Introduction
Cancer is the second major cause of mortality around
the world, second to cardiovascular diseases (1). The causes of cancer are mainly
associated with poor lifestyle behaviors, including smoking,
obesity, physical inactivity and poor diet, and changing
reproductive patterns (2). In
addition, colon cancer is considered the third most commonly
diagnosed cancer clinically in the world (3). Currently, the treatment of colon cancer
comprises mainly conventional chemotherapy and radiotherapy;
however, these therapeutic methods are often accompanied with
serious side effects (4). Therefore,
it is essential to identify novel effective and safe therapeutic
strategies for treating colon cancer. At present, phytotherapy,
which uses active anticancer agents isolated from plants to treat
cancer, has gained increased attention and has become a widely
accepted alternative drug for treating cancer (4). It is reported that etoposide and
teniposide, which are extracted from the roots and rhizomes of the
Mayapple tree, are used for treating lymphoma, bronchial and
testicular cancer (5).
Puerarin, a type of flavonoid, is the major compound
in the root of Pueraria lobata (Willd.) Ohwi (P.
lobata) (6). It has been widely
used in the treatment of cancer, cardiovascular diseases,
Parkinson's disease, Alzheimer's disease and diabetes (7). It also exerts protective effects against
fever, inflammation, hyperlipidemia and oxidative damage (7). Puerarin injection and other forms
(tablet and capsule) of puerarin have been used in clinics
extensively in China (8). The
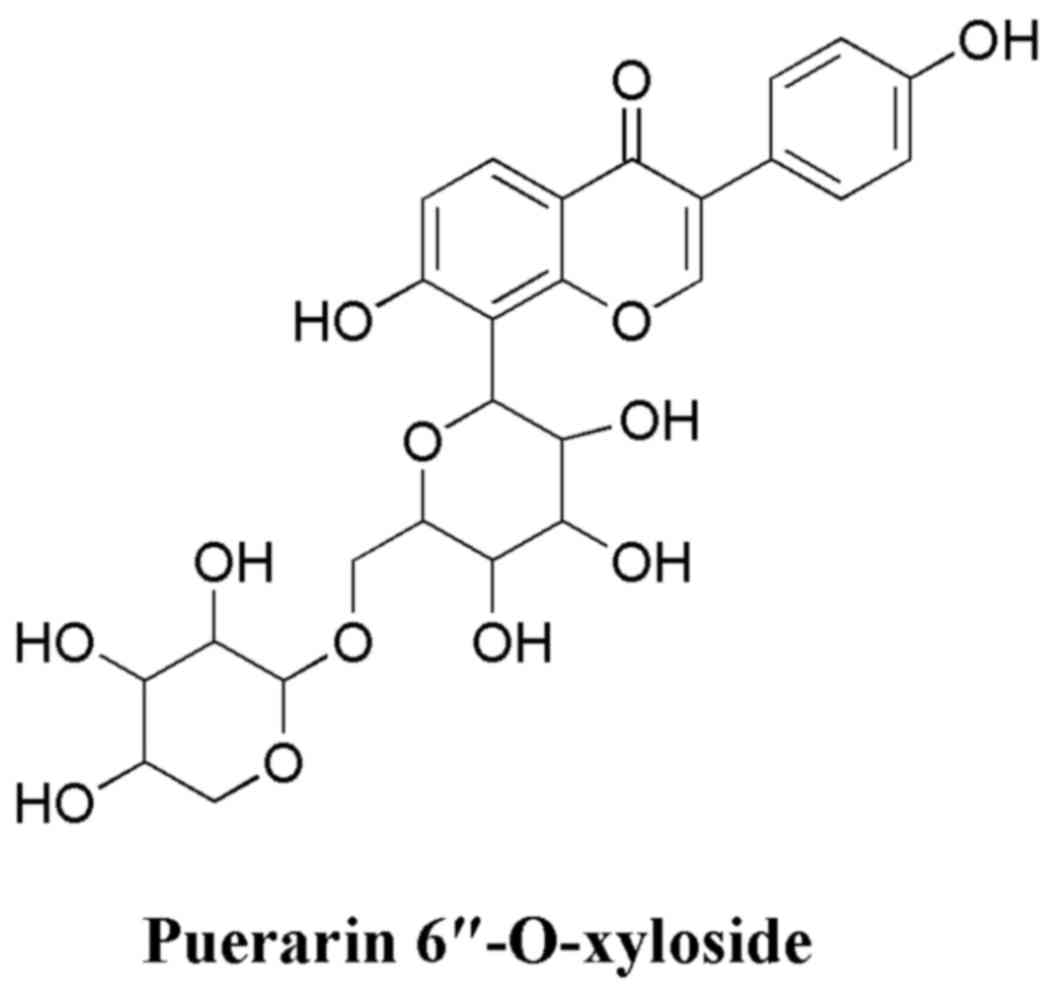
hydroxyl group at C-6′ of puerarin is often substituted with xylose
residues to form puerarin 6″-O-xyloside (PRX; chemical structure
shown in Fig. 1) with a high content
in the root of P. lobata (9).
PRX is one of the major isoflavones of P. lobata
(10). Currently, there are numerous
investigations on the activities of puerarin. It is reported that
PRX has significant antitumor activities against A549 human lung
cancer cells (10). However, to the
best of our knowledge, there is no relevant report on the effect of
PRX against colon cancer.
Therefore, the present study was designed to
systemically investigate the antitumor effects of PRX on colon
cancer in vitro and examine its possible molecular
mechanism. This may be of significant value for the further
identification of useful therapeutic agents from this plant to
treat diseases clinically.
Materials and methods
Reagents and cell lines
PRX (cat. no. JD-24146) was purchased from Shanghai
Jindow Biological Technology Co., Ltd. (Shanghai, China). The
SW480, LoVo and HCT-116 colon cancer cell lines were obtained from
the American Type Culture Collection (Manassas, VA, USA). The DMEM
and fetal bovine serum (FBS) were obtained from Invitrogen; Thermo
Fisher Scientific, Inc. (Waltham, MA, USA); the Cell Counting Kit-8
(CCK-8) was purchased from Beyotime Institute of Biotechnology
(Shanghai, China). Cleaved (c)-caspase-3 (cat. no. ab136812) and
c-caspase-9 (cat. no. ab2324) antibodies were purchased from Abcam
(Cambridge, MA, USA). B-cell lymphoma 2 (Bcl-2)-associated X
protein (Bax; cat. no. ab32503), Bcl-2 (cat. no. ab32124),
Bcl-2-associated death promoter (Bad; cat. no. ab32445), c-Jun
N-terminal kinase (JNK; cat. no. ab76125), phosphorylated (p)-JNK
(cat. no. ab4821), p-Akt (cat. no. ab38449), Akt (cat. no. ab8805),
matrix metalloproteinase (MMP)-3 (cat. no. ab38907), MMP-9 (cat.
no. ab73734) and vascular endothelial growth factor (VEGF; cat. no.
ab11939) antibodies were products of Abcam. Bicinchoninic acid
(BCA) protein assay reagent was purchased from Beyotime Institute
of Biotechnology (cat. no. P0012S). Silica-gel (100–200 mesh) was
purchased from Qingdao Haiyang Chemical Co., Ltd. (Qingdao, China).
The Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide
(PI) kit was purchased from BD Biosciences (San Jose, CA, USA). All
other chemicals used in the present study were of analytical
reagent grade.
Cell culture
The SW480, LoVo and HCT-116 colon cancer cell lines
were maintained in DMEM supplemented with 10% FBS and antibiotics
(1% penicillin and 100 µg/ml streptomycin; Beyotime Institute of
Biotechnology). The cell lines were cultured in an atmosphere
containing 5% CO2/95% air at 37°C.
Determination of cytotoxicity
The cytotoxicity was evaluated using the CCK-8
assay. A 100-µl cell suspension (5×105 cells/ml) was
seeded in 96-well plates and incubated in an atmosphere containing
5% CO2/95% air at 37°C for 24 h. The cells were then
administrated PRX at a series of concentrations (4, 8, 16, 32, 64,
128 and 256 µg/ml) and maintained for 24 h at 37°C. The control
cells were treated with 10 µl DMEM for 24 h at 37°C. Subsequently,
the CCK-8 assay was performed to determine the percentage of cell
proliferation inhibition (n=4) by detecting the optical density
(OD) at 450 nm using a microplate reader (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The half maximal inhibitory concentration
(IC50) values of PRX on the SW480, LoVo and HCT-116
cells were calculated. Additionally, SW480 cells were treated with
PRX (16, 32 and 64 µg/ml) for 12, 24, 36 and 48 h to determine the
dose-dependent and time-dependent effects of PRX. The inhibitory
rate was calculated according to the following formula:
[(ODcontrol-ODtreatment)/ODcontrol]
×100.
Analysis of apoptosis
The apoptotic effect of PRX was detected by
ACSCalibur cytometer (BD Biosciences). The SW480 cells
(5×105/ml; 2 ml) were seeded in 6-well plates for 24 h
at 37°C. Subsequently, the cells were treated with 15, 30 and 60
µg/ml PRX. After 48 h, the cells were trypsinized, washed with PBS,
centrifuged for 5 min at 500 × g at room temperature and stained
using the Annexin V-FITC/PI kit (200 ml Annexin V-FITC and 10 ml PI
for every 1×105 cells) for 5 min at room temperature in
the dark, according to the manufacturer's protocol.
Western blot analysis
The cells were treated with PRX (15, 30 and 60
µg/ml) for 24 h at 37°C. Total protein was extracted from the cells
using the cell lysis buffer for western blot analysis and
immunoprecipitation (Beyotime Institute of Biotechnology; cat. no.
P0013), and the protein concentration was determined using the BCA
protein assay reagent. Subsequently, 35 µg of protein was separated
by 12% SDS-PAGE and running buffer [0.3% Tris Base, 1.4% glycine
and 20% SDS (pH 8.3)]. The proteins were then transferred onto
polyvinylidene fluoride membranes. The membranes were blocked with
5% fat-free dry milk in 1X TBST (containing 0.1% Tween-20) for 2 h
at room temperature. The membranes were then incubated at 4°C
overnight with the following primary antibodies: Bax (dilution
1:1,000), Bcl-2 (dilution 1:1,000), Bad (dilution 1:1,000),
c-caspase-3 (dilution 1:1,000), c-caspase-9 (dilution 1:1,000), JNK
(dilution 1:1,000), p-JNK (dilution 1:1,000), p-Akt (dilution
1:1,000), Akt (dilution 1:1,000), MMP-9 (dilution 1:1,000), VEGF
(dilution 1:1,000), MMP-3(dilution 1:1,000) and GAPDH (dilution
1:2,000). Subsequently, the membranes were incubated with
horseradish peroxidase-conjugated secondary antibodies (1:2,000;
cat. no. A0286; Beyotime Institute of Biotechnology) at room
temperature for 1 h. The proteins were detected using the
chemiluminescence ECL kit (Abcam). The signals were quantitated
using ImageLab software (version 4.0; Bio-Rad Laboratories, Inc.)
on a Chemidoc Imaging instrument. To normalize for protein loading,
antibodies directed against GAPDH were used, with protein
expression levels expressed relative to GAPDH.
Statistical analysis
The significance of differences between groups was
determined by one-way analysis of variance followed by Dunnett's
multiple comparisons post hoc test using SPSS software (SPSS for
Windows 19.0; IBM SPSS, Armonk, NY, USA). The results are presented
as the mean ± standard deviation. P<0.05 was considered to
indicate a statistically significant difference.
