Introduction
Hepatocellular carcinoma (HCC) is a common
malignancy globally and the third most common cause of
cancer-associated mortality (1,2). Surgery
has become the primary treatment for HCC, with a 5-year overall
survival (OS) rate of 40–50% (3,4).
Furthermore, >50% of patients with HCC are initially diagnosed
with multiple tumors (5), reducing
the probability of receiving successful treatment for cases that
exceed the Barcelona Clinic Liver Cancer (BCLC) staging system for
radical hepatectomy, due to its high recurrence rate following
surgery (6,7); however, a previous study determined that
not all patients with multinodular HCC of an intermediate stage
would experience poor long-term survival following surgical
resection (8). Multiple HCC lesions
may originate differently from multicentric occurrence (MO) or
intrahepatic metastases (IM), which have dissimilar outcomes
(9). The origin of MO-HCC is
different from the primary lesion, while IM-HCC is derived from the
primary tumor. A number of methods and features are used to
differentiate MO-HCC from IM-HCC, including tumor location,
satellite tumors, growth from portal tumor emboli or histological
grading (10,11). In previous studies, patients with
MO-HCC were reported to have an improved outcome compared with
patients with IM-HCC following hepatectomy (12,13); thus,
determining the origin of multifocal HCC may benefit the selection
of patients for radical surgery. Additionally, a number of studies
also determined that adjuvant transcatheter arterial
chemoembolization (TACE) may be beneficial in prolonging the
survival of patients with high-risk HCC, including those with
larger size, multinodular tumors and microvascular invasion
(14–16); however, the current literature does
not indicate the type of multifocal HCC that may benefit from
adjuvant TACE. The present study was conducted to investigate the
survival benefits for patients with MO-HCC or IM-HCC who underwent
liver resection and subsequent adjuvant TACE.
Materials and methods
Selection of patients
A total of 871 patients with
pathologically-confirmed HCC underwent hepatectomy between January
2005 and December 2015 in Hepatopancreatobiliary Surgery Department
I at the Peking University Cancer Hospital and Institute (Beijing,
China). The study design was approved by the Ethical Review Board
Committee of the Beijing Cancer Hospital and Institute (Beijing,
China). A total of 107 patients (12.3%) were pathologically
diagnosed with multinodular HCC. A total of 4 patients were
excluded. Finally, 103 patients were included in the present study,
including 89 men and 14 women, with a median age of 57 years
(range, 25–78 years). According to the aforementioned
clinicopathological determination criteria, 59 cases were included
in the MO group and 44 cases were included in the IM group. In the
MO-HCC subgroup, 22 patients received hepatic resection (HR) only
(MO-HR), and 37 patients received HR plus adjuvant TACE (HRT)
(MO-HRT). The pathological stage of each nodular was reviewed and
recorded in the resected specimens by two senior pathologists in a
blinded manner using the Edmondson-Steiner staging method (6). The demographic, surgical, pathological
and survival data of all patients were collected and analyzed. The
inclusion criterion included the multinodular lesions being
pathologically confirmed as HCC. The exclusion criteria included
the following: i) Mixed HCC or cholangiocellular carcinoma; ii)
emergence of extrahepatic metastasis; iii) no R0 resection; and iv)
existence of another type of primary tumor.
Differentiation criteria for MO and IM
of multinodular HCC
A number of studies have examined the differences
between IM and MO (10–12,17). The
differentiation criteria described in the present study are based
on the Liver Cancer Study Group criteria (Japanese Society of HCC
criteria) (18). IM-HCC is defined
based on the following: i) Tumor cases that appear to have
developed from or on the basis of portal tumor emboli; ii) a large
primary tumor with multiple satellite nodules; and iii) all tumors
are histologically similar. MO-HCC is defined based on the
following: i) Each tumor occurs separately in a different hepatic
segment; and ii) the multiple tumors have different histological
grading of well-differentiated and moderately or poorly
differentiated HCC (pathological heterogeneity) (10,11,19).
Surgery
Tumor cases were confirmed using contrast-enhanced
computed tomography (CT) or magnetic resonance imaging with
vascular contrast agents. A number of patients were also assessed
using contrast-enhanced ultrasonography. Liver function tests were
performed prior to the surgery, including tests for albumin,
bilirubin, blood coagulation function and 15-min indocyanine green
clearance. Only patients with well-preserved liver function
(Child-Pugh grade A) and good performance status, with an estimated
residual liver volume >40%, underwent HR. Patients with
hepatitis B infection were treated with antiviral drugs at least 1
week prior to surgery. Liver transections were primarily performed
using the clamp method with a Peng multifunctional operative
dissector [Hangzhou Shuyou Medical Instrument Co., Ltd., Hangzhou,
China; FDA catalog no. 510(K), K040780]. An intermittent Pringle
maneuver was used during liver transection. Vascular invasion was
diagnosed if vascular involvement or tumor invasion was confirmed
by imaging or pathological studies. Hepatic resection was performed
using anatomical liver resection or partial liver resection.
Resection was considered as major when ≥3 liver segments were
removed and as minor when <3 liver segments or partial liver
parenchyma were removed.
Postsurgical outcomes and
follow-up
Postsurgical mortality was defined as mortality
within 30 days of surgery. Grade III or higher adverse events were
considered major complications, while Grade I–II adverse events
were defined as minor complications. Postsurgical hepatic
insufficiency was defined according to the International Study
Group of Liver Surgery consensus (20).
Contrast-enhanced CT or magnetic resonance imaging,
chest radiography, liver function tests and measurements of serum
α-fetoprotein levels were performed 4 weeks after surgery, and
every 3 months thereafter. Tumor recurrences were treated with
liver resection, radiofrequency ablation or TACE. Only 13 patients
did not receive the treatment following recurrence due to a fast
recurrence of the tumor (within 3 months) or severe liver cirrhosis
and liver failure.
Adjuvant TACE
Adjuvant TACE (2 cycles) was suggested to all
patients with multinodular HCC by the attending physician ~4 weeks
after surgery, when the liver function had recovered. Whether
patients followed the recommendations of the physician primarily
depended on their socioeconomic status or wishes; therefore,
adjuvant TACE was not performed in all patients, with those who
refused receiving hepatic resection only. The Seldinger technique
was performed to place a hepatic arterial catheter into the proper
hepatic artery via the femoral artery, with the patient under local
anesthesia. Hepatic angiography or CT angiography was performed to
detect any notable tumor stains in the remnant liver. Oxaliplatin
(150 mg) and leucovorin (150 mg) were infused, and fluorouracil
(1,500 mg/m2) was continuously pumped (for 24 h) through
the catheter. The dosage was determined by the body surface area
and underlying liver function. At the 1-month follow-up, a CT scan
was obtained to determine the effects of TACE.
