Introduction
Gallbladder cancer (GBC) is a highly malignant
tumor, and its pathogenesis remains unknown at present. GBC
symptoms remain inconspicuous in the early stages. When patients
exhibit remarkable symptoms, metastasis has already advanced. Lymph
node metastasis is the primary mode of transfer of GBC; the first
step involves the spread of cancer cells to the lymph nodes via
lymphatic vessels. The overall rate of lymph node metastasis in GBC
ranges from 54 to 64% (1). Early
diagnosis of gallbladder carcinoma presents difficulty, and lymph
node metastasis is also early. Therefore, treatment of GBC bears
importance for early detection of lymphatic metastasis caused by
cytokines in the blood.
Vascular endothelial growth factor-C (VEGF-C), a
member of the VEGF family, has been found to induce
lymphangiogenesis and promote lymph node metastasis in many
malignant tumors (2–4). Karpanen et al (5) observed that VEGF-C facilitates tumor
metastasis via the lymphatic vessels, and that tumor spread can be
inhibited by blocking the interaction between VEGF-C and its
receptors. Kitadai et al (6)
suggested that VEGF-C may play a role in tumor progression via
lymphangiogenesis and angiogenesis in human esophageal carcinoma.
Our previous study (7) also
demonstrated the involvement of VEGF-C in lymphangiogenesis and
angiogenesis of GBC and promotion of lymph node metastasis in
GBC.
However, insufficient information describes the
relationship between VEGF-C expression at the circulating level and
lymph node metastasis in GBC. In this study, we aimed at
characterizing the role of serum VEGF-C (sVEGF-C) in predicting
lymph node metastasis and prognosis of patients with GBC. We
observed that sVEGF-C level was significantly higher in patients
with GBC than in healthy donors. We then measured the positive
association between sVEGF-C and expression of VEGF-C in tissue
samples of human GBC. We also showed that sVEGF-C level was
significantly associated with lymphatic vessel density (LVD) and
lymph node metastasis. We demonstrated that mean survival time was
significantly shorter with high sVEGF-C than with low sVEGF-C.
Altogether, these findings suggest that sVEGF-C levels may predict
lymph node metastasis and prognosis of patients with GBC.
Patients and methods
Patients and tissue specimens
The study was performed in 51 patients with
histopathologically proven GBC and who underwent potentially
curative surgery without preoperative therapy or transfusion in the
Affiliated Union Hospital of Fujian Medical University (Fuzhou,
China) between 2005 and 2016. The patient group comprised 20 men
and 31 women with a median age of 59 years (32–84 years old). All
patients were staged clinically according to the American Joint
Committee on Cancer (AJCC; 7th edition) (8). According to operative notes and
pathological results, 27 and 16 patients presented lymph node
metastasis and distant metastasis, respectively. A total of 15
patients with chronic cholecystitis were treated by surgery in the
Affiliated Union Hospital of Fujian Medical University, and 10
healthy volunteers were used as controls. Paraffin-embedded
specimens were collected according to the protocol approved by the
Ethics Committee of the Affiliated Union Hospital of Fujian Medical
University.
Blood sVEGF-C level assay with
enzyme-linked immunoadsorbent assay (ELISA)
Peripheral venous blood samples were collected on
the day prior to surgery or anticancer therapy. Blood samples were
collected according to the protocol approved by the Ethics
Committee of the Affiliated Union Hospital of Fujian Medical
University. Serum samples were separated from the blood by
centrifugation at 2,000 r/min for 15 min and kept frozen at −80°C
until the assay. Measurement of VEGF-C was performed using the
Quantikine Human VEGF-C Immunoassay kit (R&D Systems,
Minneapolis, MN, USA) according to manufacturer's instructions. All
assays were duplicated. Sensitivity limit of the method for VEGF-C
reached 13.3 pg/ml.