Results
Inhibitory effects of PRX on colon
cancer
As shown in Fig. 2,
PRX exerted marked inhibitory effects on the three colon cell lines
(SW480, LoVo and HCT-116). The IC50 values of the SW480,
LoVo and HCT-116 cells were 37.114, 49.231 and 43.022 µg/ml,
respectively. It was shown that PRX exerted the highest
anti-proliferative effect on the SW480 cells, therefore, the SW480
cell line was selected from the three cell lines for further
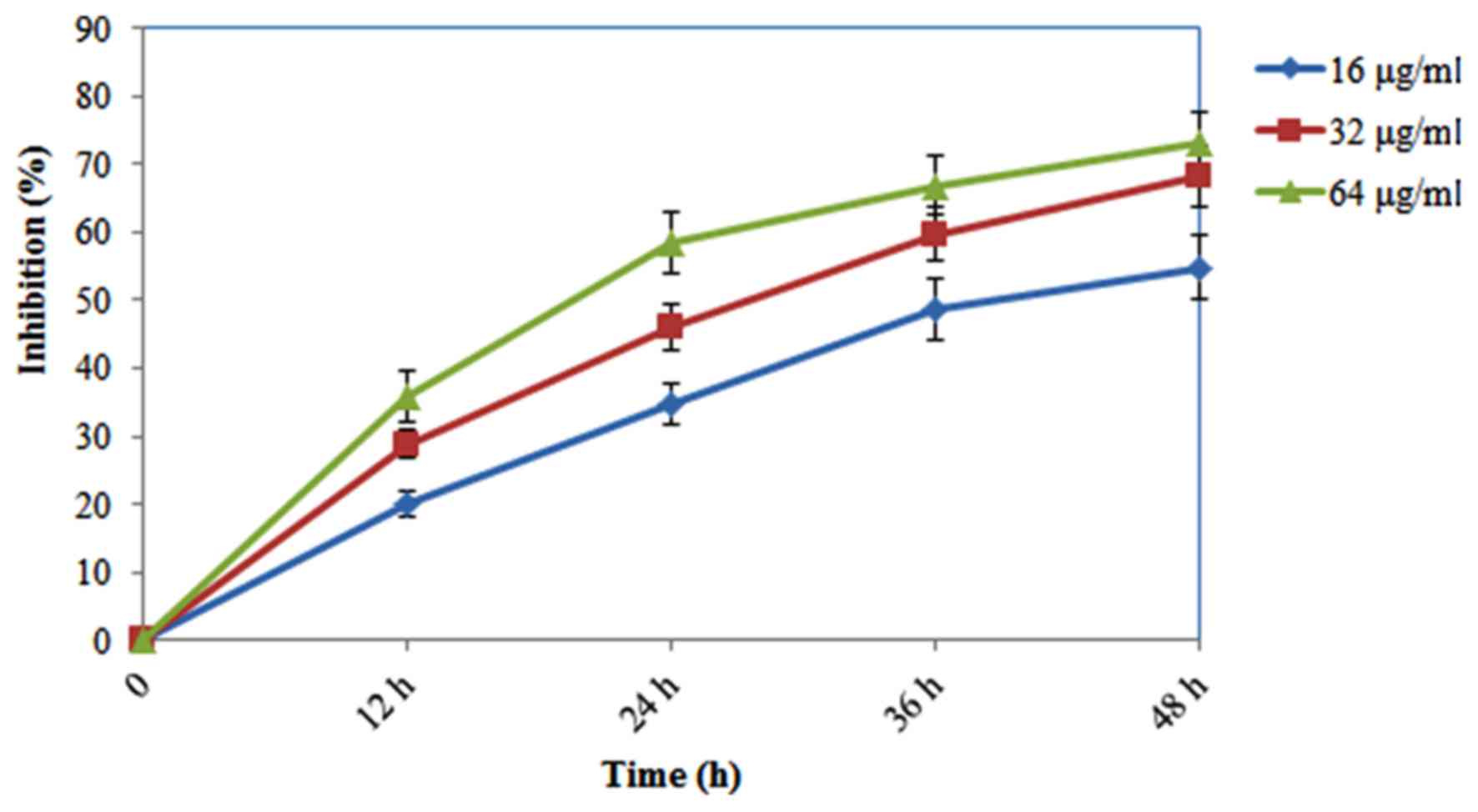
experiments. In addition, as shown in Fig. 3, PRX (16, 32 and 64 µg/ml) exhibited
dose-dependent and time-dependent cytotoxic effects against the
SW480 cells.
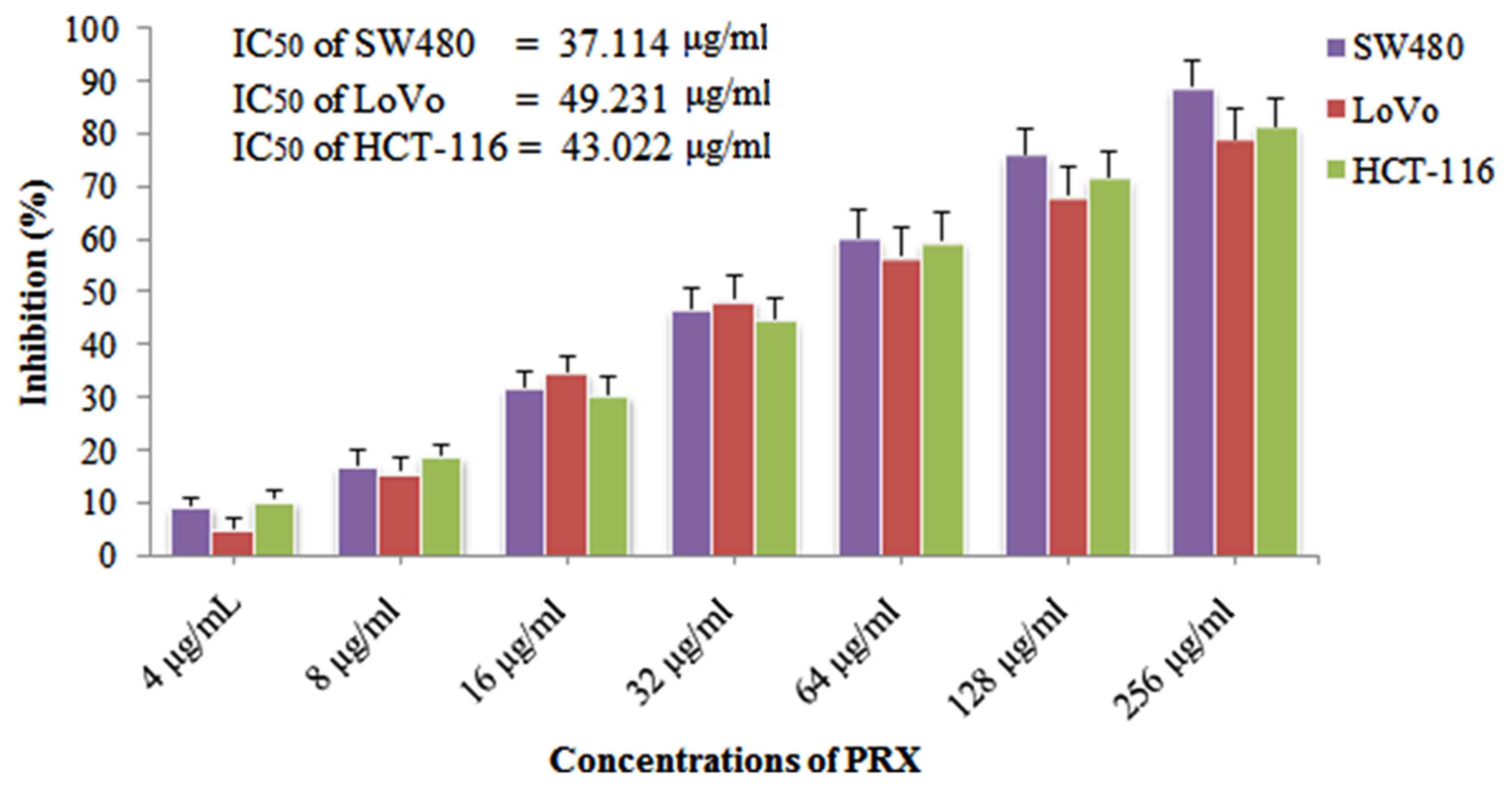 | Figure 2.Inhibitory effects of PRX on the
proliferation of colon cancer cells. SW480, LoVo and HCT-116 colon
cancer cell lines were treated with PRX (4, 8, 16, 32, 64, 128 and
256 µg/ml) for 24 h, and cell counting kit-8 assays were performed
