Introduction
Liver cancer is the third leading cause of
cancer-associated mortality worldwide (1). The incidence of liver cancer is high in
East Asia and Africa (1–4), and the prognosis is generally poor.
Hepatocellular carcinoma (HCC) is the most common form of liver
cancer (1). Advances in sequencing
technologies have enabled the examination of liver cancer genomes
at high resolution. Whole genome sequencing (WGS) and whole exome
sequencing (WES) have been used to identify genomic alterations in
liver cancers (5), and have
identified mutations that frequently occur in liver cancer. Since
cancer is heterogeneous, it is difficult for WGS or WES to identify
variants at low frequencies due to the relatively low coverage of
sequencing (<200×). Investigating liver cancer genomes using a
more sensitive method is therefore required to identify more
detailed patterns of somatic variants.
Biliary tract cancer (BTC) is the second most common
primary hepatobiliary cancer after HCC (6). BTC typically follows an aggressive
disease course associated with a poor clinical outcome. Surgery is
the only curative treatment, but the majority of patients present
with advanced disease and therefore have a limited survival
probability. Gemcitabine and cisplatin-based chemotherapy has been
the only widely accepted standard systemic therapy (7). Reports on somatic mutations in BTC are
rare, and recurrent mutations in BTC have not been identified to
date. An understanding of the molecular characteristics of BTC may
allow for the clinical development of therapies targeting
actionable alterations, with the ultimate aim of improving clinical
outcome. For instance, with the aid of next generation sequencing
(NGS), actionable mutations including those in isocitrate
dehydrogenase 1, fibroblast growth factor (FGF) receptor (FGFR)-2,
BRAF and human epidermal growth factor receptor 2 have been
identified as candidates for targeted therapeutics (8).
Hepatitis B virus (HBV) infection, cirrhosis, fatty
liver and alcohol intake are well-known risk factors for liver
cancer (2–4). It has been reported that HBV has
preferences in host genome integration sites (9–11),
contributing toward genomic instability and hepatocarcinogenesis
(12). HBV not only creates an
environment that promotes the tumorigenic process, but also directs
interactions among signaling cascades to further enhance the
process (13). For example, the
expression of viral protein HBx may promote the transcription of
insulin-like growth factor (IGF)-2, transforming growth factor
(TGF)-β, vascular endothelial growth factor (VEGF) and
yes-associated protein 1 (14–17), and
enhance mechanistic target of rapamycin (mTOR), Wnt/β-catenin and
Hedgehog signaling (17–19). However, it remains unclear whether
these risk factors have any correlation with somatic variants in
liver cancer.
The present study identified the somatic variants in
liver cancer by targeted NGS at a depth of 639×, and studied
variants relevant to HBV, cirrhosis and bilirubin levels. The
proportion of patients harboring potential targets of existing
drugs for all cancer types was also analyzed.
Patients and methods
Patients
Tumor specimens and matched blood samples were
obtained from 57 patients who were diagnosed with HCC (45) or BTC
(12) at the 302 Military Hospital of
China (Beijing, China) between December 2015 and August 2016.
Clinical information, including age, sex, HBV infection status,
hepatohistological grade, disease stage and previous treatment
history were collected. The present study included 50 male and 7
female patients with the mean age of the patients being 52.1 years,
ranging from 17 to 71 years. The present study was approved by the
Independent Ethics Committee of the 302 Military Hospital of China.
All the patients provided written informed consent for the genomic
testing used for the present study. Specimens were evaluated by
board-certified pathologists to identify tumor-bearing areas for
DNA extraction. The characteristics of the patients are presented
in Table I.
 | Table I.Clinical information of the 57
patients with liver and biliary cancer. |
Table I.
Clinical information of the 57
patients with liver and biliary cancer.
|
Characteristics | Category | Number |
|---|
| Sex | M | 50 |
|
| F | 7 |
| Age at diagnosis,
mean years ± SD |
| 52.1±10.6 |
| BMI index | <18.5 | 2 |
|
| 18.5–23.9 | 24 |
|
| >23.9 | 31 |
| Smoking | Yes | 21 |
|
| Occasionally | 3 |
|
| No | 31 |
|
| N/A | 2 |
| Alcohol | Yes | 15 |
|
| Occasionally | 5 |
|
| No | 35 |
|
| N/A | 2 |
| HBV | Yes | 42 |
|
| No | 15 |
| Alcoholic
hepatitis | Yes | 5 |
|
| No | 52 |
| Fatty liver | Yes | 3 |
|
| No | 53 |
|
| N/A | 1 |
| Cirrhosis and liver
fibrosis | Yes | 41 |
|
| No | 15 |
|
| N/A | 1 |
| Family cancer
history | Yes | 27 |
|
| No | 30 |
| Hepatectomy
surgery | Yes | 51 |
|
| No | 3 |
|
| N/A | 3 |
| ECOG score | 0 | 52 |
|
| 1 | 4 |
|
| N/A | 1 |
| Metastases | Yes | 6 |
|
| No | 50 |
|
| N/A | 1 |
| Degree of
differentiation |
Undifferentiated | 0 |
|
| Low | 5 |
|
| Middle | 49 |
|
| High | 0 |
|
| N/A | 3 |
| Largest diameter of
the | <2 | 4 |
| tumor, cm | 2–5 | 22 |
|
| 5–10 | 18 |
|
| ≥10 | 9 |
|
| NA | 4 |
| Albumin, g/l | <35 | 6 |
|
| 35–50 | 49 |
|
| >50 | 1 |
|
| N/A | 1 |
| Bilirubin,
mg/l | 1–10 | 20 |
|
| >10 | 36 |
|
| N/A | 1 |
| Platelet | <100 | 9 |
|
| 100–300 | 43 |
|
| >300 | 4 |
|
| N/A | 1 |
| AFP (ng/ml) | <25 | 34 |
|
| 25–500 | 11 |
|
| >500 | 11 |
|
| N/A | 1 |
| ALT | 0–40 | 36 |
|
| >40 | 19 |
|
| N/A | 2 |
| Child-Pugh
classification | 0/A | 52 |
|
| B/C/D | 1 |
|
| N/A | 4 |
| BCLC | 0/A | 12 |
|
| B | 23 |
|
| C | 16 |
|
| N/A | 6 |
| Stage | I | 2 |
|
| II | 28 |
|
| III | 17 |
|
| IV | 7 |
|
| N/A | 3 |
| Lesion | Primary | 52 |
|
| Secondary | 2 |
|
| N/A | 3 |
| Nodules | Single | 42 |
|
| Multiple | 14 |
|
| N/A | 1 |
| Number of
tumors | 1 | 42 |
|
| >1 | 7 |
|
| N/A | 8 |
| Microvascular
invasion (tumor thrombus) | Yes | 44 |
|
| No | 9 |
|
| N/A | 4 |
| Large vascular
invasion (tumor thrombus) | Yes | 4 |
|
| No | 49 |
|
| N/A | 4 |
| Portal vein
invasion (tumor thrombus) | Yes | 9 |
|
| No | 44 |
|
| N/A | 4 |
| Peripheral nerve
invasion | Yes | 4 |
|
| No | 49 |
|
| N/A | 4 |
Targeted NGS
Tumor tissue was obtained from biopsy or surgery
upon the initial diagnosis of cancer at the primary site, fixed in
10% neutral formalin buffer at room temperature for 48 to 72 h and
embedded in paraffin. Tumor genomic DNA was extracted from
formalin-fixed and paraffin-embedded tumor tissue blocks using
QIAamp DNA FFPE Tissue kit (Qiagen GmbH, Hilden, Germany),
according to the manufacturer's protocols. In addition, tumor
specimens were stained with 0.5% hematoxylin for 8 min and 0.5%
eosin for 15 sec at room temperature. A 4-µm section of a
hematoxylin and eosin-stained slide was reviewed for pathology and
only tissue slices with >20% of tumor cells were further
analyzed. Normal genomic DNA was extracted from peripheral blood
mononuclear cells using QIAamp DNA Blood Mini kit (Qiagen GmbH,
Hilden, Germany), according to the manufacturer's protocols.
