Introduction
Gastric cancer is the second leading cause of
cancer-associated mortality globally (1). The incidence of gastric cancer is
particularly high in Eastern Asian countries, including China,
Japan and Korea, as well as in South America (1). Of these countries, China has the highest
overall incidence (1). Gastric cancer
is a highly heterogeneous disease that can be categorized into two
major histological subtypes, intestinal and diffuse, on the basis
of the Lauren classification (2,3). It has
been reported that genomic or molecular classifications are
important for evaluating the curative effects and prognosis of
gastric cancer (4). Aberrant genetic
and molecular changes occur during oncogenesis and gastric cancer
progression, some of which are representative of therapeutic
targets, including human epidermal growth factor receptor 2
(HER-2), MET proto-oncogene, receptor tyrosine kinase (MET) and
vascular endothelial growth factor receptor (VEGFR) (5). However, despite advances in targeted
therapy, the curative effect of treatments has not improved
(6). When developing targeted
therapies for gastric cancer, somatic mutations that occur with low
frequencies may be lost to screening as certain detection
approaches have high detection limits (3).
Recent genomic studies have identified four
molecular subtypes (MSS/EMT, MSI, MSS/TP53+ and MSS/TP53-) of
gastric cancer that are associated with distinct patterns of
disease progression and prognosis (4). The Cancer Genome Atlas (TCGA) study also
reported 4 genetic subgroups (7). The
chromosomal instability (CIN) classification is present in ~50% of
cases and is associated with intestinal morphology, as well as a
high frequency and density of p53 mutations. The genomically stable
(GS) classification is present in 20% of cases and is associated
with diffuse histology, as well as CDH1 and RhoA mutations. A
further 20% of cases are MSI and are associated with hyper
mutations of somatic DNA (7).
Finally, 10% of cases are associated with Epstein Barr virus and
have a high frequency of PIK3CA and ARID1A mutations (7). It may be possible to illustrate the
mutation pattern of gastric cancer through the comprehensive
analysis of multiple studies, and molecular markers that may aid in
guiding patient management.
Historically, mutations have been profiled using
Sanger sequencing, reverse transcription-quantitative polymerase
chain reaction (RT-qPCR) and microarrays. The Sanger sequencing
method generates a precise base sequence, typically <1,000 base
pairs (bp) in one sequencing run (8).
As a result, only mutations within this short sequence can be
detected at any one time. The heterogeneity resolution rate of
Sanger sequencing is relatively high, at 15–20% (9,10).
However, Sanger sequencing for a large number of genes is expensive
and labor-intensive. qPCR has a higher resolution rate when it
comes to analyzing low frequency mutations compared with Sanger
sequencing (11). However, qPCR can
only profile one locus with known alleles in each reaction, making
it expensive and time-consuming for sequencing multiple genes.
Microarrays are an efficient parallel profiling approach for
analyzing large heterogeneous loci (12). However, the high detection limit
restricts the application of microarrays for somatic mutation
profiling. Next-generation sequencing (NGS) technologies are low
cost with good throughput and resolution rates. NGS may therefore
be used to overcome the problems associated with other sequencing
methods and provide accurate information regarding gene mutations
to aid in treatment decisions (13).
NGS can be used to investigate multiple genes simultaneously with
only small amounts of DNA that can be obtained from a variety of
specimens, including formalin-fixed, paraffin-embedded (FFPE)
tissues, and NGS has an improved sensitivity compared with routine
technologies.
It has been reported that genetic alterations result
in heterogeneous disease states in human cancer, and investigating
this heterogeneity is essential for understanding the potential
mechanisms of oncogenesis and developing effective treatments
(14). A number of studies have
reported that genetic mutations occur in oncogenes and tumor
suppressor genes in gastric cancer (15–17). A
number of genes, including TP53 and HER-2, have been identified as
driver genes in gastric cancer (18).
However, the association between somatic mutations and clinical
features has not yet been completely elucidated. It is therefore
important to profile the somatic mutation pattern of driver genes
and potential driver genes in gastric cancer. TP53 is the most
frequently mutated gene in human cancer, with alterations occurring
in ~50% of all cases of cancer (19).
TP53 serves a fundamental role in regulating cellular processes
involved in the inhibition of proliferation, and maintaining genome
integrity and stability (20). TP53
mutations have been reported in gastric cancer, suggesting that it
may be an effective biomarker for this disease (4,21,22). However, the mutation patterns of TP53
have been demonstrated to differ within the same tumor sample, and
intratumoral heterogeneity exists in TP53-inactivation mechanisms
(3). The association between TP53
mutation patterns and clinical features is obscure and it is
unknown whether TP53 mutations influence the other genes. Using
whole-exome sequencing, it was demonstrated that inactivating
mutations in cell adhesion and chromatin remodeling genes are also
frequent (16).
To improve our understanding of the genomic basis of
gastric cancer and to identify the underlying genetic
heterogeneity, NGS technologies were used to screen genetic
mutations in potential driver genes from 45 FFPE gastric
adenocarcinoma samples. In total, 1,021 cancer-related genes,
including certain known oncogenes and tumor suppressor genes, were
sequenced with a median sequencing coverage depth of 708-fold. Over
80% of the samples were demonstrated to exhibit at least one
somatic genomic alteration. In the present study, TP53 was the most
frequently mutated gene, and patients with TP53 mutations had a
significantly higher number of mutations.
Materials and methods
Patients and tissue sample
collection
A total of 45 patients with gastric cancer were
recruited from the Department of General Surgery of Nanjing Medical
University Affiliated Cancer Hospital (Nanjing, China) between
January 2013 and December 2013 (39 males and 6 females). The median
age at diagnosis was 60 years (range, 32–79 years). All patients
provided written informed consent and were diagnosed using
histology. The present study was approved by the Ethics Committee
of Jiangsu Cancer Hospital (Nanjing, China).
