Introduction
Lung cancer is the most common type of malignancy
and the leading cause of cancer-associated mortality worldwide
(1). Despite the identification of
novel molecular therapies and driver oncogenes (2), the prognosis for patients with locally
advanced or metastatic lung cancer remains poor, with an overall
5-year survival rate of <15% (3).
This highlights the urgent requirement for the identification of
novel and effective therapeutic agents.
The Janus kinase 2 (JAK2)/signal transducer and
activator of transcription 3 (STAT3) signaling pathway is involved
in oncogenesis and cancer development (4). STAT3 belongs to the STAT family of
transcription factors, which serve diverse roles in cell
proliferation, differentiation, apoptosis and ontogenesis (5). It has been suggested that STAT3 also
serves an important role in cancer angiogenesis (5,6). STAT3
expression is dysregulated in various types of hematopoietic
malignancies and solid tumors, including leukemia, lymphoma,
pancreatic cancer, breast cancer, head and neck cancer, skin
cancer, melanoma, brain glioblastoma, prostate cancer, liver cancer
and lung cancer (7,8).
Constitutive activation of the STAT3 pathway can
inhibit the apoptosis of tumor cells by upregulating anti-apoptotic
proteins, including Bcl2, apoptosis regulator (Bcl2),
Bcl-extra-large (Bcl-xl), myeloid cell leukemia-1 (Mcl-1) and
surviving (9). Other
proliferation-associated proteins, including cyclin D1 and myc, and
the pro-angiogenic vascular endothelial growth factor (VEGF), are
also regulated by the JAK-STAT3 signaling pathway (10). STAT3 and proto-oncogene c-Jun inhibit
the expression of Fas cell surface death receptor, which may
inhibit cancer cell apoptosis (11).
Numerous studies have demonstrated that the inhibition of STAT3
prevents tumor growth and causes the apoptosis of tumor cells
(12,13). A number of anti-angiogenesis
therapeutic strategies have been suggested to block STAT3 pathway
signaling, including upstream kinase inhibition, peptide or small
molecule inhibitor phosphorylation, dominant-negative STAT3
mutations and oligonucleotide decoys (14–16).
However, these methods have limitations, including poor stability
and low transduction rates, and have not performed effectively in
clinical trials (17,18). Using structure-based design, Professor
Li and his team (Ohio State University, Columbus, Ohio, USA)
developed LLL12, a non-peptide small molecule inhibitor of STAT3,
which possesses good solubility and predictable oral
bioavailability. LLL12 binds to the phosphorylated tyrosine of
STAT3 monomers, blocking dimerization and subsequent translocation
into the nucleus. This abrogates the function of STAT3 as a
transcription factor (19).
A number of studies have demonstrated that LLL12 has
potent antitumor activity in breast cancer, brain cancer,
pancreatic cancer and hepatocellular carcinoma (19–21).
However, to the best of our knowledge, the activity of LLL12 in
lung cancer has not been previously investigated. The aim of the
present study was to characterize the biological activity and
possible underlying mechanism of LLL12 in lung cancer in
vitro and in vivo.
Materials and methods
Cell lines and reagents
The human lung adenocarcinoma cell line, A549, was
purchased from the Type Culture Collection Center of Wuhan
University (Wuhan, China). The cells were maintained in RPMI-1640
medium containing 10% fetal bovine serum (Shanghai Baoman
Biotechnology Co., Ltd., Shanghai, China) at 37°C in 5%
CO2. The following primary antibodies were used for
western blotting experiments: Anti-p-STAT3 mAb (Y705; cat. no.
ab76315; 1:500 dilution), anti-STAT3 mAb (cat. no. ab5073; 1:500
dilution), anti-p-Src mAb (cat. no. ab40660; 1:500 dilution),
anti-p-ERK1/2 mAb (cat. no. ab176660; 1:500 dilution) and
anti-GAPDH mAb (cat. no. ab8245; 1:1,000 dilution) (all Abcam,
Cambridge, UK). The secondary antibody was HRP-conjugated
anti-mouse IgG (cat. no. ab222759; Abcam). LLL12 was kindly gifted
by Professor Tom Li (Ohio State University, Columbus, Ohio,
USA).
Mice
Female BALB/c nude mice were purchased from Beijing
Vital River Laboratory Animal Co. Ltd. (Beijing, China), with the
following production license number: SCXK (Beijing) 2006–0009. A
total of 25 mice, 4–6 weeks of age, weighing 18.0–22.0 g, were kept
three to five mice per cage in microisolator units and provided
with water and food in accordance with Institutional Animal Care
and Use Committee (IACUC) guidelines, at a temperature of 26–28°C
and 40–60% humidity. The present study was approved by the Ethics
Committee of Hubei Cancer Hospital (Wuhan, China).
MTT assay
A549 cells were cultured at 1×103
cells/ml in 96-well plates with RPMI-1640 medium. Cells were
cultured with 0.0, 0.625, 1.25, 2.5, 5.0 or 10.0 µM LLL12 at 37°C
for 24, 48, 72 or 96 h. A total of 50 µl 5 mg/ml MTT was added per
well. After 4 h, the formazan crystals were dissolved by adding 100
µl dimethyl sulfoxide (DMSO). The absorbance was read at 490 nm,
and the IC50 values were calculated using SigmaPlot9.0
software (Systat Software, Inc., San Jose, CA, USA). Each
experiment was repeated 3 times.
