Introduction
Colorectal cancer (CRC) is the most common gastric
and intestinal malignant tumor type. Epidemiological studies of CRC
have demonstrated that the incidence and mortality rates of CRC are
comparable with those of gastric cancer, esophageal cancer and
primary liver cancer, all of which affect the digestive system
(1). CRC is also a common cause of
cancer-associated mortality and is responsible for ~10% of cancer
cases worldwide (2,3). CRC, along with other types of cancer, is
defined by different stages depending on the presence of
cancer-positive lymph nodes. The 5-year overall survival rate of
CRC patients at stage I–II of the disease is ~90%, but for patients
with stage III of the disease it is ~60% and for stage IV only ~8%
(4,5).
Currently, surgical resection and chemotherapy remain the most
popular options for CRC treatment (6). Chemotherapy is not suitable for certain
patients, due to their age, complications or side effects.
Therefore, numerous patients suffer from relapse in stage III of
CRC (6). Extensive investigations
have been performed to identify diagnostic biomarkers for CRC or
novel molecular therapies to replace certain treatments prone to
chemoresistance (7–9). It has been reported that a substantial
number of molecules and complicated signaling pathways are involved
in the occurrence and development of CRC, including the activation
of oncogenes, inactivation of tumor suppressor genes and epigenetic
modifications (10–12). The elucidation of CRC pathogenesis,
examining the molecular dynamics and targeting of molecular
abnormalities in CRC are popular areas of research. The role of
microRNAs (miRNAs or miRs) has attracted the attention of numerous
researchers (13).
miRNAs are a class of endogenous short non-coding
RNAs that consist of 19–25 nucleotides. Bioinformatics and cloning
studies have demonstrated that miRNAs serve key functions in
regulating the expression and function of various genes and
proteins by binding to their target genes. It has been estimated
that miRNAs may regulate 1/3 of all human genes and control
hundreds of target genes (14,15).
Previous evidence has indicated that miRNAs regulate proliferation,
invasion, migration, angiogenesis and epithelial-mesenchymal
transition through degradation of target mRNAs via binding to the
3′ untranslated region (3′UTR) (16,17). Data
from microarray profiling of stage III CRC tissues have
demonstrated that certain cancer-associated miRNAs are upregulated,
while others are downregulated, compared with adjacent noncancerous
colorectal tissues. These include miR-18a, miR-1260b and miR-21
(18–22). However, extensive investigation is
required to clarify the underlying mechanism and function of these
miRNAs in CRC.
Numerous decades ago, PDCD4 was identified as a
novel tumor suppressor gene. Overexpressed PDCD4 was sufficient to
inhibit the neoplastic transformation induced by tissue plasminogen
activator (23,24). Researchers have demonstrated that one
of the major functions of PDCD4 is to inhibit the translation
process by interacting with the translation initiation factors,
eukaryotic initiation factor-4A and the eukaryotic translation
initiation factor-4 G, partly by inhibiting their helicase activity
(24,25). Furthermore, studies in cultured
ovarian cancer cells have suggested that PDCD4 suppresses
proliferation and progression of the cell cycle, in addition to
inducing apoptosis (25–27). Notably, phosphorylated Akt (p-Akt) is
functionally associated with the shuttling of PDCD4 between the
cytoplasm and the nucleus through its ability to phosphorylate
PDCD4 at positions Ser67 and Ser457 (27,28). PDCD4
is an effector of the phosphorylated 3-kinase (PI3K)/Akt signal
pathway, which activates tumorigenesis (28–32). To
the best of our knowledge, the present study is the first to
demonstrate that PDCD4 is a target of miR-1260b.
In the present study, the effect of miR-1260b
inhibitor on the proliferation and migration of the CRC cell line
HCT116 was investigated. PDCD4 was demonstrated to be a direct
target of miR-1260b through bioinformatics and dual-luciferase
reporter assay. In addition, the present study demonstrated that
miR-1260b inhibitor could enhance the chemosensitivity of CRC cells
to 5-fluorouracil (5-FU). Finally, the current study may provide
the basis for a novel therapeutic strategy for CRC by elucidating
the underlying mechanism of combined therapy.
Materials and methods
Patient recruitment and tissue sample
collection
A total of 30 pairs of fresh CRC and adjacent normal
tissues were collected from the Changzhi People's Hospital
(Changzhi, China) and the Hospital of Chinese People's Liberation
Army (Taiyuan, China) from January 2017 to January 2018. Clinical
information, including diagnosis, tumor size, pathological stage
and lymphatic metastasis, was reviewed for all enrolled patients
(n=30). Patients who had previously received chemotherapy or
radiotherapy were excluded from the present study. Among the 30
patients with CRC, the age was between 45–60 years old (median age
of 56 years) with a 0.54: 1, male to female ratio. For all
patients, samples were collected intraoperatively and flash frozen
in liquid nitrogen, then stored at −80°C until subsequent RNA
extraction and reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) analysis. The use of human tissue for mRNA
detection was approved by the Changzhi People's Hospital Ethics
Committee (Changzhi, China). All recruited patients provided
informed consent for participation.
Cell culture
The human colorectal cancer cell lines, HCT116 and
SW480, were purchased from Stem Cell Bank, Chinese Academy of
Sciences (Shanghai, China). The cells were cultured in RPM-1640
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) supplemented with
10% fetal bovine serum (Thermo Fisher Scientific, Inc., Waltham,
MA, USA) and ampicillin (20 µg/ml; Sigma-Aldrich; Merck KGaA) at
37°C with 5% CO2 in a humidified cell culture incubator
(Sanyo Tokyo Manufacturing Co., Ltd, Tokyo Japan).
Cell transfection
HCT116 cells (3×105) or SW480 cells
(2×105) were plated in 6-well plates and, once the cells
reached 70% confluency, they were transfected with 100 nM miR-1260b
inhibitor (sequence: 5′-AUGGUGGCAGUGGUGGGAU-3′) or negative control
(sequence: 5′-UACAAACGUCACGGCGUA-3′) for 4 h (GenePharma, Shanghai,
China) using Lipofectamine 2000 reagent (Thermo Fisher Scientific,
Inc.) at 37°C with 5% CO2 in a humidified cell culture
incubator, according to the manufacturer's protocol. Following
transfection, cells were cultured at 37°C until sampling. The cells
were sampled at the indicated time points for the following
experiments analysis, including the validation of cell
proliferation at 24, 48 and 72 h, and the measurement of protein or
RNA expression at 72 h.
MTT assay
The reduction of MTT by metabolically active cells
was used as a measure of proliferation. An MTT assay kit (Beyotime
Institute of Biotechnology, Shanghai, China) was used to measure
proliferation from day 1–3, following the manufacturer's protocol.