to determine the percentage of cell proliferation inhibition (n=4),
and IC50 values of PRX on SW480, LoVo and HCT-116 cells
were calculated. PRX, puerarin 6″-O-xyloside; IC50, half
maximal inhibitory concentration. |
Pro-apoptotic effect of PRX on SW480
cells
The results of the cell viability experiment
indicated that PRX exerted notable antitumor activity against the
colon cancer cells. To determine whether the anticancer activity of
PRX was associated with apoptosis, the apoptosis induced by PRX was
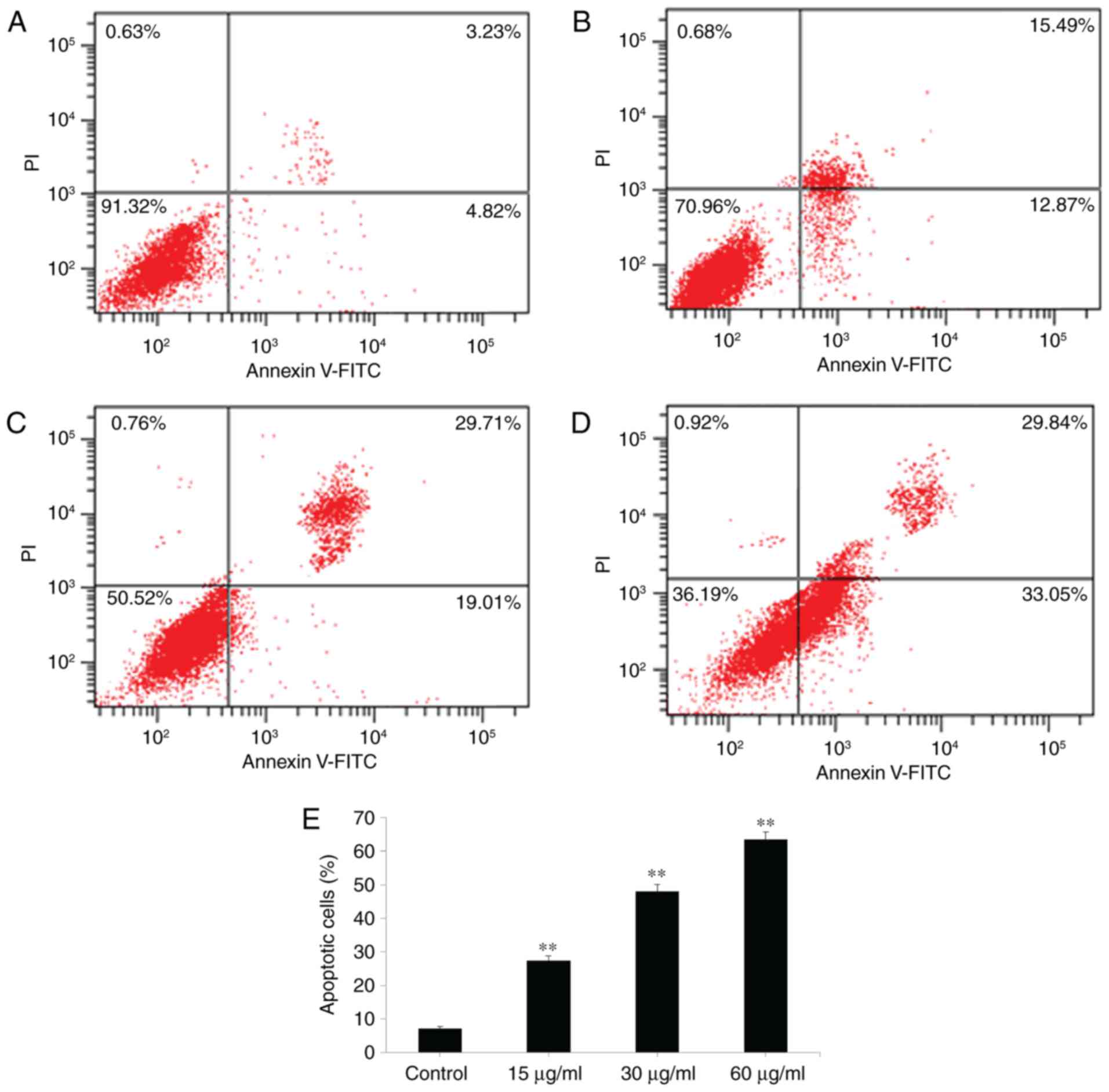
determined by staining with Annexin V-FITC/PI followed by flow
cytometric analysis. It was found, as shown in Fig. 4A-E, that the number of apoptotic cells
was increased gradually by treating the cells with increased
concentrations of PRX (15, 30 and 60 µg/ml). These results showed
that PRX induced the death of colon cancer cells due to the
induction of apoptosis.
