Introduction
Pancreatic cancer is one of the most common types of
cancer that leads to increased mortality (1). Although significant improvements have
been made in the diagnosis and treatment of pancreatic cancer in
the last few decades, the prognosis of pancreatic cancer remains
poor due to its aggressiveness and early systemic dissemination
(2). The majority of patients with
pancreatic cancer is diagnosed at an advanced stage of disease and
is not eligible for curative resection, thus leading to high
mortality rate (3). Therefore, it is
imperative to investigate the molecular mechanisms underlying the
development of pancreatic cancer in order to identify biological
markers for the early diagnosis and the development of novel
therapeutic agents in pancreatic cancer.
Long non-coding RNAs (lncRNAs) are a class of RNA
molecules >200 nucleotides in length that do not encode proteins
(4). Accumulating evidence has
suggested that lncRNAs exhibit important roles in a number of
fundamental cellular processes, including cell growth, apoptosis
and differentiation by regulating gene expression at the
transcriptional or post-transcriptional level (5,6). Previous
studies have demonstrated that several lncRNAs may be dysregulated
in various types of human cancer (7,8).
Pseudogenes including small ubiquitin-like modifier 1 pseudogene
(SUMO1P3) are considered as a separate class of lncRNAs (9).
SUMO1P3 was originally identified as a potential
biomarker for the diagnosis of gastric cancer in 2013 (9). Furthermore, increased expression of
SUMO1P3 has been reported in bladder cancer tissues, and it is
significantly associated with a greater histological grade and
advanced tumor-node metastasis (TNM) stage (10). An in vitro study further
demonstrated that knockdown of SUMO1P3 inhibited bladder cancer
cell proliferation and induced bladder cancer cell apoptosis
(10). However, the biological
function of SUMO1P3 in pancreatic cancer and its potential
molecular mechanisms remain unclear.
In the present study, the expression of SUMO1P3 in
pancreatic cancer tissues and the paired adjacent normal pancreatic
tissues was evaluated, and its effects on epithelial-mesenchymal
transition (EMT). The objective of the present study was to clarify
the biological function and potential mechanism of SUMO1P3 in the
development of pancreatic cancer.
Materials and methods
Patient tissue samples
A total of 48 pairs of pancreatic cancer tissues and
paired adjacent normal tissues were collected from patients
undergoing surgical resection at The Pancreatic Cancer Center,
Fenjinting Hospital between January 2009 and October 2013 (mean age
63.3±8.86, age range, 37–71). All of the enrolled patients in the
present study underwent surgery during which samples were
collected. All specimens were frozen and stored in liquid nitrogen
until further use. No patients received preoperative anticancer
treatment, including chemotherapy or radiation prior to specimen
collection. The present study was conducted with the approval of
the Ethics and Research Committees of Fenjinting Hospital (Jiangsu,
China) and was performed in accordance with the Declaration of
Helsinki. All patients provided written informed consent prior to
their participation in the present study. The clinical
characteristics of all the patients are summarized in Table I.
 | Table I.Association between the expression of
SUMO1P3 and the clinicopathological characteristics of patients
with pancreatic cancer. |
Table I.
Association between the expression of
SUMO1P3 and the clinicopathological characteristics of patients
with pancreatic cancer.
|
|
| SUMO1P3 expression,
n |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
parameters | n | High | Low | P-value |
|---|
|
| 48 | 33 | 15 |
|
| Age, years |
|
|
| 0.633 |
|
<60 | 19 | 12 | 7 |
|
| ≥60 | 29 | 21 | 8 |
|
| Sex |
|
|
| 0.416 |
|
Female | 17 | 13 | 4 |
|
| Male | 31 | 20 | 11 |
|
| Tumor size, cm |
|
|
| 0.019 |
|
<4 | 30 | 17 | 13 |
|
| ≥4 | 18 | 16 | 2 |
|
| Histological
differentiation |
|
|
| 0.205 |
| Well and
moderate | 33 | 21 | 12 |
|
| Poor | 15 | 12 | 3 |
|
| Location |
|
|
| 0.371 |
|
Head-neck | 27 | 19 | 8 |
|
|
Body-tail | 21 | 14 | 7 |
|
| Lymph node
metastasis |
|
|
| 0.028 |
|
Negative | 35 | 22 | 13 |
|
|
Positive | 13 | 11 | 2 |
|
| TNM stage |
|
|
| 0.034 |
| I–II | 41 | 26 | 15 |
|
| III | 7 | 7 | 0 |
|
Cell culture
The human pancreatic cancer cell lines BxPC-3,
PANC-1, MiaPaCa-2, and ASPC-1 and the normal pancreatic ductal
epithelial cell line HPDE6-C7 were purchased from the American Type
Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured
in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 50 U/ml
penicillin and 0.1 mg/ml streptomycin. Cells were cultured at 37°C
in a humidified atmosphere containing 5% CO2.