Sequencing libraries for each sample were prepared followed by
target capturing for 372 genes that are frequently rearranged in
cancer. Massive parallel sequencing was then performed using
Illumina NextSeq 500 (Illumina, Inc., San Diego, CA, USA), and
samples with a mean sequencing depth of 639× were analyzed.
Data processing and analysis
Sequence data were mapped to the human genome (hg19)
using BWA aligner v0.7.12 (http://bio-bwa.sourceforge.net/). PCR duplicate read
removal and sequence metric collection were performed using Picard
1.130 (https://broadinstitute.github.io/picard/) and Samtools
0.1.19 (http://samtools.sourceforge.net/). Base substitution
analysis, indel analysis, copy number analysis and rearrangement
analysis were performed using variants called pipelines developed
by 3D Medicines, Inc (Shanghai, China). All variants were verified
by visually checking Integrative Genomics Viewer images. The
differences in somatic variants between HCC and BTC were assessed
by Student's t-test. For signaling pathway enrichment analysis,
Student's t-tests were performed to calculate P-values. P<0.05
was considered to indicate a statistically significant
difference.
Results
Somatic alterations in HCC and
BTC
In the present study, 57 primary HCC/BTC tumors and
matched blood mononuclear cells were analyzed by DNA sequencing
targeting 372 cancer-related genes (Fig.
1). The average base pair coverage was above 500× and 200× for
tumor tissues and blood mononuclear cells, respectively. In total,
263 variants were detected in 139 genes (Fig. 1A). A total of 69.1% of the mutated
genes were identified in only 1 patient, and the number of genes
altered in more than 6 patients (10%) was 6 (4.3%), indicating a
diverse somatic mutation pattern in the tested samples (Fig. 1B). Of all 344 variants detected, 67.2,
18.6, 8.4 and 5.5% were single nucleotide variants (SNVs), copy
number variants (CNVs), small insertions or deletions (INDELs) and
splices, respectively (Fig. 1C). Only
one gene fusion (diglyceride acyltransferase-ATM
serine/threonine kinase) was identified. The detected SNVs, CNVs,
INDELs and splices exhibited preferences to different sets of
genes, and only one gene [tumor protein p53 (TP53)] was identified