A total of 45 human gastric cancer tissues were
analyzed using target capture and NGS. Germ-line DNA from matched
adjacent non-cancerous tissues was used as a reference sequence to
detect somatic alterations.
Gastric tumor specimens were harvested from three
anatomical locations: 15 from the antrum (33.33%), 19 from the body
(42.22%) and 11 from the cardia (24.44%). At least two senior
pathologists from the department of Pathology, Jiangsu Cancer
Hospital (Nanjing, China), reviewed all histopathological diagnoses
independently. In the present study, 14 (31.11%) cases were
classified as diffuse-type, 26 (57.78%) as intestinal-type, and 5
(11.11%) as mixed intestinal and diffuse histology. The clinical
stage was determined according to 7th National Comprehensive Cancer
Network guidelines (23). Of the 45
samples collected, 5 (11.11%) were determined to be stage I, 6
(13.33%) were stage II, and 34 (75.56%) were stage III. Patients
had a median follow-up time of 39 months.
DNA extraction and target capture
sequencing
Human gastric cancer tissues were fixed in 10%
formalin up to 24 h at room temperature. Genomic DNA was isolated
from FFPE tumor samples and matched non-cancerous tissues using the
QIAamp DNA FFPE Tissue kit (Qiagen GmbH, Hilden, Germany),
according to the manufacturer's protocol. The DNA concentration was
measured using the Qubit dsDNA HS (High Sensitivity) assay kit in
the Qubit fluorometer (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). To test the DNA integrity, 200 ng extracted DNA
was loaded onto the 1% agarose gel with λ-Hind III digest
DNA marker (Takara Biotechnology Co., Ltd., Dalian, China). The DNA
samples that were longer than the second largest bonds (9,416 bp)
of λ-Hind III digest DNA marker were considered as
integrated samples and were used for subsequent analysis.
A panel was designed, including exons of 1,017
genes, as well as introns, promoters and fusion regions of 24
genes. In brief, the panel comprised recurrent mutated genes in
gastric cancer as recorded in the COSMIC database (http://cancer.sanger.ac.uk/cosmic), oncogenes and
tumor suppressor genes associated with tumorigenesis and metastasis
in gastric cancer (16,24), and genes associated with other cancer
types as recorded in the TCGA network (https://cancergenome.nih.gov/).
Library constructions were prepared using protocols
recommended in the Illumina TruSeq DNA Library Preparation kit
(Illumina, Inc., San Diego, CA, USA) using 1 µg DNA isolated from
FFPE tumor samples. DNA was sheared prior to using an
ultrasonoscope with a peak of 250 bp, followed by end repair.
Fragments were then ligated to the Illumina-indexed adapters
according to the standard library construction protocol.
Custom-designed biotinylated oligonucleotide probes (Roche
NimbleGen, Inc., Madison, WI, USA) covering ~1.1 M bp of 1,021
genes were used to capture target sequences in the libraries. DNA
sequencing was performed with 2×150 bp paired-end reads on the
HiSeq 3000 sequencing system (Illumina, Inc.).
Sequencing data analysis
Terminal adaptor sequences and low-quality reads
were removed from the raw data. The clean reads were aligned to the
human genome build GRCh37 using BWA software version 0.7.12-r1039
(25,26). Somatic single nucleotide variants
(SNVs) and somatic small insertions and deletions (Indels) were
generated using MuTect version 1.1.4 (27) and GATK version 3.4–46-gbc02625
(28), respectively. Candidate
somatic mutations were SNVs and Indels where the variant allele
fraction (VAF) was ≥2% and there were ≥5 high-quality reads (Phred
score ≥30, mapping quality ≥13, and without paired-end reads bias)
containing the target base. The candidate mutations were annotated
to genes using ANNOVAR software (29)
to identify the mutated protein-coding position and exclude
intronic and silent changes. Missense, nonsense, frameshift, spans,
splicing, cds-del, cds-ins, stop-gain and stop-loss mutations were
retained. CONTRA v2.0.8 (30) was
used to detect copy number variants (CNVs) and a manual visual
inspection step was performed to further remove artifactual
changes.
Statistical analysis of clinical and
genetic data
All statistical analyses were performed using SPSS
version 19.0 (IBM Corp., Armonk, NY, USA). Unpaired Student's
t-tests and χ2 tests were used to compare the mutation
status of TP53 genes between different groups with different
clinical and pathological characteristics. Mann-Whitney U tests
were used to compare the overall number of mutations between the
group of TP53-WT (wild-type) and TP53-Mut (mutated type). The
mutation number data are presented as the mean ± interquartile
range. Pearson's correlation analysis was used to evaluate the
correlation between the number of mutations and patient age. The
statistic of overall survival (OS) were carried out using the
Kaplan-Meier method, and differences between groups were evaluated
using the log-rank test. To determine which independent factors had
a significant impact on OS, Cox proportional hazards regression
analysis was performed. P<0.05 was considered to indicate a
statistically significant difference.