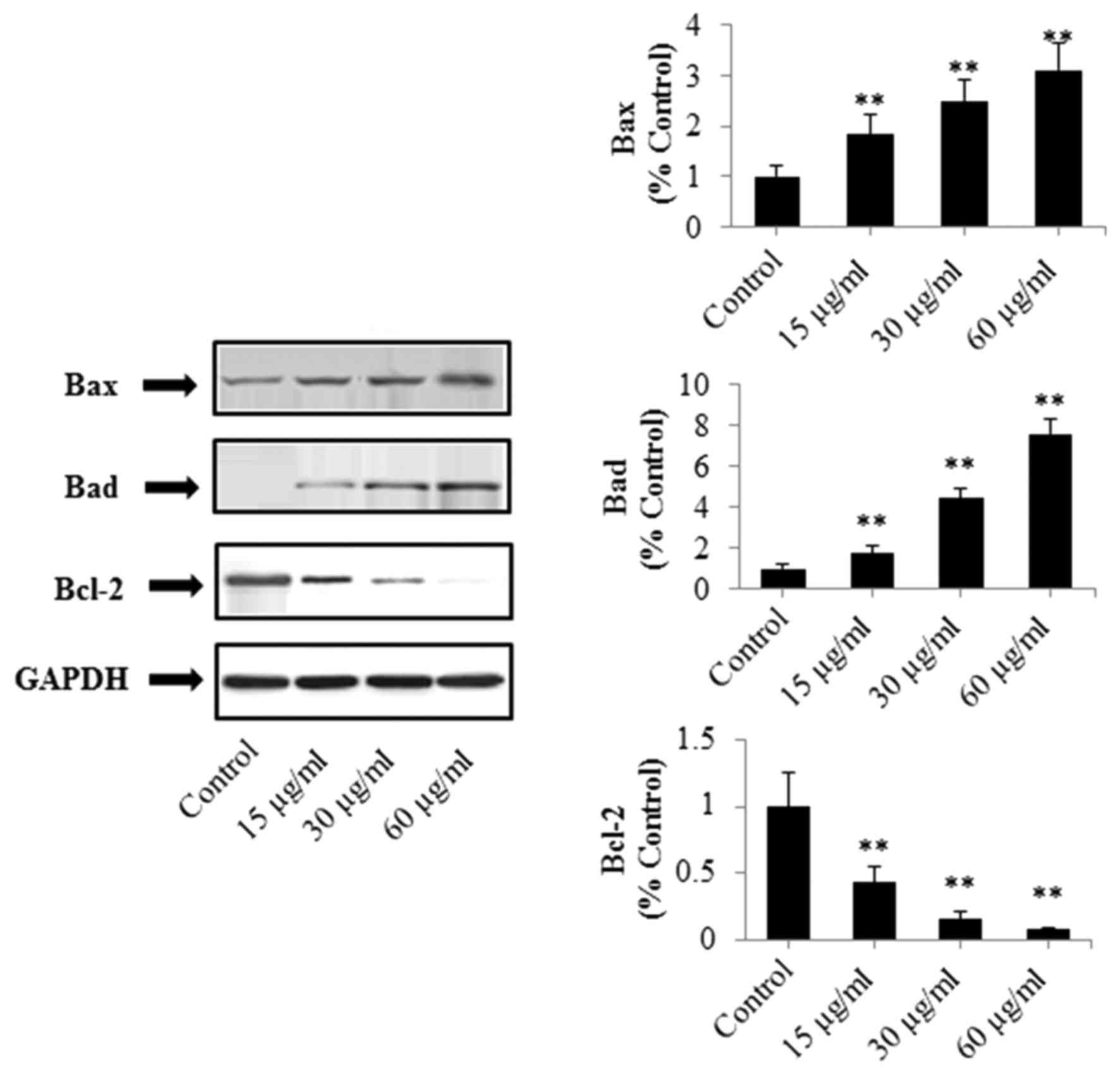
Effects of PRX on the protein
expression of caspase-3, caspase-9, Bad, Bax and Bcl-2 in SW480
cells
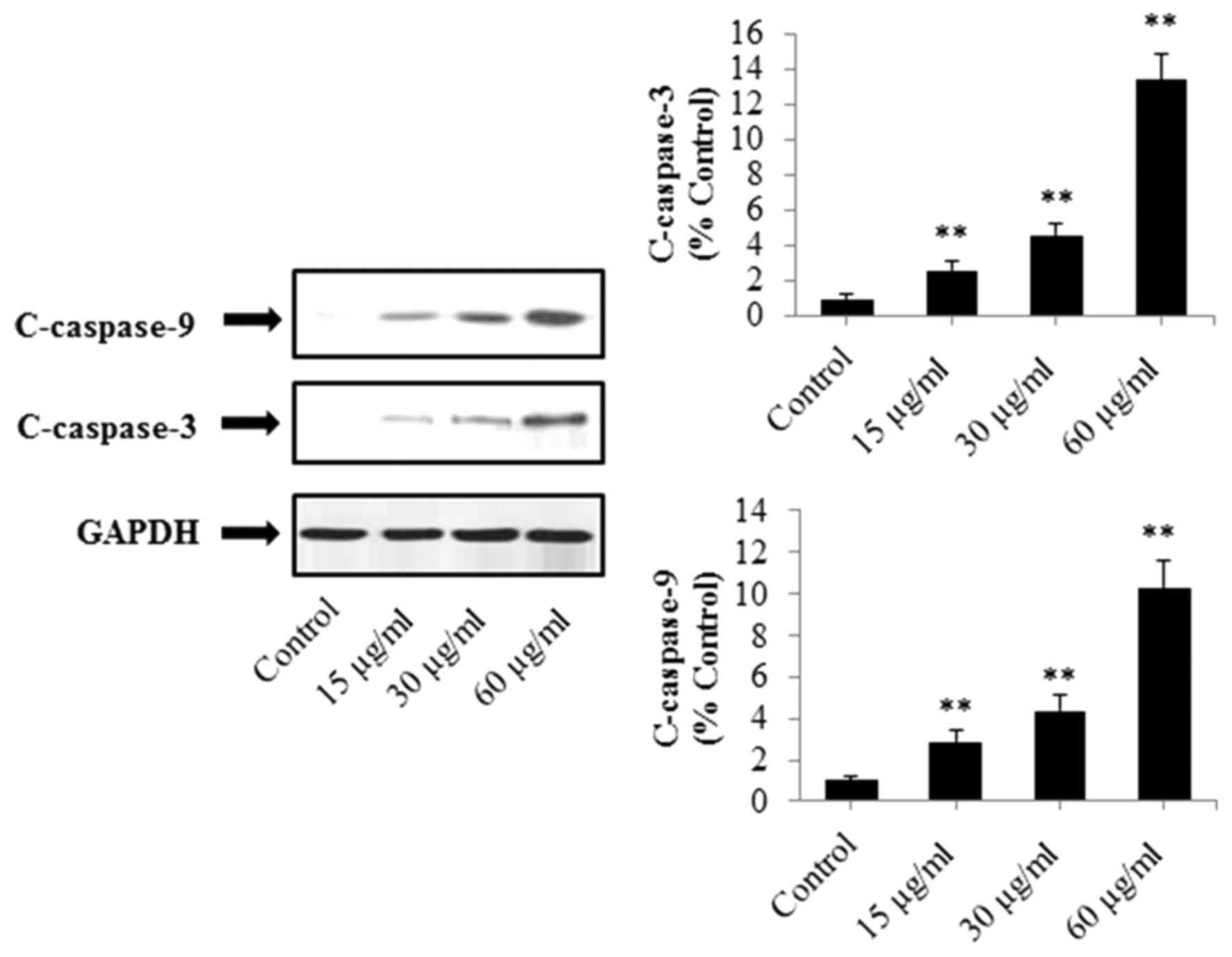
As shown in Fig. 5,
the expression levels of caspase-3 and caspase-9 were upregulated
gradually following treatment with increased concentrations of PRX
(15, 30 and 60 µg/ml; P<0.01). In addition, as shown in Fig. 6, the expression levels of Bad and Bax
were significantly increased in a concentration-dependent manner,
whereas that of Bcl-2 was significantly decreased (P<0.01).
These results demonstrated that PRX-induced apoptosis of SW480
cells may be associated with the mitochondria-mediated apoptotic
pathway.