Small interfering (si)RNA
transfection
siRNAs that targeted SUMO1P3 and a scrambled
negative control were purchased from Shanghai GenePharma Co., Ltd.
(Shanghai, China). The target sequence of siSUMO1P3 was
5′-TGGCCCTGATGTTCTAGCATGTGAT-3′. The RNAs were introduced into
cells at a final concentration of 50 nM. Transfections were
performed using the Lipofectamine 3000 kit (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's protocol. The knockdown efficiency was assessed by
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) 48 h after transfection.
RNA extraction and RT-qPCR
Total RNA from tissues and cells was isolated using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. Total RNA was reverse
transcribed into cDNA using PrimeScript RT Reagent kit (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. qPCR was performed using SYBR Premix Ex Taq (Takara
Biotechnology Co., Ltd., Dalian, China), according to the
manufacturer's protocol. The primer sequences were as follows:
SUMO1P3, 5′-ACTGGGAATGGAGGAAGA-3′ (forward) and
5′-TGAGAAAGGATTGAGGGAAAAG-3′ (reverse); GAPDH,
5′-CGCTCTCTGCTCCTCCTGTTC-3′ (forward) and
5′-ATCCGTTGACTCCGACCTTCAC-3′ (reverse). The thermocycling
conditions were as follows: Initial denaturation at 95°C for 10
min, followed by 40 cycles at 95°C for 15 sec and extension at 60°C
for 1 min. Relative expression of PHGDH was normalized to the
expression of GAPDH. Relative expression of PHGDH was calculated
using the 2−ΔΔCq method (11). All experiments were performed in
triplicate. RT-qPCR was performed using the ABI PRISM 7500 PCR
System (Applied Biosystems; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. The median value was the
cutoff between low and high SUMO1P3 expression in patients with
pancreatic cancer. The median value was included in the low
group.
Cell proliferation and colony
formation assay
Following transfection with si-SUMO1P3 or
si-negative control (NC), BxPC-3 panc-1 cells cell proliferation
was accessed using Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc., Kumamoto, Japan), according to the
manufacturer's protocol. A total of 2,000 cells were plated into
96-well plates. Following culture for 24 h, 100 µl CCK-8 was added
into wells and the viable cells were evaluated at a wavelength of
450 nm. For colony formation assay, 500 cells per plate were seeded
in 6-well plates and incubated for two weeks. Then, colonies were
fixed with 4% paraformaldehyde at room temperature for 15 min and
stained with crystal violet. A total of five fields were randomly
selected and cells were counted under a light microscope at low
magnification (×100). Colonies that contained >50 cells were
designated as survivors.
Cell migration and invasion assay
To measure the migratory ability of pancreatic
cancer cells, a wound-healing assay was performed. BXPC-3 and
PANC-1 cells were cultured in a 6-well plate until they reached
100% confluence. The monolayer cells were scratched using a 200 µl
sterile pipette tip to create the wound. Cells were cultured with
DMEM without FBS at 37°C for 24 h. The migration of cells across
the gap wound was measured using light microscopy (×100
magnification). The invasive ability of pancreatic cancer cells was
assessed using a Matrigel-coated Transwell chamber (BD Biosciences,
San Jose, CA, USA). A total of 2×104 cells in 100 µl of
serum-free medium were plated in the upper chamber. DMEM medium
supplemented with 10% FBS was plated in the lower chamber.
Following incubation for 24 h at 37°C in a humidified atmosphere
containing 5% CO2. The non-invading cells on the upper
surface of the well were scraped off with a cotton swab, and the
invading cells on the lower surface were stained with 4% crystal
violet at room temperature for 10 min and counted using a light
microscope (×200 magnification). Each experiment was performed in
triplicate.
Western blot analysis
Cell proteins were extracted with ice-cold lysis
buffer (1 mM EDTA, pH 8.0, 50 mM Tris/HCl, pH 7.4, 150 mM NaCl, 1%
NP-40, 0.1% SDS and 0.5% sodium deoxycholate, pH 7.4). After 30 min
on ice, cell debris was removed by centrifugation at 15,000 × g for
15 min at 4°C. A basic protein quantification kit (BioVision, Inc.,
CA, USA) was used to determine total protein concentration. Equal
amounts of the protein (20–40 µg/lane) were separated by SDS-PAGE
(10% gels) and transferred onto polyvinylidene difluoride (PVDF)
membranes and the blots were blocked for 1 h using 5% fat-free milk
at room temperature. The membranes were incubated with the
following primary antibodies: Anti-vimentin (5741; Cell Signaling
Technology, Inc., Danvers, MA, USA; 1:1,000 dilution);
Anti-neuronal (N)-cadherin (13116; Cell Signaling Technology;
1:1,000 dilution); Anti-β-catenin (8480; Cell Signaling Technology;
1:1,000 dilution); Anti-Epithelial (E)-cadherin (3195; Cell
Signaling Technology; 1:1,000 dilution); and anti-β-actin antibody
(sc-58673, Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
overnight at 4°C. Following primary incubation, membranes were
incubated with secondary antibodies (cat. no. AB6721; 1:3,000;
Abcam) for 1 h at room temperature Finally, protein bands were
developed with Amersham ECL Western Blotting Detection reagent (GE
Healthcare Life Sciences, Little Chalfont, UK) and visualized using
a gel imaging analysis system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) and further analyzed using Image Lab software
(version 3.0; Bio-Rad Laboratories, Inc.).