VEGF-C and D2-40 expression with
immunohistochemical staining
Human GBC specimens were collected after operation
and were fixed in 10% formaldehyde solution and embedded in
paraffin. The paraffin-embedded tissues were cut into 4 mm serial
sections and mounted on glass slides. Tissue sections were
deparaffinized with xylene, dehydrated in ethanol, and incubated
with 3% hydrogen peroxidase (H2O2) for 20 min
to block endogenous peroxidase activity. After washing with
phosphate-buffered saline (PBS), the tissue sections were
antigen-retrieved by heating in a microwave for 13 min in a citric
acid buffer solution (pH 6.0). Tissue sections were blocked by 15
min incubation with 5% rabbit serum, followed by an overnight
incubation with VEGF-C polyclonal goat anti-human antibody (1:80;
Santa Cruz Biotechnology, Santa Cruz, CA, USA) and D2-40 monoclonal
mouse anti-human antibody (1:100; Maixin-Bio, Fuzhou, China) in
humidified boxes at 4°C. The sections were then washed with PBS for
5 min and incubated with an UltraSensitive S-P kit (Maixin-Bio)
according to manufacturer's instructions. After exposure to stable
III, 3-diaminobenzidine for 5–10 min, the slides were
counterstained with hematoxylin and eosin, dehydrated, and mounted.
For the negative control, PBS was used instead of the primary
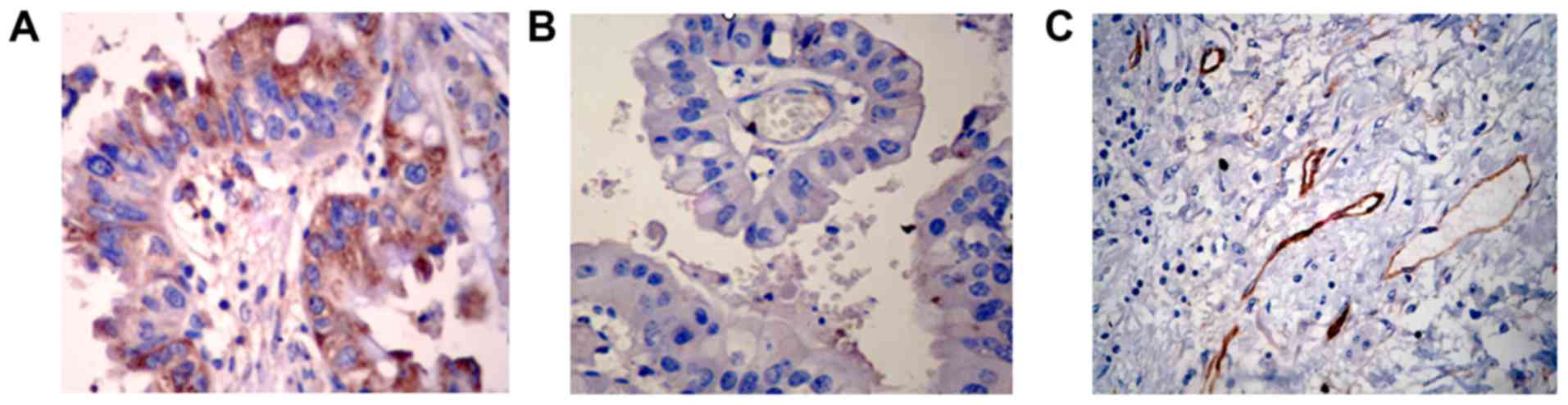
antibody. VEGF-C expression was semiquantitatively evaluated to be
negative and positive based on a previously described scoring
system (Fig. 1A and B) (9). Average of D2-40-positive was assessed
according to the method described by Takammi I (Fig. 1C) (10).
Each slide was first scanned at magnification, ×100 to determine
three ‘hot spots’, which were defined as areas with the maximum
number of positive vessels, and all the immunostained vessels at
magnification, ×400 were counted to determine the positive vessel
density. The average number of positive vessel in the five selected
areas was calculated for each case.