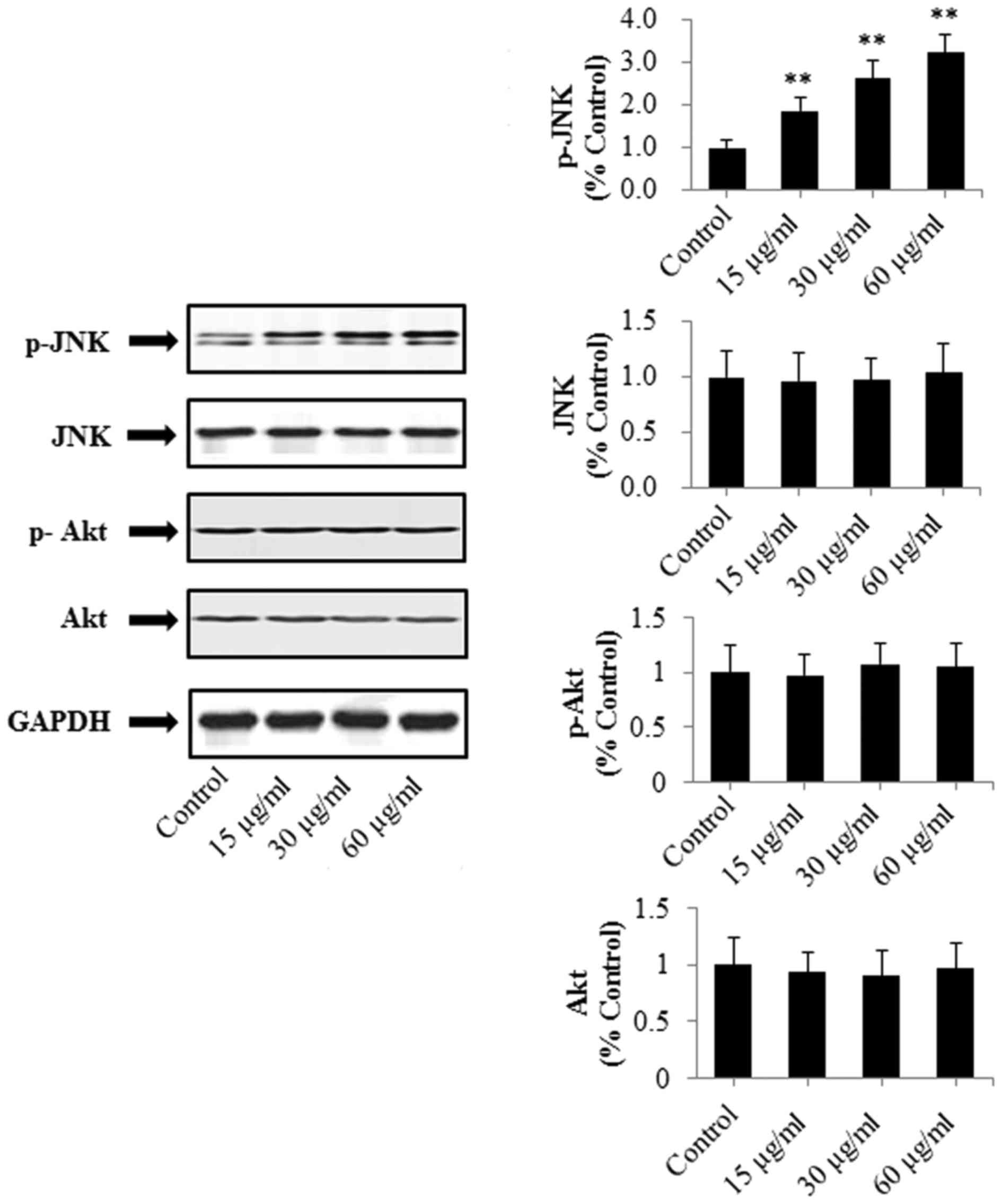
Effects of PRX on the protein levels
of PRX on JNK, p-JNK, p-Akt and Akt in SW480 cells
To examine other potential mechanisms underlying the
effect of PRX on the SW480 cells, the expression levels of proteins
associated with the JNK/Akt signal pathway were detected, including
JNK, p-JNK, p-Akt and Akt. As shown in Fig. 7, PRX had no significant effect on JNK,
p-Akt or Akt (P>0.05). However, PRX (15, 30 and 60 µg/ml)
significantly upregulated the expression of p-JNK (P<0.01) in a
dose-dependent manner, compared with that in the control group.
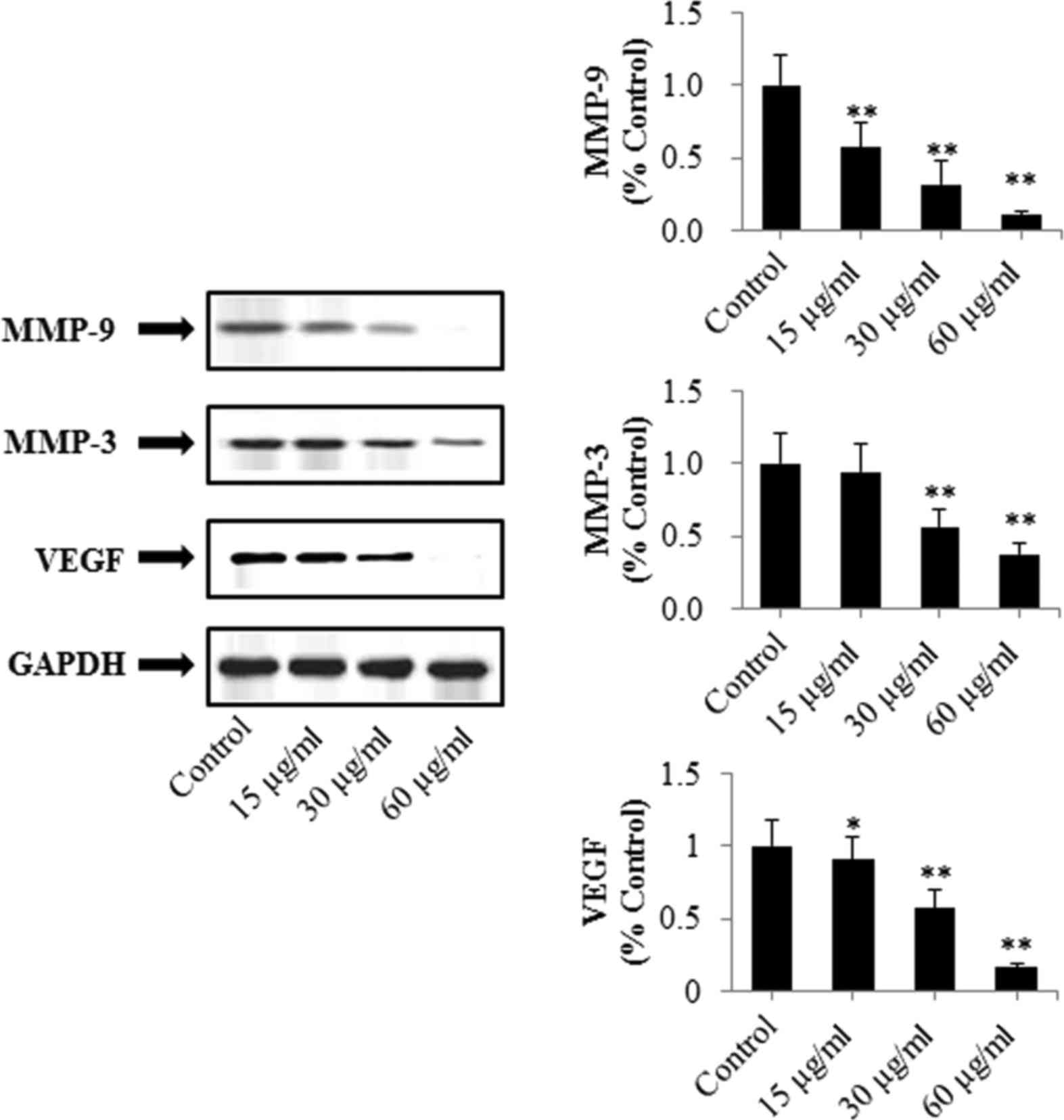
Effects of PRX on the protein
expression of MMP-9, VEGF and MMP-3 in SW480 cells
To examine other possible mechanisms underlying the
antitumor activities of PRX on SW480 cells, the expression of
proteins associated with tumor invasion and metastasis were
detected in the present study. The effects of PRX on the expression
levels of MMP-3, MMP-9 and VEGF are shown in Fig. 8. It was found that PRX significantly
decreased the expression levels of MMP-9 and VEGF in a
concentration-dependent manner (P<0.01). Additionally, the
expression of MMP-3 was downregulated following treatment with PRX
(30 and 60 µg/ml, P<0.01).
Discussion
The present study is the first, to the best of our
knowledge, to systemically investigate the antitumor effect of PRX
on colon cancer cell lines in vitro. The results showed that
PRX exerted significant anticancer effects against colon cancer
cells in vitro through the induction of
mitochondria-mediated intrinsic apoptosis and through inhibiting
the invasion and metastasis of tumor cells.
It has been reported that plant-derived medicines
are safer than synthetic drugs (11),
and they have also been demonstrated to be effective in the
treatment of various diseases, particularly those that cannot be
treated by modern synthetic drugs (12). Therefore, the aim of the present study
was to investigate the antitumor activities of PRX isolated from
the root of the P. lobata.
Uncontrolled cell proliferation and insufficient
apoptosis may be considered primary causes of cancer (13). Apoptosis is a physiological cell
suicide process and is regulated by a series of biochemical events
that eventually result in cell death (14,15). In
addition, apoptosis is considered to be an ideal target for cancer
therapy (16). In the present study,
PRX exhibited significantly pro-apoptotic effects against SW480
cells. Mitochondria-mediated apoptosis is considered to be a major
apoptotic pathway (17). Previous
investigations have demonstrated that caspase family proteins are
important in apoptosis and inflammatory responses (18). Caspase-9 is considered to be the
initiating caspase in the caspase cascade reaction, and activated
by cytochrome c (19).
Caspase-3 activated by caspase-9 is a crucial death protease and is
considered to be a bio-marker for identifying whether cells are