Wound-healing assay
A wound-healing assay was performed, in which
2×104 cells/well were cultured in RPMI-1640 supplemented
with 10% fetal calf serum. LLL12 (at the IC50
concentration: 5.0 µmol/l) DMSO or PBS (blank control) was added to
each well and incubated at 37°C for 24 h. A P10 pipette tip was
used to create a 0.5-cm wound in the cell monolayer. The cells were
then cultured in serum-free medium for 24 h prior to washing in
PBS. Images of the cells were captured at 0 and 24 h under an
inverted fluorescence microscope (magnification, ×100). All
experiments were performed in triplicate.
Migration assays
A Transwell assay was also performed, in which
2×105 cells treated with LLL12 (at the IC50
concentration: 5.0 µmol/l), DMSO or PBS (blank control) in
serum-free medium, were seeded into the upper chambers of 6-well
plates containing polycarbonate Transwell inserts (pore diameter, 5
µm; Corning Costar; Corning Incorporated, Corning, NY, USA).
Soluble stromal-cell derived factor 1 (PeproTech, Inc., Rocky Hill,
NJ, USA) was added at a concentration of 100 ng/ml to the lower
chamber in DMEM medium supplemented with 10% fetal calf serum. The
plates were incubated at 37°C for 18 h. Images were captured of the
cells that had migrated to the lower surface of the Transwell
membrane and then the cells were counted under a light microscope
at magnification, ×200. All experiments were performed in
triplicate.
Western blot analysis
Cells were treated with DMSO or LLL12 (at the
IC50 concentration: 5.0 µmol/l) for 24 h prior to
protein isolation. The cells were washed twice with cold PBS, then
lysed using radioimmunoprecipitation assay buffer [10 mM Tris (pH
8.0), 150 mM NaCl, 1% sodium deoxycholate, 0.1% sodium dodecyl
sulfate (SDS), 1% Triton X-100, 10 µg/ml leupeptin, 10 µg/ml
aprotinin and 1 mM phenylmethylsulfonyl fluoride]. The cell lysates
were centrifuged at 13,000 × g at 4°C for 20 min. The protein
concentration was determined by bicinchoninic acid protein assay
(Thermo Scientific, IL, USA). Protein (40 µg per lane) was
separated by 0.1% SDS-PAGE, and transferred onto Hybond
polyvinylidene difluoride membranes. The membranes were blocked
with Tris-buffered saline buffer (20 mM Tris-HCl, 150 mM NaCl, pH
7.4) containing 5% non-fat milk for 1 h at 25°C, then incubated
with the aforementioned primary antibodies for 2 h at room
temperature. Incubation with the horseradish peroxidase-conjugated
secondary antibody followed, prior to washing. The membranes were
imaged using Scanner STORM 860 (GE Healthcare, Chicago, IL, USA).
The relative expression levels of p-Src (Tyr418), p-STAT3 (Y705),
STAT3, p-ERK1/2 (T202/Y204) proteins were quantitated using Matrox
Inspector software (version 2.1; Matrox Electronic Systems Ltd.
Dorval, QC, Canada).
Mouse xenograft model
The 25 mice were injected subcutaneously into the
left flank with 5×106 A549 cells. Once the tumors
reached 100–150 mm3 in size, the mice were randomly
assigned into the high-dose (20 mg/kg LLL12, administered at a
concentration of 4 mg/ml), low-dose (10 mg/kg LLL12, administered
at a concentration of 2 mg/ml) or control (administered with an
equal volume of DMSO) group. There were 5 mice in each group, and
treatment was administered by daily intraperitoneal injection.
Tumor growth was determined by caliper measurements of the length
(L) and width (W), taken every other day. Tumor volume was
calculated using the following formula: Volume = (π/6) × (L ×
W2). The mice were weighed daily for the 21-day
treatment period. After 21 days, the mice were sacrificed and
tumor, liver and kidney tissues, as well as blood, were collected
for further experimentation. Blood cell counts were analyzed using
animal blood analyzers (HEMAVET Veterinary Multi-species Hematology
System; Drew Scientific Inc., Miami Lakes, FL, USA).
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical analysis was performed using GraphPad Prism
(version 5.0; GraphPad Software, Inc., La Jolla, CA, USA).