to have all four types of mutations in different individuals
(Fig. 1D). Out of 129 genes that had
SNVs, 107 genes (82.9%) had SNVs only, and 16 genes (12.4%)
occurred in chromosome 3 (P=0.0018). There were 18 genes harboring
INDELs, and 5 genes (27.8%) had INDELs exclusively. For the 34
genes with CNVs, 24 (70.6%) were not detected to have SNVs, INDELs
or splices, and 6 genes (17.6%) occurred in chromosome 12
(P=0.018).
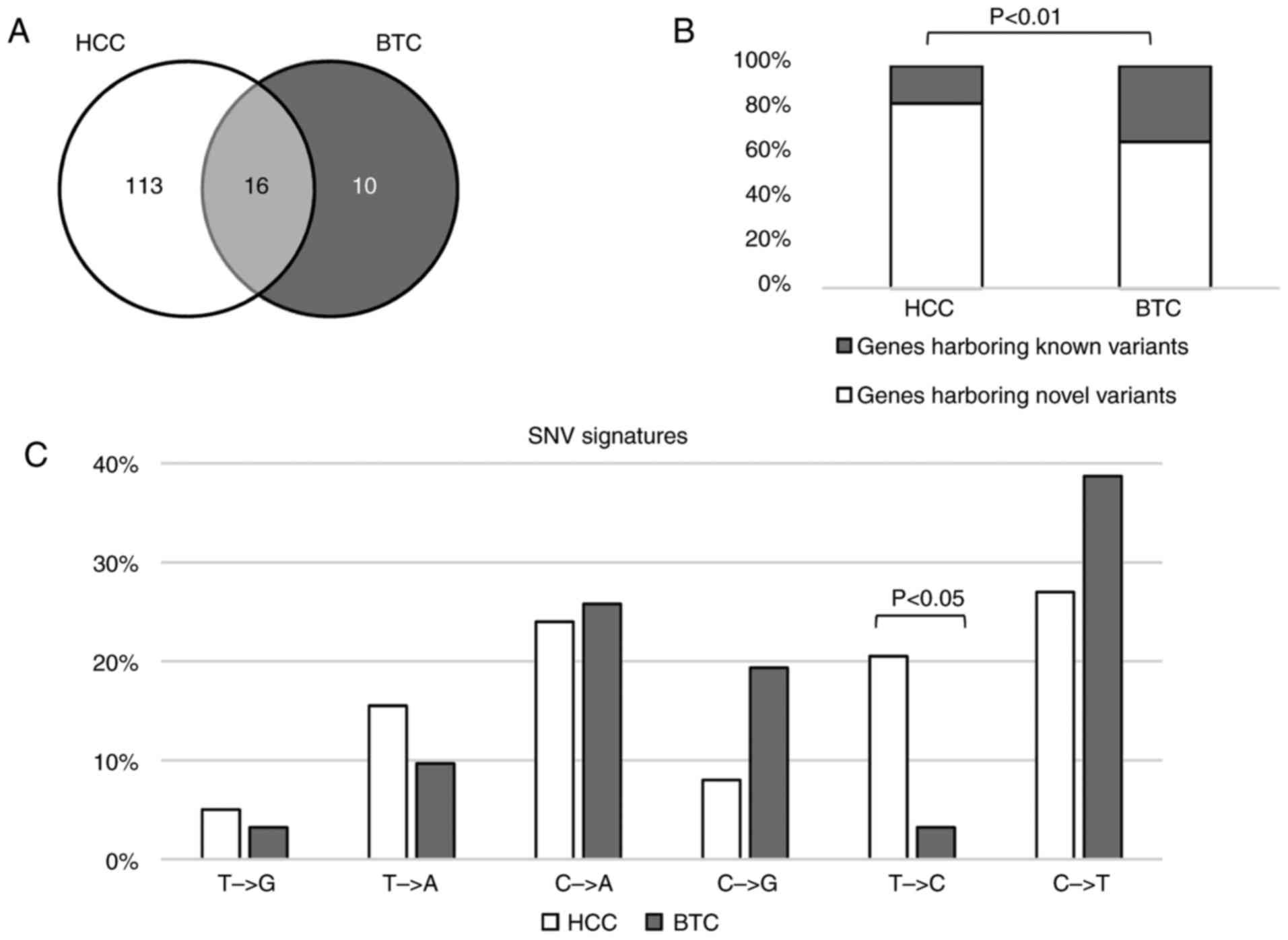
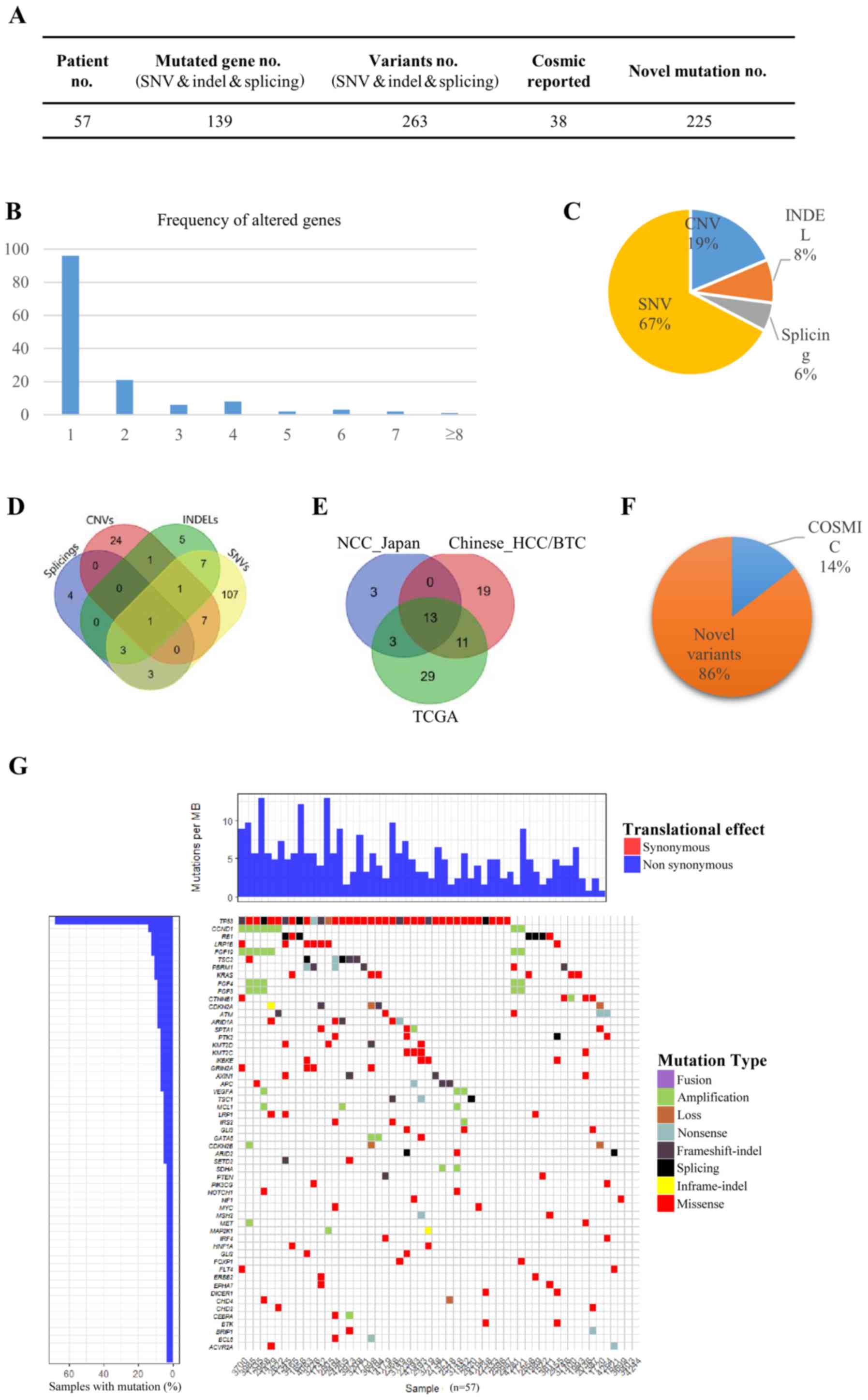 | Figure 1.Somatic alterations in HCC and BTC.
(A) In total, 263 variants were detected in 139 genes in the 57
patients with HCC/BTC. Of all variants detected, 225 were novel
variants. (B) The comparison of mutated genes among TCGA, NCC_JP
and the current HCC/BTC datasets. (C) The frequency of altered
genes in the patients. (D) Of all 344 variants detected, 67.2,
18.6, 8.4 and 5.5% were SNVs, CNVs, INDELs and splices,
respectively. (E) Genes with different types of mutation. (F) Of
all variants detected, 85.6% had not previously been reported in
the COSMIC database. (G) The heat-map for different types of
variants in the patients. HCC, hepatocellular carcinoma; BTC,
biliary tract cancer; SNVs, single nucleotide variants; CNVs, copy
number variants; INDELs, small insertions or deletions. |
To determine whether the mutated genes in the
patients with HCC/BTC were similar to those in published datasets,
recurrent genes were compared in the current dataset. The Cancer
Genome Atlas (TCGA) dataset and the National Cancer Center Japan
(NCC_JP) dataset. Notably, only 13 genes were shared across all
three datasets (Fig. 1E). TP53
(64.9%) and catenin-β1 (CTNNB1; 7.0%) were two of the genes that
appeared among the most frequently mutated genes in all three
datasets. This finding is consistent with the data reported by Kan
et al (5). Low-density
lipoprotein receptor-related protein 1B (LRP1B; 12.3%) and
retinoblastoma gene (RB1; 12.3%) were another two genes that were
frequently mutated in the tested samples. Notably, the mutation
rate of LRP1B in TCGA dataset was markedly higher compared with
that in the NCC_JP dataset, whereas RB1 exhibited the opposite
pattern. These results indicated diverse HCC/BTC variant patterns
among different populations.