Statistical analysis
All results are shown as mean ± standard deviation
and were analyzed using GraphPad Prism version 6 (GraphPad
Software, Inc., La Jolla, CA, USA) from at least three independent
experiments. The χ2 test was performed to explore the
associations between SUMO1P3 level and clinicopathological factors.
The Kaplan-Meier method was used to calculate the survival curve,
and log-rank test to determine statistical significance.
Multivariate analysis was applied to determine the independent
indicator for overall survival of patients with pancreatic cancer.
The differences between groups were analyzed using one-way analysis
of variance (ANOVA) followed by the Student-Newman-Keuls test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of SUMO1P3 is upregulated
in pancreatic cancer tissues and cells
To explore the role of SUMO1P3 in pancreatic cancer,
the relative expression level of SUMO1P3 in 48 pairs of pancreatic
cancer tissues and adjacent non-tumor tissues was examined by
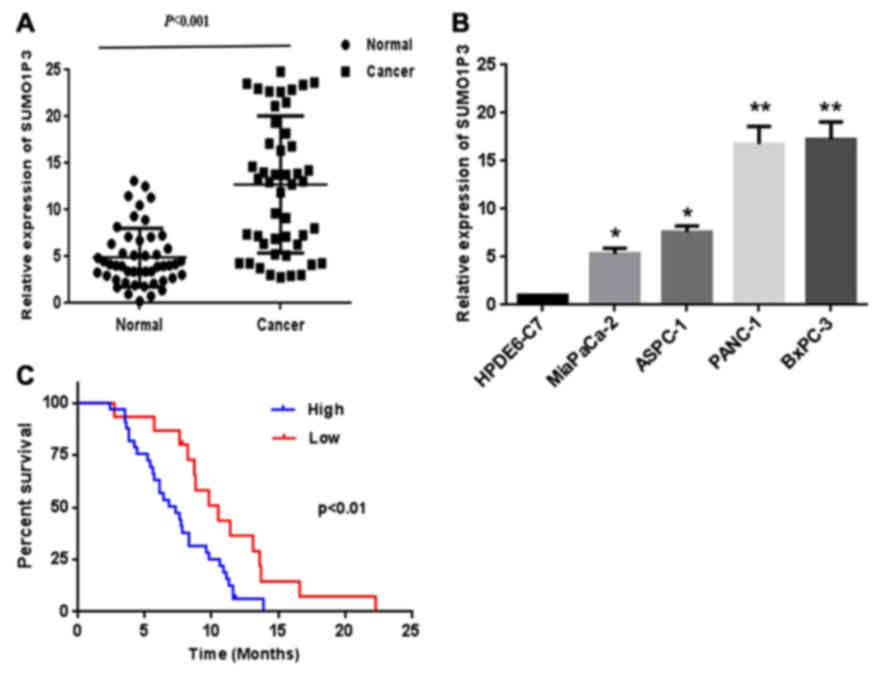
RT-qPCR analysis. As presented in Fig.
1A, increased expression of SUMO1P3 was identified in
pancreatic cancer tissues compared with corresponding adjacent
non-tumor tissues. The expression of SUMO1P3 was assessed in 4
human pancreatic cancer cell lines and normal pancreatic ductal
epithelial cell line HPDE6-C7. The results revealed that pancreatic
cancer cell lines demonstrated a higher expression of SUMO1P3
compared with that in the normal pancreatic ductal epithelial cell
(Fig. 1B).
Increased expression of SUMO1P3 is
associated with the progression and poor prognosis of patients with
pancreatic cancer
The present study further investigated the
association between SUMO1P3 expression and clinicopathological
factors in 48 patients with pancreatic cancer (Table I). The results revealed that the
increased expression of SUMO1P3 was significantly associated with
tumor size (P=0.019), lymph node metastasis (P=0.028) and TNM stage
(P=0.034). However, there was no significant association between
SUMO1P3 expression and age, sex, histological differentiation or
tumor location. Kaplan-Meier survival curves (Fig. 1C) demonstrated that the survival of
patients with pancreatic cancer with a lower expression of SUMO1P3
is significantly improved compared with that of the higher
expression group (the median value was the cutoff between low and
high SUMO1P3 expression). Furthermore, multivariate analysis
indicated that increased expression of SUMO1P3 was an independent
indicator for overall survival of patients with pancreatic cancer
(Table II).
 | Table II.Cox multivariate regression analysis
of the association of prognostic factors in pancreatic cancer. |
Table II.