Statistical analysis
Data are expressed as mean ± standard deviation for
continuous variables. Student's t-test was used to analyze
continuous variables for two groups and one-way ANOVA for more than
two groups. Chi-square test was used to compare categorical
variables. Kaplan-Meier survival analysis was used to estimate
survival time, and Mantel's long-rank test was used to compare
differences in survival time. Statistical analyses were performed
using the SPSS software (version 17.0; SPSS Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Relatively high sVEGF-C levels in GBC
patient group
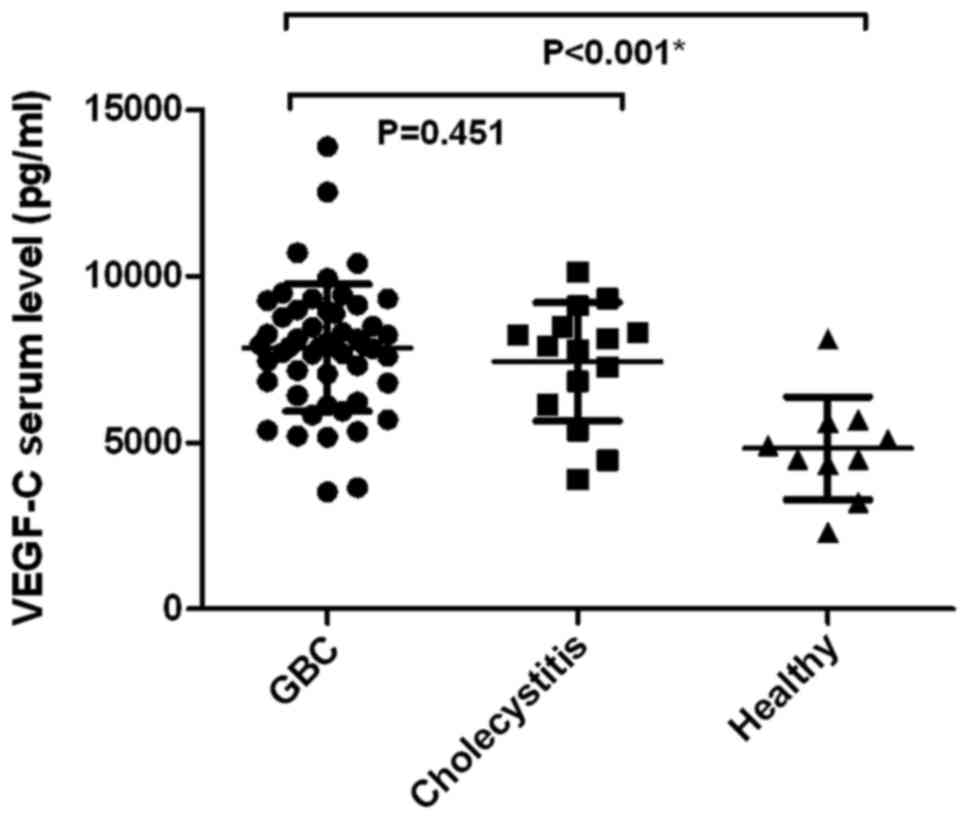
Preoperative sVEGF-C level in patients with GBC
totaled 7846.17±1915.6 pg/ml (median ± standard deviation), which
was significantly higher than that in healthy volunteers
(4828.1±1547.12 pg/ml; P=0.00001) (Fig.
2). However, no statistical significance was observed in the
preoperative sVEGF-C level in patients with GBC and chronic
cholecystitis (7425.33±1784.76 pg/ml, P=0.451) (Fig. 2).
Association among sVEGF-C levels,
VEGF-C expression, LVD, and clinicopathological characteristics of
GBC
Table I summarizes the
associations among sVEGF-C levels, VEGF-C expression, LVD, and
clinicopathological characteristics in patients with GBC. sVEGF-C
level was associated with lymph node metastasis (P=0.001). However,
no significant association was observed between sVEGF-C and
factors, such as age, sex, tumor size, histological type,
histological grade, tumor depth, distant metastasis, and stage.
VEGF-C expression in human GBC tissue was significantly correlated
with lymph node metastasis (P=0.02) and tumor size (≤5 and >5
cm; P=0.04) but showed no correlation with age, sex, histological
type, histological grade, tumor depth, distant metastasis, and
stage. LVD in human GBC tissue was significantly correlated with
lymph node metastasis (P=0.02) but presented no correlation with
age, sex, tumor size, histological type, histological grade, tumor
depth, distant metastasis, and stage.
 | Table I.Associations between sVEGF-C, VEGF-C
expression, LVD and the clinicopathological characteristics of
gallbladder cancer. |
Table I.