Comparisons between groups were analyzed using Duncan's multiple
range test, The correlation analysis was conducted using simple
linear regression analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
LLL12 inhibits the proliferation of
human lung cancer cells
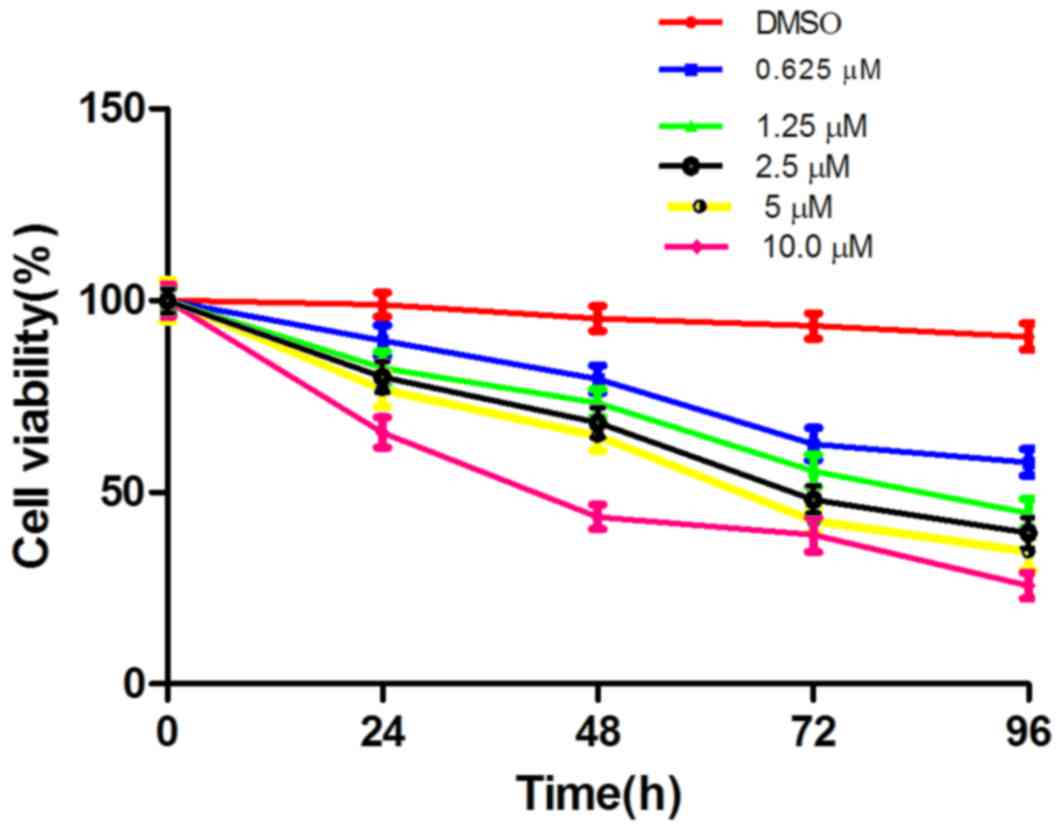
The effect of LLL12 on A549-cell proliferation was
assessed by MTT assay. This revealed dose- and time-dependent
inhibition of cell proliferation with LLL12 treatment (Fig. 1). The IC50 value at 72 h
was 5.0 µmol/l, and this concentration was selected for use in
subsequent experiments.
LLL12 inhibits the migration of human
lung cancer cells
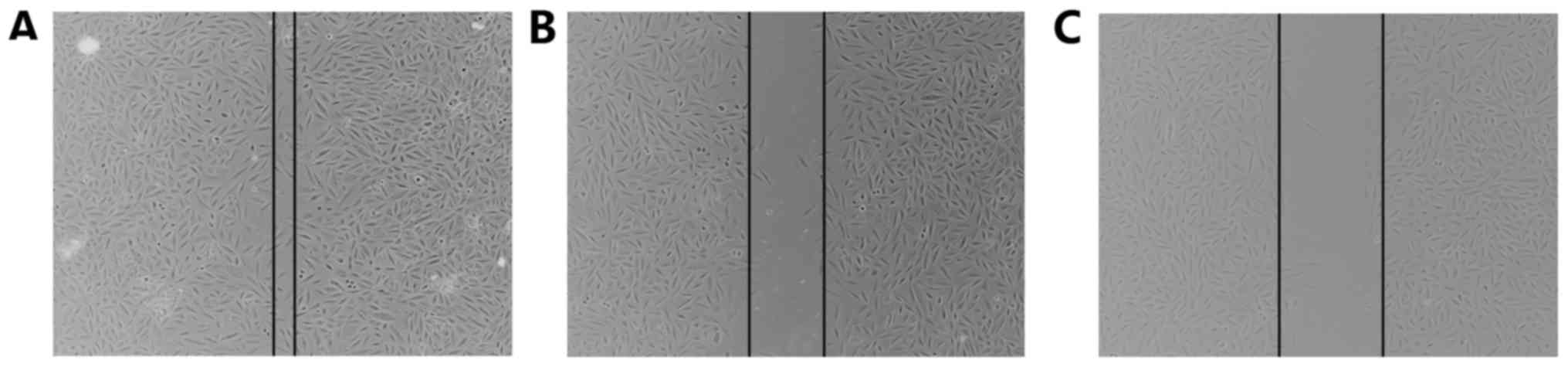
Migration was analyzed using a wound-healing assay
and a Transwell migration assay. Following treatment with LLL12,
