Introduction
Chondroitin sulfate (CS) is a class of
glycosaminoglycan (GAG), which is mainly present in the
extracellular matrix and on the cell surface (1). CS plays very important roles in
morphogenesis and tissue development (2). CS also shows an immunomodulatory effect
and has been reported to be involved in tumor progression (3,4). For
example, in colorectal cancer, the GAG disaccharide content and
composition were altered (5).
Additionally, in endometrial epithelial cancer, CS promoted cell
proliferation and migration (3).
Chondroitin synthase-1 (CHSY1) is one of the six enzymes
responsible for the biosynthesis of CS in mammalian cells (6). CHSY1is a protein with 802 amino acids
and is located in the chromosome 15q26.3 region. CHSY1 is important
for normal development. For example, the methylation level of CHSY1
is associated with T cell differentiation (7). CHSY1 is also necessary for bone
development (8), and loss of CHSY1
causes temtamy preaxial brachydactyly syndrome (9).
However, evidence suggests an oncogenic function of
CHSY1 during tumorigenesis. For example, CHSY1 is required for the
interaction of myeloma cells with osteoclasts (10). The abnormal expression of CHSY1 has
been found in malignant soft tissue sarcomas (11). Furthermore, knockdown of CHSY1
increased the expression of JAG2, a critical molecule in
glioblastoma cells (12). Forced
expression of CHSY1 enhanced cell migration, invasion, and EMT in
hepatocellular carcinoma (13). As a
result, CHSY1 was proposed to promote tumor progression. However,
in colorectal cancer, CHSY1 expression showed a significant
increase in stage I tumor tissues compared to that in the normal
control group. In stage II or III tumor tissues, expression of
CHSY1 was comparable or slightly lower than that in control tissues
(14). However, the actual function
of CHSY1 in colorectal cancer remains unknown.
According to cancer reports by Chen, colorectal
cancer is one of the most four malignant cancers in China. The
estimated number of new cases was 376,300 and the number of new
deaths was ~191,000 (15). Colorectal
cancer is also a common malignant cancer in the USA. As reported,
both the new incidence and new mortality of colorectal cancer
patients accounted for ~8% of all cancers in 2017 (16). Furthermore, the 5-year survival rate
of metastatic colorectal cancer patients was <15% (17). However, surgical resection remains the
most commonly used therapy for colorectal cancer (17). Unfortunately, ~50% of colorectal
cancer patients undergo recurrence and metastasis following surgery
(18). Therefore, it is necessary to
determine the mechanisms underlying colorectal cancer and develop
new strategies to win the war against colorectal cancer.
In this study, to investigate the role of CHSY1 in
colorectal cancer, we determined the clinical level of CHSY1 in
tumor tissues and evaluated the effects of CHSY1 on cell growth and
cell apoptosis. Then, we demonstrated that nuclear factor (NF) κB
and caspase-3/7 signaling were regulated by CHSY1.
Materials and methods
Cell lines and cell culture
Human colorectal cancer cell lines, including RKO,
HCT116, SW480 and the human immortal colon epithelial cell line
NCM460, were obtained from the Shanghai Cell Bank of Chinese
Academy of Science (Shanghai, China) and maintained in RPMI-1640
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (Sangon Biotech, Shanghai,
China), 100 units/ml penicillin and 0.1 mg/ml streptomycin at 37°C
in a 5% CO2 incubator.
Patient tissues and ethics
statement
A total of 21 tumor tissues and the adjacent normal
tissues were collected from Jiangxi Province People's Hospital
(Nanchang, China) between 2009 and 2012.
All study procedures were approved by the
Institutional Review Board of Jiangxi Provincial People's Hospital,
and a written informed consent form was collected from each
patient.
Immunohistochemistry assay (IHC)
The IHC assay was carried out as report before
(19). Briefly, tissue sections of 4
µm were deparaffinized, rehydrated, and subjected to antigen
retrieval by boiling in sodium citrate buffer (10 mmol/l; pH 6.0).