Briefly, 5×103 HCT116 or SW480 cells in the logarithmic
phase were seeded into 96-well plates in triplicate. A volume of
200 µl/well miR-1260b, 5-FU or insulin-like growth factor 1 (IGF1)
was added to the plate when cells were completely adherent at 24 h,
and the cells were cultured at 37°C (5% CO2) for a
further 48 h. MTT (20 µl; 5 mg/ml; (Beyotime) solution was added to
each well at 24, 48 and 72 h post miR-1260b inhibitor or negative
control transfection. The cells were incubated for an additional 4
h. At the end of incubation, the supernatants were removed with a
pipette. Prior to reading under a microplate reader, 150 µl
dimethyl sulfoxide (Sigma-Aldrich; Merck KGaA) was added into each
well. The proliferation rate was calculated by measuring optical
density (OD) at a wavelength of 480 nm.
Transwell cell migration assay
The in vitro cell migration assay was
performed according to the method used by Justus et al
(33). Briefly, 100 µl HCT116 cells
(3×106 cells/well) transfected with miR-1260b inhibitor
or negative control were plated in the upper chamber of a transwell
(Corning Incorporated, Corning, NY, USA) inserted in a 24-well
plate. When the cells had settled, 600 µl in RPMI-1640 media
supplemented with 30% fetal bovine serum, was added into the lower
chamber of the 24-well plate. At 48 h, the cells that had not
migrated from the top of the membrane were removed carefully and
the migrated cells were fixed with 4% paraformaldehyde at room
temperature for 30 min. Removing the paraformaldehyde, the cells
were stained with 1 mg/ml crystal violet solution (Thermo Fisher
Scientific) at room temperature for 4 h. Following washing 3 times
with pure water, the cells in 4 different fields of view were
counted under a light microscope at the magnification of ×10 and
the average number of cells was determined. Since HCT116 cells were
more sensitive to miR-1260b inhibitor than SW480 cells, HCT116
cells were selected for subsequent experiments.
Flow cytometric analysis of
apoptosis
Apoptotic rate was analyzed using the Annexin V-FITC
kit and propidium iodide (PI) (Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. Briefly, 1×106
HCT116 cells were plated in 100-mm dishes (Corning Incorporated,
Corning, NY, USA) and then transfected with miR-1260b inhibitor or
mock vector or 5-FU. At 48 h, the cells were lysed and collected
following two washes with cold PBS and re-suspended in 500 µl
binding buffer. Then, cells were stained with annexin V-fluorescein
isothiocyanate (FITC) and PI for an additional 5 min incubation at
room temperature prior to assessment with a flow cytometer (BD,
Biosciences, Inc). The data analysis was used ModFit LT™
(version 5.0, Verity Software House, Topsham, ME, USA). For each
group, the samples were measured in triplicate.
Predicted target analysis of
miR-1260b
MiRecords (http://c1.accurascience.com/miRecords/) is an online
database of animal miRNA-target interactions containing validated
and predicted targets. The predicted targets are based on the
results of multiple miRNA target predication tools, including
DIANA-microT, MicroInspector and miTarget. The potential targets of
miR-1260b were predicted using two algorithms on MiRecords (version
4).
3′UTR-luciferase reporter gene
assay
PDCD4-WT (wildtype) and PDCD4-MT (mutant) were
purchased from Genewiz and carried the PDCD4 sequence containing
the wild-type or mutant 3′UTR predicted of the miR-1260b binding
sites. A total of 1×105 HCT116 cells were seeded to each
well in a 24-well plate and cultured at 37°C (5% CO2)
overnight. HCT-116 cells were then transfected with 2.5 ng/µl PDCD4
vector or mutant vector using Lipofectamine 2000 reagent (Thermo
Fisher Scientific, Inc.), according to the manufacturer's protocol.
After 4 h, 50 nM of miR-1260b mimic, control or 5-FU was
transfected into the cells which were at ~50% confluence. After 48
h, cells were lysed and the luciferase activities were analyzed
using a dual luciferase assay kit (Promega, Madison, WI, USA) and
the normalized luciferase activity was calculated with the ratio of
firefly luciferase activity to Renilla luciferase activity.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
HCT116 cells were collected and total RNA was
isolated from these cells or from tumor tissues using A
PicoPure™ RNA Isolation kit (Arcturus, Sunnyvale, CA,
USA), according to the manufacturer's protocol. First, 2 µg of mRNA
was reverse transcribed into cDNA using SuperScript™ III
first-strand synthesis system kit (Thermo Fisher Scientific, Inc.),
according to manufacturer's protocols. Then, qPCR was performed
with TaqMan (Thermo Fisher Scientific, Inc.) primers for miR1260b
(Cat# 4426961, Thermo Fisher Scientific, Inc.) and GAPDH
(cat#4331182, Thermo Fisher Scientific, Inc.). The Vii™
7 system (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used
to run the qPCR procedure, according to the manufacturer's
protocol. The qPCR procedure includes two stages: Hold stage and
PCR stage. The briefly procedure as follows: Step 1: Heating from
25 to 95°C at a rate of 1.6°C/s, holding for 2 min at 50°C. Step 2:
Heating from 50 to 95°C at a rate of 1.6°C/s, holding for 10 min at
95°C. For qPCR stage: Step 1: Initial denaturation at 95°C for 15
s. Step 2: Annealing extension at 60°C for 1 min. The temperature
was reduced from 95 to 60°C at the rate of 1.6°C/s; the
denaturation and extension stages were repeated for 40 cycles. The
expression of mRNA was quantified using the 2−ΔΔCq
method (34) and normalized to the
internal reference gene, GAPDH.
Western blot analysis
Following treatment with miR1260b inhibitor with or
without 5-FU for 48 h, HCT116 cells were collected. The cells were
lysed in radioimmunoprecipitation assay buffer (Sigma-Aldrich;
Merck KGaA). Protein concentration was quantified using a BCA
Protein Assay kit (Beyotime Institute of Biotechnology). Total
protein (30 µg) from each group was loaded and resolved by 10%
Tris-SDS-PAGE. Following electrophoresis, the gel was
electro-transferred to polyvinylidene fluoride membranes (Thermo
Fisher Scientific, Inc.). The following blocking and antibody
incubations were performed according to the iBind kit (Thermo
Fisher Scientific, Inc.) manufacturer's protocol. Briefly, primary
antibodies were incubated at room temperature for overnight.
Following 3 times washing with iBind™
Flex/iBind™ Flex FD solution (Cat#SLF2020, Thermo Fisher
Scientific, Inc.), the secondary antibodies were used to incubate
the membranes at room temperature for 4 h. The primary antibodies
were as follows: Anti-p-Akt (cat# 4060, dilution, 1:300; Cell
Signalling Technology, Inc., Danvers, MA, USA), anti-PDCD4 (cat#
9535, dilution, 1:300; Cell Signaling Technology, Inc.)
anti-phosphorylated-extracellular-signal-regulated kinase (p-ERK).
(cat# sc-81492, dilution, 1:1,000; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) and anti-β-actin (cat# 58673, dilution, 1:1,000;
Santa Cruz Biotechnology, Inc.). Horseradish peroxidase-conjugated
IgG secondary antibodies were purchased from Santa Cruz
Biotechnology with the dilution of 1:3,000 and the detail
information of secondary antibodies as following: Anti-rabbit (cat#
sc-2004), anti-mouse (cat# sc-2005) and anti-goat (cat# sc-2020).