Study endpoints
The primary endpoint of the study was to evaluate 1,
3 and 5-year OS and disease-free survival (DFS) rates in the IM and
MO groups. The secondary endpoint was to evaluate the OS benefits
and safety of hepatectomy plus postsurgical adjuvant TACE. The
tertiary endpoint was to identify whether tumor number was a
prognostic factor affecting the staging and long-term outcomes of
multinodular HCC.
Statistical analysis
Continuous variables are presented as the mean ±
standard deviations or medians with interquartile ranges, and
discreet variables are presented as numbers with percentages.
Categorical variables were compared using the χ2 test,
and continuous variables were compared using a Student's t-test or
non-parametric Mann-Whitney U test. Survival rates were obtained by
the Kaplan-Meier method and were compared using the log-rank test.
OS and DFS were calculated from the date of hepatectomy to the time
of mortality/recurrence or the last time of follow-up. Variables
that were statistically significant in the univariate analysis
(P<0.05) were included in the multivariate analysis using a Cox
proportional hazards model. All patients were followed up until
mortality or until June 1, 2016. P<0.05 was considered to
indicate a statistically significant difference. The analysis was
performed using SPSS version 21.0 statistical software (IBM Corp.,
Armonk, NY, USA).
Results
Grouping based on clinicopathological
features
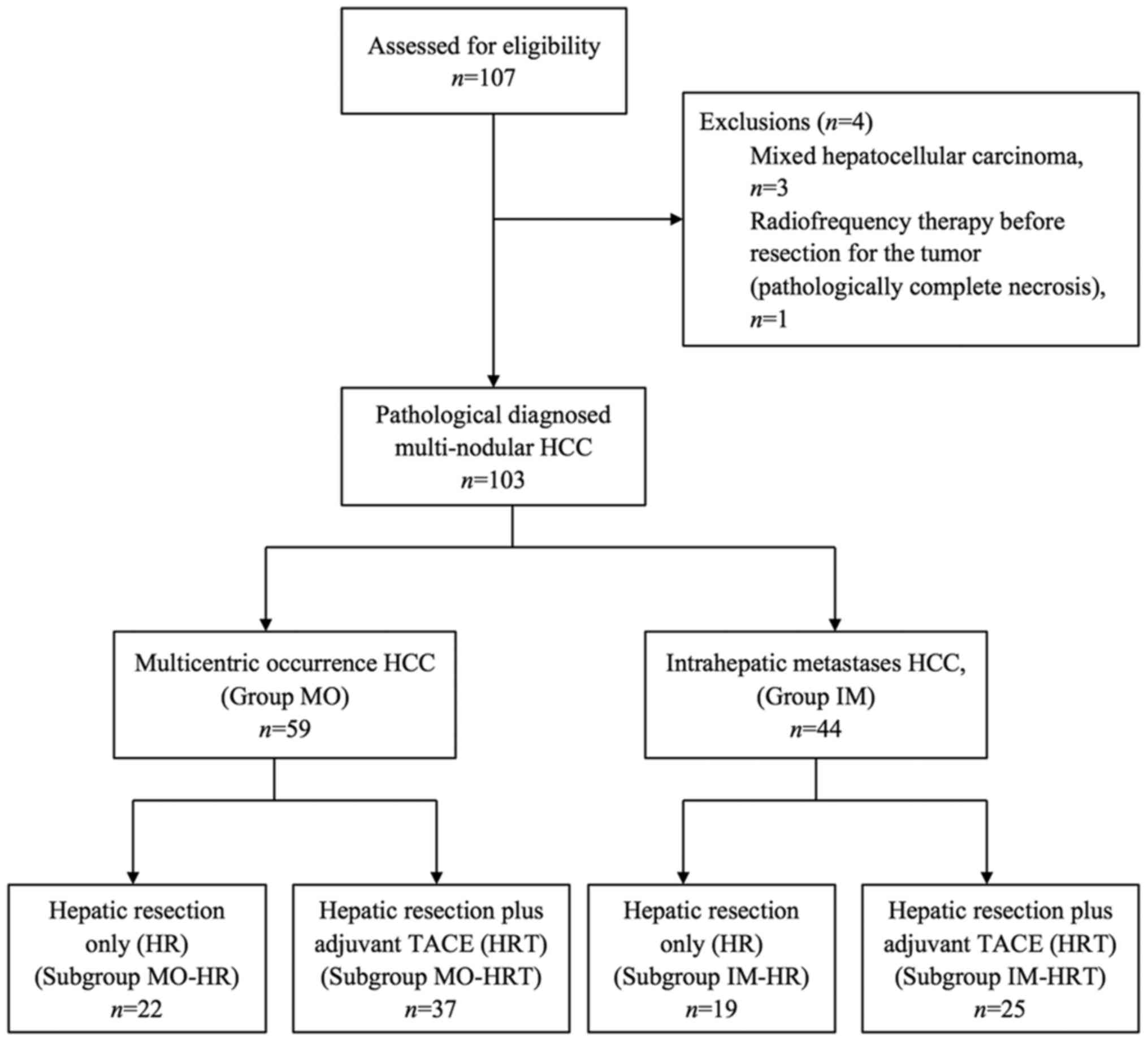
The flowchart for the present study is depicted in
Fig. 1. In total, 107 patients with
multinodular tumors who underwent liver resection with or without
adjuvant TACE were evaluated. A total of 4 patients were excluded,
including 3 patients with mixed HCC and 1 patient who received
radiofrequency therapy prior to tumor resection; subsequently, the
tumor was pathologically demonstrated to have undergone complete
necrosis. None of the patients were lost to follow-up, thus 103
patients were included in the present study, including 89 men and
14 women, with a median age of 57 years (range, 25–78 years).
According to the aforementioned clinicopathological determination
criteria, 59 cases were included in the MO group and 44 cases were
included in the IM group for further analysis.
Clinicopathological and surgical
features of the patients
For further analysis, the clinicopathological
variables of the two groups were investigated (Table I). Tumor size (the largest tumor in
one patient) (P=0.008), portal tumor emboli (P=0.031), pathological
heterogeneity (P=0.002), location of segment and satellite tumors
(P<0.001) differed significantly between the two groups. The
surgical outcomes and postsurgical treatments are presented in
Table II. There were no significant
differences in surgical time, surgical blood loss or type of
surgery between the two groups. Surgical morbidities, including
hepatic insufficiency, ascites and biliary fistula, and the
proportion of patients receiving adjuvant therapy were also similar
between the two groups.
 | Table I.Patient demographic and
clinicopathological characteristics. |
Table I.