Associations between sVEGF-C, VEGF-C
expression, LVD and the clinicopathological characteristics of
gallbladder cancer.
|
|
|
|
| VEGF-C |
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Parameters | Total | sVEGF-C (pg/ml) | P-value | + | − | P-value | LVD (/400 HP) | P-value |
|---|
| Age |
|
|
|
|
|
|
|
|
| <60
years | 27 | 7800.8±1915.1 |
| 20 | 7 |
| 7.6±4.0 |
|
| ≥60
years | 24 | 7897.2±1956.0 | 0.86 | 12 | 12 | 0.08 | 6.2±2.8 | 0.15 |
| Sex |
|
|
|
|
|
|
|
|
|
Female | 31 | 7934.0±1921.7 |
| 19 | 12 |
| 7.4±3.7 |
|
| Male | 20 | 7710.0±1947.7 | 0.69 | 13 | 7 | 0.79 | 6.2±3.4 | 0.27 |
| Tumor size |
|
|
|
|
|
|
|
|
| ≤5
cm | 40 | 8089.3±1858.8 |
| 28 | 12 |
| 7.0±3.5 |
|
| >5
cm | 11 | 6962.1±1943.4 | 0.08 | 4 | 7 | 0.04a | 6.7±3.8 | 0.85 |
| Histological
type |
|
|
|
|
|
|
|
|
|
Adeno | 23 | 7541.7±1271.1 |
| 12 | 11 |
| 7.0±3.5 |
|
|
Others | 28 | 8096.3±2309.8 | 0.31 | 20 | 8 | 0.16 | 6.9±3.7 | 0.94 |
| Histological
grading |
|
|
|
|
|
|
|
|
| Poor | 16 | 8035.6±2084.3 |
| 10 | 6 |
| 6.9±3.6 |
|
|
Moderate | 15 | 8231.3±2594.8 |
| 8 | 7 |
| 6.7±4.2 |
|
|
Well | 20 | 7510.8±1252.4 | 0.59 | 14 | 6 | 0.6 | 6.9±3.4 | 0.31 |
| Tumor depth |
|
|
|
|
|
|
|
|
|
Tis-T2 | 15 | 7999.4±1913.1 |
| 11 | 4 |
| 6.1±2.6 |
|
|
T3-T4 | 36 | 7782.3±1940.1 | 0.72 | 21 | 15 | 0.31 | 7.3±3.9 | 0.30 |
| Lymphatic
metastasis |
|
|
|
|
|
|
|
|
|
Yes | 27 | 8650.0±1959.9 |
| 21 | 6 |
| 8.0±3.8 |
|
| No | 24 | 6941.8±1422.3 | 0.001a | 11 | 13 | 0.02a | 5.7±2.9 | 0.02a |
| Distant
metastasis |
|
|
|
|
|
|
|
|
| M0 | 35 | 7860.4±1694.1 |
| 23 | 12 |
| 6.7±3.3 |
|
| M1 | 16 | 7814.9±2392.7 | 0.94 | 9 | 7 | 0.52 | 7.4±4.1 | 0.55 |
| Stage |
|
|
|
|
|
|
|
|
|
0–II | 12 | 7642.3±1561.5 |
| 9 | 3 |
| 6.0±2.9 |
|
|
III–IV | 39 | 7908.9±2026.2 | 0.68 | 23 | 16 | 0.32 | 7.2±3.7 | 0.33 |
sVEGF-C levels exhibited positive
correlation with VEGF-C expression and LVD in GBC
We analyzed the relationship between sVEGF-C levels
and VEGF-C expression in tumor tissues. As the median of
preoperative sVEGF-C level in patients with GBC reached 7846.17
pg/ml, we divided the samples into two groups with a mean value of
7846 pg/ml. Considering the mean value of 7846 pg/ml, we divided
the samples into two groups (>7846, ≤7846 pg/ml) (Table II). The positive rate of VEGF-C
expression in human GBC tissue with higher sVEGF-C (>7846 pg/ml)
group totaled 89.3%, and that in human GBC tissue with lower
sVEGF-C (≤7846 pg/ml) group measured 30.4%. Both differences showed
statistical significance at P<0.01. At the same time, a
statistically significant decrease in LVD was noted in the
lower-sVEGF-C group (5.0+2.7) compared with the higher-sVEGF-C
group (8.5+3.4) (P<0.01).
 | Table II.sVEGF-C exhibits positive correlation
with VEGF-C expression and LVD in gallbladder cancer. |
Table II.
sVEGF-C exhibits positive correlation
with VEGF-C expression and LVD in gallbladder cancer.