The signals were detected with SuperSigal® west femto
maximum sensitivity substrate (Thermo Fisher Scientific, Inc), and
the specific protein bands were captured with Bio-Rad
Lab™ 2.0 software (Bio-Rad Laboratories, Inc.).
Statistical analysis
GraphPad Prism7 (GraphPad Software, Inc., La Jolla,
CA, USA) was used to perform the two-tailed Student's t-test or
one-way ANOVA followed by Dunnett's test to determine the
difference between groups. P<0.01 and P<0.05 was considered
to indicate a statistically significant difference. All experiments
were performed in triplicate and data are presented as the mean ±
standard deviation.
Results
miR-1260b inhibitor inhibits CRC-cell
proliferation and migration
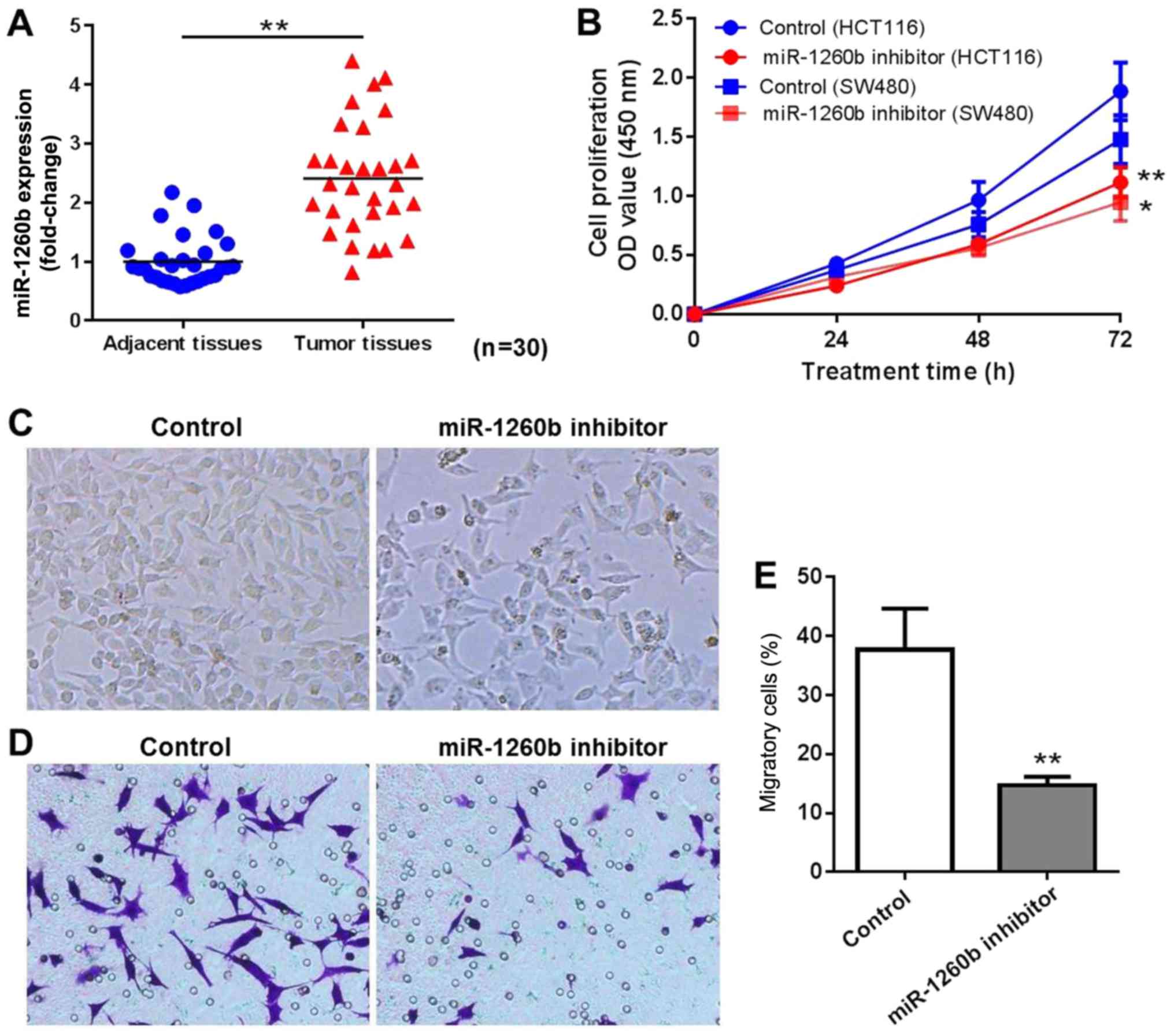
A total of 30 patients diagnosed with CRC were
enrolled in the present study. The miR-1260b expression level in
CRC tissues and adjacent normal tissues was determined by RT-qPCR.
The results indicated that miR-1260b was overexpressed in CRC
tissues compared with adjacent normal tissues (P<0.01; Fig. 1A). In order to explore the biological
role of miR-1260b in CRC cells, miR-1260b inhibitor or negative
control were transiently transfected into HCT116 or SW480 cells.
Subsequently, the proliferation and migration of HCT116 cells was
detected at 24, 48 and 72 h using an MTT assay and migration assay,
respectively. As indicated in Fig.
1B, miR-1260b inhibitor significantly inhibited proliferation
of HCT116 and SW480 cells, particularly at 72 h. Since HCT116 cells
were more sensitive to miR-1260b inhibitor compared with SW480
cells, HCT116 cells were selected for subsequent experiments. The
cell density was decreased in miR-1260b inhibitor transfected
HCT-116 cells group compared with that in the control group
(Fig. 1C). Similarly, a reduced
number of cells was observed in the migration assays following
transfection with miR-1260b inhibitor (Fig. 1D and E). These results indicated that
miR-1260b inhibitor exerted anti-proliferation and anti-migration
effects on HCT116 cancer cells.