Patient demographic and
clinicopathological characteristics.
|
Characteristics | MO-HCC (n=59) | IM-HCC (n=44) | P-value |
|---|
| Age, years |
|
| 0.245 |
| Mean ±
SD | 57.05±8.39 | 54.84±10.81 |
|
| Sex, n (%) |
|
| 0.773 |
|
Male | 50 (84.7) | 39 (88.6) |
|
|
Female | 9
(15.3) | 5 (11.4) |
|
| Albumin, g/l |
|
| 0.529 |
| Mean ±
SD | 44.31±3.74 | 43.80±4.44 |
|
| Total bilirubin,
µmol/l |
|
| 0.991 |
| Median
(IQR) | 14.80 (8.30) | 14.80 (7.95) |
|
| Platelet count,
×109/l |
|
| 0.736 |
| Median
(IQR) | 126 (60) | 130 (67.75) |
|
| Prothrombin time,
s |
|
| 0.472 |
| Median
(IQR) | 11.90 (1.30) | 11.65 (1.15) |
|
| Liver cirrhosis, n
(%) |
|
| 1.000 |
|
Yes | 57 (96.6) | 42 (95.5) |
|
| No | 2 (3.4) | 2 (4.5) |
|
| Underlying
hepatitis, n (%) |
|
| 0.223 |
|
HBV | 50 (84.7) | 40 (90.9) |
|
|
HCV | 5 (8.5) | 0 (0.0) |
|
|
Both | 3 (5.1) | 2 (4.5) |
|
|
None | 1 (1.7) | 2 (4.5) |
|
| Tumor number, n
(%) |
|
|
<0.001a |
| 2 | 52 (88.1) | 21 (47.7) |
|
|
>2 | 7 (11.9) | 23 (52.3) |
|
| Tumor size (largest
tumor), mm |
|
| 0.008a |
| Median
(IQR) | 38.0 (38.0) | 56.5 (43.0) |
|
| Tumor size (second
largest tumor), mm |
|
| 0.328 |
| Median
(IQR) | 15.0 (20.0) | 12.0 (15.0) |
|
| AFP, ng/ml |
|
| 0.622 |
| Median
(IQR) | 21.83 (306.95) | 35.35 (686.17) |
|
| Microvascular
invasion, n (%) |
|
| 0.051 |
|
Yes | 13 (22.0) | 18 (40.9) |
|
| No | 46 (78.0) | 26 (59.1) |
|
| Portal tumor
emboli, n (%) |
|
| 0.031a |
|
Yes | 0 (0.0) | 4 (9.1) |
|
| No | 59
(100.0) | 40 (90.9) |
|
| Edmonson grade,
n |
|
| 0.866 |
| I | 6 | 4 |
|
| II | 37 | 30 |
|
|
III | 16 | 10 |
|
| Pathological
heterogeneity, n (%) |
|
| 0.002a |
|
Yes | 40 (81.6) | 41 (93.2) |
|
| No | 19 (18.4) | 3 (6.8) |
|
| Location of
segment, n (%) |
|
|
<0.001a |
|
Same | 10 (16.9) | 32 (72.7) |
|
|
Different | 49 (83.1) | 12 (27.2) |
|
| Satellite tumors, n
(%) |
|
|
<0.001a |
|
Yes | 58 (98.3) | 22 (50.0) |
|
| No | 1 (1.7) | 22 (50.0) |
|
 | Table II.Surgical and postsurgical
treatment. |
Table II.
Surgical and postsurgical
treatment.
| Variables | MO (n=59) | IM (n=44) | P-value |
|---|
| Surgical time,
min |
|
| 0.393 |
| Mean ±
SD | 167.1±50.1 | 176.1±8.5 |
|
| Surgical blood
loss, ml |
|
| 0.332 |
| Median
(IQR) | 200 (300) | 200 (287.5) |
|
| Type of surgery, n
(%) |
|
| 0.064 |
|
Minor | 42 (71.2) | 23 (52.3) |
|
|
Major | 17 (28.8) | 21 (47.7) |
|
| Surgical
morbidities, n (%)a | 8 (13.55) | 8 (18.2) | 0.827 |
| Hepatic
insufficiency | 5 (8.5) | 7 (15.9) | 0.353 |
| Biliary
fistula | 3 (5.1) | 1 (2.3) | 0.634 |
| Adjuvant TACE, n
(%) |
|
| 0.684 |
|
Yes | 37 (62.7) | 25 (56.8) |
|
| No | 22 (37.3) | 19 (43.2) |
|
Survival analysis
The median duration of follow-up was 34 months
(range, 5–123 months) for the MO group and 25 months (range, 3–49
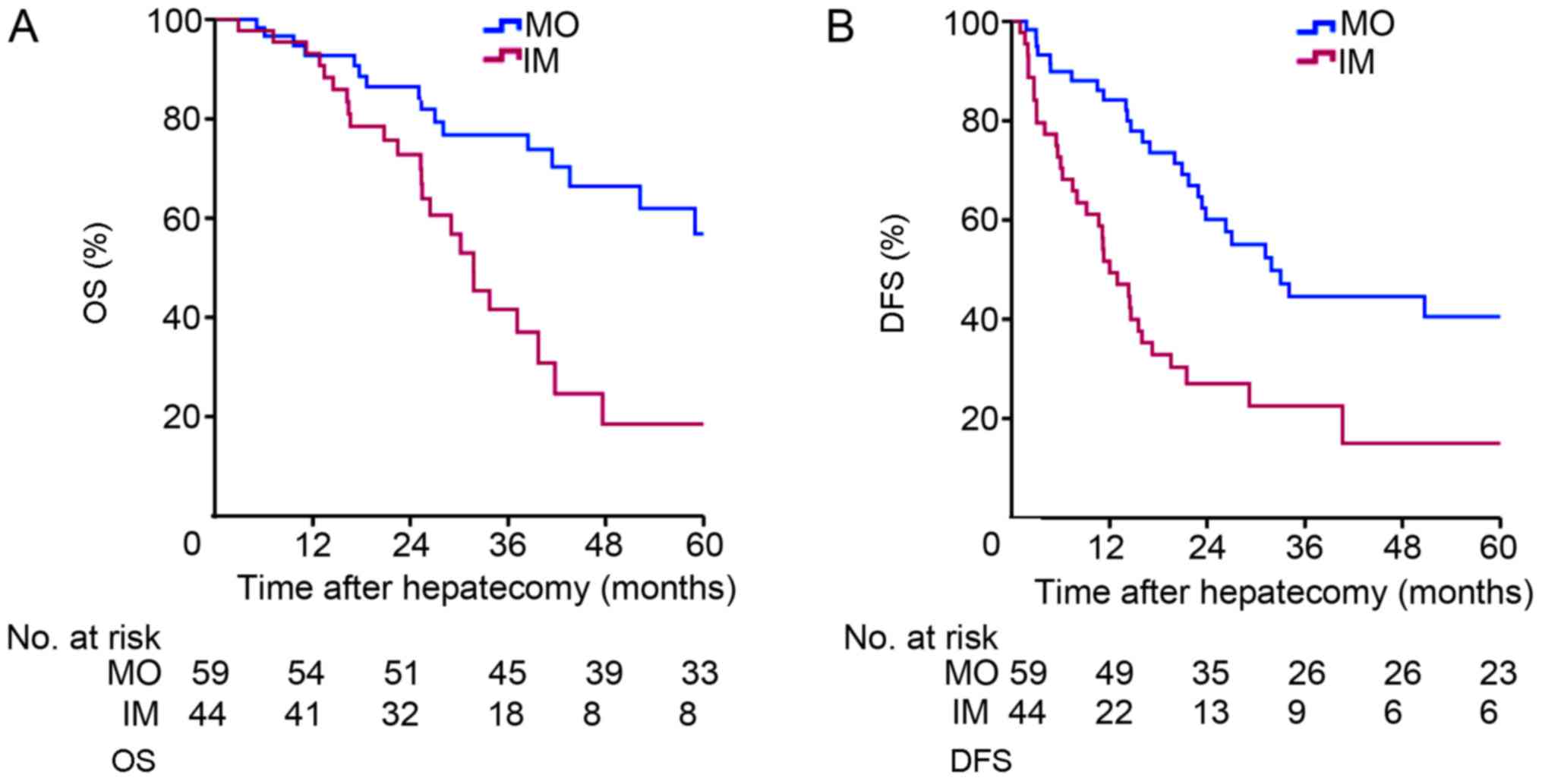
months) for the IM group. The Kaplan-Meier survival curves for the
two groups are depicted in Fig. 2.