the wound width was compared with that of the control group, and
was found to be larger (Fig. 2). In
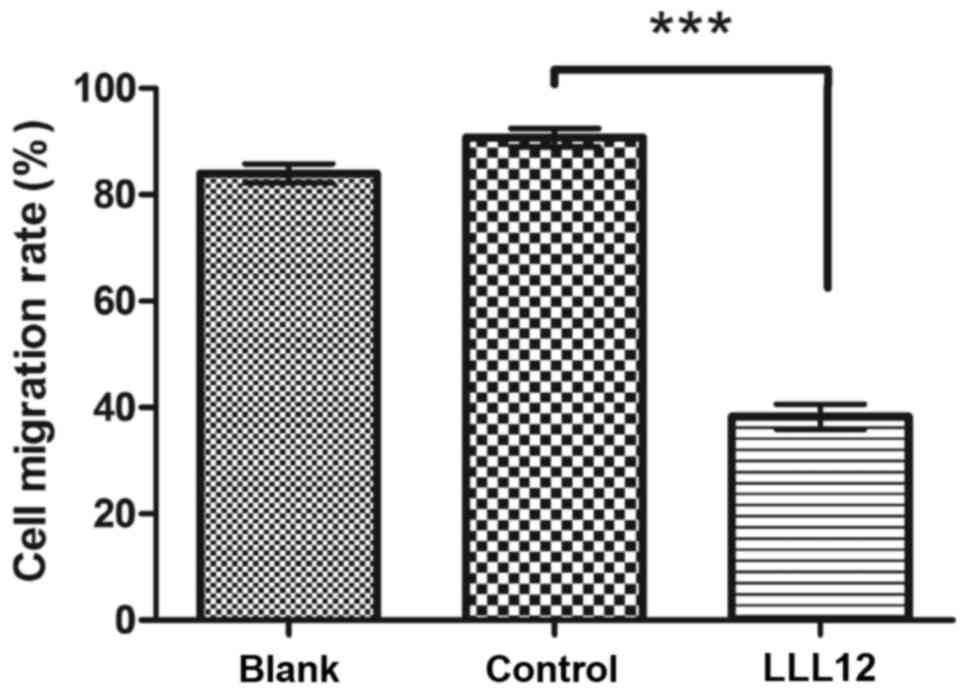
the Transwell assay, the cell number in the bottom compartment was
lower in the group treated with LLL12 compared with that in the
control group (Fig. 3). These results
suggest that LLL12 could inhibit cell migration in human lung
cancer cells.
LLL12 inhibits STAT3 phosphorylation
in human lung cancer cells
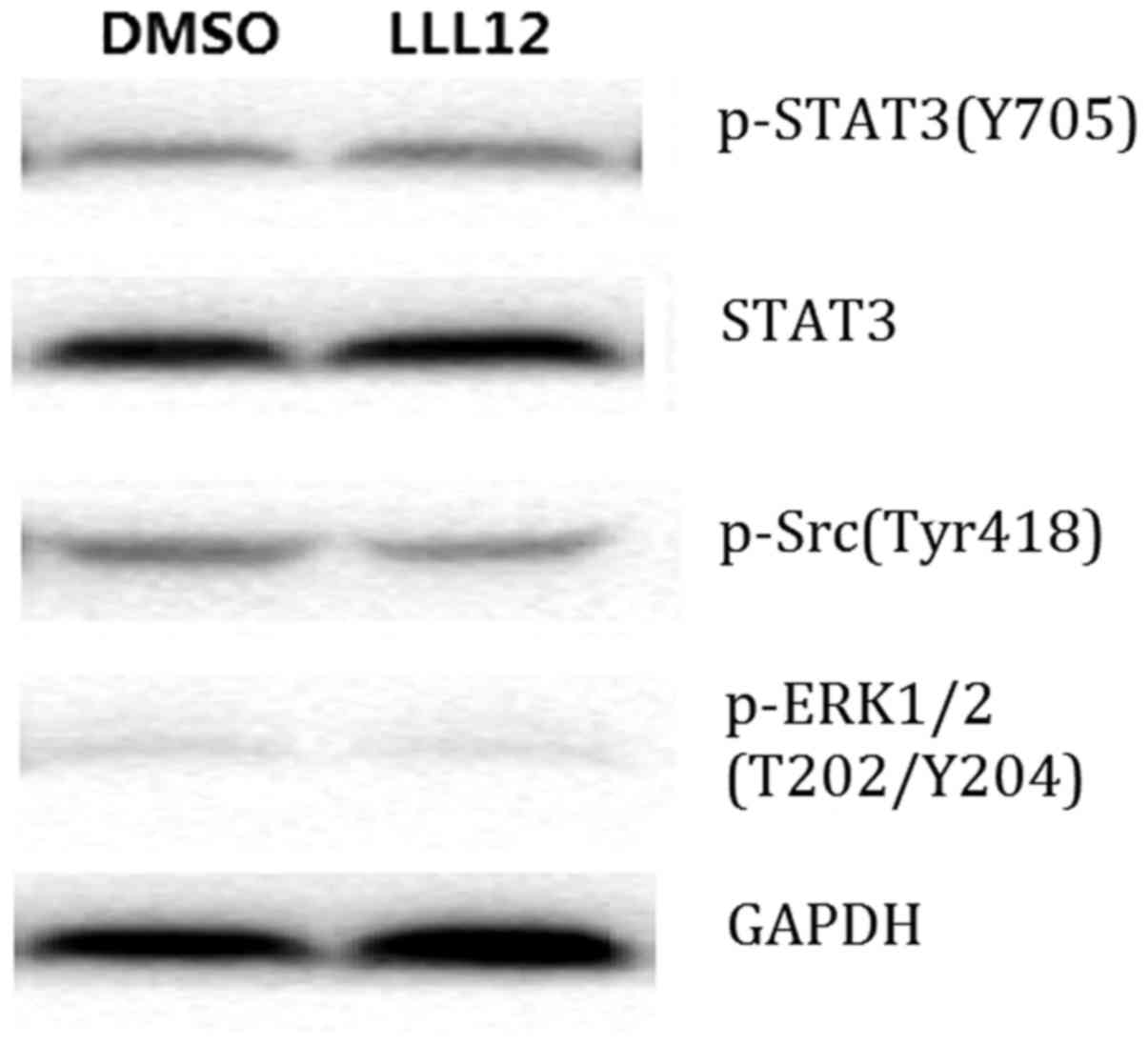
Western blot analysis demonstrated that LLL12
treatment reduced the protein expression level of p-STAT3 and STAT3
significantly compared with that of the control group. However,
there was no significant difference in the protein expression level
of p-Src or p-ERK1/2 between groups (Fig.