miR-1260b induces CRC-cell
proliferation by targeting PDCD4
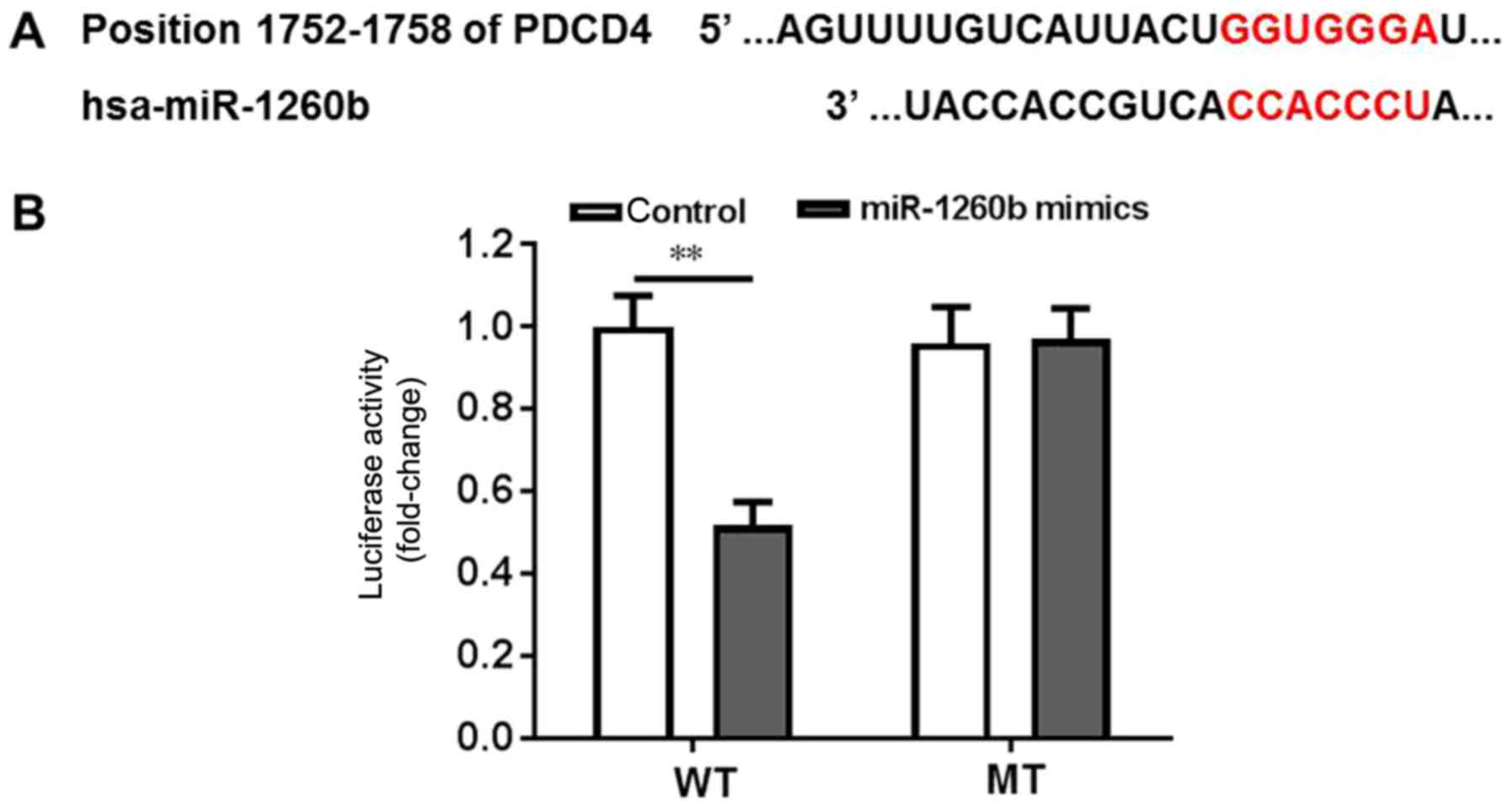
Bioinformatics tools were used to clarify the
underlying mechanisms of anti-proliferation and anti-migration
effects of miR-1260b on human CRC cells. PDCD4 exhibited the
highest prediction score. It is well known that PDCD4 is a
suppressor of the apoptosis signalling pathway (35,36).
Therefore, it was important to validate whether PDCD4 is a direct
target of miR-1260b. Wild-type and mutant PDCD4-3′UTR expression
plasmids were constructed, which were fused with a luciferase
reporter gene (Fig. 2A). The results
indicated that miR-1260b could significantly inhibit the luciferase
activity of wild-type PDCD4 compared with the control (P<0.01).
However, there was no difference between the control group and the
miR-1260 treatment group when PDCD4 was mutated (Fig. 2B). Thus, these findings suggested that
PDCD4 was a direct target of miR-1260b.
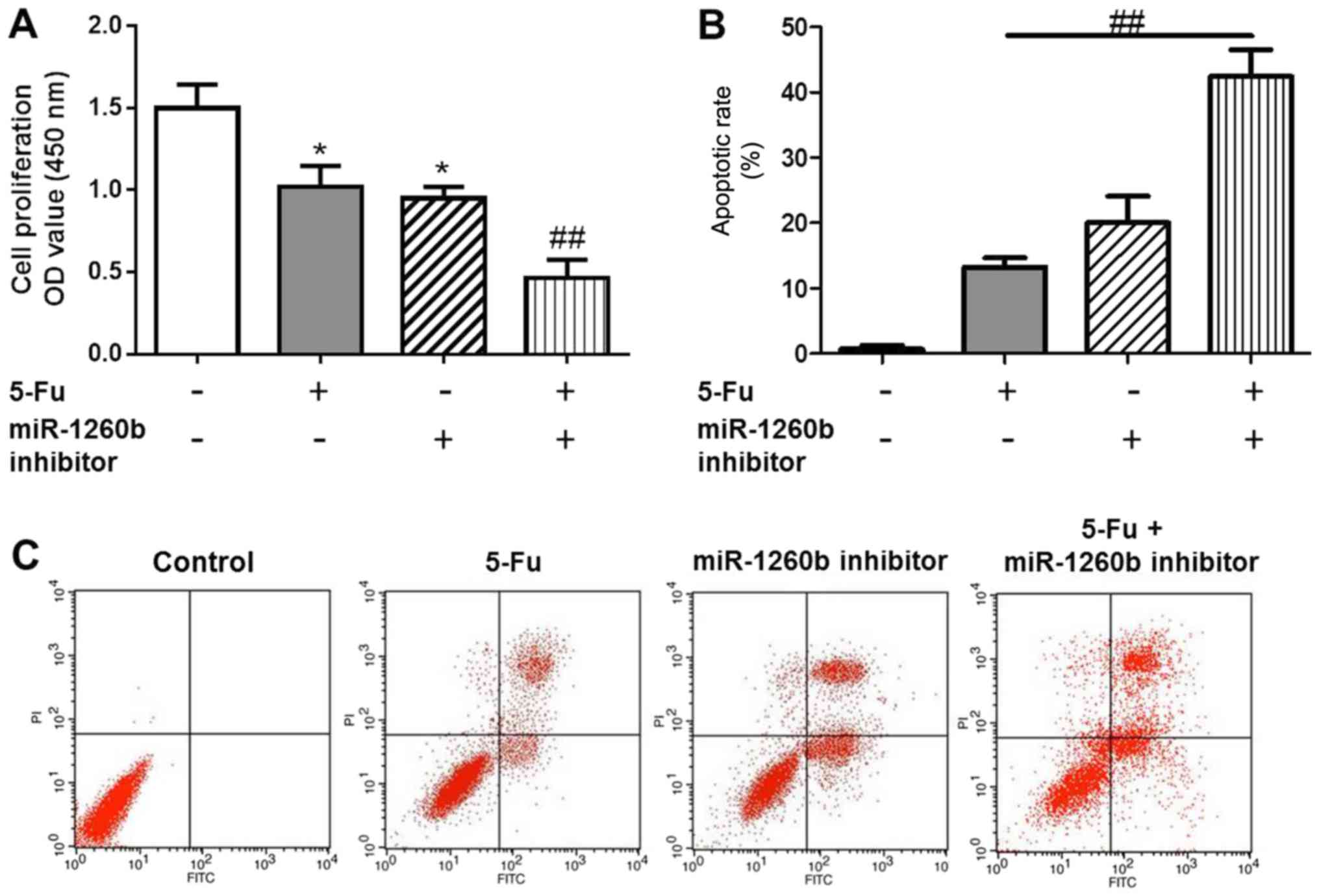
miR-1260b inhibitor enhances
5-FU-induced apoptotic rate in HCT116 cells
Apoptotic cells were detected with PI/Annexin V
staining, in order to investigate the biological function of the
miR-1260b inhibitor on CRC-cell proliferation and migration. In
order to determine whether there was a synergistic effect with
treatment with of miR-1260b inhibitor and 5-FU on the
anti-proliferation and induction of apoptosis effects, the MTT and
flow cytometry assays were conducted following 5-FU treatment alone
or in combination with miR-1260b inhibitor. The results indicated
that 5-FU alone or miR-1260b inhibitor alone significantly
decreased the proliferation rate of HCT116 cells compared with the
vehicle control group. However, the proliferation rate was lowest
in the combination group (P<0.05 vs. control group, P<0.01
vs. 5-FU alone treatment group (Fig.