Results
Target capture and NGS of human
gastric cancer
In the present study, a panel of 1,021 genes was
investigated. A median sequencing coverage depth of 708-fold (from
14- to 1,609-fold) was achieved for 45 gastric cancer tissues. At
least one somatic genomic alteration, including SNVs, Indels or
CNVs, was detected in 37/45 (82.4%) patients. The maximum number of
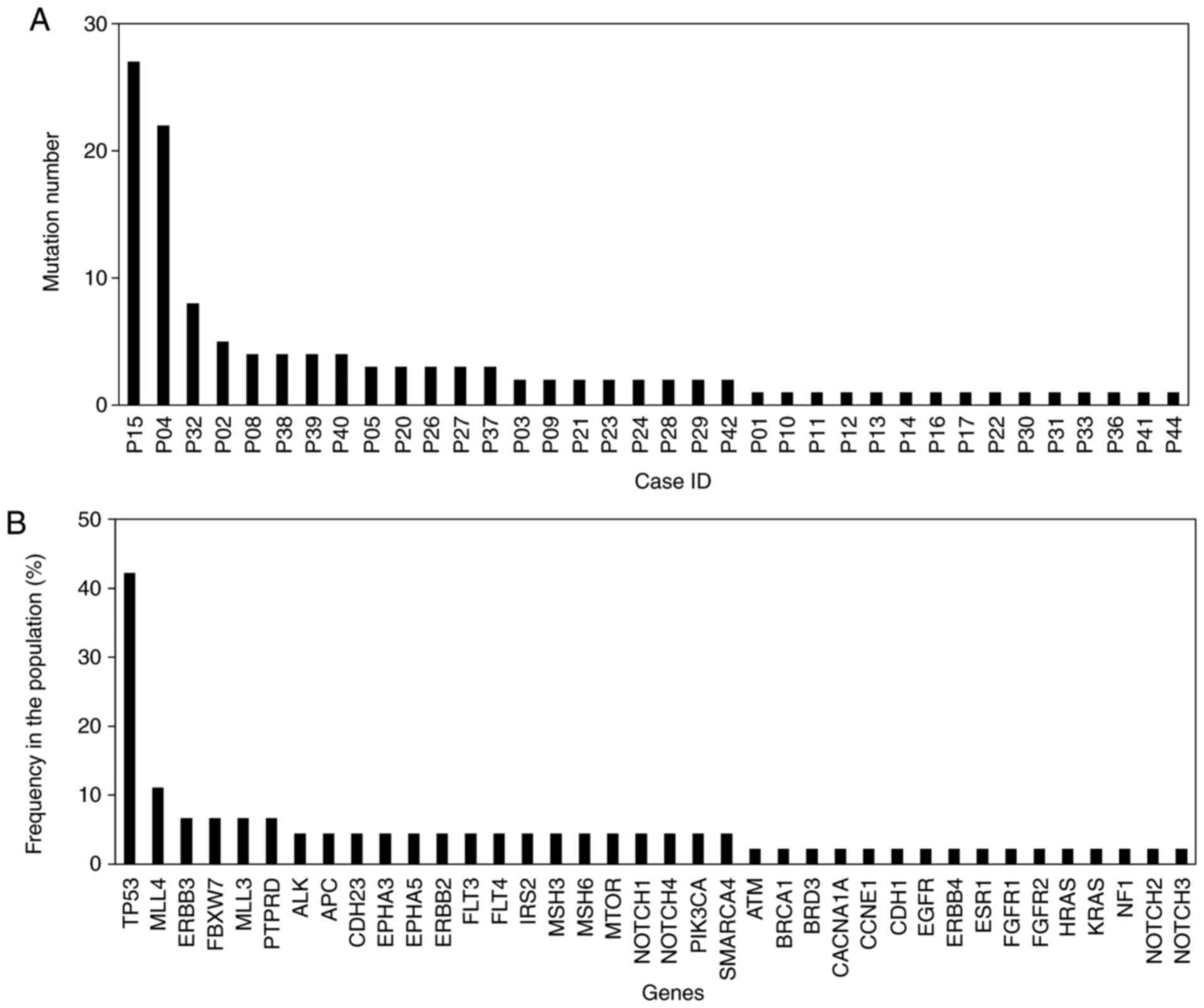
mutations identified in one tumor sample was 27 (Fig. 1A). There were a total of 121 mutations
in this cohort and the highest mutation rate was 73.4% (TP53
p.R273C; Fig. 1B). Furthermore,
somatic mutations in the TP53 gene were the most frequently
identified genetic alterations, occurring in 19/45 (42.2%) samples.
Somatic mutations in the MLL4 gene were the second most frequent,
occurring in 5/45 (11.1%) samples (Fig.
1B). Mutations were identified in the ERBB3, FBXW7 and MLL3
genes in more than one case. Finally, six other mutated genes
(MTOR, NOTCH1, PIK3CA, KRAS, ERBB4 and EGFR) were identified in one
case each (Fig. 1B). CNVs were
detected in 8 patients and ten genes (Table I). CNVs of ERBB2, which was the most
frequent gene, occurred in 3 patients.
 | Table I.Extent and distribution of copy
number variants. |
Table I.
Extent and distribution of copy
number variants.