|
| VEGF-C |
|
|
|
|---|
|
|
|
|
|
|
|---|
| sVEGF-C
(pg/ml) | + | − | P-value | LVD (/400 HP) | P-value |
|---|
| >7846 | 25 | 3 |
| 8.5±3.4 |
|
| ≤7846 | 7 | 16 | <0.001a | 5.0±2.7 |
<0.001a |
Correlation among sVEGF-C level,
VEGF-C expression, LVD, and patient survival in GBC
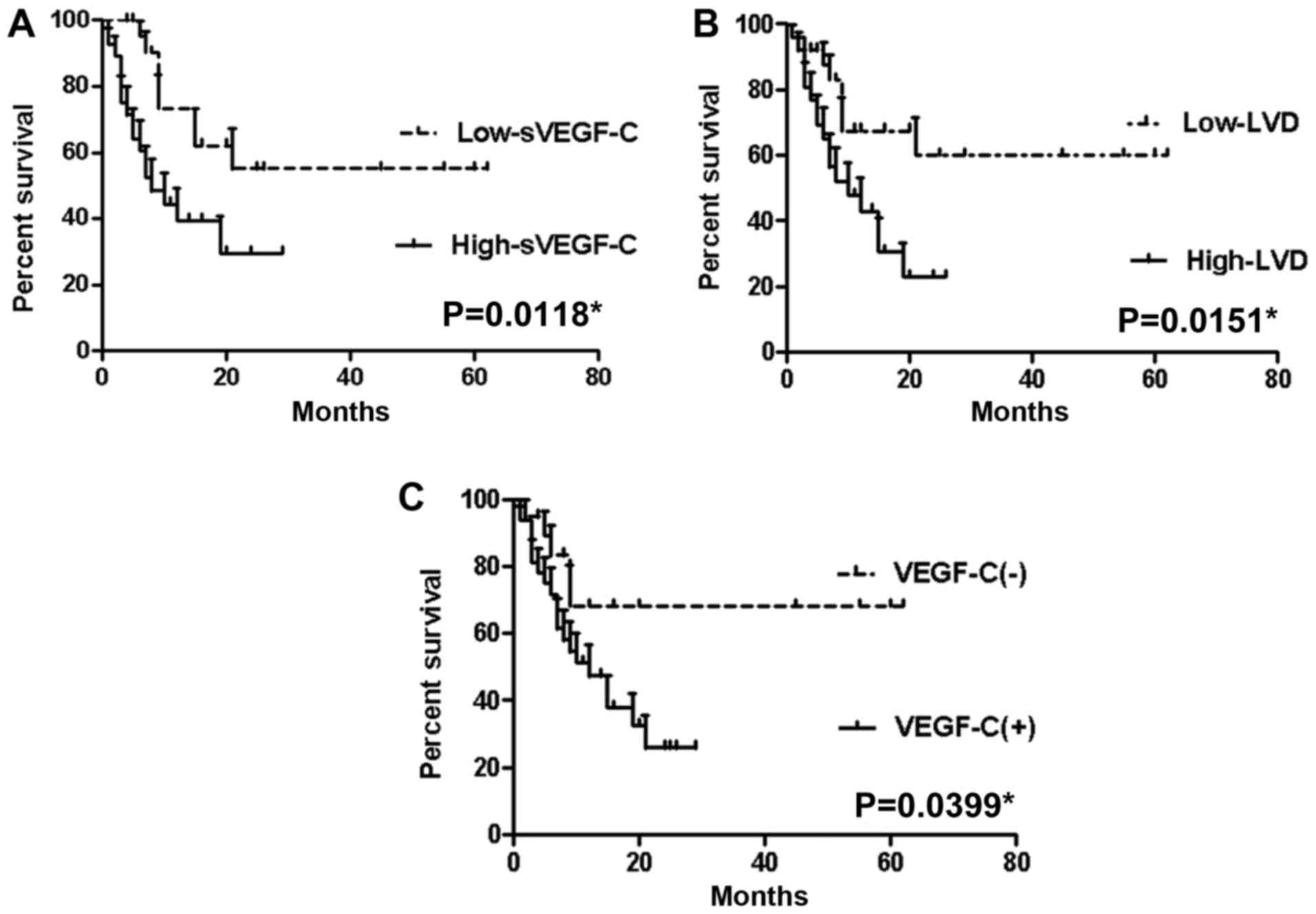
In this study, one-year survival rate was 39.2%.