The 1-, 3- and 5-year OS rates were 92.7, 76.8 and 56.8%,
respectively, for the MO group, and 93.1, 41.6 and 18.5%,
respectively, for the IM group (P=0.001; Fig. 2A). The 1-, 3- and 5-year DFS rates
were 84.1, 44.6 and 40.5%, respectively, for the MO group, and
51.7, 22.5 and 15.0%, respectively, for the IM group (P<0.001;
Fig. 2B). There was a significant
difference between the two groups in terms of DFS and OS.
Multivariate Cox proportional hazards regression analyses
identified >2 tumors, no adjuvant TACE and IM-HCC as independent
prognostic factors for OS in patients with multifocal HCC (Table III).
 | Table III.Univariate and multivariate Cox
proportional hazards regression analyses of factors associated with
overall survival of patients (n=103) with multinodular
hepatocellular carcinoma. |
Table III.
Univariate and multivariate Cox
proportional hazards regression analyses of factors associated with
overall survival of patients (n=103) with multinodular
hepatocellular carcinoma.
|
| Univariate | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factor | P-value | HR (95% CI) | P-value |
|---|
| Tumor number
(2/>2) |
<0.001a | 2.985
(1.425–6.251) | 0.004a |
| Tumor size
(<50/≥50 mm) | 0.215 |
|
|
| AFP (<100/≥100
ng/ml) | 0.144 |
|
|
| Microvascular
invasion (no/yes) | 0.102 |
|
|
| Portal tumor emboli
(no/yes) | 0.639 |
|
|
| Liver cirrhosis
(no/yes) | 0.652 |
|
|
| MO/IM |
<0.001a | 2.311
(1.087–4.914) | 0.031a |
| Adjuvant TACE
(no/yes) |
<0.001a | 0.331
(0.163–0.676) | 0.002a |
Subgroup analysis by treatment
method
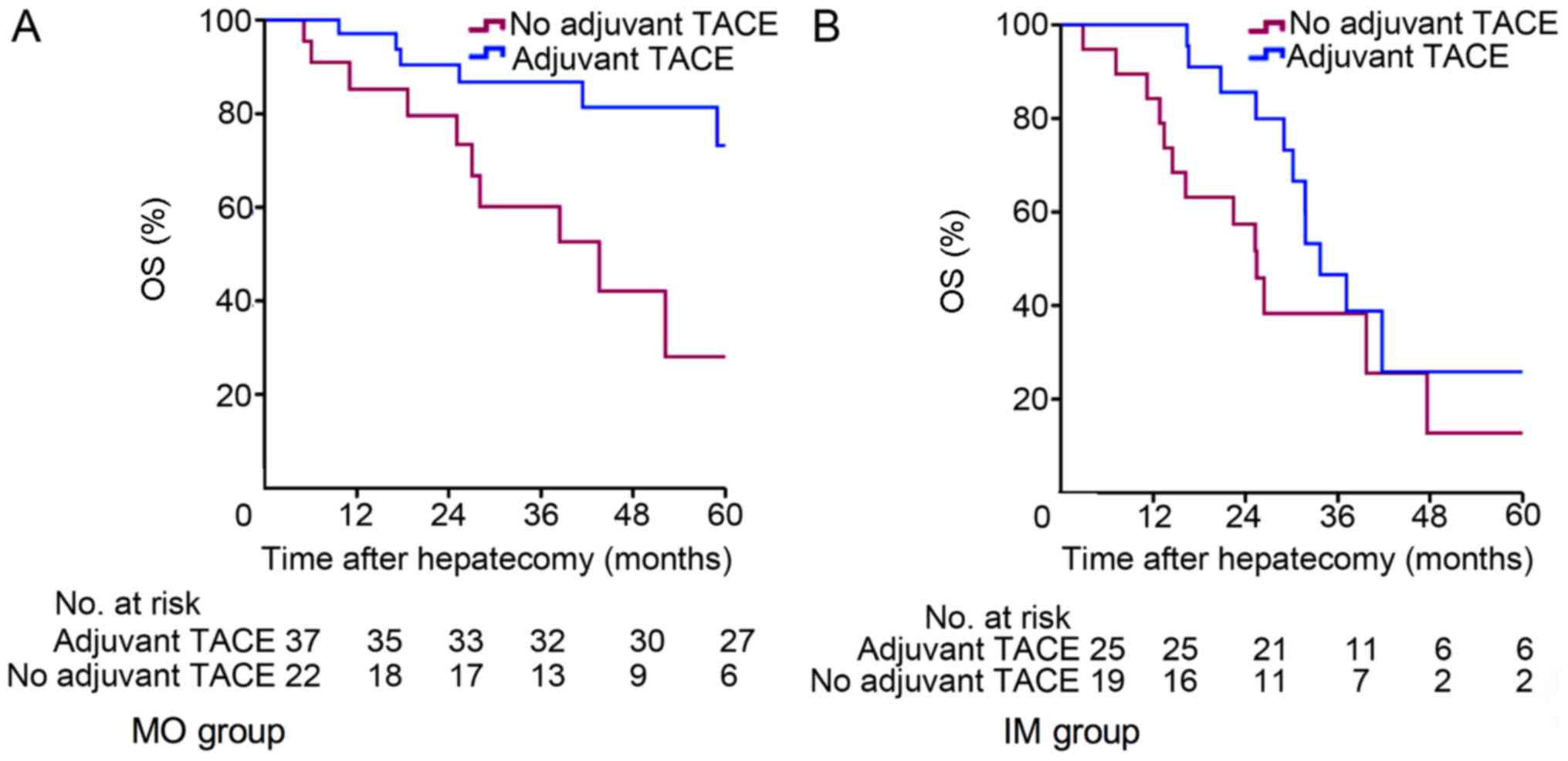
Patients with multifocal HCC were further classified
according to whether they underwent HR only or HRT. In the MO-HCC
subgroup, 22 patients received HR only (MO-HR), and 37 patients
received HRT (MO-HRT). The Kaplan-Meier survival curves for the
patients are depicted in Fig. 3. The
1-, 3- and 5-year OS rates were 97.1, 86.8 and 73.2%, respectively,
for the MO-HRT group, and 85.2, 60.1 and 28.0%, respectively, for
the MO-HR group (P=0.019; Fig. 3A),
and there was a significant difference between the two groups.