4). This suggests that LLL12 specifically affects STAT3.
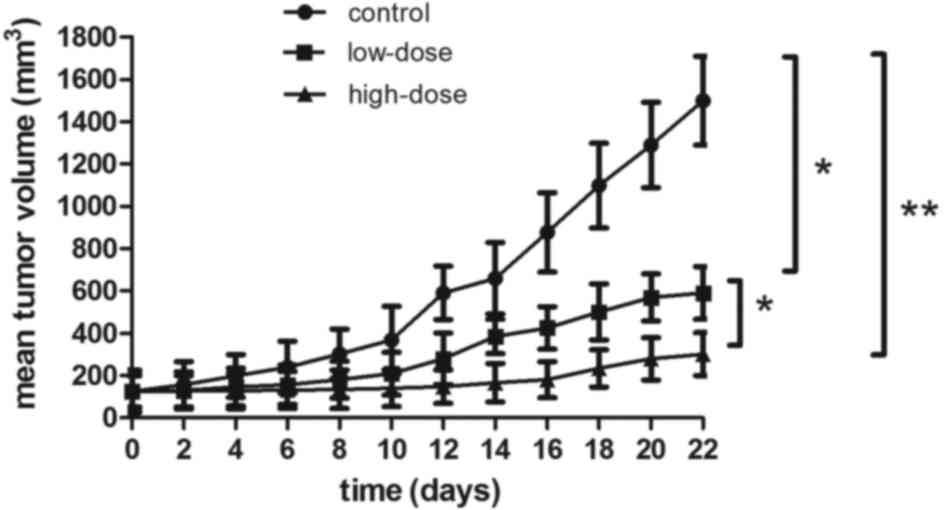
LLL12 suppresses tumor growth in
vivo
The antitumor effect of LLL12 in vivo was
analyzed using a xenograft mouse model. Lung tumor growth was
reduced in the groups treated with LLL12 compared with that in the
control group. The final tumor volume was significantly lower in
the high-dose group compared with that in the low-dose group
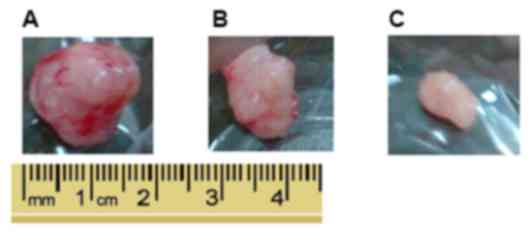
(Figs. 5 and 6). Fig. 6A
represents the largest tumor diameter obtained. There was no
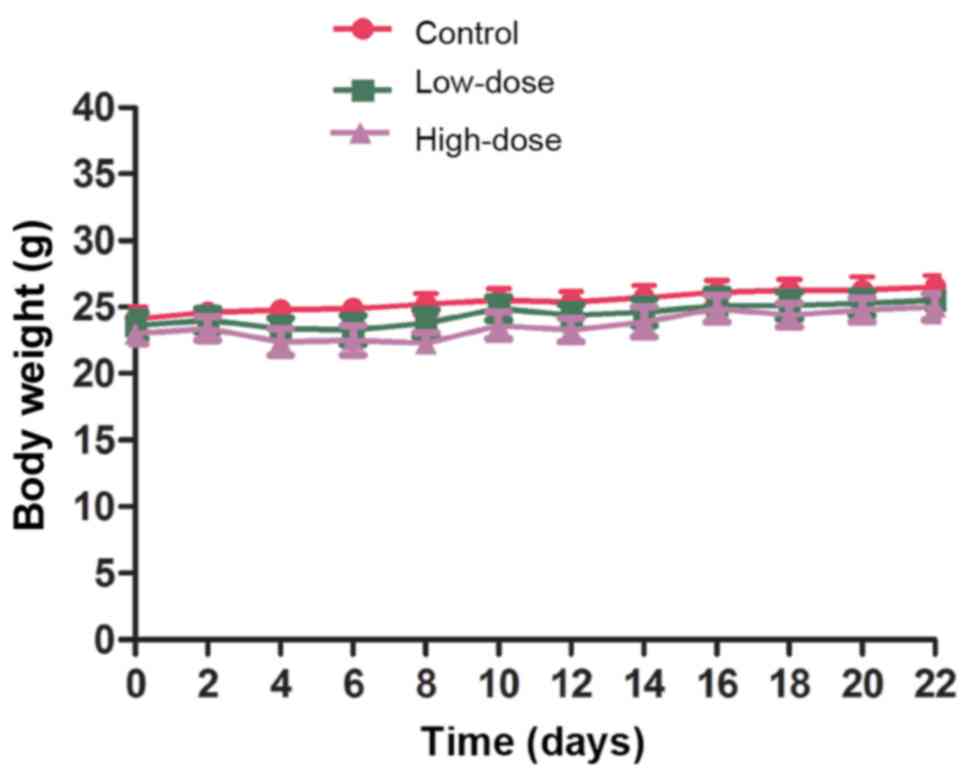
significant difference in the body weight of the mice among any of
the groups (Fig. 7).