Cox multivariate regression analysis
of the association of prognostic factors in pancreatic cancer.
|
|
| 95% CI |
|---|
|
|
|
|
|---|
| Factors | P-value | Lower | Upper |
|---|
| SUMO1P3 expression,
high/low | 0.027 | 0.371 | 0.814 |
| Age, years | 0.092 | 0.315 | 1.224 |
| Sex | 0.831 | 0.272 | 1.283 |
| Tumor size, cm | 0.063 | 0.329 | 1.072 |
| Histological
differentiation | 0.744 | 0.295 | 1.316 |
| Location | 0.083 | 0.255 | 1.097 |
| Lymph node
metastasis | 0.062 | 0.216 | 1.013 |
| TNM stage | 0.012 | 0.217 | 0.711 |
Knockdown of SUMO1P3 impairs
proliferation of BXPC-3 and PANC-1 cells in vitro
In order to explore the potential biological
function of SUMO1P3 in the development of pancreatic cancer,
SUMO1P3 expression was silenced in BXPC-3 and PANC-1 cells. As
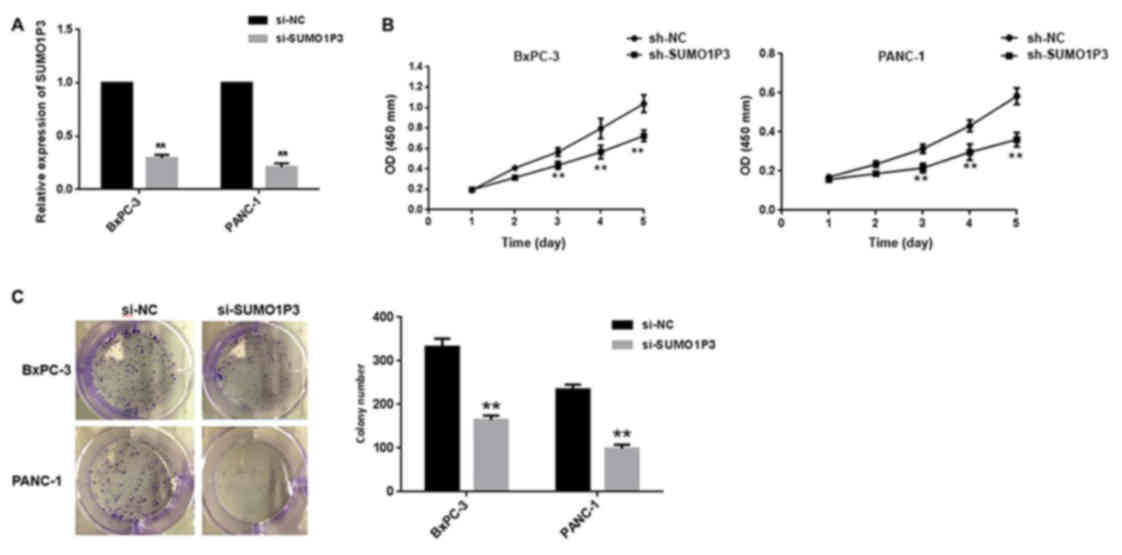
presented in Fig. 2A, mRNA expression
level of SUMO1P3 was significantly decreased in cells transfected
with SUMO1P3 siRNA compared with the control group. Knockdown of
SUMO1P3 significantly suppressed the proliferative ability of
BXPC-3 and PANC-1 cells (Fig. 2B), as
assessed using a CCK-8 assay. Furthermore, a colony formation assay
was also used to evaluate the cell proliferation ability. Knockdown
of SUMO1P3 significantly decreased the colony formation ability of
BXPC-3 and PANC-1 cells (Fig.
2C).