Of all the variants detected, 85.6% had not
previously been reported in the Catalogue Of Somatic Mutations In
Cancer (COSMIC) database (Fig. 1A and
F). These novel variants were distributed across 96.5% (55/57)
of the patients and as 95.0% (132/139) of the mutated genes,
suggesting the existence of a dynamic mutation behavior in HCC/BTC
(Fig. 1G).
HCC vs. BTC
To identify the differences in somatic variants in
HCC and BTC, the mutated genes in these two types of cancer were
initially compared. As depicted in Fig.
2A, 16 genes were mutated in HCC and BTC. Of all the mutated
genes, 11.5% of the genes were mutated in HCC and BTC. In the HCC
samples, 83.2% of the mutated genes harbored novel variants, which
was significantly higher than that in the BTC samples (65.9%;
binomial P<0.01; Fig. 2B).
It has been reported that SNV signatures are diverse
in different types of cancer (20).
To determine the mutation signature differences in HCC and BTC, the
nucleotide alteration patterns between HCC and BTC were calculated
and compared. SNVs in these two types of cancer shared similar
alteration signatures except for T>C, which more frequently
occurred in HCC (Fig. 2C).
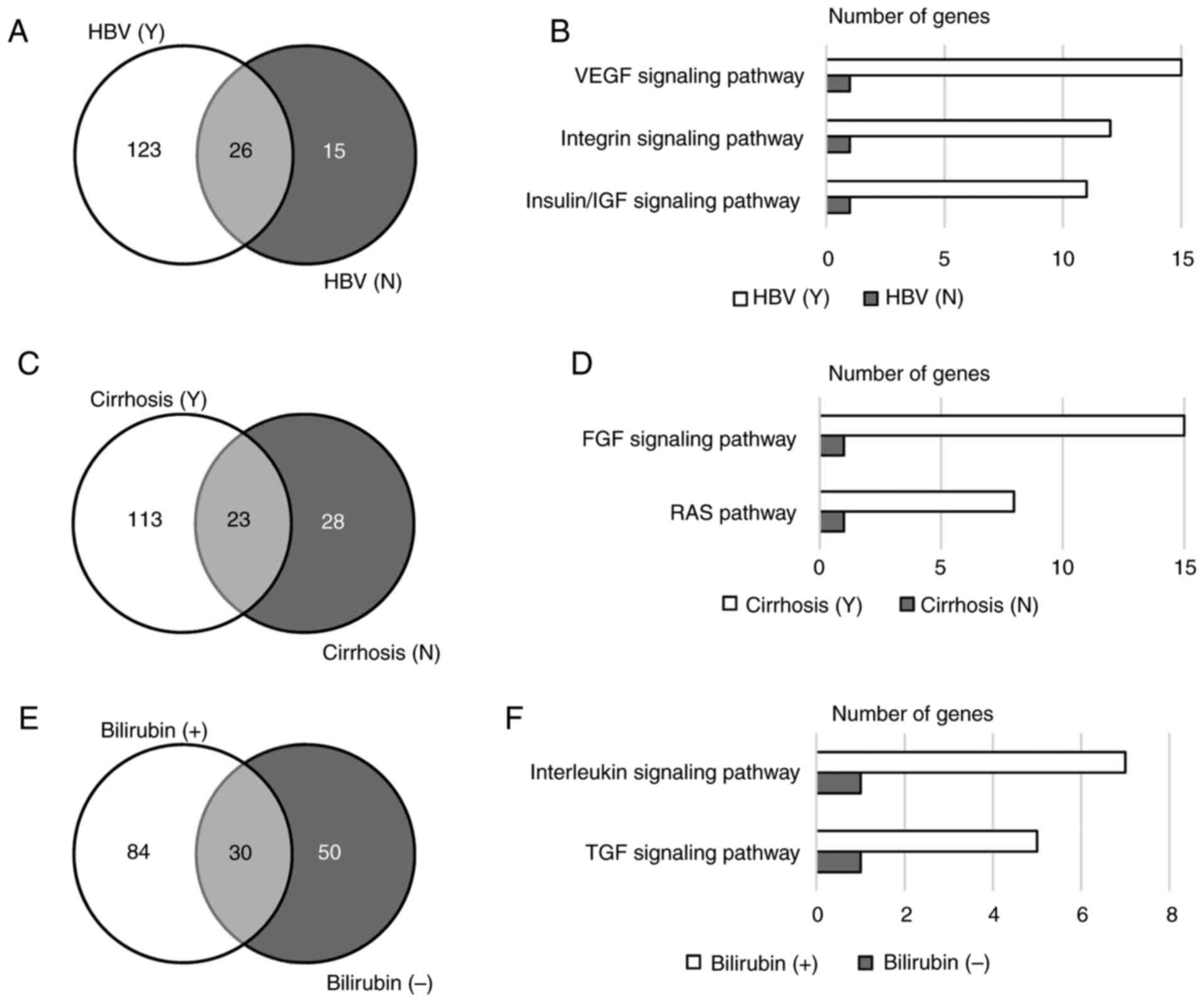
Variants relevant to HBV
Among the 57 tested patients, 42 were HBV carriers
and 15 were not (Table I). Only 26
(16.4%) genes were mutated in patients regardless of their HBV
infection status (Fig. 3A). Gene
ontology analysis revealed that genes mutated in HBV carriers were
enriched in the VEGF, integrin and insulin/IGF signaling pathways
(P<0.001; Fig. 3B). Among the
genes involved in these signaling pathways, KRAS proto-oncogene and
phosphatase and tensin homolog are the genes that were mutated in
HBV carriers and non-HBV carriers. Other genes in these signaling
pathways that were exclusively mutated in HBV carriers included
VEGFA, RAF1, protein tyrosine kinase 2, protein kinase c iota type
(PRKCI), phospholipase Cγ2 (PLCG2), polycystin 2,
phosphoinositide-3-kinase (PIK3) regulatory subunit 2, PIK3
catalytic subunit-γ (PIK3CG), PIK3C-δ (PIK3CD), PIK3C-α (PIK3CA),
PIK3C2-β (PIK3C2B), mitogen-activated protein kinase (MAPK) kinase
1 (MAP2K1), MAPK kinase kinase 1 (MAP3K1), HRAS proto-oncogene,
Fms-related tyrosine kinase 4, LCK proto-oncogene, insulin receptor
substrate 2, IGF2, IGF1 receptor, TSC1 and TSC2. In addition, the
average number of variants per sample in HBV carriers was higher
than that in non-HBV carriers, and this trend was more significant
with novel variants (Table II).