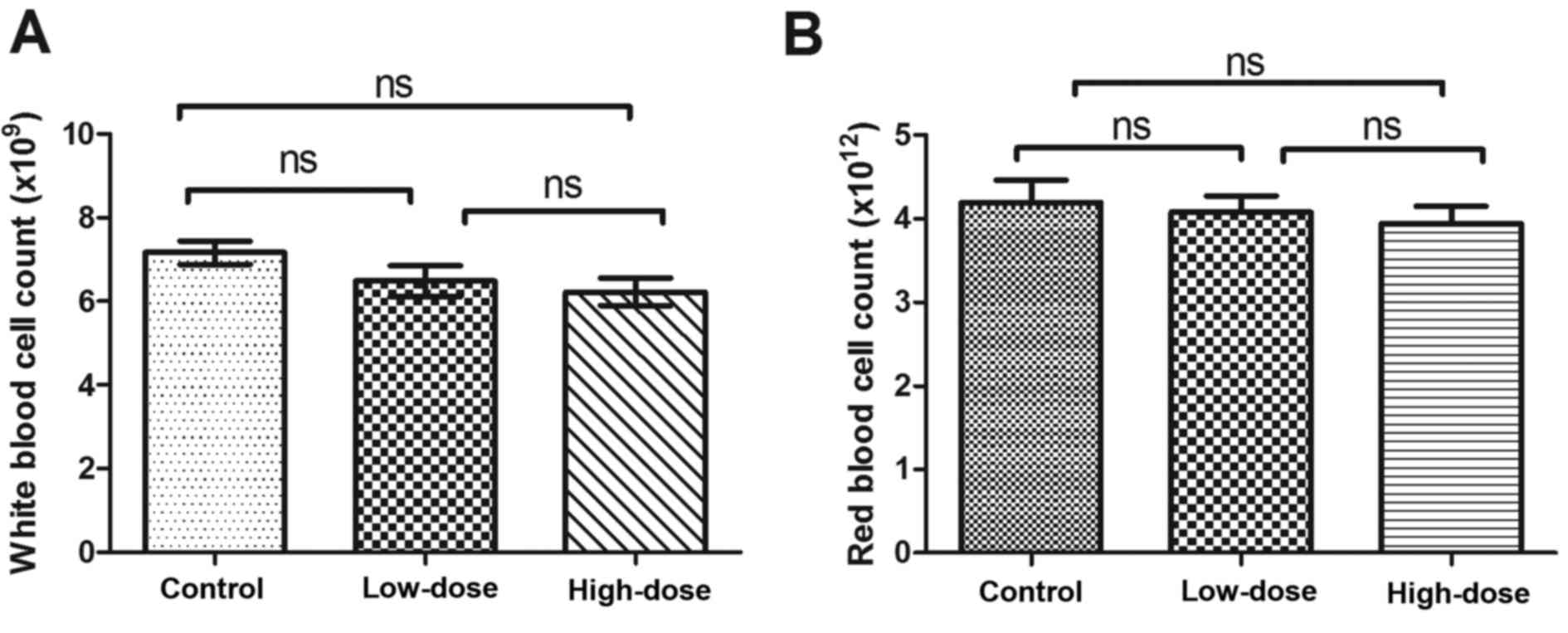
Safety assessment of LLL12
treatment
During the 21 days of LLL12 administration, mice
consumed food and water as normal without vomiting, diarrhea or a
significant reduction in body weight. There was no significant
difference in white or red blood cell number between groups
(Fig. 8).
Discussion
STAT3 is an important member of the JAK-STAT
signaling pathway, and is activated by phosphorylation of a single
tyrosine residue located at position 705. STAT3 is phosphorylated
when a cytokine or growth factor activates JAK (22). STAT3 has been reported to regulate
tumor cell proliferation, differentiation and apoptosis, as well as
angiogenesis (5,19,23).
Studies have demonstrated that abnormal activation of STAT3 is
closely associated with the development of multiple types of
cancer, as well as the prognosis of patients with these types of
cancer, including head and neck squamous cell carcinoma and
prostate cancer (15,24). Morikawa et al (25) suggested that the activation and high
expression of p-STAT3 in colorectal cancer may be associated with
adverse clinical outcome, indicating its potential role as a
prognostic biomarker or therapeutic target. It has also been
suggested that cancer-initiating cells may be more sensitive to
STAT3 inhibitors (26). Furthermore,
it has been reported that the expression of p-STAT3 correlates with
tumor differentiation, TNM stage and survival time in gastric
cancer, suggesting that STAT3 is a prognostic factor for the
disease (27). This is supported by
other studies in which the expression levels of STAT3 and p-STAT3
were demonstrated to be closely associated with the progression of
gastric cancer, osteosarcoma and esophageal cancer (27–29).