Knockdown of SUMO1P3 inhibits cell
migration and invasion
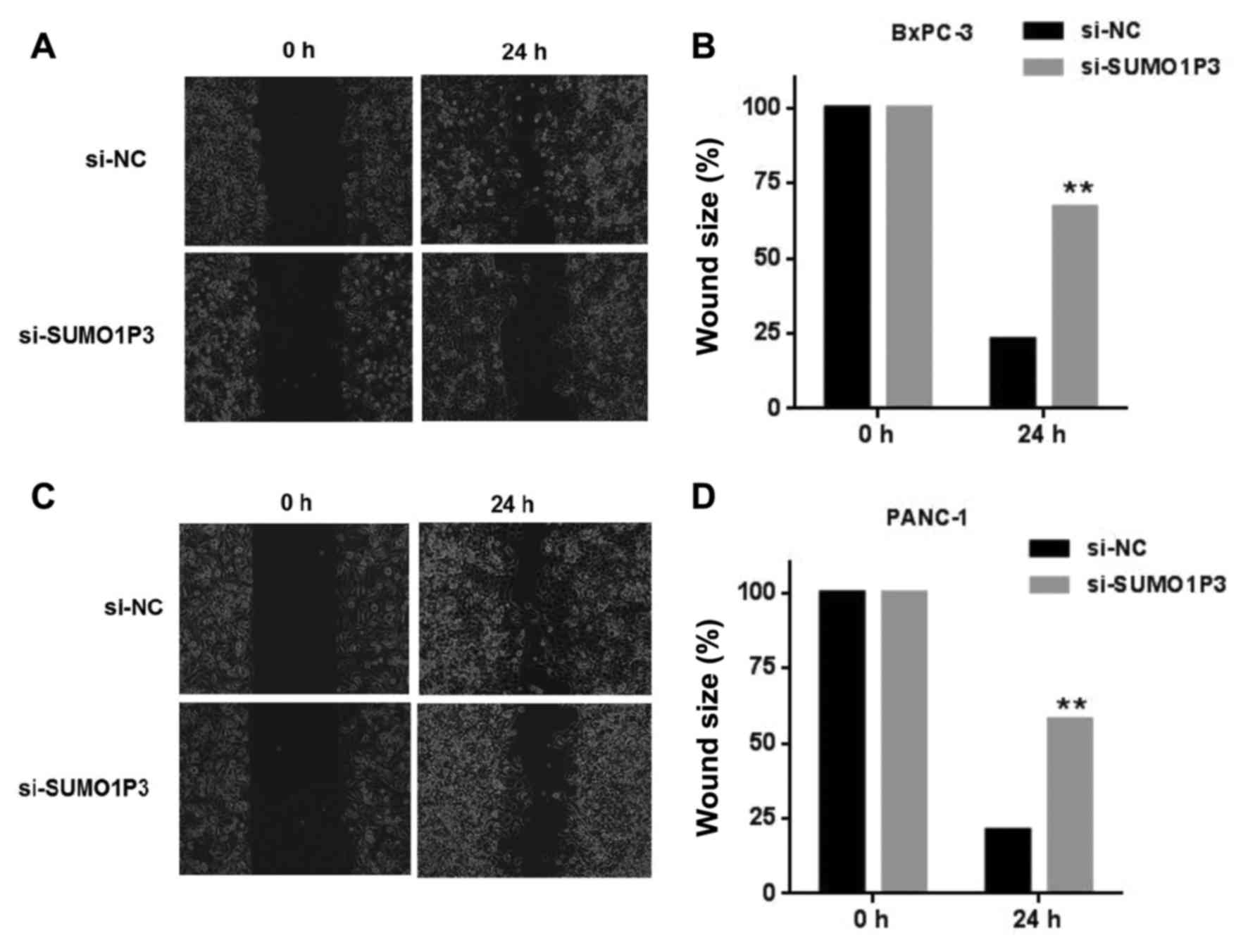
Wound healing and Transwell assays were performed in
order to measure the migratory and invasive ability of pancreatic
cancer BxPC-3 cells and PANC-1 cells. Knockdown of SUMO1P3
suppressed the migration ability of BxPC-3 (Fig. 3A and B) and PANC-1 (Fig. 3C and D) cells. Knockdown of SUMO1P3
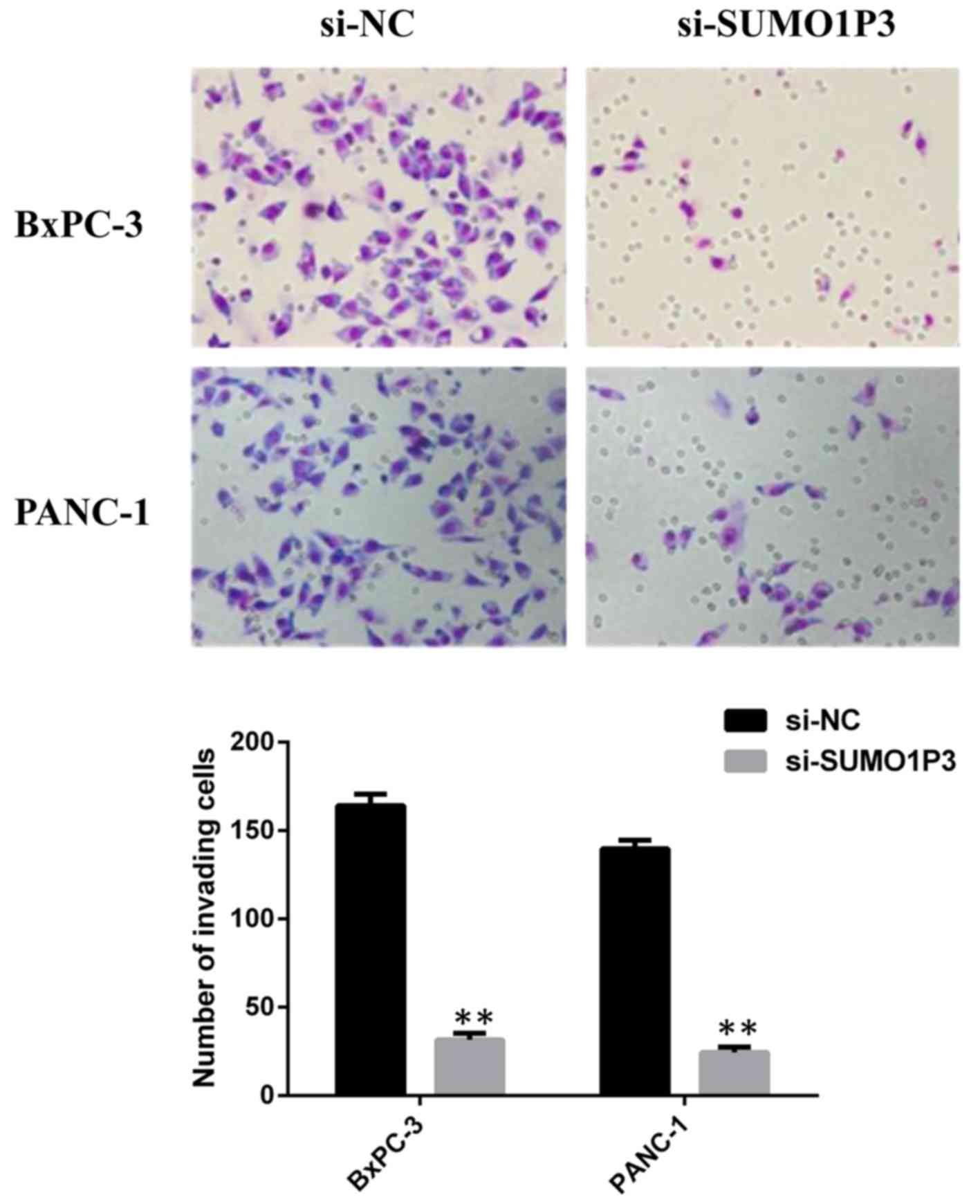
led a significant inhibition of the invasive ability of BxPC-3 and
PANC-1 cells compared with that in the si-NC group (Fig. 4).