These results indicated that patients with HBV harbored more
mutations than those without HBV, and that HBV infection may
correlate with mutations in angiogenesis and cell cycle-related
signaling pathways.
 | Table II.Association between clinical
characteristics and number of mutations. |
Table II.
Association between clinical
characteristics and number of mutations.
| Characteristic | Category | Sample number | Variants
number | Novel variants
number | Variants per
sample | Novel variants per
sample |
|---|
| Sex | Male | 50 | 244 | 195 | 4.9 | 3.9 |
|
| Female | 7 | 35 | 30 | 5.0 | 4.3 |
| Smoking | Yes | 21 | 94 | 75 | 4.5 | 3.6 |
|
|
Occasionally/no | 34 | 181 | 148 | 5.3 | 4.4 |
| Alcohol | Yes | 15 | 66 | 52 | 4.4 | 3.5 |
|
|
Occasionally/no | 40 | 209 | 171 | 5.2 | 4.3 |
| HBV | Yes | 42 | 232 | 196 | 5.5 | 4.7 |
|
| No | 15 | 47 | 29 | 3.1 | 1.9 |
| Alcoholic
hepatitis | Yes | 5 | 15 | 10 | 3.0 | 2.0 |
|
| No | 52 | 264 | 215 | 5.1 | 4.1 |
| Fatty liver | Yes | 3 | 13 | 10 | 4.3 | 3.3 |
|
| No | 53 | 261 | 211 | 4.9 | 4.0 |
| Cirrhosis and liver
fibrosis | Yes | 41 | 210 | 174 | 5.1 | 4.2 |
|
| No | 15 | 64 | 47 | 4.3 | 3.1 |
| Family cancer
history | Yes | 27 | 146 | 116 | 5.4 | 4.3 |
|
| No | 30 | 133 | 109 | 4.4 | 3.6 |
| Metastases | Yes | 6 | 25 | 20 | 4.2 | 3.3 |
|
| No | 50 | 249 | 201 | 5.0 | 4.0 |
| Degree of
differentiation |
Undifferentiated/low | 5 | 11 | 7 | 2.2 | 1.4 |
|
| Middle/high | 49 | 251 | 205 | 5.1 | 4.2 |
| Largest diameter of
the tumor, cm | <5 | 26 | 125 | 102 | 4.8 | 3.9 |
|
| ≥5 | 27 | 124 | 99 | 4.6 | 3.7 |
| Bilirubin,
mg/l | 1–10 | 20 | 108 | 90 | 5.4 | 4.5 |
|
| >10 | 36 | 166 | 131 | 4.6 | 3.6 |
| AFP, ng/ml | <25 | 34 | 165 | 128 | 4.9 | 3.8 |
|
| ≥25 | 22 | 109 | 93 | 5.0 | 4.2 |
| ALT | 0–40 | 36 | 197 | 160 | 5.5 | 4.4 |
|
| >40 | 19 | 72 | 56 | 3.8 | 3.0 |
| Child-Pugh
classification | 0/A | 52 | 256 | 208 | 4.9 | 4.0 |
|
| B/C/D | 1 | 0 | 0 | 0.0 | 0.0 |
| BCLC | 0/A | 12 | 64 | 55 | 5.3 | 4.6 |
|
| B/C | 39 | 197 | 159 | 5.1 | 4.1 |
| Stage | I/II | 30 | 173 | 142 | 5.8 | 4.7 |
|
| III/IV | 24 | 100 | 79 | 4.2 | 3.3 |
| Lesion | Primary | 52 | 258 | 208 | 5.0 | 4.0 |
|
| Secondary | 2 | 8 | 7 | 4.0 | 3.5 |
| Nodules | Single | 42 | 198 | 158 | 4.7 | 3.8 |
|
| Multiple | 14 | 76 | 63 | 5.4 | 4.5 |
| Number of
tumors | 1 | 42 | 198 | 158 | 4.7 | 3.8 |
|
| >1 | 7 | 39 | 30 | 5.6 | 4.3 |
| Microvascular
invasion (tumor thrombus) | Yes | 44 | 215 | 176 | 4.9 | 4.0 |
|
| No | 9 | 40 | 30 | 4.4 | 3.3 |
| Large vascular
invasion (tumor thrombus) | Yes | 4 | 10 | 7 | 2.5 | 1.8 |
|
| No | 49 | 245 | 199 | 5.0 | 4.1 |
| Portal vein
invasion (tumor thrombus) | Yes | 9 | 25 | 19 | 2.8 | 2.1 |
|
| No | 44 | 230 | 187 | 5.2 | 4.3 |
| Peripheral nerve
invasion | Yes | 4 | 7 | 2 | 1.8 | 0.5 |
|
| No | 49 | 248 | 204 | 5.1 | 4.2 |
Variants associated with
cirrhosis/liver fibrosis
Patients with liver carcinoma are frequently
diagnosed with cirrhosis, which has a strong correlation with HBV
infection and chronic hepatitis (21). In the present study, 41 (71.9%)
patients were diagnosed with cirrhosis. Patients with cirrhosis and
those without cirrhosis shared 23 mutated genes, including KRAS
(Fig. 3C). Genes exclusively mutated
in patients with cirrhosis were enriched in the FGF signaling
pathway and the RAS pathway (P<0.01; Fig. 3D). These two signaling pathways share
numerous genes, including PIK3CG, PIK3CD, PIK3CA, MAP3K1, MAP2K1,
RAF1, KRAS and HRAS, but not FGF3, FGF4, FGFR1, FGFR substrate 2,
PIK3C2B, PLCG2 and PRKCI, which are involved in the FGF signaling
pathway only. Similar to HBV carriers, patients with cirrhosis also
harbored significantly more novel variants than those without
cirrhosis (Table II).