Multivariate Cox proportional hazards regression analyses
identified no adjuvant TACE as an independent prognostic factor for
OS in patients with MO-HCC (Table
IV). In the IM-HCC subgroup, 19 patients received HR only
(IM-HR), and 25 patients received HRT (IM-HRT). The 1-, 3- and
5-year OS rates were 100.0, 46.6 and 25.9%, respectively, for the
IM-HRT group, and 84.2, 38.3 and 25.5%, respectively, for the IM-HR
group (P=0.132; Fig. 3B), with no
significant difference between the two groups.
 | Table IV.Univariate and multivariate Cox
proportional hazards regression analyses of factors associated with
overall survival of patients (n=59) with multicentric occurrence
hepatocellular carcinoma. |
Table IV.
Univariate and multivariate Cox
proportional hazards regression analyses of factors associated with
overall survival of patients (n=59) with multicentric occurrence
hepatocellular carcinoma.
|
| Univariate | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factor | P-value | HR (95% CI) | P-value |
|---|
| Tumor number
(2/>2) | 0.264 |
|
|
| Tumor size
(<50/≥50 mm) | 0.307 |
|
|
| AFP (<100/≥100
ng/ml) | 0.371 |
|
|
| Microvascular
invasion (no/yes) | 0.200 |
|
|
| Liver cirrhosis
(no/yes) | 0.698 |
|
|
| Adjuvant TACE
(no/yes) | 0.026a | 0.300
(0.108–0.833) | 0.021a |
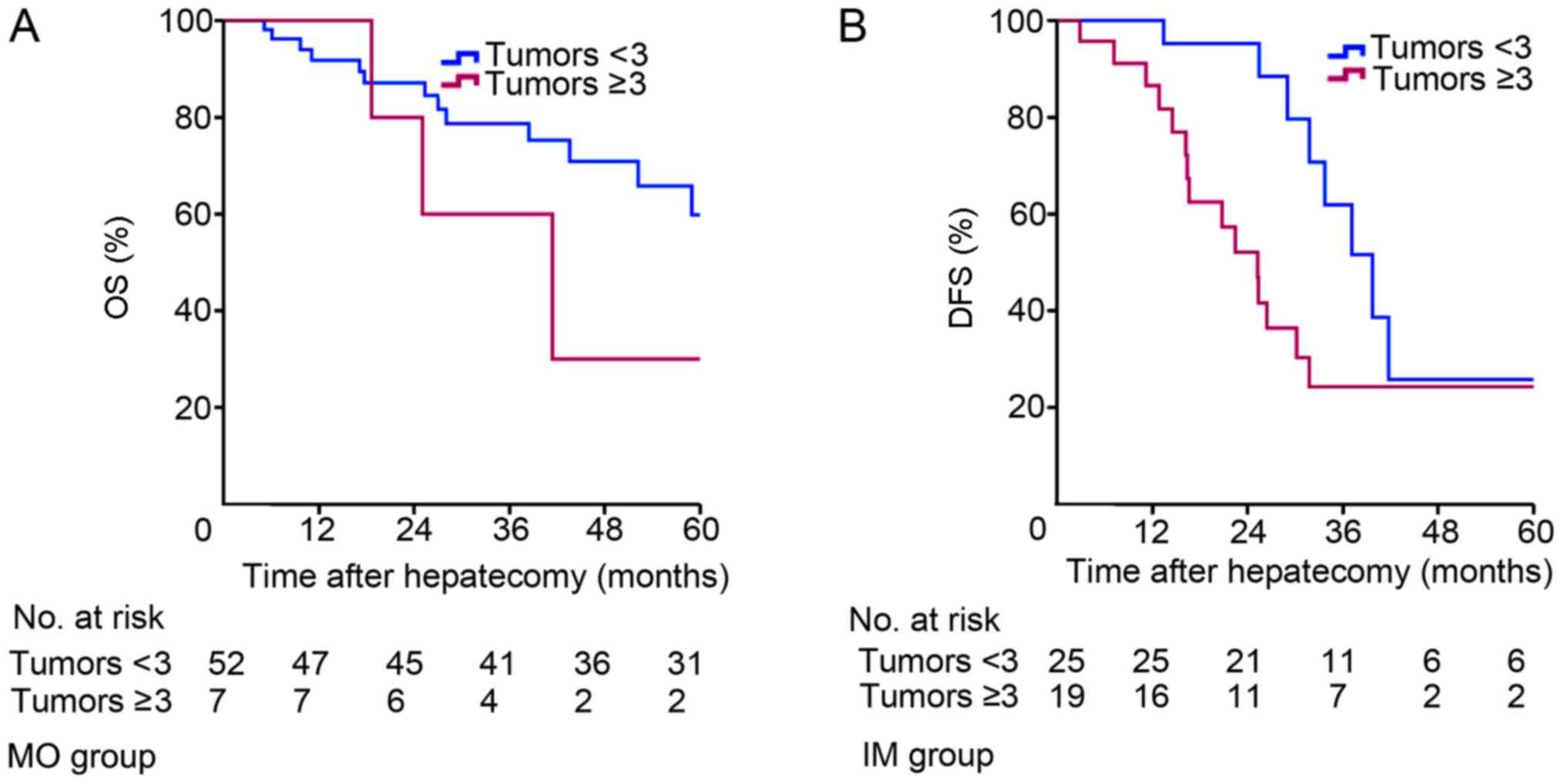
Subgroup analysis by tumor number
In order to verify whether tumor number is a
prognostic factor affecting the staging and treatment of
multinodular HCC, the patients were classified according to whether
they had <3 or ≥3 tumors (Table
V). The Kaplan-Meier survival curves for the patients are
depicted in Fig. 4. In the MO-HCC
subgroup, 52 patients had <3 tumors and 7 patients had ≥3
tumors. The 1-, 3- and 5-year OS rates were 100.0, 60.0 and 30.0%,
respectively, for the MO-HCC subgroup with <3 tumors, and 91.8,
78.7 and 59.8%, respectively, for the MO-HCC subgroup with ≥3
tumors (P=0.300; Fig. 4A). There was
no significant difference between the two groups. Additionally, 21
patients with IM-HCC had <3 tumors and 23 patients with IM-HCC
had ≥3 tumors. The 1-, 3- and 5-year OS rates were 95.2, 88.4 and
25.8%, respectively, for the IM-HCC subgroup with <3 tumors, and
86.5, 52.1 and 24.3%, respectively, for patients with ≥3 tumors
(P=0.022; Fig. 4B). There was a
significant difference between the two groups.