3A). This synergistic effect was also observed in apoptosis.
These data indicated that miR-1260b inhibitor with 5-FU treatment
significantly induced apoptosis of HCT116 cells, which may be due
to miR-1260b enhancing the chemosensitivity of HCT116 cells to 5-FU
[P<0.01 vs. 5-FU alone treatment group (Fig. 3B)]. Flow cytometry results
demonstrated that miR-1260b inhibitor could induce apoptosis of
HCT116 cells (Fig. 3C).
miR-1260b inhibitor enhances the
chemosensitivity of HCT-116 cells to 5-FU due to downregulation of
PDCD4 expression
In order to investigate the anti-proliferation
mechanisms of miR-1260b inhibitor in HCT116 cells, the expression
of apoptosis-associated proteins was evaluated, as well as that of
the target protein PDCD4. It was identified that PDCD4 expression
was significantly decreased in the 5-FU (P<0.05), miR-1260b
(P<0.01) or combination (P<0.01) groups compared with the
vehicle control group (Fig. 4A and
B). In addition, downregulation trends were identified for the
apoptosis-associated proteins, p-Akt and p-ERK, following treatment
with miR-1260b inhibitor or combined miR-1260b and 5-FU (Fig. 4A, C and D). These results indicated
that miR-1260b increased HCT116-cell apoptotic rate via blocking
the p-Akt and p-ERK pathways.
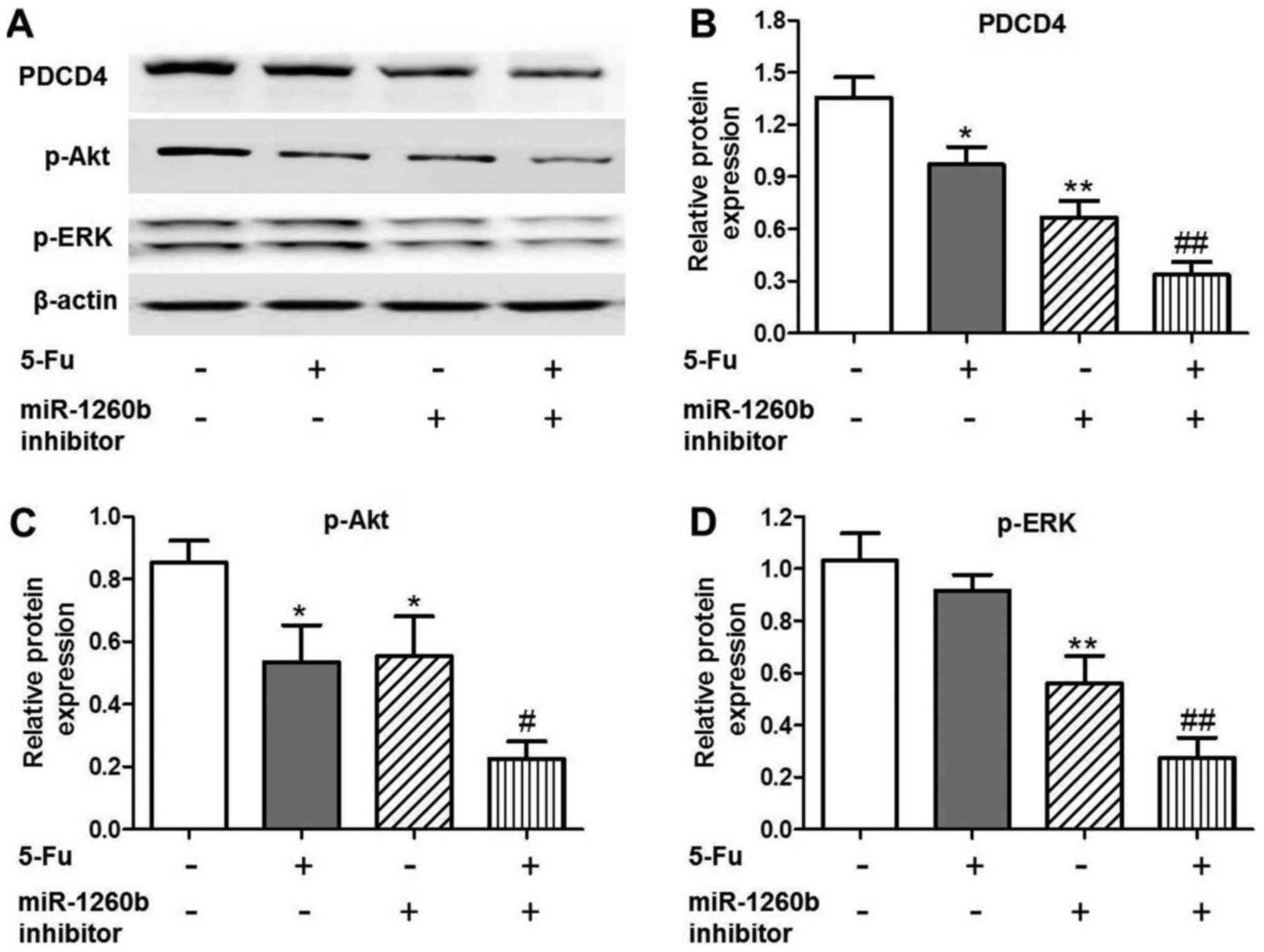 | Figure 4.PDCD4, p-Akt and p-ERK protein
expression levels are decreased in HCT116 cells treated with
miR1260b inhibitor and/or 5-FU. (A) Protein expression levels of
PDCD4, p-AKT and p-ERK following treatment with 5-FU and/or
miR-1260b inhibitor, as detected by western blotting. (B)
Quantification of the protein expression levels of PDCD4
(*P<0.05, **P<0.01 vs. control group; ##P<0.01
vs. 5-Fu alone treatment group). (C) Quantification of the protein
expression levels of p-Akt (*P<0.05, **P<0.01 vs. control
group, #P<0.01 vs. 5-FU alone treatment group). (D)
Quantification of the protein expression levels of p-ERK
(**P<0.01 vs. control group, ##P<0.01 vs. 5-FU
alone treatment group). PDCD4, programmed cell death receptor 4;
p-phosphorylated; ERK, extracellular-signal-regulated kinase; 5-FU,
5-fluorouracil; miR, microRNA. |
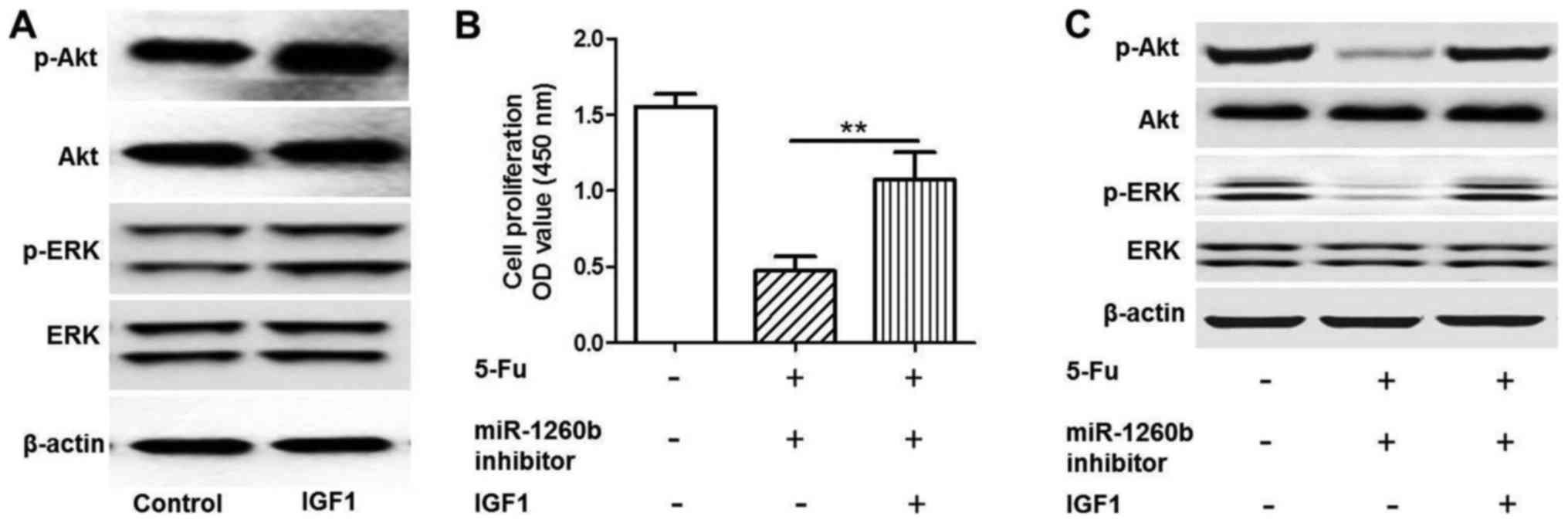
miR-1260b inhibitor enhances the
chemosensitivity of HCT116 cells to 5-FU via downregulation of the
PI3K/Akt pathway
As indicated in Fig.
5, a rescue experiment was conducted by adding insulin-like
growth factor 1 (IGF1) to activate the PI3K/Akt pathway. Protein
expression levels of p-Akt and p-ERK were detected when HTC116
cells were treated with IGF1 by western blot analysis (Fig. 5A). The MTT assay demonstrated that
IGF1 could significantly increase HCT116 proliferation rate
compared with the 5-FU and miR-1260b combination treatment group
(P<0.01; Fig. 5B). Western
blotting indicated that the p-Akt and p-ERK protein expression
levels in the IGF-1 group were increased compared with the 5-FU and