Variants relevant to bilirubin
level
In the present study, 20 patients had a normal level
of bilirubin, while 36 patients exhibited abnormal bilirubin levels
(Table II). Genes mutated in these
two populations were different, although 18.3% of the genes were
shared by the two groups (Fig. 3E).
Altered genes in patients with abnormal bilirubin levels were
enriched in the TGF and interleukin signaling pathways (P<0.05;
Fig. 3F). There was no correlation
between mutation burden and bilirubin abnormality (Table II).
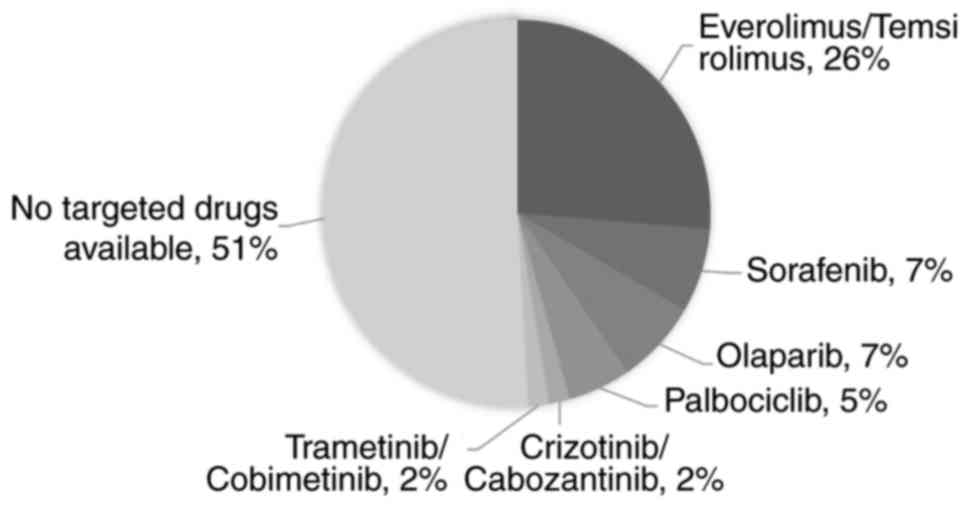
Variants relevant to targeted
drugs
To identify the number of patients with liver and
biliary carcinoma that harbored drug-targetable mutations, the
mutated genes that have been approved to be the targets of existing
drugs were analyzed. As depicted in Fig.
4, 7% of the patients could possibly benefit from sorafenib,
which is the only drug that has been approved to treat patients
with advanced HCC (22). A total of
42% of the patients had mutations that were targets of
everolimus/temsirolimus (26%), olaparib (7%), palbociclib (5%),
crizotinib/cabozantinib (2%) or trametinib/cobimetinib (2%).
Notably, the number of patients with mutations targetable by
everolimus/temsirolimus was markedly higher than the number of
patients with mutations targetable by sorafenib, indicating that
these drugs may have promise in HCC/BTC therapy.
Discussion
HCC and BTC are two different types of tumor. They
are morphologically distinct from each other, and vary markedly in
clinical features: For example, jaundice is a prominent symptom of
BTC but not of HCC; and carbohydrate antigen 19-9 is a widely used
marker in diagnosing BTC, while α-fetoprotein is the gold standard
in HCC diagnostics. At the molecular level, carcinogenesis is a
complex process resulting from the accumulation of genetic
alterations of various cancer-driver genes. The majority of cancer
types, including HCC and BTC, are genetically and biologically
heterogeneous, which causes the response of patients with the same
type of cancer to differ when administered with the same treatment,
due to substantial variations in the molecular mutations underlying
carcinogenesis. Accumulating studies have identified the presence
of common molecular mutations beyond those of the traditionally
viewed histological tumor subtypes (23). It has been demonstrated that the
expression of SLC22A1 variants may affect the response of HCC and
cholangiocarcinoma to sorafenib (24). Additionally, a recent study identified
that Asian patients with either HCC or cholangiocarcinoma, though
clinically treated as separate entities, share common molecular
subtypes with similar actionable drivers (25). Clinically, numerous trials are ongoing
for HCC and BTC, including a single arm phase II trial of
gemcitabine and oxaliplatin with erlotinib (Tarceva) for the
treatment of patients with HCC and BTC (NCT00832637), a phase II
trial of BBI503 in adult patients with advanced hepatobiliary
cancer (NCT02232633), and phase I and II trials of combined immune
checkpoint inhibition in combination with ablative therapies in
patients with HCC or BTC (NCT02821754).
In the human genome, there are six patterns of base
substitution (C>A, C>G, C>T, T>A, T>C and T>G).
Base substitution patterns are affected by exogenous or endogenous
mutagens, including oxidative stress, exposure to chemicals or
ultraviolet radiation and defects in the DNA repair machinery
(26). It has been reported that
C>T and T>C substitutions are dominant in HCC (27). In line with these findings, the
results of the present study demonstrated that C>T (27%), C>A
(24%) and T>C (21%) were the top 3 base substitutions in HCC
cases (Fig. 2C). In addition, T>A
transitions also frequently occurred (16%) in the HCC cases, which
to the best of our knowledge has not been reported previously. A
previous study revealed that C>T transitions were dominant in
cholangiocarcinoma with liver fluke (28). Consistent with the results of this
previous study, the most frequently occurring base substitutions in
the present study were C>T transitions (39%). Furthermore, 26%
of the base substitutions were C>A transitions, indicating that
C>A alterations may be another signature of BTC substitutions.
Unlike HCC, the T>C transitions were rarely identified in BTC
(P<0.05), revealing distinct mutation signatures in HCC and
BTC.
A previous WGS study of 88 HCC cases identified an
average somatic mutation rate of 3.69 per Mb, which is mid-range
among all types of cancer (5). In the
present study, the average somatic mutation rate was 4.89 per
sample, which is equal to 3.95 per Mb. Notably, HBV-positive cases
exhibited a significantly higher mutation rate (5.5 per sample),
compared with HBV-negative cases (3.1 per sample; Table II). This difference was even greater
for newly identified variants (4.7 vs. 1.9 per sample). As HBV
infection causes genome instabilities and is associated with a poor
prognosis of liver cancer, these findings confirmed that the
presence of HBV infection is associated with the frequencies of
mutations that lead to the complexity of liver cancer.