 | Table V.Subgroup analysis classified by tumor
number. |
Table V.
Subgroup analysis classified by tumor
number.
| Tumor number | MO (n=59) | IM (n=44) | P-value |
|---|
| 2 lesions | 52 | 21 |
<0.001a |
| >2 lesions | 7 | 23 |
|
| 3 lesions | 5 | 8 |
|
| 4 lesions | 1 | 6 |
|
| 5 lesions | 1 | 8 |
|
| 6 lesions | 0 | 1 |
|
Discussion
Multifocal tumors are common in HCC (21,22). A
previous survey demonstrated that approximately half of all
patients with HCC are diagnosed with multiple lesions (23). Despite advances in resection and
ablation techniques, the recurrence rate following initial
treatment remains high, and the prognosis of patients with
multifocal HCC following surgical resection is generally
unfavorable (24,25). According to BCLC staging, palliative
treatment, including TACE, in the only appropriate treatment option
for the majority of patients with multifocal HCC, with a median
survival time of <20 months and a 5-year survival rate of 6–20%
(26).
Currently, it is accepted that multifocal HCC may be
classified into two types: IM and MO (27,28).
IM-HCC is primarily considered as a metastatic lesion from the
central tumor; therefore, tumor cases are frequently at an advanced
stage, and the prognosis is generally unfavorable. By contrast,
MO-HCC tumors are derived independently from each other, resulting
in an improved prognosis compared with that of metastatic disease.
Therefore, it is important to distinguish between these two tumor
types. As early as the 1990s, surgeons distinguished IM-HCC from
MO-HCC primarily using clinicopathological features (13,17,29). In
the present study, patients were classified with multifocal HCC
according to the Japanese Society of HCC criteria, and it was
determined that 57% were patients with MO-HCC, which is similar to
the results of a previous study (13). Portal tumor emboli, pathological
heterogeneity, location of segments and satellite tumors differed
significantly between the two groups, which is consistent with the
differentiation criteria for IM and MO. Previously, a number of
other approaches have been developed to differentiate between these
two HCC types, including profiling of integrated hepatitis B virus
(HBV) DNA by polymerase chain reaction and southern blotting, loss
of heterozygosity analysis of specific microsatellite loci and
next-generation sequencing (28,30,31). In a
previous study, seven candidate genes with notable differential
expression in 2 patients were selected and validation studies were
performed using paired tumor/non-tumor tissues from 174 patients
with HBV-HCC. Subsequently, the expression of threonine and
tyrosine kinase was identified as a novel adverse prognostic factor
of HBV-HCC (28). Furthermore,
clinicopathological features are the most convenient method to
distinguish between the two types of multifocal HCC. Using this
criteria, it was determined that patients with MO-HCC have an
improved benefit (5-year OS rate of 56.8% and a DFS rate of 40.5%)
from liver resection compared with that of patients with IM-HCC
(5-year OS rate of 18.5% and a DFS rate of 15.0%). Multivariate
analyses identified IM-HCC as an independent prognostic factor for
OS in patients with multifocal HCC. Additionally, surgery for IM
and MO was not associated with increased surgical time or blood
loss. All surgical morbidities were Clavien-Dindo grade I and II,
indicating that surgical resection for multinodular HCC is a safe
treatment option. This result is consistent with that of a previous
study (32).
Of the patients with HCC, ~70% experience recurrence
within 5 years; therefore, reducing the rate of postsurgical
recurrence is a key factor in prolonging long-term survival in
patients with HCC (33,34). Currently, the accepted method for
reducing the recurrence rate of cancer following surgery is
adjuvant therapy; however, adjuvant TACE therapy is not recommended
for HCC following radical surgery according to the previous
guidelines (6,7), as not all patients benefit from it.
Previously, a number of studies indicated that adjuvant
postsurgical TACE can reduce the postsurgical recurrence rate in
high-risk patients with HCC (35–38).
Although a randomized controlled trial indicated that postsurgical
adjuvant therapy had a minimal effect on outcomes, it was probable
that patients with early-stage HCC were included in the study
(39). In the present study, it was
determined that adjuvant TACE was an independent prognostic factor
for OS in patients with multifocal HCC; however, it remains unknown
if the MO and IM groups can benefit from adjuvant TACE therapy
following surgery. A further subgroup analysis demonstrated that
postsurgical adjuvant TACE significantly prolonged long-term
survival in patients with MO-HCC; however, OS was not significantly
prolonged in patients with IM-HCC who underwent adjuvant TACE.
Based on these results, patients with MO-HCC should be actively
treated with adjuvant TACE to maximize the benefit of surgery. By
contrast, since the prognosis of patients with IM-HCC was
significantly worse following hepatectomy, postsurgical adjuvant
TACE did not result in the same survival benefit for patients with
IM-HCC as it did for patients with MO-HCC. Thus, adjuvant TACE may
be more beneficial for patients with MO-HCC.
Until now, the BCLC staging system and American
Association for the Study of Liver Disease/European Association for
the Study of the Liver guidelines classified patients with >3
tumors as stage B (6,7). TACE is recommended for these patients as
the first-line treatment; however, in the present study, it was
determined that the prognosis of patients with MO-HCC with ≥3
tumors was not worse compared with that of patients with <3
tumors following hepatectomy. For the patients with IM-HCC, an
increased tumor number indicated a worse prognosis. A previous
study demonstrated that the long-term survival rate of patients
with MO-HCC following surgical resection was similar to that of
patients with single lesions (10).