| ID | Gene | Transcripts | Chromosome | Copy number |
|---|
| P01 | MDM2 | NM_002392.4 | chr12 | 6.3 |
| P01 | ERBB3 | NM_001982.3 | chr12 | 3.2 |
| P01 | AURKA | NM_198433.1 | chr20 | 5.6 |
| P01 | GNAS | NM_001077488.2 | chr20 | 10.7 |
| P01 | VEGFA | NM_001025366.2 | chr6 | 4.7 |
| P14 | VEGFA | NM_001025366.2 | chr6 | 7.7 |
| P14 | EGFR | NM_005228.3 | chr7 | 5.5 |
| P16 | ERBB2 | NM_001005862.1 | chr17 | 25.6 |
| P23 | PIK3CA | NM_006218.2 | chr3 | 2.3 |
| P32 | ERBB2 | NM_001005862.1 | chr17 | 347.7 |
| P41 | ERBB2 | NM_001005862.1 | chr17 | 2.1 |
| P43 | CCNE1 | NM_001238.2 | chr19 | 7.7 |
| P44 | ERBB3 | NM_001982.3 | chr12 | 3.0 |
| P44 | STK11 | NM_000455.4 | chr19 | 1.8 |
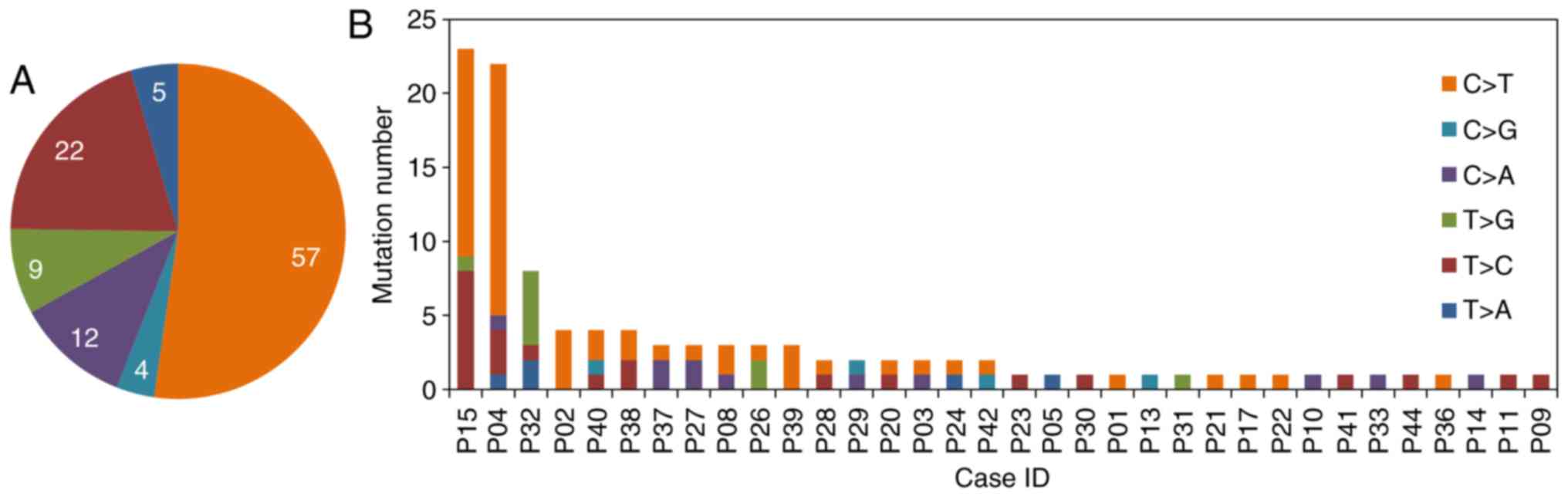
In the coding regions, the average number of
mutations per sample was 3 (mutation range, 1–27). The somatic SNVs
list is presented in Fig. 2A. The
most frequently occurring variant was the percentage of C>T
across the coding regions, at 52.3%. The number of SNVs per patient
genome varied greatly (Fig. 2B).
TP53 mutations are associated with
clinicopathological subtypes of gastric cancer
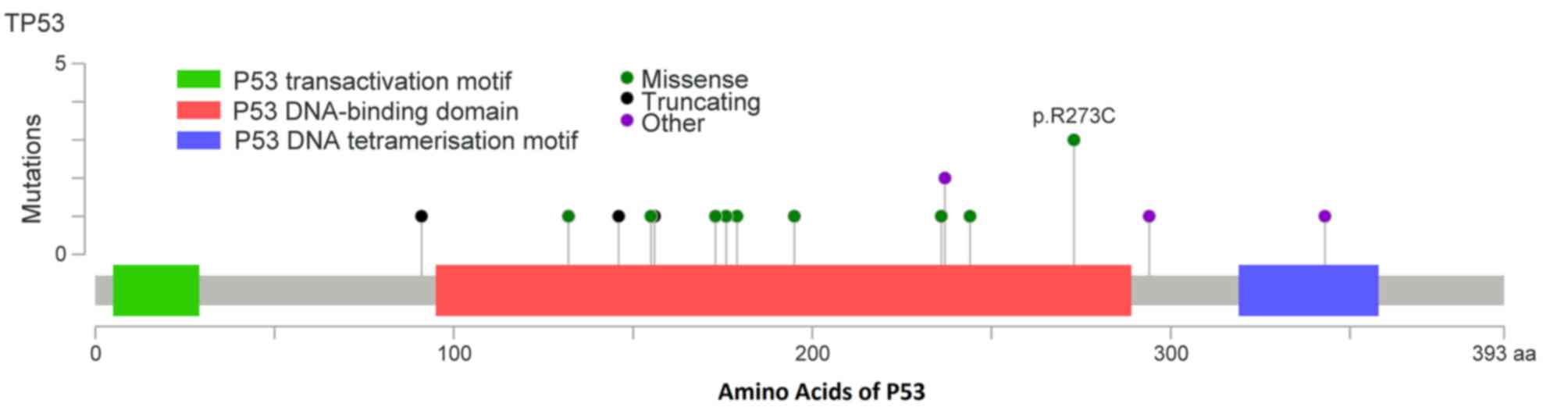
TP53 was the most frequently mutated gene, with 12
missense, 4-frame shift, 2 non-sense and 1 splice-5 mutation
(Table II). Changes in TP53 protein
are summarized in Fig. 3. Mutational
hotspots were identified in codons 91, 132, 146, 155, 156, 173,
176, 179, 195, 236, 244, 237, 273, 294 and 343. TP53 mutation rates
were then compared with the clinicopathological subtypes of GC.