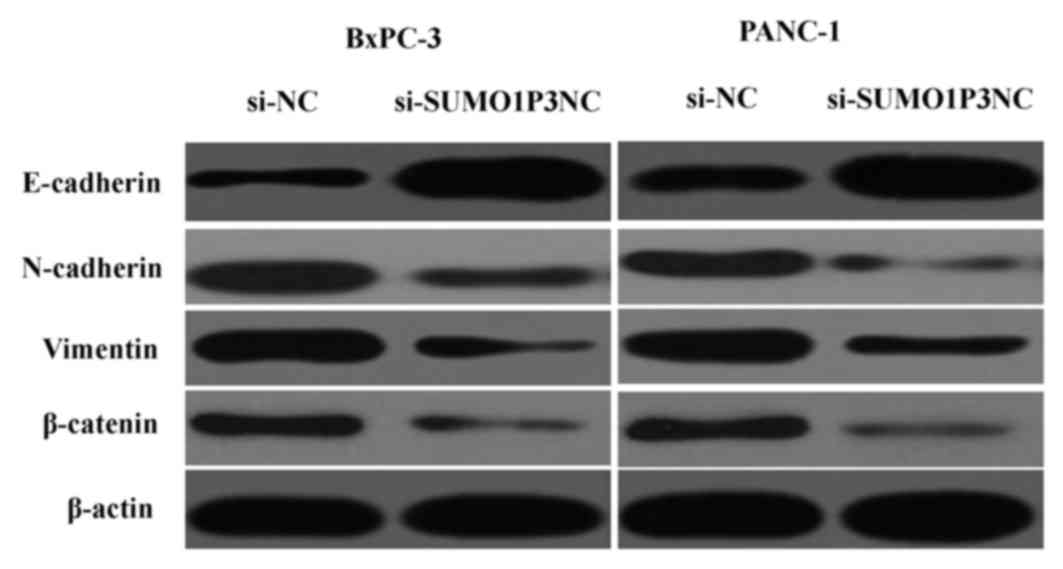
SUMO1P3 is associated with EMT
Since EMT is essential for tumor progression and
metastasis (12), the effects of
SUMO1P3 on EMT were analyzed by western blotting. The results
showed that downregulation of SUMO1P3 resulted in upregulation of
epithelial markers (E-cadherin) and downregulation of mesenchymal
markers (N-cadherin, vimentin and β-catenin) (Fig. 5). Taken together, these findings
reveal that SUMO1P3 may be a positive regulator of EMT, with an
important biological function in the development of pancreatic
cancer.