Furthermore, another study indicated that resection may be the
treatment of choice for HCC even if patients have >4 tumors
(40). It is probable that single
lesions in MO-HCC originate independently from each other with
early stage grading; therefore, even if >3 tumors are present,
surgical resection is suitable for these patients. However, the
lesions are metastases from one lesion in IM-HCC, which are similar
to distant metastasis; therefore, an increased number of tumors is
indicative of a later stage. Although the study sample was small,
the present results indicated that HCC staging should not rely on
tumor status alone, and that IM and MO carcinogenesis should also
be taken into account. Furthermore, tumor number should not be the
primary factor considered when selecting the MO-HCC treatment.
There are a number of limitations to the present
study. Firstly, since multinodular HCC with >3 tumors or with 2
tumors, 1 of which is >3 cm, are classified as BCLC stage B, it
was controversial to perform surgery. Thus, a limited number of
these patients were included in the study, although no less than
the number included in previous studies (12,13).
Larger cohort studies may be necessary to confirm the results.
Secondly, the patients included in the present study were patients
with HCC in China, and 92.2% of patients had HBV-associated HCC. In
western countries, the most common causes of HCC are hepatitis C
and alcohol (6). The different
etiologies may cause HCC origin differences, and thus affect the
ratio of IM and MO. To confirm the conclusions of the present
study, further multicenter perspective studies should be performed
with a larger cohort.
In conclusion, the present data demonstrated that
patients with MO-HCC benefit from HRT, while the same treatment has
a minimal effect on the survival of patients with IM-HCC.
Accordingly, liver resection plus TACE is recommended for MO-HCC,
and TACE, targeted drugs or other palliative treatment should be
considered for IM-HCC. Prospective multicenter studies with a
larger sample are required in the future to further confirm the
present results.
Acknowledgements
Not applicable.
Funding
This study was funded by grants from the Chinese
State Key Project for Basic Research (973) (no. 2014CBA02001).
Availability of data and materials
The datasets used or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DX, XL and BX participated in the design of the
study. DX and LW collected the data. DX and XL analyzed and
interpreted the data. DX, XL and BX prepared the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Beijing Cancer Hospital.
Patient consent for publication
Informed consent statement: Patients were not
required to give informed consent to the study, as the analysis
used anonymous clinical data that were obtained after each patient
agreed to treatment by verbal consent. Individuals cannot be
identified according to the data presented.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Maluccio M and Covey A: Recent progress in
understanding, diagnosing, and treating hepatocellular carcinoma.
CA Cancer J Clin. 62:394–399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhong JH, Ke Y, Gong WF, Xiang BD, Ma L,
Ye XP, Peng T, Xie GS and Li LQ: Hepatic resection associated with
good survival for selected patients with intermediate and
advanced-stage hepatocellular carcinoma. Ann Surg. 260:329–340.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Torzilli G, Belghiti J, Kokudo N, Takayama
T, Capussotti L, Nuzzo G, Vauthey JN, Choti MA, De Santibanes E,
Donadon M, et al: A snapshot of the effective indications and
results of surgery for hepatocellular carcinoma in tertiary
referral centers: Is it adherent to the EASL/AASLD
recommendations?: An observational study of the HCC East-West study
group. Ann Surg. 257:929–937. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hsu CY, Liu PH, Hsia CY, Lee YH, Nagaria
TS, Lee RC, Lin HC and Huo TI: Surgical resection is better than
transarterial chemoembolization for patients with hepatocellular
carcinoma beyond the milan criteria: A prognostic nomogram study.
Ann Surg Oncol. 23:994–1002. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
European Association For The Study Of The
Liver, ; European Organisation For Research And Treatment Of
Cancer, . EASL-EORTC clinical practice guidelines: Management of
hepatocellular carcinoma. J Hepatol. 56:908–943. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bruix J and Sherman M; American
Association for the Study of Liver Diseases, : Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kudo M, Izumi N, Kokudo N, Matsui O,
Sakamoto M, Nakashima O, Kojiro M and Makuuchi M; HCC Expert Panel
of Japan Society of Hepatology, : Management of hepatocellular
carcinoma in Japan: Consensus-based clinical practice guidelines
proposed by the Japan society of hepatology (JSH) 2010 updated
version. Dig Dis. 29:339–364. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Feo F and Pascale RM: Multifocal
hepatocellular carcinoma: Intrahepatic metastasis or multicentric
carcinogenesis? Ann Transl Med. 3(4)2015.PubMed/NCBI
|
|
10
|
Yasui M, Harada A, Nonami T, Takeuchi Y,
Taniguchi K, Nakao A and Takagi H: Potentially multicentric
hepatocellular carcinoma: Clinicopathologic characteristics and
postoperative prognosis. World J Surg. 21:860–865. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shimada M, Hamatsu T, Yamashita Y,
Rikimaru T, Taguchi K, Utsunomiya T, Shirabe K and Sugimachi K:
Characteristics of multicentric hepatocellular carcinomas:
Comparison with intrahepatic metastasis. World J Surg. 25:991–995.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang J, Li Q, Sun Y, Zheng H, Cui Y, Li H,
Zhou H and Hao X: Clinicopathologic features between multicentric
occurence and intrahepatic metastasis of multiple hepatocellular
carcinomas related to HBV. Surg Oncol. 18:25–30. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li SL, Su M, Peng T, Xiao KY, Shang LM, Xu
BH, Su ZX, Ye XP, Peng N, Qin QL, et al: Clinicopathologic
characteristics and prognoses for multicentric occurrence and
intrahepatic metastasis in synchronous multinodular hepatocellular
carcinoma patients. Asian Pac J Cancer Prev. 14:217–223. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun JJ, Wang K, Zhang CZ, Guo WX, Shi J,
Cong WM, Wu MC, Lau WY and Cheng SQ: Postoperative adjuvant
transcatheter arterial chemoembolization after R0 hepatectomy
improves outcomes of patients who have hepatocellular carcinoma
with microvascular invasion. Ann Surg Oncol. 23:1344–1351. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dong ZR, Zhang PF, Wang CH, Zhang C, Cai
JB, Shi GM, Ke AW, Sun HC, Qiu SJ, Zhou J and Fan J: Postoperative
adjuvant transcatheter arterial chemoembolization for resectable
multiple hepatocellular carcinoma beyond the Milan criteria: A
retrospective analysis. Am J Cancer Res. 5:450–457. 2014.PubMed/NCBI
|
|
16
|
Liu C, Sun L, Xu J and Zhao Y: Clinical
efficacy of postoperative adjuvant transcatheter arterial
chemoembolization on hepatocellular carcinoma. World J Surg Oncol.