HBV is a DNA virus with a genome that integrates
into the host genome. Studies have demonstrated that HBV
integration in the telomerase reverse transcriptase,
myeloid/lymphoid lineage 4, lysine methyltransferase 2b, cyclin E1
and fibronectin 1 genes is frequent in cases of HCC (11,29,30). It
was reported that genes involved in the WNT/CTNNB1 and Janus
kinase/signal transducer and activator of transcription pathways
were frequently mutated in HBV-positive HCC cases (30). By contrast to these findings, the
present study demonstrated that genes in the VEGF, integrin and
insulin/IGF signaling pathways were frequently mutated in
HBV-positive cases, but not in HBV-negative cases. Notably, a
previous study reported that the expression of the HBV protein HBx
can upregulate VEGF and hypoxia-inducible factor-1α to induce the
angiogenesis response (31). It was
also reported that the HBx protein may serve roles in cell
spreading by modulating the balance of cell adhesion (32). The results of the present study
suggested that HBV infection may induce angiogenesis and cell
adhesion changes through genomic alterations and expression of
virus proteins. Further studies are required to determine the
mechanisms underlying mutations in angiogenesis and cell adhesion
genes caused by HBV infection. Frequent mutations in these
signaling pathways in HBV-positive patients suggested that drugs
targeting these pathways may be worthy of clinical trials in
HBV-positive HCC patients.
At present, surgery remains the most effective
treatment for patients with liver cancer. Sorafenib, a drug
targeting VEGFA, has been approved for the treatment of advanced
HCC (22). However, the results of
the present study indicated that few patients (7%) may benefit from
this drug. Notably, approximately half (42%) of the patients in the
present study harbored target mutations of drugs that have been
approved to treat other types of cancer. Certain drug targets,
including mTOR pathway genes, occurred more frequently than the
targets of sorafenib. Further clinical studies are warranted to
evaluate the efficacies of these drugs in patients with liver
cancer. Notably, 51% of patients in the present study did not
harbor targets of any drugs. Studies with a larger cohort may
elucidate the biomarkers that are more widespread in patients with
liver cancer.
In recent years, advances in immunotherapy have
provided novel therapeutic strategies for cancer patients with
complicated cases. Immunotherapeutics, including antibodies that
block programmed death receptor-1 (PD-1)/programmed death-ligand 1
(PD-L1) may induce durable responses across numerous types of tumor
(33–36). However, the majority of
biomarker-unselected patients will not respond to immunotherapy;
therefore, there is an unmet requirement to determine biomarkers
that will identify patients more likely to respond to PD-1/PD-L1
blockade, as well as other immunotherapeutics. Cancer is caused by
the accumulation of somatic mutations, and high tumor mutational
burden (TMB) may be a response biomarker for PD-1/PD-L1 blockade in
tumors. It was demonstrated in non-small cell lung cancer that a
higher TMB was associated with improved objective response, durable
clinical benefit and progression-free survival (37). However, it is unclear whether TMB
serves as a useful biomarker for predicting response to
immunotherapy in HCC. In a recent phase 1/2 clinical trial
(NCT01658878), the PD-1 inhibitor nivolumab was assessed for safety
and efficacy in patients with advanced HCC. A manageable safety
profile and durable objective responses indicated the potential of
nivolumab for the treatment of advanced HCC (38). As indicated in the present study, HCC
and BTC exhibit relatively high TMB compared with other tumors, and
HBV carriers have an even higher mutation load. Therefore, further
clinical studies are required to evaluate the efficacies of
immunotherapy in HCC and BTC, particularly in HBV-positive HCC
patients.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BLZ analyzed the somatic alterations in HCC and BTC
and wrote the manuscript. XJ collected the samples and clinical
information of the patients and wrote the manuscript. LXY
identified the differences in somatic variants in HCC and BTC and
wrote the manuscript. YG performed gene ontology analysis. C-HX
conducted drug-targetable mutation analysis. JL collected the
samples and clinical information of the patients. DXZ performed the
pathway analysis. YL made correlation analysis between the
informatic data and clinical information. GHD performed
bioinformatics analysis of the targeted NGS data. JYS contributed
to data interpretation and wrote the manuscript. GHL made
correlation analysis between the informatic data and clinical
information. GLL discussed the results and contributed toward data
interpretation. PY detected the bilirubin, albumin and AFP levels
of the patients. RLW performed bioinformatics analysis of the
targeted NGS data. JZW conducted the HBV tests. PHY collected the
samples and clinical information of the patients. JY detected the
bilirubin, albumin and AFP levels of the patients. JYL constructed
the sequencing libraries and performed NGS. JJX extracted genomic
DNA from tumor tissue and peripheral blood mononuclear cell. SGZ
conceived the study and participated in its design and
coordination. HT conceived the study and participated in its
design. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Independent
Ethics Committee of the 302 Military Hospital of China. All the
patients provided written informed consent for the genomic testing
used for the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
El-Serag HB: Epidemiology of viral
hepatitis and hepatocellular carcinoma. Gastroenterology.
142:1264–1273.e1. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shaib Y and El-Serag HB: The epidemiology
of cholangiocarcinoma. Semin Liver Dis. 24:115–125. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kan Z, Zheng H, Liu X, Li S, Barber TD,
Gong Z, Gao H, Hao K, Willard MD, Xu J, et al: Whole-genome
sequencing identifies recurrent mutations in hepatocellular
carcinoma. Genome Res. 23:1422–1433. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hennedige TP, Neo WT and Venkatesh SK:
Imaging of malignancies of the biliary tract-an update. Cancer
Imaging. 14:142014.PubMed/NCBI
|
|
7
|
Jain A, Kwong LN and Javle M: Genomic
profiling of biliary tract cancers and implications for clinical
practice. Curr Treat Options Oncol. 17:582016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jain A and Javle M: Molecular profiling of
biliary tract cancer: A target rich disease. J Gastrointest Oncol.
7:797–803. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fujimoto A, Totoki Y, Abe T, Boroevich KA,
Hosoda F, Nguyen HH, Aoki M, Hosono N, Kubo M, Miya F, et al:
Whole-genome sequencing of liver cancers identifies etiological
influences on mutation patterns and recurrent mutations in
chromatin regulators. Nat Genet. 44:760–764. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang Z, Jhunjhunwala S, Liu J, Haverty
PM, Kennemer MI, Guan Y, Lee W, Carnevali P, Stinson J, Johnson S,
et al: The effects of hepatitis B virus integration into the
genomes of hepatocellular carcinoma patients. Genome Res.
22:593–601. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sung WK, Zheng H, Li S, Chen R, Liu X, Li
Y, Lee NP, Lee WH, Ariyaratne PN, Tennakoon C, et al: Genome-wide
survey of recurrent HBV integration in hepatocellular carcinoma.