miR-1260b combination group (Fig.
5C). These results demonstrated that IGF1 rescued the effect
induced by miR-1260b inhibitor and 5-FU. Therefore, it was revealed
that the underlying mechanism of miR-1260b inhibitor induced
suppression of HCT116 proliferation involved in blocking the
PI3K/Akt pathway.
Discussion
Previous research has been conducted regarding CRC
prevention and diagnosis, as well as the biological, genetic or
epigenetic changes of CRC, in order to develop novel biomarkers or
therapies for CRC treatment (7,11).
Amounting evidence has revealed that aberrant miRNA expression
serves a functional role in CRC initiation and progression
(16). In a previous review of miRNA
expression studies, it was reported that ≥164 miRNAs have been
demonstrated to be significantly dysregulated in CRC compared with
adjacent normal tissues, including miR-17-92 cluster, miR-31,
miR181b, miR-21, miR-135a/b and miR-224 (16). Multiple miRNAs have been reported to
be associated with CRC initiation, development and progression,
including miR-21, which is expressed during the transition from
adenoma to advanced carcinoma (37).
Therefore, miR-21 has been identified as an important oncogenic
miRNA with roles in tumor initiation, progression and metastasis.
However, an association between miR-1260b and CRC has also been
reported (21). Navarro-Quiroz et
al (38), reported that miR-1260b
was overexpressed in human blood dendritic cells. Hirata et
al (22) demonstrated that
miR-1260b could promote renal cancer cell proliferation and
invasion. In addition, the group identified that genistein could
achieve its anti-tumor effect via downregulation of miR-1260b
expression and targeting of Smad4 in prostate carcinoma (39). It has been reported that miR-1260b is
associated with the development of non-small-cell lung cancer
(40). In addition, miR-1260b
expression has been demonstrated to be increased in CRC tissues,
particularly in patients with positive lymph nodes (21). Therefore, miR-1260b may be involved in
a variety of cancer cell activities. Furthermore, Nordentoft et
al (41) studied the association
between miRNA and chemosensitivity in advanced bladder cancer by
RNA profiling, and indicated that overexpression of miR-138 could
increase the chemosensitivity of RT4 and CLR2169 bladder cancer
cells to cisplatin. Valeri et al (42) reported that elevated miR-21 could
induce chemoresistance to 5-FU in colon cancer cell lines. In
summary, the patients of miR-1260 in chemotherapy response provides
a novel direction for CRC investigation.
The present study focused on the underlying
mechanism of miR-1260b regulation of CRC-cell proliferation and
invasion, as well as its function in the chemosensitivity of CRC to
5-FU. It was identified that miR-1260b was expressed at higher
levels in CRC tissues compared with adjacent normal tissues.