14:1002016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takenaka K, Adachi E, Nishizaki T,
Hiroshige K, Ikeda T, Tsuneyoshi M and Sugimachi K: Possible
multicentric occurrence of hepatocellular carcinoma: A
clinicopathological study. Hepatology. 19:889–894. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liver Cancer Study Group of Japan, .
Primary liver cancer in Japan. Clinicopathologic features and
results of surgical treatment. Ann Surg. 211:277–287.
1990.PubMed/NCBI
|
|
19
|
Matsumoto Y, Fujii H, Matsuda M and Kono
H: Multicentric occurrence of hepatocellular carcinoma: Diagnosis
and clinical significance. J Hepatobiliary Pancreat Surg.
8:435–440. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rahbari NN, Garden OJ, Padbury R,
Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo
RP, Christophi C, et al: Posthepatectomy liver failure: A
definition and grading by the International Study Group of Liver
Surgery (ISGLS). Surgery. 149:713–724. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Oikawa T, Ojima H, Yamasaki S, Takayama T,
Hirohashi S and Sakamoto M: Multistep and multicentric development
of hepatocellular carcinoma: Histological analysis of 980 resected
nodules. J Hepatol. 42:225–229. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ng IO, Guan XY, Poon RT, Fan ST and Lee
JM: Determination of the molecular relationship between multiple
tumour nodules in hepatocellular carcinoma differentiates
multicentric origin from intrahepatic metastasis. J Pathol.
199:345–353. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chan AC, Chan SC, Chok KS, Cheung TT, Chiu
DW, Poon RT, Fan ST and Lo CM: Treatment strategy for recurrent
hepatocellular carcinoma: Salvage transplantation, repeated
resection, or radiofrequency ablation? Liver Transpl. 19:411–419.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gluer AM, Cocco N, Laurence JM, Johnston
ES, Hollands MJ, Pleass HC, Richardson AJ and Lam VW: Systematic
review of actual 10-year survival following resection for
hepatocellular carcinoma. HPB (Oxford). 14:285–290. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hasegawa K, Kokudo N, Makuuchi M, Izumi N,
Ichida T, Kudo M, Ku Y, Sakamoto M, Nakashima O, Matsui O and
Matsuyama Y: Comparison of resection and ablation for
hepatocellular carcinoma: A cohort study based on a Japanese
nationwide survey. J Hepatol. 58:724–729. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bolondi L, Burroughs A, Dufour JF, Galle
PR, Mazzaferro V, Piscaglia F, Raoul JL and Sangro B: Heterogeneity
of patients with intermediate (BCLC B) Hepatocellular Carcinoma:
Proposal for a subclassification to facilitate treatment decisions.
Semin Liver Dis. 32:348–359. 2012.PubMed/NCBI
|
|
27
|
Xue R, Li R, Guo H, Guo L, Su Z, Ni X, Qi
L, Zhang T, Li Q, Zhang Z, et al: Variable intra-tumor genomic
heterogeneity of multiple lesions in patients with hepatocellular
carcinoma. Gastroenterology. 150:998–1008. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Miao R, Luo H, Zhou H, Li G, Bu D, Yang X,
Zhao X, Zhang H, Liu S, Zhong Y, et al: Identification of
prognostic biomarkers in hepatitis B virus-related hepatocellular
carcinoma and stratification by integrative multi-omics analysis. J
Hepatol. 61:840–849. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kumada T, Nakano S, Takeda I, Sugiyama K,
Osada T, Kiriyama S, Sone Y, Toyoda H, Shimada S, Takahashi M and
Sassa T: Patterns of recurrence after initial treatment in patients
with small hepatocellular carcinoma. Hepatology. 25:87–92. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yamamoto T, Kajino K, Kudo M, Sasaki Y,
Arakawa Y and Hino O: Determination of the clonal origin of
multiple human hepatocellular carcinomas by cloning and polymerase
chain reaction of the integrated hepatitis B virus DNA. Hepatology.
29:1446–1452. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Morimoto O, Nagano H, Sakon M, Fujiwara Y,
Yamada T, Nakagawa H, Miyamoto A, Kondo M, Arai I, Yamamoto T, et
al: Diagnosis of intrahepatic metastasis and multicentric
carcinogenesis by microsatellite loss of heterozygosity in patients
with multiple and recurrent hepatocellular carcinomas. J Hepatol.
39:215–221. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim PT, Jang JH, Atenafu EG, Fischer S,
Greig PD, McGilvray ID, Wei AC, Gallinger S and Cleary SP: Outcomes
after hepatic resection and subsequent multimodal treatment of
recurrence for multifocal hepatocellular carcinoma. Br J Surg.
100:1516–1522. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Taura K, Ikai I, Hatano E, Fujii H, Uyama
N and Shimahara Y: Implication of frequent local ablation therapy
for intrahepatic recurrence in prolonged survival of patients with
hepatocellular carcinoma undergoing hepatic resection: An analysis
of 610 patients over 16 years old. Ann Surg. 244:265–273. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Poon RT, Fan ST, Lo CM, Ng IO, Liu CL, Lam
CM and Wong J: Improving survival results after resection of
hepatocellular carcinoma: A prospective study of 377 patients over
10 years. Ann Surg. 234:63–70. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Llovet JM and Bruix J: Systematic review
of randomized trials for unresectable hepatocellular carcinoma:
Chemoembolization improves survival. Hepatology. 37:429–442. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Llovet JM, Real MI, Montana X, Planas R,
Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, et al:
Arterial embolisation or chemoembolisation versus symptomatic
treatment in patients with unresectable hepatocellular carcinoma: A
randomised controlled trial. Lancet. 359:1734–1739. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lo CM, Ngan H, Tso WK, Liu CL, Lam CM,
Poon RT, Fan ST and Wong J: Randomized controlled trial of
transarterial lipiodol chemoembolization for unresectable
hepatocellular carcinoma. Hepatology. 35:1164–1171. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cheng X, Sun P, Hu QG, Song ZF, Xiong J
and Zheng QC: Transarterial (chemo)embolization for curative
resection of hepatocellular carcinoma: A systematic review and
meta-analyses. J Cancer Res Clin Oncol. 140:1159–1170. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yin L, Li H, Li AJ, Lau WY, Pan ZY, Lai
EC, Wu MC and Zhou WP: Partial hepatectomy vs. transcatheter
arterial chemoembolization for resectable multiple hepatocellular
carcinoma beyond Milan Criteria: A RCT. J Hepatol. 61:82–88. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nojiri K, Tanaka K, Takeda K, Ueda M,
Matsuyama R, Taniguchi K, Kumamoto T, Mori R and Endo I: The
efficacy of liver resection for multinodular hepatocellular
carcinoma. Anticancer Res. 34:2421–2426. 2014.PubMed/NCBI
|