Mean survival time of patients with high sVEGF-C levels (>7846
pg/ml) measured 9.32±7.18 months, whereas that of with low sVEGF-C
level (<7846 pg/ml) reached 23.39±20.40 months (P=0.0118;
Fig. 3A). As the median of
microlymphatic density in patients with GBC was 7/400 HP, we
divided the samples into two groups with mean values of <7/400
and >7/400 HP. Mean survival rates of patients in high (≥7/400
HP) LVD and low (<7/400 HP) LVD groups amounted to 10.08±6.92
and 21.48±20.61 months, respectively (P=0.0151; Fig. 3B). Mean survival time was shorter in
the VEGF-C-positive expression group than in the negative
expression group (11.25±7.87 vs. 23.11±22.92 months; P=0.0339;
Fig. 3C).
Discussion
GBC, a common malignancy with high mortality, refers
to the fifth most common cancer among gastrointestinal cancers
(11) and accounts for 80–90% of
biliary tract cancers (12). Only
several effective diagnostic measures and classical symptoms exist,
and most patients with GBC are treated at the late stages of the
disease, resulting in poor overall prognosis. Lymph node metastasis
is the most common form of GBC progression. Given that cancer
spread via the lymphatics characterizes the early stages, lymph
node metastasis also acts as an important prognostic factor in GBC
(13). Thus, identifying a method
that can exactly assess lymph node metastasis of GBC before
operation bears importance. VEGF-C, an important member of the VEGF
family, has been identified as a lymphangiogenic factor. This
factor induces formation of lymphatic ducts and promotes lymph node
metastasis in combination with VEGF receptor (VEGFR)-3 (14). VEGF-C has also been correlated with
lymphatic metastasis in numerous carcinoma cases (7,15–17). Tumor cells secrete high levels of
VEGF-C and induce growth of tumor lymphatic vessels. Therefore,
sVEGF-C levels may be used as tumor markers to predict lymph node
metastasis and prognostic factors for patients with GBC.
To our knowledge, this study was the first to
demonstrate that sVEGF-C levels form a positive correlation with
VEGF-C expression and LVD and may predict lymph node metastasis and
prognosis of patients with GBC. In this study, we used ELISA
techniques to analyze preoperative sVEGF-C levels in 51 patients
with GBC, 15 patients with chronic cholecystitis, and 10 healthy
volunteers. Preoperative sVEGF-C level in patients with GBC was
significantly higher than that in healthy volunteers. These
discoveries suggest that sVEGF-C level is lower in the normal
gallbladder when transitioning into cancerous cells, and tumor
cells secrete high levels of VEGF-C, thereby inducing the growth of
tumor lymphatic vessels. Our findings agree with those of studies
on lung cancer (18), esophageal
cancer (19), and colorectal cancer
(20). However, no statistical
significance was observed in preoperative sVEGF-C levels between
patients with GBC and those with chronic cholecystitis. This
finding is consistent with other studies indicating that VEGF-C is
involved in the formation of inflammatory lymphatic vessels through
VEGFR3 in chronic bronchitis (21).
Our early research also showed no statistical significance in the
expression of VEGF-C between paraffin-embedded tissue specimens of
GBC and chronic cholecystitis (7).
This finding may imply that VEGF-C is constantly involved in the
formation of lymphatic vessels in carcinogenesis of chronic
cholecystitis and easy detection of lymph node metastasis in the
early stage of GBC.
This study has analyzed the relationship among
sVEGF-C levels, VEGF-C expression, and LVD in GBC. We observed that
the positive rate of VEGF-C expression in human GBC tissue with
higher sVEGF-C was higher than that with lower sVEGF-C. A
statistically significant increase in LVD was noted in
higher-sVEGF-C group when compared with the lower-sVEGF-C group.
Our results are similar to those of previous research, in which
Mathur et al (22) observed
that sVEGF-C was related to VEGF-C expression in advanced cervical
cancer. These results indicated that sVEGF-C level featured a
positive correlation with VEGF-C expression, LVD in GBC, and that
the amount of VEGF-C in serum coincided with the expression of
VEGF-C in tissue of patients with GBC. We further analyzed the
associations among sVEGF-C levels, VEGF-C expression, LVD, and
clinicopathological characteristics of GBC. We observed that the
levels of sVEGF-C were related to lymph node metastasis, and that
VEGF-C expression in human GBC tissues was significantly correlated
with lymph node metastasis and tumor size. LVD in human GBC tissues
was also significantly correlated with lymph node metastasis. These
findings are consistent with those of other studies (18), which revealed that sVEGF-C level was
higher in patients with non-small-cell lung cancer with lymph node
metastasis than in those without lymph node metastasis. These
results suggest that sVEGF-C levels may predict lymph node
metastasis of patients with GBC.