Furthermore, inhibition of HCT116-cell proliferation and invasion
by the miR-1260b inhibitor demonstrated, as well as revealed an
increased apoptotic rate. In addition, PDCD4 was identified as a
validated target of miR-1260b. PDCD4 protein expression level was
markedly decreased by miR-1260b inhibitor, along with downregulated
p-Akt and p-ERK protein. PDCD4 is a 64 kDa protein that is
preferentially expressed in tumor promoter-resistant cells, but has
been indicated to be suppressed in tumor promoter-sensitive cells
undergoing neoplastic transformation (23). PDCD4 protein expression has been
suggested to be increased during apoptosis in response to different
inducers, including retinoic acid, and has been demonstrated to be
regulated by topoisomerase inhibitors, cyclooxygenase-2 inhibitors,
Myb and Akt (43–46). Lankat-Buttgereit et al
(46) demonstrated that the
suppression of PDCD4 protein expression could enhance the release
of CgA and SgII, which have been identified as diagnostic markers
of neuroendocrine tumors. The stimulation of these markers by low
levels of PDCD4 was demonstrated to mediated by activation of Akt
via the PI3K pathway. Notably, Mudduluru et al (47) reported that p-Akt expression and
translocation of PDCD4 from the nucleus to the cytoplasm was
inversely correlated with PDCD4 levels in colon carcinoma samples.
However, above phenomena was not demonstrated in the normal
adjacent tissue. These findings indicate that the interplay between
PDCD4 and p-Akt serves a critical function in tumor
suppression.
In the present study, it was identified that
miR-1260b inhibitor could enhance the chemotherapy response of
HCT116 cells to 5-FU via blocking the PI3K/Akt pathway. These
trends were disrupted by treatment with IGF1, which serves as an
activator of the PI3K/Akt pathway. Depending on the cell type,
previous studies have demonstrated that IGF1 promotes neuronal
survival, maturation and cell cycle progression, by activating the
PI3K/Akt and/or Ras/MAPK pathway (48,49). These
findings may provide a novel combination therapy for future CRC
treatment.
Acknowledgements
None.
Funding
No funding received.
Availability of data and material
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ, JC, LZ, YD and WY managed the experimental
design, tissue collection and experimental execution. XZ, BY, YG,
YW and NM analyzed and interpreted the data and executed the
experiments. JZ reviewed and approved the final draft of this
manuscript before submission. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Ethics approval for the study was given by The
Changzhi People's Hospital ethics committee. All recruited patients
provided informed consent for participation.
Patient consent for publication
All patients agreed to publication and a written
informed consent was obtained from all participants.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kraus S, Nabiochtchikov I, Shapira S and
Arber N: Recent advances in personalized colorectal cancer
research. Cancer Lett. 347:15–21. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
O Connell JB, Maggard MA and Ko CY: Colon
cancer survival rates with the new American Joint Committee on
Cancer 6th edition staging. J Natl Cancer Inst. 96:1420–1425. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferlay J, Shin HR, Bray F, Forman D,
Mather C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nagtegaal ID, Quirke P and Schmoll HJ: Has
the new TNM classification for colorectal cancer improved care? Nat
Rev Clin Oncol. 9:119–123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Watanabe T: Biomarker for high-risk
patients with stage II colon cancer. Lancet Oncol. 14:1247–1248.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chua YJ and Zalcberg JR: Progress and
challenges in the adjuvant treatment of stage II and III colon
cancers. Expert Rev Anticancer Ther. 8:595–604. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grady WM and Pritchard CC: Molecular
alterations and biomarkers in colorectal cancer. Toxicol Pathol.
42:124–139. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ye JJ and Cao J: MicroRNAs in colorectal
cancer as markers and targets: Recent advances. World J
Gastroenterol. 20:4288–4299. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma J, Dong C and Ji C: MicroRNA and drug
resistance. Cancer Gene Ther. 17:523–531. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oqin S, Chan AT, Fuchs CS and Giovannucci
E: Molecular pathological epidemiology of colorectal neoplasia: An
emerging transdisciplinary and interdisciplinary field. Gut.
60:397–411. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Okugawa Y, Grady WM and Goel A: Epigenetic
alterations in colorectal cancer: Emerging biomarkers.
Gastroenterology. 149:1204–1225.e12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luo Y, Tsuchiya KD, ll Park D, Fausel R,
Kanngurn S, Welcsh P, Dzieciatkowski S, Wang J and Grady WM: RET is
a potential tumor suppressor gene in colorectal cancer. Oncogene.
32:2037–2047. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wiemer EA: The role of microRNAs in
cancer: No small matter. Eur J Cancer. 43:1529–1544. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hernando E: microRNAs and cancer: Role in
tumorigenesis, patient classification and therapy. Clin Transl
Oncol. 9:155–160. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kosaka N, Iguchi H, Yoshioka Y, Takeshita
F, Matsuki Y and Ochiya T: Secretory mechanisms and intercellular
transfer of microRNAs in living cells. J Boil Chem.
285:17442–17452. 2010. View Article : Google Scholar
|
|
16
|
Zhou JJ, Zheng S, Sun LF and Zheng L:
MicroRNA regulation network in colorectal cancer metastasis. World
J Biol Chem. 5:301–307. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vilarr E, Tabernero J and Gruber SB:
Micromanaging the classification of colon cancer: The role of the
microRNAome. Clin Cancer Res. 17:7207–7209. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cummins JM, He Y, Leary RJ, Paqliarini R,
Diaz LA Jr, Sjiblom T, Barad O, Bentwich Z, Szafranska AE,
Labourier E, et al: The colorectal microRNAome. Proc Natl Acad Sci
USA. 103:3687–3692. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hollis M, Nair K, Vyas A, Chaturvedi LS,
Gambhir S and Vyas D: MicroRNAs protential utility in colon cancer:
Early detection, prognosis and chemosensitivity. World J
Gastroenterol. 21:8284–8292. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Di Leva G, Cheung DG and Croce CM: miRNA
clusters as therapeutic targets for hormone-resistant. Expert Rev
Endocrinol Metab. 10:607–617. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu DR, Guan QL, Limin X, Gao MT, Jiang L
and Kang HX: MiR-1260b is a potential prognostic biomarker in
colorectal cancer. Med Sci Monit. 22:2417–2423. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hirata H, Ueno K, Nakajima K, Tabatatai
ZL, Hinoda Y, Ishii N and Dahiya R: Genistein downregulates
onco-miR-1260b and inhibits Wnt-signalling in renal cancer cells.
Br J Cancer. 108:2070–2078. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Afonia O, Juste D, Das S, Matsuhashi S and
Samuels HH: Induction of PDCD4 tumor suppressor gene expression by
RAR agonists, antagonist in breast cancer cells. Oncogene.
23:8135–8145. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jansen AP, Camalier CE, Stark C and
Colburn NH: Characterization of programmed cell death 4 in multiple
human cancers reveals a novel enhancer of drug sensitivity. Mol
Cancer Ther. 3:103–110. 2004.PubMed/NCBI
|
|
25
|
Wei N, Liu SS, Chan KK and Ngan HY: Tumour
suppressive function and modulation of programmed cell death 4
(PDCD4) in ovarian cancer. PLoS One. 7:e303112012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yin K, Liu MH, Zhang M, Wang F, Fen M, Liu
ZJ, Yuan Y, Gao S, Yang L, Zhang W, et al: miR-208a-3p suppresses
cell apoptosis by targeting PDCD4 in gastric cancer. Oncotarget.