Age, sex, histology, Lauren subtype, differentiation, venous and
lymphatic invasion, staging and lymph node, liver, peritoneal and
other distant metastases were risk factors included in this
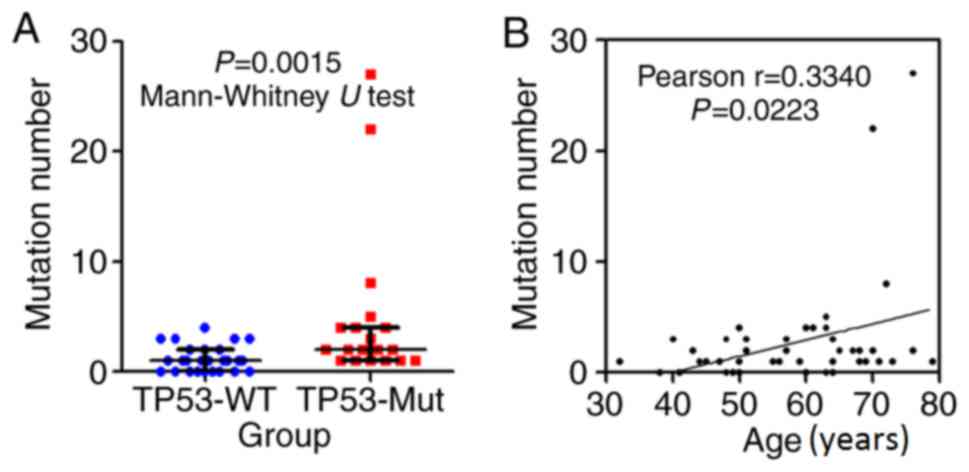
analysis. Two main trends were observed: i) The patients with TP53
mutations had a higher overall number of mutations (Fig. 4A); and ii) the number of mutations was
significantly associated with patient age (P=0.0223, Fig. 4B).
 | Table II.Tumor protein P53 mutation type. |
Table II.
Tumor protein P53 mutation type.
| Case ID | Protein_Change | Mutation_Type |
|---|
| P02 | p.M237I |
Missense_Mutation |
| P03 | p.R273C |
Missense_Mutation |
| P04 | p.R273C |
Missense_Mutation |
| P05 | p.R156Pfs*25 |
Frame_Shift_Ins |
| P11 | p.C176F |
Missense_Mutation |
| P14 | p.G244C |
Missense_Mutation |
| P15 | p.E294Sfs*51 |
Frame_Shift_Del |
| P17 | p.W146* |
Nonsense_Mutation |
| P23 | p.E343Gfs*2 |
Frame_Shift_Del |
| P24 | p.W91* |
Nonsense_Mutation |
| P28 | p.Y236C |
Missense_Mutation |
| P29 | . | Splice_Site |
| P31 | p.T155P |
Missense_Mutation |
| P32 | p.V173G |
Missense_Mutation |
| P38 | p.H179R |
Missense_Mutation |
| P39 | p.M237Cfs*10 |
Frame_Shift_Del |
| P40 | p.R273C |
Missense_Mutation |
| P41 | p.I195T |
Missense_Mutation |
| P44 | p.K132R |
Missense_Mutation |
Although there was no evidence to suggest that TP53
mutation positivity was associated with age, sex, histology, Lauren
subtype, differentiation, staging or distant metastasis, patients
with TP53 mutations exhibited less venous invasion (10.5 vs. 46.2%;
P=0.011), less perineural invasion (47.4 vs. 88.8%; P=0.019) and
less severe lymph node metastasis (N0-2 vs. N3-4; 15.8 vs. 46.2%;
P=0.033; Table III). It was also
reported that patients with the TP53 mutation had an improved
overall survival (P=0.109; data not shown). However, these results
are contradictory to those of previous reports (22,31).
 | Table III.Patient clinicopathological
characteristics between TP53 mutation+ and TP53- gastric
cancer. |
Table III.
Patient clinicopathological
characteristics between TP53 mutation+ and TP53- gastric
cancer.
| Characteristic | TP53 mutation+ | TP53 mutation- |
|---|
| Number | 19 | 26 |
| Median age
(range) | 63 (47–76) | 55.5 (32–79) |
| Sex |
|
Male | 17 | 22 |
|
Female | 2 | 4 |
| Lauren type |
|
Intestinal | 13 | 13 |
|
Diffuse | 4 | 10 |
|
Mixed | 2 | 3 |
| pT stage |
| T1 | 2 | 1 |
| T2 | 2 | 2 |
| T3 | 2 | 1 |
| T4 | 13 | 22 |
| pN stage |
| N0 | 7 | 5 |
| N1 | 5 | 3 |
| N2 | 4 | 6 |
| N3 | 3 | 12 |
| AJCC stage (7th,
ed.) |
| I | 3 | 2 |
| II | 4 | 2 |
|
III | 12 | 22 |
| IV | 0 | 0 |
| Venous
invasion |
|
Positive | 2 | 12 |
|
Negative | 17 | 14 |
| Perineural
invasion |
|
Positive | 9 | 21 |
|
Negative | 10 | 5 |
| Recurrence |
|
Positive | 10 | 18 |
|
Negative | 9 | 8 |
Discussion
In the present study, genomic alterations in 45
gastric cancer samples were detected using target capture and NGS
analysis. Somatic mutations were detected in 82.4% of patients,
indicating that gastric cancer is a highly heterogeneous disease.
C:G to T:A transitions were more common than other
single-nucleotide alterations. It has previously been reported that
N-methyl-N′-nitro-N-nitrosoquanidine and N-nitroso compounds
identified in food are able to induce C to T transitions (32) and are considered to be gastric
carcinogens. These foods such as pickles vegetables and salted meat
are commonly consumed in Chinese populations, increasing the risk
of gastric carcinogenesis (33). The
results of the present study supported previous reports that C to T
transitions are typical of TP53 mutations in gastric
carcinogenesis. Such mutations are considered to be the result of
interactions between underlying genetic factors and environmental
predisposing factors (32).