Discussion
Numerous studies have demonstrated that several
lncRNAs serve a critical function in the development and
progression of various types of cancer, including pancreatic cancer
(13). For example,
lncRNA-plasmacytoma variant translocation 1 functions as an
endogenous sponge by competing with microRNA (miR)-488 to regulate
Serpin Family E Member 1 mRNA Binding Protein 1 and therefore
promote cell proliferation and migration in pancreatic cancer
(14). LncRNA-taurine-upregulated
gene 1 may enhance the cell proliferation and migration in
pancreatic cancer by regulating EMT (15). Linc00673 may regulate non-small cell
lung cancer proliferation, migration, invasion and EMT by
functioning as an endogenous sponge by competing with miR-150-5p
(16).
LncRNA SUMO1P3 has been previously reported to be
upregulated and may serve as a potential therapeutic target in
several types of cancer (9–10). In the present study, SUMO1P3 was
demonstrated to be upregulated in pancreatic cancer tissues
compared with paired adjacent non-tumor tissue and increased
expression of SUMO1P3 was significantly associated with tumor size,
lymph node metastasis and TNM stage. Furthermore, the survival of
patients with pancreatic cancer with a lower expression of SUMO1P3
was significantly improved compared with that of the higher
expression group. Furthermore, knockdown of SUMO1P3 inhibited cell
proliferation, migration and invasion. These results suggested that
SUMO1P3 may serve an important role in the development of
pancreatic cancer.
EMT is a vital pathological progress in the
development and progression in numerous types of human cancer,
including pancreatic cancer (15,16).
During this process, cancer cells lose cell polarity and cell-cell
adhesion and acquire mesenchymal characteristics, including
motility and invasiveness (17,18).
Cadherin switch (loss E-cadherin and gain of N-cadherin expression)
represents an important characteristic in EMT (19). E-cadherin, a canonical epithelial
marker, has been widely accepted as a critical suppressor of
motility and invasiveness of epithelial cells in numerous types of
cancer (20). Transcriptional
downregulation or genomic deletion of E-cadherin expression may
result in key pathological changes in tumor cells (21). However, N-cadherin may promote
invasion and distal metastasis in various types of human cancer
(22). Vimentin and β-catenin are
well-established markers for EMT (23,24).
Therefore, the present study further assessed
whether the biological function of SUMO1P3 on pancreatic cancer
cells was via EMT induction. The results demonstrated that
downregulation of SUMO1P3 led to upregulation of epithelial markers
(E-cadherin) and downregulation of mesenchymal markers (N-cadherin,
vimentin and β-catenin). These results revealed that SUMO1P3 may
function as an oncogene in pancreatic cancer by regulating EMT.
In conclusion, the expression of SUMO1P3 was
increased in pancreatic cancer tissues and cell lines, and
increased expression of SUMO1P3 was associated with the malignant
status and poor prognosis in patients with pancreatic cancer.
Knockdown of SUMO1P3 suppressed cell proliferation, migration and
invasion in pancreatic cancer by regulating EMT in
vitro.
Acknowledgements
Not applicable.
Funding
The present study was supported by The Fund for 333
Engineering Project in Jiangsu Province (grant no. BRA2017266).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on request.
Authors' contributions
CT and YJ performed the majority of the research and
were the major contributors in writing the manuscript. CT, YJ and
SS prepared experimental materials and reviewed the article. CT and
SS made substantial contributions to the design of the work,
drafting the manuscript and revising it critically for important
intellectual content. SS gave final approval of the version to be
published.
Ethics approval and consent to
participate
The present study was conducted with the approval of
the Ethics and Research Committees of Fenjinting Hospital (Jiangsu,
China) and was performed in accordance with the Declaration of
Helsinki. All patients provided written informed consent prior to
their participation in the present study.
Patient consent for publication
All patients provided written informed consent for
the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu W, Xu B, Yao Y, Yu X, Cao H, Zhang J,
Liu J and Sheng H: Overexpression and biological function of IQGAP3
in human pancreatic cancer. Am J Transl Res. 8:5421–5432.
2016.PubMed/NCBI
|
|
3
|
Yao W, Ji S, Qin Y, Yang J, Xu J, Zhang B,
Xu W, Liu J, Shi S, Liu L, et al: Profilin-1 suppresses
tumorigenicity in pancreatic cancer through regulation of the
SIRT3-HIF1α axis. Mol Cancer. 13:1872014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xiao H, Tang K, Liu P, Chen K, Hu J, Zeng
J, Xiao W, Yu G, Yao W, Zhou H, et al: LncRNA MALAT1 functions as a
competing endogenous RNA to regulate ZEB2 expression by sponging
miR-200s in clear cell kidney carcinoma. Oncotarget. 6:38005–38015.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsai MC, Manor O, Wan Y, Mosammaparast N,
Wang JK, Lan F, Shi Y, Segal E and Chang HY: Long noncoding RNA as
modular scaffold of histone modification complexes. Science.