7:67321–67332. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shen F, Mo M, Chen L, An S, Tan X, Fu Y,
Rezaei K, Wang Z, Zhang L and Fu SW: MicroRNA-21 down-regulates Rb1
expression by targeting PDCD4 in retinoblastoma. J Cancer.
5:804–812. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Foley NH, Bray IM, Tivnan A, Bryan K,
Murphy DM, Buckley PG, Ryan J, O'Meara A, O'Sullivan M and
Stallings RL: MicroRNA-184 inhibits neuroblastoma cell survival
through targeting the serine/threonine kinase AKT2. Mol Cancer.
9:832010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gao N, Budhraja A, Cheng S, Liu EH, Chen
J, Yang Z, Chen D, Zhang Z and Shi X: Phenethyl isothiocyanate
exhibits antileukemic activity in vitro and in vivo by inactivation
of Akt and activation of JNK pathways. Cell Death Dis. 2:e1402011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu X, Zhen Y, Yang H, Wang H, Zhou Y, Wang
E, Marincola FM, Mai C, Chen Y and Wei H: Loss of connective tissue
growth factor as an unfavorable prognosis factor activates miR-18b
by PI3K/AKT/C-Jun and C-Myc and promotes cell growth in
nasopharyngeal carcinoma. Cell Death Dis. 4:e6342013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang WQ, Zhang H, Wang HB, Sun YG, Peng
ZH, Zhou G, Yang SM, Wang RQ and Fang DC: Programmed cell death 4
(PDCD4) enhances the sensitivity of gastric cancer cells to
TRAIL-induced apoptosis by inhibiting the PI3K/Akt signaling
pathway. Mol Diagn Ther. 14:155–161. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ivanov VN, Krasilnikov M and Ronai Z:
Regulation of Fas expression by STAT3 and c-Jun is mediated by
phosphatidylinositol 3-kinase-AKT signaling. J Biol Chem.
277:4932–4944. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Justus CR, Leffler N, Ruiz-Echevarria M
and Yang LV: In vitro cell migration and invasion assay. J Vis Exp.
1–Jun;2014. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Livak KJ and Schmitten TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2011.
View Article : Google Scholar
|
|
35
|
Liwak U, Thakor N, Jordan LE, Roy R, Lewis
SM, Pardo OE, Seckl M and Holcik M: Tumor suppressor PDCD4
repressed internal ribosome entry site-mediated translation of
antiapoptotic proteins and is regulated by S6 kinase 2. Mol Cell
Biol. 32:1818–1829. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang S, Li J, Jiang Y, Xu Y and Oin C:
Programmed cell death 4 (Pdcd4) suppresses metastatic potential of
human hepatocellular carcinoma cells. J Exp Clin Cancer Res.
28:712009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Asangani IA, Rasheed SA, Nikolova DA,
Leupold JH, Colburn NH, Post S and Allgayer H: MicroRNA-21 (miR-21)
post-transcriptionally downregulates tumor suppressor Pdcd4 and
stimulates invasion, intravasation and metastasis in colorectal
cancer. Oncogene. 27:2128–2136. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Navarro-Quiroz E, Pacheco-Lugo L,
Navarro-Quiroz R, Lorenzi H, España-Puccini P, Díaz-Olmos Y,
Almendrales L, Olave V, Gonzalez-Torres H, Diaz-Perez A, et al:
Profiling analysis of circulating microRNA in peripheral blood of
patients with class IV lupus nephritis. PLoS One. 12:e01879732017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hirata H, Hinoda Y, Shahryari V, Deng G,
Tanaka Y, Tabatabai ZL and Dahiya R: Genistein down regulates
onco-miR-1260b and upregulates sFRP1 and Smad4 via demethylation
and histone modification in prostate cancer cells. Br J Cancer.
110:1645–1654. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu L, Li L, Li J, Li H, Shen Q, Ping J, Ma
Z, Zhong J and Dai L: Overexpression of miR-1260b in non-samll cell
lung cancer is associated with lymph node metastasis. Aging Dis.
6:478–485. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nordentoft I, Birkenkamp-Demtroder K,
Agerbak M, Thedorescu D, Ostenfeld MS, Hartmann A, Borre M, Ørntoft
TF and Dyrskjøt L: miRNAs associated with chemo-sensitivity in cell
lines and in advanced bladder cancer. BMC Med Genomics. 5:402012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Valeri N, Gasparini P, Braconi C, Paone A,
Lovat F, Fabbri M, Sumani KM, Alder H, Amadori D, Patel T, et al:
MicroRNA-21 induces resistance to 5-fluorouracil by down-regulating
human DNA MutS homolog 2 (hMSH2). Proc Natl Acad Sci USA.
107:20198–21103. 2010. View Article : Google Scholar
|
|
43
|
Zhang Z and DuBois RN: Detection of
differentially expressed genes in human colon carcinoma cells
treated with a selective COX-2 inhibitor. Oncogene. 20:4450–4456.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Palamarchuk K, Efanov A, Maximov V,
Aqeilan RI and Croce CMP: Akt phosphorylates and regulates Pdcd4
tumor suppressor protein. Cancer Res. 65:11282–11286. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bitomsky N, Bohm M and Klempnauer KH:
Transformation suppressor protein Pdcd4 interferes with
JNK-mediated phosphorylation of c-Jun and recruitment of the
coactivator p300 by c-Jun. Oncogene. 23:7484–7493. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lankat-Buttgereit B, Muller S, Schmidt H,
Parhofer KG, Gress TM and Göke R: Knockdown of Pdcd4 results in
induction of proprotein convertase 1/3 and potent secretion of
chromogranin A and secretogranin II in a neuroendocrine cell line.
Bio Cell. 100:703–715. 2008. View Article : Google Scholar
|
|
47
|
Mudduluru G, Medved F, Grobholz R, Jost C,
Gruber A, Leupold JH, Post S, Jansen A, Colburn NH and Allgayer H:
Loss of Pdcd4 expression marks adenoma-carcinoma transition,
correlates inversely with pAkt and is a new and independent
prognostic factor in resected colorectal cancer. Cancer.
110:1697–1707. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Otaegi G, Yusta-Boyo MJ, Vergaño-Vera E,
Méndez-Gómez HR, Carrera AC, Abad JL, González M, de la Rosa EJ,
Vicario-Abejón C and de Pablo F: Modulation of the PI 3-kinase-Akt
signalling pathway by IGF-I and PTEN regulates the differentiation
of neural stem/precursor cells. J Cell Sci. 119:2739–2748. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cui QL and Almazan G: IGF-1 induced
oligodendrocyte progenitor proliferation requires PI3K/Akt, MEK/ERK
and Src-like tyrosine kinases. J Neurochemistry. 217:361–370.
2009.
|