Nat Genet. 44:765–769. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao LH, Liu X, Yan HX, Li WY, Zeng X,
Yang Y, Zhao J, Liu SP, Zhuang XH, Lin C, et al: Genomic and
oncogenic preference of HBV integration in hepatocellular
carcinoma. Nat Commun. 7:129922016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang P, Markowitz GJ and Wang XF: The
hepatitis B virus-associated tumor microenvironment in
hepatocellular carcinoma. Natl Sci Rev. 1:396–412. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang S, Hu W, Hu J, Wu S, Li J, Luo Y, Cao
M, Zhou H and Jiang X: Hepatitis B virus X protein promotes P3
transcript expression of the insulin-like growth factor 2 gene via
inducing hypomethylation of P3 promoter in hepatocellular
carcinoma. Liver Int. 35:608–619. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pan J, Clayton M and Feitelson MA:
Hepatitis B virus X antigen promotes transforming growth
factor-beta1 (TGF-beta1) activity by up-regulation of TGF-beta1 and
down-regulation of alpha2-macroglobulin. J Gen Virol. 85:275–282.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yen CJ, Lin YJ, Yen CS, Tsai HW, Tsai TF,
Chang KY, Huang WC, Lin PW, Chiang CW and Chang TT: Hepatitis B
virus X protein upregulates mTOR signaling through IKKβ to increase
cell proliferation and VEGF production in hepatocellular carcinoma.
PLoS One. 7:e419312012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang T, Zhang J, You X, Liu Q, Du Y, Gao
Y, Shan C, Kong G, Wang Y, Yang X, et al: Hepatitis B virus X
protein modulates oncogene Yes-associated protein by CREB to
promote growth of hepatoma cells. Hepatology. 56:2051–2059. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hsieh A, Kim HS, Lim SO, Yu DY and Jung G:
Hepatitis B viral X protein interacts with tumor suppressor
adenomatous polyposis coli to activate Wnt/β-catenin signaling.
Cancer Lett. 300:162–172. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim HY, Cho HK, Hong SP and Cheong J:
Hepatitis B virus X protein stimulates the Hedgehog-Gli activation
through protein stabilization and nuclear localization of Gli1 in
liver cancer cells. Cancer Lett. 309:176–184. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alexandrov LB, Nik-Zainal S, Wedge DC,
Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A,
Børresen-Dale AL, et al: Signatures of mutational processes in
human cancer. Nature. 500:415–421. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang JD, Kim WR, Coelho R, Mettler TA,
Benson JT, Sanderson SO, Therneau TM, Kim B and Roberts LR:
Cirrhosis is present in most patients with hepatitis B and
hepatocellular carcinoma. Clin Gastroenterol Hepatol. 9:64–70.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dotto GP and Rustgi AK: Squamous cell
cancers: A unified perspective on biology and genetics. Cancer
Cell. 29:622–637. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Herraez E, Lozano E, Macias RI, Vaquero J,
Bujanda L, Banales JM, Marin JJ and Briz O: Expression of SLC22A1
variants may affect the response of hepatocellular carcinoma and
cholangiocarcinoma to sorafenib. Hepatology. 58:1065–1073. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chaisaingmongkol J, Budhu A, Dang H,
Rabibhadana S, Pupacdi B, Kwon SM, Forgues M, Pomyen Y,
Bhudhisawasdi V, Lertprasertsuke N, et al: Common molecular
subtypes among asian hepatocellular carcinoma and
cholangiocarcinoma. Cancer Cell. 32:57–70.e3. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Helleday T, Eshtad S and Nik-Zainal S:
Mechanisms underlying mutational signatures in human cancers. Nat
Rev Genet. 15:585–598. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Totoki Y, Tatsuno K, Yamamoto S, Arai Y,
Hosoda F, Ishikawa S, Tsutsumi S, Sonoda K, Totsuka H, Shirakihara
T, et al: High-resolution characterization of a hepatocellular
carcinoma genome. Nat Genet. 43:464–469. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ong CK, Subimerb C, Pairojkul C, Wongkham
S, Cutcutache I, Yu W, McPherson JR, Allen GE, Ng CC, Wong BH, et
al: Exome sequencing of liver fluke-associated cholangiocarcinoma.
Nat Genet. 44:690–693. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dong H, Zhang L, Qian Z, Zhu X, Zhu G,
Chen Y, Xie X, Ye Q, Zang J, Ren Z and Ji Q: Identification of
HBV-MLL4 integration and its molecular basis in chinese
hepatocellular carcinoma. PLoS One. 10:e01231752015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li X, Zhang J, Yang Z, Kang J, Jiang S,
Zhang T, Chen T, Li M, Lv Q, Chen X, et al: The function of
targeted host genes determines the oncogenicity of HBV integration
in hepatocellular carcinoma. J Hepatol. 60:975–984. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee YY, Mok MT and Cheng AS: Dissecting
the pleiotropic actions of HBx mutants against hypoxia in
hepatocellular carcinoma. Hepatobiliary Surg Nutr. 3:95–97.
2014.PubMed/NCBI
|
|
32
|
Lara-Pezzi E, Majano PL, Yáñez-Mó M,
Gómez-Gonzalo M, Carretero M, Moreno-Otero R, Sánchez-Madrid F and
López-Cabrera M: Effect of the hepatitis B virus HBx protein on
integrin-mediated adhesion to and migration on extracellular
matrix. J Hepatol. 34:409–415. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Robert C, Long GV, Brady B, Dutriaux C,
Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C,
Kalinka-Warzocha E, et al: Nivolumab in previously untreated
melanoma without BRAF mutation. N Engl J Med. 372:320–330. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Garon EB, Rizvi NA, Hui R, Leighl N,
Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L,
et al: Pembrolizumab for the treatment of non-small-cell lung
cancer. N Engl J Med. 372:2018–2028. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Motzer RJ, Escudier B, McDermott DF,
George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G,
Plimack ER, et al: Nivolumab versus everolimus in advanced
renal-cell carcinoma. N Engl J Med. 373:1803–1813. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rizvi NA, Hellmann MD, Snyder A, Kvistborg
P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al: Cancer
immunology. Mutational landscape determines sensitivity to PD-1
blockade in non-small cell lung cancer. Science. 348:124–128. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
El-Khoueiry AB, Sangro B, Yau T, Crocenzi
TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, et al:
Nivolumab in patients with advanced hepatocellular carcinoma
(CheckMate 040): An open-label, non-comparative, phase 1/2 dose
escalation and expansion trial. Lancet. 389:2492–2502. 2017.
View Article : Google Scholar : PubMed/NCBI
|