At the genetic level, TP53 is the most commonly
mutated gene in human cancer (19).
TP53 negatively regulates the cell cycle and induces DNA repair
under environmental pressure, serving as a tumor suppressor gene
(34). It was demonstrated that
mutant TP53 acquires oncogenic properties that are entirely
independent of wild-type TP53 (35,36). TP53
gain-of-function actively contributes toward various stages of
tumor progression whilst also increasing resistance to anticancer
treatments. It may therefore be beneficial to develop molecules
that are able to recover wild-type TP53 activity and remove TP53
gain-of-function mutations from the cell. To this end, a number of
studies have been performed (37–39). It is
challenging to translate these approaches into a clinical setting;
and a more accessible approach may be to target the downstream
pathways mediating mutant TP53 activity. Progress has been made in
this area, and a number of clinically approved drugs that target
MET, EGFR and cholesterol synthesis pathways are already available
(6,40). However, further research is
required.
In the present study, it was demonstrated that TP53
gene mutation was the most frequent genetic change associated with
gastric cancer in a Chinese population, which is consistent with
the results of previous reports (16,41).
According to the TCGA database, TP53 mutations occur in 36.4–55% of
gastric cancer cases (42). In the
present study, TP53 mutations were observed in 42.2% of gastric
cancer cases, which is lower than in patients from Singapore
(41), but similar to patients from
Hong Kong and Japan (22,43). In addition, patients with TP53
mutations had a significantly higher number of mutations overall,
which suggested that the TP53 gene may serve a crucial role in the
maintenance of genome integrity and stability. Zang et al
have used whole-exome sequencing to identify frequent inactivating
mutations in cell adhesion and chromatin remodeling genes in
addition to TP53 mutations (41).
TP53 has been referred to as ‘the guardian of the genome’ (20). Genome instability generates genetic
diversity, and expedites the cell mutations and accumulation of
mutated genes required to initiate tumorigenesis (44). There are conflicting results with
respect to the prevalence of TP53 mutations, as well as their
association with clinicopathological features in gastric cancer
(45). The present study demonstrated
that the patients with a TP53 mutation had less venous invasion and
perineural invasion, as well as less severe lymphoid metastasis
(N3-4). The role of TP53 as a biomarker for predicting prognoses in
gastric cancer was also investigated. The follow-up data
demonstrated that mutations in TP53 were associated with increased
survival, although there was no statistical difference. We
hypothesized that this may be due to the small sample size and
short follow-up time.
TP53, ERBB2, ERBB3, ERBB4, EGFR, MTOR and c-MET gene
alterations are emerging as potential clinical biomarkers (37). Novel somatic gene targets, ARIDLA,
FAT4 and MLL, are of increasing interest and importance (32). In the present study, mutations were
also identified in the histone methyltransferase gene MLL3 (3/45)
and the MLL4 (5/45) gene in gastric cancer samples. MLL genes
encode histone methyltransferases that are essential for the proper
expression of a number of genes (46). It has been suggested that mutational
and expressional alterations of MLL genes are involved in various
human malignancies (47–49). Somatic mutations in the MLL3 gene have
been identified in colorectal cancer (48). Amplification of the MLL4 gene has also
been revealed in glioblastomas and pancreatic cancer (49). Chen et al (16) uncovered mutations in the MLL4 gene
through whole-genome sequencing of two Chinese patients with
gastric cancer. Je et al (50)
indicated that frame shift mutations of MLL, MLL2, MLL3 and MLL5
genes and loss of expression of MLL3 protein are common in gastric
cancer with high microsatellite instability (MSI-H). In the present
study, novel MLL4 frame shift mutations were identified in gastric
cancer. Activating signal cointegrator-2 (ASC-2) complex, named
ASCOM, contains histone H3K4 methyltransferase MLL3 or its paralog
MLL4 (51). Notably, in vivo
and in vitro observations revealed that ASCOM acts as a
crucial TP53 coactivator, and is necessary for H3K4-trimethylation
and the expression of endogenous TP53 target genes in response to
DNA damage (51). These findings
implicated MLL3/4 in the TP53 tumor suppression pathway. Taken
together, these data suggested that MLL gene mutations may
contribute toward oncogenesis in gastric cancer.
In summary, NGS analysis of 1,021 genes in FFPE
tissue specimens from 45 gastric adenocarcinomas was successfully
performed. At least one somatic genomic alteration was detected in
37/45 (82.4%) patients, and somatic mutations in the TP53 gene were
most frequent. Patients with a TP53 mutation had a significantly
higher number of mutations overall, indicating that the TP53 gene
may serve a crucial role in the maintenance of genome integrity and
stability. Tumor heterogeneity is a clear hallmark of gastric
cancer. In the era of precision medicine, further research is
required to investigate intratumoral heterogeneity, and develop an
understanding of the potential mechanisms of tumorigenesis and
potential therapeutic approaches.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81502678), the
‘Jiangsu Provincial Medical Youth Talent’ program of the project of
Invigorating Health Care through Science, Technology Education, and
the Young Talents Program of Jiangsu Cancer Hospital.
Availability of data and materials
The datasets used during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
XP participated in study design, coordination data
analysis and drafted the manuscript. XJ participated in
experimental performance and coordination data analysis. RZ
participated in experimental performance. ZZ, YZ, WP, and NS
participated in study design. XX and LX participated in study
design and reviewed all histopathological diagnoses. PL
participated in coordination data analysis. JL and JT conceived of
the study, participated in its design, revised the manuscript and
made final proof. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Jiangsu Cancer Hospital (Nanjing, China). The
reference number of the ethics approval statement is 2013LS-6. All
patients provided written informed consent to participate in this
study.
