth century definition in the era of
genomics: Linitis plastica. World J Surg Oncol. 15:1232017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chiyo T, Kobara H, Mori H, Katsuki N, Haba
R and Masaki T: Submucosal endoscopic sampling for indefinite
gastric linitis plastica infiltrating into the submucosal layer. J
Gastrointestin Liver Dis. 24:375–378. 2015.PubMed/NCBI
|
|
9
|
Luo Y, Gao P, Song Y, Sun J, Huang X, Zhao
J, Ma B, Li Y and Wang Z: Clinicopathologic characteristics and
prognosis of Borrmann type IV gastric cancer: A meta-analysis.
World J Surg Oncol. 14:492016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou XX, Ji F, Xu L, Li L, Chen YP, Lu JJ,
Wang CW and Huang W: EUS for choosing best endoscopic treatment of
mesenchymal tumors of upper gastrointestinal tract. World J
Gastroenterol. 17:1766–1771. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mortensen MB, Edwin B, Hünerbein M,
Liedman B, Nielsen HO and Hovendal C: Impact of endoscopic
ultrasonography (EUS) on surgical decision-making in upper
gastrointestinal tract cancer: An international multicenter study.
Surg Endosc. 21:431–438. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Carter JE, Nelson JJ, Eves M and Boudreaux
C: Diagnosis of linitis plastica-type gastric adenocarcinoma by
endoscopic ultrasound-guided fine needle aspiration: A case report.
Acta Cytol. 52:725–728. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Larghi A, Verna EC, Ricci R, Seerden TC,
Galasso D, Carnuccio A, Uchida N, Rindi G and Costamagna G:
EUS-guided fine-needle tissue acquisition by using a 19-gauge
needle in a selected patient population: A prospective study.
Gastrointest Endosc. 74:504–510. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kanno A, Ishida K, Hamada S, Fujishima F,
Unno J, Kume K, Kikuta K, Hirota M, Masamune A, Satoh K, et al:
Diagnosis of autoimmune pancreatitis by EUS-FNA by using a 22-gauge
needle based on the International Consensus Diagnostic Criteria.
Gastrointest Endosc. 76:594–602. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sendino O, Fernández-Esparrach G, Solé M,
Colomo L, Pellisé M, Llach J, Navarro S, Bordas JM and Ginès A:
Endoscopic ultrasonography-guided brushing increases cellular
diagnosis of pancreatic cysts: A prospective study. Dig Liver Dis.
42:877–881. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li C, Oh SJ, Kim S, Hyung WJ, Yan M, Zhu
ZG and Noh SH: Macroscopic Borrmann type as a simple prognostic
indicator in patients with advanced gastric cancer. Oncology.
77:197–204. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim JI, Kim YH, Lee KH, Kim SY, Lee YJ,
Park YS, Kim N, Lee DH, Kim HH, Park DJ, et al: Type-specific
diagnosis and evaluation of longitudinal tumor extent of borrmann
type IV gastric cancer: CT versus gastroscopy. Korean J Radiol.
14:597–606. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pellicano R, Bruno M, Fagoonee S,
Ribaldone DG, Fasulo R and De Angelis C: Endoscopic ultrasound in
the preoperative staging of gastric cancer: Key messages for
surgeons. Minerva Chir. 70:417–427. 2015.PubMed/NCBI
|
|
19
|
Caletti G, Fusaroli P, Togliani T, Bocus P
and Roda E: Endosonography in gastric lymphoma and large gastric
folds. Eur J Ultrasound. 11:31–40. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vetro C, Chiarenza A, Romano A, Amico I,
Calafiore V, Di Raimondo C, Coppolino F and Di Raimondo F:
Prognostic assessment and treatment of primary gastric lymphomas:
How endoscopic ultrasonography can help in tailoring patient
management. Clin Lymphoma Myeloma Leuk. 14:179–185. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shan GD, Xu GQ and Li YM: Endoscopic
ultrasonographic features of gastric linitis plastica in fifty-five
Chinese patients. J Zhejiang Univ Sci B. 14:844–848. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Blackham AU, Swords DS, Levine EA, Fino
NF, Squires MH, Poultsides G, Fields RC, Bloomston M, Weber SM,
Pawlik TM, et al: Is linitis plastica a contraindication for
surgical resection: A multi-institution study of the U.S. gastric
cancer collaborative. Ann Surg Oncol. 23:1203–1211. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pedrazzani C, Marrelli D, Pacelli F, Di
Cosmo M, Mura G, Bettarini F, Rosa F, de Manzoni G and Roviello F:
Gastric linitis plastica: Which role for surgical resection?