Various studies showed that sVEGF-C levels can
predict tumor prognosis. Gisterek (23) et al observed that high sVEGF-C
level was related to poor prognosis in patients with breast tumors.
Wang (24) et al have shown
the association of sVEGF-C and prognosis of patients with gastric
cancer. In this study, mean survival time of patients with high
sVEGF-C level was significantly shorter than that with low sVEGF-C
level. We have further analyzed that mean survival time was shorter
in the VEGF-C-positive expression group than in the negative
expression group. Mean survival time of patients with high LVD was
also significantly shorter than that with low LVD. These studies
suggested that sVEGF-C, VEGF-C, and LVD can be useful in predicting
prognosis of patients with GBC as high sVEGF-C levels in patients
with increases LVD, thereby promoting lymph node metastasis and
resulting in poor outcomes.
We concluded that a preoperative high-sVEGF-C level
is related to VEGF-C expression in human GBC tissues, LVD, and
lymph node metastasis. These findings suggest that preoperative
sVEGF-C level serves as a useful biomarker for assessment of the
need for anti-lymphatic metastasis therapy. We also deduced that
preoperative VEGF-C level may reflect malignancies, such as lymph
node metastasis, and may predict prognosis in patients with
GBC.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Youth Foundation
of Fujian Provincial Health and Family Planning Commission (grant
no. 2014-1-49), Fujian Provincial Scientific and Technological
Innovation Joint Fund Project (grant no. 2017Y9029) and The
National Natural Science Foundation (grant no. 81672468) in
China.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LJ and LX conceived and designed the study. LJ, ML,
XC, LX, FS and YC assisted with the collection of the data. LJ, ML,
XC, LX and FS performed the data analysis and interpretation of the
results. LJ wrote the manuscript. All authors approved the final
version of the manuscript for publication.
Ethics approval and consent to
participate
All procedures performed in the study involving
human participants were in accordance with the protocol approved by
the Ethics Committee of the Affiliated Union Hospital of Fujian
Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Muratore A, Polastri R and Capussotti L:
Radical surgery for gallbladder cancer: Current options. Eur J Surg
Oncol. 26:438–443. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schoppmann SF, Fenzl A, Nagy K, Unger S,
Bayer G, Geleff S, Gnant M, Horvat R, Jakesz R and Birner P: VEGF-C
expressing tumor-associated macrophages in lymph node positive
breast cancer: Impact on lymphangiogenesis and survival. Surgery.
139:839–846. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mattila MM, Ruohola JK, Karpanen T,
Jackson DG, Alitalo K and Härkönen PL: VEGF-C induced
lymphangiogenesis is associated with lymph node metastasis in
orthotopic MCF-7 tumors. Int J Cancer. 98:946–951. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tanaka T, Ishiguro H, Kuwabara Y, Kimura
M, Mitsui A, Katada T, Shiozaki M, Naganawa Y, Fujii Y and Takeyama
H: Vascular endothelial growth factor C (VEGF-C) in esophageal
cancer correlates with lymph node metastasis and poor patient
prognosis. J Exp Clin Cancer Res. 29:832010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Karpanen T, Egeblad M, Karkkainen MJ, Kubo
H, Ylä-Herttuala S, Jäättelä M and Alitalo K: Vascular endothelial
growth factor C promotes tumor lymphangiogenesis and intralymphatic
tumor growth. Cancer Res. 61:1786–1790. 2001.PubMed/NCBI
|
|
6
|
Kitadai Y, Amioka T, Haruma K, Tanaka S,
Yoshihara M, Sumii K, Matsutani N, Yasui W and Chayama K:
Clinicopathological significance of vascular endothelial growth
factor (VEGF)-C in human esophageal squamous cell carcinomas. Int J
Cancer. 93:662–666. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang L, Chen YL, She FF, Tang NH, Li XJ
and Wang XX: Expressions of VEGF-C and VEGF-D and their correlation
with lymphangiogenesis and angiogenesis in gallbladder carcinoma.
Zhonghua Zhong Liu Za Zhi. 32:190–195. 2010.(In Chinese).