Patient consent for publication
All patients provided written informed consent for
publication of any associated data and accompanying images involved
in this manuscript.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hu B, El Hajj N, Sittler S, Lammert N,
Barnes R and Meloni-Ehrig A: Gastric cancer: Classification,
histology and application of molecular pathology. J Gastrointest
Oncol. 3:251–261. 2012.PubMed/NCBI
|
|
3
|
Wong SS, Kim KM, Ting JC, Yu K, Fu J, Liu
S, Cristescu R, Nebozhyn M, Gong L, Yue YG, et al: Genomic
landscape and genetic heterogeneity in gastric adenocarcinoma
revealed by whole-genome sequencing. Nat Commun. 5:54772014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cristescu R, Lee J, Nebozhyn M, Kim KM,
Ting JC, Wong SS, Liu J, Yue YG, Wang J, Yu K, et al: Molecular
analysis of gastric cancer identifies subtypes associated with
distinct clinical outcomes. Nat Med. 21:449–456. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Deng N, Goh LK, Wang H, Das K, Tao J, Tan
IB, Zhang S, Lee M, Wu J, Lim KH, et al: A comprehensive survey of
genomic alterations in gastric cancer reveals systematic patterns
of molecular exclusivity and co-occurrence among distinct
therapeutic targets. Gut. 61:673–684. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wong H and Yau T: Molecular targeted
therapies in advanced gastric cancer: Does tumor histology matter?
Therap Adv Gastroenterol. 6:15–31. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cancer Genome Atlas Research Network, .
Comprehensive molecular characterization of gastric adenocarcinoma.
Nature. 513:202–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim Y and Yeung ES: DNA sequencing with
pulsed-field capillary electrophoresis in poly(ethylene oxide)
matrix. Electrophoresis. 18:2901–2908. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bar-Eli M, Ahuja H, Gonzalez-Cadavid N,
Foti A and Cline MJ: Analysis of N-RAS exon-1 mutations in
myelodysplastic syndromes by polymerase chain reaction and direct
sequencing. Blood. 73:281–283. 1989.PubMed/NCBI
|
|
10
|
Collins SJ, Howard M, Andrews DF, Agura E
and Radich J: Rare occurrence of N-ras point mutations in
Philadelphia chromosome positive chronic myeloid leukemia. Blood.
73:1028–1032. 1989.PubMed/NCBI
|
|
11
|
Gentle A, Anastasopoulos F and McBrien NA:
High-resolution semi-quantitative real-time PCR without the use of
a standard curve. BioTechniques. 31:502, 504–506, 508. 2001.
View Article : Google Scholar
|
|
12
|
Schena M, Shalon D, Heller R, Chai A,
Brown PO and Davis RW: Parallel human genome analysis:
Microarray-based expression monitoring of 1000 genes. Proc Natl
Acad Sci USA. 93:10614–10619. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Meyerson M, Gabriel S and Getz G: Advances
in understanding cancer genomes through second-generation
sequencing. Nat Rev Genet. 11:685–696. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Andrechek ER, Cardiff RD, Chang JT, Gatza
ML, Acharya CR, Potti A and Nevins JR: Genetic heterogeneity of
Myc-induced mammary tumors reflecting diverse phenotypes including
metastatic potential. Proc Natl Acad Sci USA. 106:16387–16392.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin Y, Wu Z, Guo W and Li J: Gene
mutations in gastric cancer: A review of recent next-generation
sequencing studies. Tumour Biol. 36:7385–7394. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen K, Yang D, Li X, Sun B, Song F, Cao
W, Brat DJ, Gao Z, Li H, Liang H, et al: Mutational landscape of
gastric adenocarcinoma in Chinese: Implications for prognosis and
therapy. Proc Natl Acad Sci USA. 112:1107–1112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu Z, Huo X, Ye H, Tang C, Nandakumar V,
Lou F, Zhang D, Dong H, Sun H, Jiang S, et al: Genetic mutation
analysis of human gastric adenocarcinomas using ion torrent
sequencing platform. PLoS One. 9:e1004422014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nadauld LD and Ford JM: Molecular
profiling of gastric cancer: Toward personalized cancer medicine. J
Clin Oncol. 31:838–839. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ozaki T and Nakagawara A: p53: The
attractive tumor suppressor in the cancer research field. J Biomed
Biotechnol. 2011:6039252011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lane DP: Cancer. p53, guardian of the
genome. Nature. 358:15–16. 1992. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bellini MF, Cadamuro AC, Succi M, Proença
MA and Silva AE: Alterations of the TP53 gene in gastric and
esophageal carcinogenesis. J Biomed Biotechnol. 2012:8919612012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tahara T, Shibata T, Okamoto Y, Yamazaki
J, Kawamura T, Horiguchi N, Okubo M, Nakano N, Ishizuka T, Nagasaka
M, et al: Mutation spectrum of TP53 gene predicts
clinicopathological features and survival of gastric cancer.
Oncotarget. 7:42252–42260. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ajani JA, D'Amico TA, Almhanna K, Bentrem
DJ, Chao J, Das P, Denlinger CS, Fanta P, Farjah F, Fuchs CS, et
al: Gastric cancer, version 3.2016, NCCN clinical practice
guidelines in oncology. J Natl Compr Cancer Netw. 14:1286–1312.
2016. View Article : Google Scholar
|
|
24
|
Wang K, Yuen ST, Xu J, Lee SP, Yan HH, Shi
ST, Siu HC, Deng S, Chu KM, Law S, et al: Whole-genome sequencing
and comprehensive molecular profiling identify new driver mutations
in gastric cancer. Nat Genet. 46:573–582. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li H and Durbin R: Fast and accurate short
read alignment with Burrows-Wheeler transform. Bioinformatics.