Gastric Cancer. 15:56–60. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cardoso R, Coburn N, Seevaratnam R,
Sutradhar R, Lourenco LG, Mahar A, Law C, Yong E and Tinmouth J: A
systematic review and meta-analysis of the utility of EUS for
preoperative staging for gastric cancer. Gastric Cancer. 15 Suppl
1:S19–S26. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park CH, Park JC, Kim EH, Jung DH, Chung
H, Shin SK, Lee SK and Lee YC: Learning curve for EUS in gastric
cancer T staging by using cumulative sum analysis. Gastrointest
Endosc. 81:898–905.e1. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cantor MJ, Davila RE and Faigel DO: Yield
of tissue sampling for subepithelial lesions evaluated by EUS: A
comparison between forceps biopsies and endoscopic submucosal
resection. Gastrointest Endosc. 64:29–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Komanduri S, Keefer L and Jakate S:
Diagnostic yield of a novel jumbo biopsy ‘unroofing’ technique for
tissue acquisition of gastric submucosal masses. Endoscopy.
43:849–855. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu YM and Yang XJ: Endoscopic
ultrasound-guided cutting of holes and deep biopsy for diagnosis of
gastric infiltrative tumors and gastrointestinal submucosal tumors
using a novel vertical diathermic loop. World J Gastroenterol.
23:2795–2801. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ahn JB, Ha TK, Lee HR and Kwon SJ: An
insufficient preoperative diagnosis of borrmann type 4 gastric
cancer in spite of EMR. J Gastric Cancer. 11:59–63. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Buscaglia JM, Nagula S, Jayaraman V,
Robbins DH, Vadada D, Gross SA, DiMaio CJ, Pais S, Patel K, Sejpal
DV, et al: Diagnostic yield and safety of jumbo biopsy forceps in
patients with subepithelial lesions of the upper and lower GI
tract. Gastrointest Endosc. 75:1147–1152. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tae HJ, Lee HL, Lee KN, Jun DW, Lee OY,
Han DS, Yoon BC, Choi HS and Hahm JS: Deep biopsy via endoscopic
submucosal dissection in upper gastrointestinal subepithelial
tumors: A prospective study. Endoscopy. 46:845–850. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Philipper M, Hollerbach S, Gabbert HE,
Heikaus S, Böcking A, Pomjanski N, Neuhaus H, Frieling T and
Schumacher B: Prospective comparison of endoscopic
ultrasound-guided fine-needle aspiration and surgical histology in
upper gastrointestinal submucosal tumors. Endoscopy. 42:300–305.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hollerbach S, Juergensen C, Hocke M,
Freund U, Wellmann A and Burmester E: EUS-FNA: How to improve
biopsy results? An evidence based review. Z Gastroenterol.
52:1081–1092. 2014.(In German).
|
|
34
|
Larghi A, Fuccio L, Chiarello G, Attili F,
Vanella G, Paliani GB, Napoleone M, Rindi G, Larocca LM, Costamagna
G, et al: Fine-needle tissue acquisition from subepithelial lesions
using a forward-viewing linear echoendoscope. Endoscopy. 46:39–45.
2014.PubMed/NCBI
|
|
35
|
Rong L, Kida M, Yamauchi H, Okuwaki K,
Miyazawa S, Iwai T, Kikuchi H, Watanabe M, Imaizumi H and Koizumi
W: Factors affecting the diagnostic accuracy of endoscopic
ultrasonography-guided fine-needle aspiration (EUS-FNA) for upper
gastrointestinal submucosal or extraluminal solid mass lesions. Dig
Endosc. 24:358–363. 2012. View Article : Google Scholar : PubMed/NCBI
|