329:689–693. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fatica A and Bozzoni I: Long non-coding
RNAs: New players in cell differentiation and development. Nat Rev
Genet. 15:7–21. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Prensner JR and Chinnaiyan AM: The
emergence of lncRNAs in cancer biology. Cancer Discov. 1:391–407.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li CH and Chen Y: Targeting long
non-coding RNAs in cancers: Progress and prospects. Int J Biochem
Cell Biol. 45:1895–1910. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mei D, Song H, Wang K, Lou Y, Sun W, Liu
Z, Ding X and Guo J: Up-regulation of SUMO1 pseudogene 3 (SUMO1P3)
in gastric cancer and its clinical association. Med Oncol.
30:7092013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhan Y, Liu Y, Wang C, Lin J, Chen M, Chen
X, Zhuang C, Liu L, Xu W, Zhou Q, et al: Increased expression of
SUMO1P3 predicts poor prognosis and promotes tumor growth and
metastasis in bladder cancer. Oncotarget. 7:16038–16048. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin C, Zhao GC, Xu YD, Wang DS, Jin DY, Ji
Y, Lou WH and Wu WC: Increased expression of αTubulin is associated
with poor prognosis in patients with pancreatic cancer after
surgical resection. Oncotarget. 7:60657–60664. 2016.PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu X, Sood AK, Dang CV and Zhang L: The
role of long noncoding RNAs in cancer: The dark matter matters.
Curr Opin Genet Dev. 48:8–15. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao L, Kong H, Sun H, Chen Z, Chen B and
Zhou M: LncRNA-PVT1 promotes pancreatic cancer cells proliferation
and migration through acting as a molecular sponge to regulate
miR-448. J Cell Physiol. 233:4044–4055. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qin CF and Zhao FL: Long non-coding RNA
TUG1 can promote proliferation and migration of pancreatic cancer
via EMT pathway. Eur Rev Med Pharmacol Sci. 21:2377–2384.
2017.PubMed/NCBI
|
|
16
|
Lu W, Zhang H, Niu Y, Wu Y, Sun W, Li H,
Kong J, Ding K, Shen HM, Wu H, et al: Long non-coding RNA linc00673
regulated non-small cell lung cancer proliferation, migration,
invasion and epithelial mesenchymal transition by sponging
miR-150-5p. Mol Cancer. 16:1182017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang C, Xie D, Cui J, Li Q, Gao Y and Xie
K: FOXM1c promotes pancreatic cancer epithelial-to-mesenchymal
transition and metastasis via upregulation of expression of the
urokinase plasminogen activator system. Clin Cancer Res.
20:1477–1488. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ma R, Chen J, Jiang S, Lin S, Zhang X and
Liang X: Up regulation of NAT10 promotes metastasis of
hepatocellular carcinoma cells through epithelial-to-mesenchymal
transition. Am J Transl Res. 8:4215–4223. 2016.PubMed/NCBI
|
|
19
|
Tam WL and Weinberg RA: The epigenetics of
epithelial-mesenchymal plasticity in cancer. Nat Med. 19:1438–1449.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu JF, Mao L, Bu LL, Ma SR, Huang CF,
Zhang WF and Sun ZJ: C4.4A as a biomarker of head and neck squamous
cell carcinoma and correlated with epithelial mesenchymal
transition. Am J Cancer Res. 5:3505–3515. 2015.PubMed/NCBI
|
|
21
|
Guo F, Kerrigan Parker BC, Yang D, Hu L,
Shmulevich I, Sood AK, Xue F and Zhang W: Post-transcriptional
regulatory network of epithelial-to-mesenchymal and
mesenchymal-to-epithelial transitions. J Hematol Oncol. 7:192014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shan ZZ, Yan XB, Yan LL, Tian Y, Meng QC,
Qiu WW, Zhang Z and Jin ZM: Overexpression of Tbx3 is correlated
with Epithelial-Mesenchymal Transition phenotype and predicts poor
prognosis of colorectal cancer. Am J Cancer Res. 5:344–353.
2014.PubMed/NCBI
|
|
23
|
Dai C, Cao J, Zeng Y, Xu S, Jia X and Xu
P: E-cadherin expression as a prognostic factor in patients with
ovarian cancer: A meta-analysis. Oncotarget. 8:81052–81061. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang X, Liu G, Kang Y, Dong Z, Qian Q and
Ma X: N-cadherin expression is associated with acquisition of EMT
phenotype and with enhanced invasion in erlotinib-resistant lung
cancer cell lines. PLoS One. 8:e576922013. View Article : Google Scholar : PubMed/NCBI
|