25:1754–1760. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
1000 Genomes Project Consortium, ;
Abecasis GR, Altshuler D, Auton A, Brooks LD, Durbin RM, Gibbs RA,
Hurles ME and McVean GA: A map of human genome variation from
population-scale sequencing. Nature. 467:1061–1073. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cibulskis K, Lawrence MS, Carter SL,
Sivachenko A, Jaffe D, Sougnez C, Gabriel S, Meyerson M, Lander ES
and Getz G: Sensitive detection of somatic point mutations in
impure and heterogeneous cancer samples. Nat Biotechnol.
31:213–219. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
McKenna A, Hanna M, Banks E, Sivachenko A,
Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly
M and DePristo MA: The genome analysis toolkit: A mapreduce
framework for analyzing next-generation DNA sequencing data. Genome
Res. 20:1297–1303. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang K, Li M and Hakonarson H: ANNOVAR:
Functional annotation of genetic variants from high-throughput
sequencing data. Nucleic Acids Res. 38:e1642010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li J, Lupat R, Amarasinghe KC, Thompson
ER, Doyle MA, Ryland GL, Tothill RW, Halgamuge SK, Campbell IG and
Gorringe KL: CONTRA: Copy number analysis for targeted
resequencing. Bioinformatics. 28:1307–1313. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lim BH, Soong R, Grieu F, Robbins PD,
House AK and Iacopetta BJ: p53 accumulation and mutation are
prognostic indicators of poor survival in human gastric carcinoma.
Int J Cancer. 69:200–204. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tatsuta M, Itoh T, Okuda S, Taniguchi H
and Tamura H: Effect of prolonged administration of gastrin on
experimental carcinogenesis in rat stomach induced by
N-methyl-N'-nitro-N-nitrosoguanidine. Cancer Res. 37:1808–1810.
1977.PubMed/NCBI
|
|
33
|
Lin SH, Li YH, Leung K, Huang CY and Wang
XR: Salt processed food and gastric cancer in a Chinese population.
Asian Pac J Cancer Prev. 15:5293–5298. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lane D and Levine A: p53 Research: The
past thirty years and the next thirty years. Cold Spring Harb
Perspect Biol. 2:a0008932010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Oren M and Rotter V: Mutant p53
gain-of-function in cancer. Cold Spring Harb Perspect Biol.
2:a0011072010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Muller PA and Vousden KH: p53 mutations in
cancer. Nat Cell Biol. 15:2–8. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bykov VJ, Issaeva N, Shilov A, Hultcrantz
M, Pugacheva E, Chumakov P, Bergman J, Wiman KG and Selivanova G:
Restoration of the tumor suppressor function to mutant p53 by a
low-molecular-weight compound. Nat Med. 8:282–288. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bykov VJN, Issaeva N, Zache N, Shilov A,
Hultcrantz M, Bergman J, Selivanova G and Wiman KG: Reactivation of
mutant p53 and induction of apoptosis in human tumor cells by
maleimide analogs. J Biol Chem. 292:196072017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lambert JM, Gorzov P, Veprintsev DB,
Söderqvist M, Segerbäck D, Bergman J, Fersht AR, Hainaut P, Wiman
KG and Bykov VJ: PRIMA-1 reactivates mutant p53 by covalent binding
to the core domain. Cancer Cell. 15:376–388. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zang ZJ, Cutcutache I, Poon SL, Zhang SL,
McPherson JR, Tao J, Rajasegaran V, Heng HL, Deng N, Gan A, et al:
Exome sequencing of gastric adenocarcinoma identifies recurrent
somatic mutations in cell adhesion and chromatin remodeling genes.
Nat Genet. 44:570–574. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang K, Kan J, Yuen ST, Shi ST, Chu KM,
Law S, Chan TL, Kan Z, Chan AS, Tsui WY, et al: Exome sequencing
identifies frequent mutation of ARID1A in molecular subtypes of
gastric cancer. Nat Genet. 43:1219–1223. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fenoglio-Preiser CM, Wang J, Stemmermann
GN and Noffsinger A: TP53 and gastric carcinoma: A review. Hum
Mutat. 21:258–270. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ruthenburg AJ, Allis CD and Wysocka J:
Methylation of lysine 4 on histone H3: Intricacy of writing and
reading a single epigenetic mark. Mol Cell. 25:15–30. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Parsons DW, Li M, Zhang X, Jones S, Leary
RJ, Lin JC, Boca SM, Carter H, Samayoa J, Bettegowda C, et al: The
genetic landscape of the childhood cancer medulloblastoma. Science.
331:435–439. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sjöblom T, Jones S, Wood LD, Parsons DW,
Lin J, Barber TD, Mandelker D, Leary RJ, Ptak J, Silliman N, et al:
The consensus coding sequences of human breast and colorectal
cancers. Science. 314:268–274. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Huntsman DG, Chin SF, Muleris M, Batley
SJ, Collins VP, Wiedemann LM, Aparicio S and Caldas C: MLL2, the
second human homolog of the Drosophila trithorax gene, maps to
19q13.1 and is amplified in solid tumor cell lines. Oncogene.
18:7975–7984. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Je EM and Lee SH, Yoo NJ and Lee SH:
Mutational and expressional analysis of MLL genes in gastric and
colorectal cancers with microsatellite instability. Neoplasma.
60:188–195. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lee J, Kim DH, Lee S, Yang QH, Lee DK, Lee
SK, Roeder RG and Lee JW: A tumor suppressive coactivator complex
of p53 containing ASC-2 and histone H3-lysine-4 methyltransferase
MLL3 or its paralogue MLL4. Proc Natl Acad Sci USA. 106:8513–8518.
2009. View Article : Google Scholar : PubMed/NCBI
|