In vitro studies have demonstrated that LLL12
can inhibit the proliferation and induce the apoptosis of various
types of cancer cells (19,20,30,31). LLL12
has also been reported to inhibit the formation of tumor blood
vessels by downregulating VEGF, matrix metallopeptidase 9 and
fibroblast growth factor 1 expression (23). LLL12 has been demonstrated to induce
the apoptosis of HCC cells in vitro and to induce cell cycle
arrest at the G2/M phase (21). It
was also reported that LLL12 inhibited liver cancer growth in a
mouse model (21). Furthermore, LLL12
has been demonstrated to inhibit the proliferation and migration of
osteosarcoma cells in vivo and in vitro (32,33).
In the present study, the in vitro results
indicated that LLL12 could inhibit the proliferation of A549 cells
in a dose- and time-dependent manner. Metastasis is an important
feature of malignant tumors, and a better understanding of the
effect of LLL12 on metastasis would be valuable for future research
and development of the inhibitor. In the wound-healing assays, the
wound width remained wider after 24 h in cells treated with LLL12
compared with the control. The Transwell assay supported this
result by demonstrating that cell migration was decreased following
treatment with LLL12 compared with the control. These results
suggest that LLL12 could inhibit the migration of human lung cancer
cells.
The present study aimed to elucidate the mechanism
of LLL12 activity in lung cancer. The results demonstrated that the
protein expression levels of STAT3 and p-STAT3 were significantly
reduced in cells treated with LLL12 compared with those in the
control. However, there was no significant difference in the
protein expression level of p-Src or p-ERK1/2 between any groups.
These results suggest that LLL12 downregulates the protein
expression level of STAT3 and its phosphorylation, which may result
in inhibition of proliferation in A549 cells.
The tumor volume in mice treated with LLL12 was
significantly reduced compared with that of the control group.
Furthermore, tumor volume in the high-dose group was significantly
reduced compared with that in the low-dose group. There was no
difference in body weight between any groups, and no toxicity was
observed. The in vivo study revealed that LLL12 could
inhibit cancer tumor growth in a dose-dependent manner. The
significant antitumor effect of LLL12 in mice may be associated
with downregulated STAT3 expression and phosphorylation. The
present study indicates that further studies should focus on the
clinical development of LLL12 for the treatment of lung cancer.
Acknowledgements
The authors would like to thank Professor Tom Li
(Ohio State University, Columbus, Ohio, USA) for providing
LLL12.
Funding
This work was supported by funding from the Focus on
the Plan Projects of Wuhan Science and Technology Bureau (grant no.
201161038347).
Availability of data and materials
The datasets used or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SH and YN conceived and designed the study. YN and
YL performed the experiments. YN wrote the paper and SH reviewed
and edited the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Hubei Cancer Hospital (Wuhan, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pao W and Girard N: New driver mutations
in non-small-cell lung cancer. Lancet Oncol. 12:175–180. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reka AK, Goswami MT, Krishnapuram R,
Standiford TJ and Keshamouni VG: Molecular cross-regulation between
PPAR-γ and other signaling pathways: Implications for lung cancer
therapy. Lung Cancer. 72:154–159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Haura EB, Turkson J and Jove R: Mechanisms
of disease: Insights into the emerging role of signal transducers
and activators of transcription in cancer. Nat Clin Pract Oncol.
2:315–324. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kamran MZ, Patil P and Gude RP: Role of
STAT3 in cancer metastasis and translational advances. BioMed Res
Int. 2013:4218212013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jatiani SS, Baker SJ, Silverman LR and
Reddy EP: Jak/STAT pathways in cytokine signaling and
myeloproliferative disorders: Approaches for targeted therapies.
Genes Cancer. 1:979–993. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu H and Jove R: The STATs of cancer—new
molecular targets come of age. Nat Rev Cancer. 4:97–105. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Looyenga BD, Hutchings D, Cherni I,
Kingsley C, Weiss GJ and Mackeigan JP: STAT3 is activated by JAK2
independent of key oncogenic driver mutations in non-small cell
lung carcinoma. PloS One. 7:e308202012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xie TX, Wei D, Liu M, Gao AC, Ali-Osman F,
Sawaya R and Huang S: Stat3 activation regulates the expression of
matrix metalloproteinase-2 and tumor invasion and metastasis.
Oncogene. 23:3550–3560. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu H, Pardoll D and Jove R: STATs in
cancer inflammation and immunity: A leading role for STAT3. Nat Rev
Cancer. 9:798–809. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carpenter RL and Lo HW: STAT3 target genes
relevant to human cancers. Cancers (Basel). 6:897–925. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Debnath B, Xu S and Neamati N: Small
molecule inhibitors of signal transducer and activator of
transcription 3 (Stat3) protein. J Med Chem. 55:6645–6668. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Masciocchi D, Gelain A, Villa S,
Meneghetti F and Barlocco D: Signal transducer and activator of
transcription 3 (STAT3): A promising target for anticancer therapy.