undergoing apoptosis (20,21). Additionally, the Bcl-2 family proteins
are crucial in mitochondria-mediated apoptosis, and are considered
the initial regulatory step in the induction of mitochondrial
apoptosis (22). In the Bcl-2 family,
Bcl-2, Bax and Bad are considered to be apoptosis-associated
proteins. The function of Bcl-2 is to bind and suppress the other
pro-apoptotic relevant proteins of the Bcl-2 family; however, Bax
and Bad directly promote the release of cytochrome c into
the cytoplasm and inhibit anti-apoptotic Bcl-2 proteins (23). The results of the present study
demonstrated that treatment of SW480 cells with PRX resulted in a
significant upregulation in the expression levels of of
c-caspase-3, c-caspase-9, Bad and Bax, and the downregulation of
Bcl-2. These findings showed that PRX induced mitochondria-mediated
apoptosis in the SW480 colon cancer cell line.
JNKs can bind to and phosphorylate c-Jun on Ser-63
and Ser-73 within its transcriptional activation domain. p-JNK can
modify the activity of proteins that reside in the mitochondria or
act in the nucleus (24). In
addition, JNK can lead to cell apoptosis via triggering the release
of cytochrome c from the mitochondria into the cytoplasm and
triggering the caspase cascade reactions (24,25). Akt
is a serine/threonine-specific protein kinase, which is important
in apoptosis and cell proliferation. Activated Akt inhibits several
pro-apoptotic factors, including Bad and procaspase-9 (23). The results of the present study
indicated that PRX significantly upregulated the expression levels
of p-JNK, which indicated that the mitochondria-mediated apoptosis
induced by PRX may be associated with the Akt/JNK signaling
pathway.
MMPs are important in the degradation of
extracellular matrix (ECM) components, which is important in tumor
invasion and metastasis (26,27). MMP-3 and MMP-9 are the important
proteases involved in cancer invasion and metastasis. MMP-9,
activated by MMP-3, can destroy and degrade ECM on the surface of
the tumor, and eventually accelerate the invasion and metastasis of
the tumor (28). In addition, MMP-9
promotes the growth and diffusion of tumors (28). It has been reported that VEGF is one
of the key components of wound healing by promoting angiogenesis
(29). VEGF can promote the formation
of MMPs and the growth and metastasis of tumor via accelerating the
formation of new vessels (30).
Therefore, the expression of MMP-3, MMP-9 and VEGF can be used to
determine the possibility of growth and metastasis of tumors. In
the present study, the results showed the downregulation of MMP-3,
MMP-9 and VEGF, indicating that PRX inhibited SW480 colon cancer
cell invasion and metastasis.
In conclusion, the present study demonstrated that
PRX exerted notable antitumor effects against colon cancer cell
lines through the induction of mitochondria-mediated intrinsic
apoptosis, and inhibition of tumor invasion and metastasis
Acknowledgements
Not applicable.