PubMed/NCBI
|
|
8
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen Y, Jiang L, She F, Tang N, Wang X, Li
X, Han S and Zhu J: Vascular endothelial growth factor-C promotes
the growth and invasion of gallbladder cancer via an autocrine
mechanism. Mol Cell Biochem. 345:77–89. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takanami I: Lymphatic microvessel density
using D2-40 is associated with nodal metastasis in non-small cell
lung cancer. Oncol Rep. 15:437–442. 2006.PubMed/NCBI
|
|
11
|
Varshney S, Butturini G and Gupta R:
Incidental carcinoma of the gallbladder. Eur J Surg Oncol. 28:4–10.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lazcano-Ponce EC, Miquel JF, Muñoz N,
Herrero R, Ferrecio C, Wistuba II, Alonso de Ruiz P, Aristi Urista
G and Nervi F: Epidemiology and molecular pathology of gallbladder
cancer. CA Cancer J Clin. 51:349–364. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shimada H, Endo I, Togo S, Nakano A, Izumi
T and Nakagawara G: The role of lymph node dissection in the
treatment of gallbladder carcinoma. Cancer. 79:892–899. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Joukov V, Pajusola K, Kaipainen A, Chilov
D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N and Alitalo K: A
novel vascular endothelial growth factor, VEGF-C, is a ligand for
the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases.
EMBO J. 15:290–298. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ito Y, Shibata MA, Eid N, Morimoto J and
Otsuki Y: Lymphangiogenesis and axillary lymph node metastases
correlated with VEGF-C expression in two immunocompetent mouse
mammary carcinoma models. Int J Breast Cancer. 2011:8671522011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maula SM, Luukkaa M, Grénman R, Jackson D,
Jalkanen S and Ristamäki R: Intratumoral lymphatics are essential
for the metastatic spread and prognosis in squamous cell carcinomas
of the head and neck region. Cancer Res. 63:1920–1926.
2003.PubMed/NCBI
|
|
17
|
Oh SJ, Jeltsch MM, Birkenhäger R, McCarthy
JE, Weich HA, Christ B, Alitalo K and Wilting J: VEGF and VEGF-C:
Specific induction of angiogenesis and lymphangiogenesis in the
differentiated avian chorioallantoic membrane. Dev Biol.
188:96–109. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tamura M and Ohta Y: Serum vascular
endothelial growth factor-C level in patients with primary nonsmall
cell lung carcinoma: A possible diagnostic tool for lymph node
metastasis. Cancer. 98:1217–1222. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kozlowski M, Kowalczuk O, Milewski R,
Chyczewski L, Niklinski J and Laudański J: Serum vascular
endothelial growth factors C and D in patients with oesophageal
cancer. Eur J Cardiothorac Surg. 38:260–267. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu T and Chen D: Serum vascular
endothelial growth factor-C and vascular endothelial growth factor
level in patients with colorectal carcinoma and clinical
significance. J Huazhong Univ Sci Technolog Med Sci. 26:329–331.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Baluk P, Tammela T, Ator E, Lyubynska N,
Achen MG, Hicklin DJ, Jeltsch M, Petrova TV, Pytowski B, Stacker
SA, et al: Pathogenesis of persistent lymphatic vessel hyperplasia
in chronic airway inflammation. J Clin Invest. 115:247–257. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mathur SP, Mathur RS, Gray EA, Lane D,
Underwood PG, Kohler M and Creasman WT: Serum vascular endothelial
growth factor C (VEGF-C) as a specific biomarker for advanced
cervical cancer: Relationship to insulin-like growth factor II
(IGF-II), IGF binding protein 3 (IGF-BP3) and VEGF-A [corrected].
Gynecol Oncol. 98:467–483. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gisterek I, Matkowski R, Lacko A,
Sedlaczek P, Szewczyk K, Biecek P, Halon A, Staszek U, Szelachowska
J, Pudelko M, et al: Serum vascular endothelial growth factors a, C
and d in human breast tumors. Pathol Oncol Res. 16:337–344. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang TB, Deng MH, Qiu WS and Dong WG:
Association of serum vascular endothelial growth factor-C and
lymphatic vessel density with lymph node metastasis and prognosis
of patients with gastric cancer. World J Gastroenterol.
13:1794–1798. 2007. View Article : Google Scholar : PubMed/NCBI
|