Future Med Chem. 3:567–597. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hedvat M, Huszar D, Herrmann A, Gozgit JM,
Schroeder A, Sheehy A, Buettner R, Proia D, Kowolik CM, Xin H, et
al: The JAK2 inhibitor AZD1480 potently blocks Stat3 signaling and
oncogenesis in solid tumors. Cancer Cell. 16:487–497. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sen M, Thomas SM, Kim S, Yeh JI, Ferris
RL, Johnson JT, Duvvuri U, Lee J, Sahu N, Joyce S, et al:
First-in-human trial of a STAT3 decoy oligonucleotide in head and
neck tumors: Implications for cancer therapy. Cancer Discov.
2:694–705. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alas S and Bonavida B: Inhibition of
constitutive STAT3 activity sensitizes resistant non-Hodgkin's
lymphoma and multiple myeloma to chemotherapeutic drug-mediated
apoptosis. Clin Cancer Res. 9:316–326. 2003.PubMed/NCBI
|
|
17
|
Sledz CA and Williams BR: RNA interference
in biology and disease. Blood. 106:787–794. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stein CA: The experimental use of
antisense oligonucleotides: A guide for the perplexed. J Clin
Invest. 108:641–644. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin L, Hutzen B, Li PK, Ball S, Zuo M,
DeAngelis S, Foust E, Sobo M, Friedman L, Bhasin D, et al: A novel
small molecule, LLL12, inhibits STAT3 phosphorylation and
activities and exhibits potent growth-suppressive activity in human
cancer cells. Neoplasia. 12:39–50. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu A, Liu Y, Li PK, Li C and Lin J: LLL12
inhibits endogenous and exogenous interleukin-6-induced STAT3
phosphorylation in human pancreatic cancer cells. Anticancer Res.
31:2029–2035. 2011.PubMed/NCBI
|
|
21
|
Zuo M, Li C, Lin J and Javle M: LLL12, a
novel small inhibitor targeting STAT3 for hepatocellular carcinoma
therapy. Oncotarget. 6:10940–10949. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mankan AK and Greten FR: Inhibiting signal
transducer and activator of transcription 3: Rationality and
rationale design of inhibitors. Exp Opin Investig Drugs.
20:1263–1275. 2011. View Article : Google Scholar
|
|
23
|
Bid HK, Oswald D, Li C, London CA, Lin J
and Houghton PJ: Anti-angiogenic activity of a small molecule STAT3
inhibitor LLL12. PloS One. 7:e355132012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bishop JL, Thaper D and Zoubeidi A: The
multifaceted roles of STAT3 signaling in the progression of
prostate cancer. Cancers (Basel). 6:829–859. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Morikawa T, Baba Y, Yamauchi M, Kuchiba A,
Nosho K, Shima K, Tanaka N, Huttenhower C, Frank DA, Fuchs CS and
Ogino S: STAT3 expression, molecular features, inflammation
patterns, and prognosis in a database of 724 colorectal cancers.
Clin Cancer Res. 17:1452–1462. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin L, Liu A, Peng Z, Lin HJ, Li PK, Li C
and Lin J: STAT3 is necessary for proliferation and survival in
colon cancer-initiating cells. Cancer Res. 71:7226–7237. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xiong H, Du W, Wang JL, Wang YC, Tang JT,
Hong J and Fang JY: Constitutive activation of STAT3 is predictive
of poor prognosis in human gastric cancer. J Mol Med(Berl).
90:1037–1046. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ryu K, Choy E, Yang C, Susa M, Hornicek
FJ, Mankin H and Duan Z: Activation of signal transducer and
activator of transcription 3 (Stat3) pathway in osteosarcoma cells
and overexpression of phosphorylated-Stat3 correlates with poor
prognosis. J Orthopaed Res. 28:971–978. 2010.
|
|
29
|
Schoppmann SF, Jesch B, Friedrich J,
Jomrich G, Maroske F and Birner P: Phosphorylation of signal
transducer and activator of transcription 3 (STAT3) correlates with
Her-2 status, carbonic anhydrase 9 expression and prognosis in
esophageal cancer. Clin Exp Metastasis. 29:615–624. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ball S, Li C, Li PK and Lin J: The small
molecule, LLL12, inhibits STAT3 phosphorylation and induces
apoptosis in medulloblastoma and glioblastoma cells. PloS One.
6:e188202011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin L, Benson DM Jr, DeAngelis S, Bakan
CE, Li PK, Li C and Lin J: A small molecule, LLL12 inhibits
constitutive STAT3 and IL-6-induced STAT3 signaling and exhibits
potent growth suppressive activity in human multiple myeloma cells.
Int J Cancer. 130:1459–1469. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Onimoe GI, Liu A, Lin L, Wei CC, Schwartz
EB, Bhasin D, Li C, Fuchs JR, Li PK, Houghton P, et al: Small
molecules, LLL12 and FLLL32, inhibit STAT3 and exhibit potent
growth suppressive activity in osteosarcoma cells and tumor growth
in mice. Invest New Drugs. 30:916–926. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Couto JI, Bear MD, Lin J, Pennel M, Kulp
SK, Kisseberth WC and London CA: Biologic activity of the novel
small molecule STAT3 inhibitor LLL12 against canine osteosarcoma
cell lines. BMC Vet Res. 8:2442012. View Article : Google Scholar : PubMed/NCBI
|