Funding
The present study was supported by Zhejiang
Provincial Natural Science Foundation (grant no. LY12H27009).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BBW conceived and designed the study; XLZ and JSM
performed the experiments; XLZ and BBW analyzed the data; BBW and
XLZ wrote the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Global Burden of Disease Cancer
Collaboration, ; Fitzmaurice C, Dicker D, Pain A, Hamavid H,
Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R, et
al: The Global Burden of Cancer 2013. JAMA Oncol. 1:505–527. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Daaboul HE, Daher CF, Bodman-Smith K,
Taleb RI, Shebaby WN, Boulos J, Dagher C, Mroueh MA and El-Sibai M:
Antitumor activity of β-2-himachalen-6-ol in colon cancer is
mediated through its inhibition of the PI3K and MAPK pathways. Chem
Biol Interact. 275:162–172. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cragg GM and Newman DJ: Plants as a source
of anti-cancer agents. J Ethnopharmacol. 100:72–79. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen R, Xue J and Xie M: Puerarin prevents
isoprenaline-induced myocardial fibrosis in mice by reduction of
myocardial TGF-β1 expression. J Nutr Biochem. 23:1080–1085. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou YX, Zhang H and Peng C: Puerarin: A
review of pharmacological effects. Phytother Res. 28:961–975. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Q, Wu T, Chen X, Ni J, Duan X, Zheng
J, Qiao J, Zhou L and Wei J: Puerarin injection for unstable angina
pectoris. Cochrane Database Syst Rev. 19:CD0041962006.
|
|
9
|
Song W, Li YJ, Qiao X, Qian Y and Ye M:
Chemistry of the Chinese herbal medicine Puerariae Radix (Ge-Gen):
A review. J Chin Pharm Sci. 23:347–360. 2014. View Article : Google Scholar
|
|
10
|
Chen T, Chen H, Wang Y and Zhang J: In
vitro and in vivo antitumour activities of puerarin 6“-O-xyloside
on human lung carcinoma A549 cell line via the induction of the
mitochondria-mediated apoptosis pathway. Pharm Biol. 54:1793–1799.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fokunang CN, Ndikum V, Tabi OY, Jiofack
RB, Ngameni B, Guedje NM, Tembe-Fokunang EA, Tomkins P, Barkwan S,
Kechia Fz, et al: Traditional medicine: Past, present and future
research and development prospects and integration in the national
health system of Cameroon. Afr J Tradit Complement Altern Med.
8:284–295. 2011.PubMed/NCBI
|
|
12
|
Xie Q, Yang Y, Wang Z, Chen F, Zhang A and
Liu C: Resveratrol-4-O-D-(2′-galloyl)-glucopyranoside isolated from
Polygonum cuspidatum exhibits anti-hepatocellular carcinoma
viability by inducing apoptosis via the JNK and ERK pathway.
Molecules. 19:1592–1602. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mattern J and Volm M: Imbalance of cell
proliferation and apoptosis during progression of lung carcinomas.
Anticancer Res. 24:4243–4246. 2004.PubMed/NCBI
|
|
14
|
Liu YL, Tang LH, Liang ZQ, You BG and Yang
SL: Growth inhibitory and apoptosis inducing by effects of total
flavonoids from Lysimachia clethroides Du by in human chronic
myeloid leukemia K562 cells. J Ethnopharmacol. 131:1–9. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kroemer G, Galluzzi L, Vandenabeele P,
Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS,
Golstein P, Green DR, et al: Classification of cell death:
Recommendations of the nomenclature committee on cell death. Cell
Death Differ. 16:3–11. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Okada H and Mak TW: Pathways of apoptotic
and non-apoptotic death in tumor cells. Nat Rev Cancer. 4:592–603.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu P, Shi L, Song M and Meng Y: Antitumor
activity of paederosidic acid in human non-small cell lung cancer
cells via inducing mitochondria-mediated apoptosis. Chem Biol
Interact. 269:33–40. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu J, Jiang S, Li Y, Li M, Cheng Q, Zhao
D, Yang B, Jia Z, Wang L and Song L: Caspase-3 serves as an
intracellular immune receptor specific for lipopolysaccharide in
oyster Crassostrea gigas. Dev Comp Immunol. 61:1–12. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao N, Budhraja A, Cheng S, Yao H, Zhang Z
and Shi X: Induction of apoptosis in human leukemia cells by grape
seed extract occurs via activation of c-Jun NH2-terminal kinase.
Clin Cancer Res. 15:140–149. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang XK, Xu MY, Xu GS, Zhang YL and Xu ZX:
In vitro and in vivo antitumor activity of scutebarbatine A on
human lung carcinoma A549 cell lines. Molecules. 19:8740–8751.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Peng W, Wu JG, Jiang YB, Liu YJ, Sun T, Wu
N and Wu CJ: Antitumor activity of
4-O-(2ˮ-O-acetyl-6ˮ-O-p-coumaroyl-β-D-glucopyranosyl)-p-coumaric
acid against lung cancers via mitochondrial-mediated apoptosis.
Chem Biol Interact. 233:8–13. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chipuk JE, McStay GP, Bharti A, Kuwana T,
Clarke CJ, Siskind LJ, Obeid LM and Green DR: Sphingolipid
metabolism cooperates with BAK and BAX to promote the mitochondrial
pathway of apoptosis. Cell. 148:988–1000. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Budhraja A, Gao N, Zhang Z, Son YO, Cheng
S, Wang X, Ding S, Hitron A, Chen G, Luo J and Shi X: Apigenin
induces apoptosis in human leukemia cells and exhibits
anti-leukemic activity in vivo. Mol Cancer Ther. 11:132–142. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dhanasekaran DN and Reddy EP: JNK
signaling in apoptosis. Oncogene. 48:6245–6251. 2008. View Article : Google Scholar
|
|
25
|
Wang K, Zhang C, Bao J, Jia X, Liang Y,
Wang X, Chen M, Su H, Li P, Wan JB and He C: Synergistic
chemopreventive effects of curcumin and berberine on human breast
cancer cells through induction of apoptosis and autophagic cell
death. Sci Rep. 6:260642016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang Y, Goldberg ID and Shi YE: Complex
roles of tissue inhibitors of metalloproteinases in cancer.
Oncogene. 21:2245–2252. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vihinen P, Ala-aho R and Kähäri VM: Matrix
metalloproteinases as therapeutic targets in cancer. Curr Cancer
Drug Targets. 5:203–220. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Luo HZ, Huang ZH, Yu YL, Chen H, Zhou SH
and Deng QY: Expression of MMP-9 in colon cancer and its clinical
significance. J Oncol. 15:552–553. 2009.
|
|
29
|
Traub S, Morgner J, Martino MM, Höning S,
Swartz MA, Wickström SA, Hubbell JA and Eming SA: The promotion of
endothelial cell attachment and spreading using FNIII10 fused to
VEGF-A165. Biomaterials. 34:5958–5968. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang ZG and Zhu HG: Vascular endothelial
growth factor (VEGF) and pancreatic cancer. J Dig Surg. 4:149–151.
2005.
|