Then the sections were incubated with CHSY1 primary antibody
(ab153813; 1:400 dilution; Abcam, Cambridge, MA, USA) for 60 min at
room temperature and stained with 3,3-diaminobenzidine followed by
counterstaining with hematoxylin and mounted. The stains were
scored according to: (a) percentage of immune-positive cells, 1,
0–30%; 2, >30–70%; 3, >70%; and (b) staining intensity, 1,
weak; 2, moderate and 3, strong. The final score of each slide was
(a) × (b).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) and cDNA was
synthesized with a PrimeScript First Strand cDNA Synthesis kit
(Takara, Dalian, China) according to the manufacturer's
instructions. Next, 1 µl of cDNA was used as a template for the
RT-qPCR assay with SYBR Green reagent on a 7500 Fast Real-Time PCR
System (Applied Biosystems, Foster City, CA, USA). The primers
designed for CHSY1 gene were as follows:
Forward, 5′-GCTATCACATTACACCCCAACA-3′ and reverse,
5′-AACTCCCATTCCAGAATCTCCT-3′
GAPDH was selected as an internal control and the
primers were as follows: Forward, 5′-TGACTTCAACAGCGACACCCA-3′ and
reverse, 5′-CACCCTGTTGCTGTAGCCAAA-3′
The protocol for RT-qPCR was as follows:
Denaturation at 95°C for 20 sec, (denaturation at 95°C for 5 sec,
extension at 60°C for 30 sec) for 40 cycles.
The expected PCR products of CHSY1 and GAPDH were
236 and 121 bp, respectively. All samples were examined in
triplicate. The relative level of the target gene was calculated
using the 2−ΔΔCq as described previously (20). The expression level of CHSY1 was
considered as high when the fold change of CHSY1 in tumor tissues
vs. that in normal control was >2. Otherwise, it was considered
as low.
Construction of recombinant lentiviral
vector and transduction
The shRNA fragment targeting human theCHSY1
gene (GenBank no. NM_014918) was designed, synthesized, and
inserted into a lentivirus expression plasmid pGV115-GFP. The shRNA
sequence was as follows: 5-ACATTGTCATGCAGGTCAT-3. Then, the
lentivirus particle carrying this shRNA fragment (shCHSY1) was
prepared.
After the lentivirus particle was prepared,
approximately 2×105 RKO cells/well were cultured in
6-well plates and infected with shCHSY1 lentivirus or control
lentivirus (shCtrl) at a multiplicity of infection (MOI) of 20.
Then, the treated cells were incubated in a 5% CO2
incubator at 37°C for 5 days. After 72 h of infection, cells were
observed under a fluorescence microscope (MicroPublisher 3.3RTV;
Olympus, Tokyo, Japan). After 5 days of infection, the knockdown
efficiency of CHSY1 was determined using RT-qPCR and western
blotting technologies.
Cell proliferation assay
Cell growth viability was monitored on a Cellomics
ArrayScan™ VT1 HCS automated reader (Cellomics Inc.,
Pittsburgh, PA, USA). Briefly, RKO cells infected with lentivirus
were seeded into 96-well plates (2,000 cells/well) and incubated
for 5 days at 37°C in a 5% CO2 incubator, and the cell
number was calculated each day for 5 days according to the GFP
expression intensity. Each experiment was performed in
triplicate.
MTT assay
SW480 cells or RKO cells treated with shCHSY1
lentivirus or shCtrl were seeded into 96-well plates at 6,000
cells/well and cultured for 48 h at 37°C in a 5% CO2
incubator. Then MTT reagent (5 mg/ml; Sangon Biotech) was added
into each well and cultured for another 4 h. The absorbance value
at 490 nm was detected on a microplate spectrophotometer.
Apoptosis analysis
The cell apoptosis rate was determined with Annexin
V-APC staining by flow cytometry. Briefly, RKO cells (5,000
cells/well) were cultured in 6-well plates. After 48 h of
lentivirus infection, cells were collected and washed twice with
ice-cold PBS. Then, cells were adjusted to 1×106/ml with
1X staining buffer (Sangon Biotech), of which 100 µl of the cell
suspension was stained with 5 µl Annexin V-APC (BD Biosciences, San
Diego, CA, USA) for 15 min at room temperature in the dark. Then,
the cells were analyzed on a flow cytometer. Each experiment was
performed independently three times.
Caspase-3/7 activity assay
To detect the activity of caspase-3/7, we seeded the
RKO cells into 96-well plates and infected them with the lentivirus
as described above. After infection for 48 h, the activity of
caspase-3/7 was determined with a Caspase-Glo 3/7 kit (Promega
Corp., Madison, WI, USA) according to the manufacturer's
instructions.
Western blot analysis
After 48 h of lentivirus infection, approximately
1×106 cells were collected and lysed with lysis buffer
(50 mM Tris, pH 7.4, 150 mM NaCl, 1% SDS, 1 mM EDTA, and 1% NP-40)
containing 1 mM PMSF (Sangon Biotech) for 30 min on ice. Then, the
lysates were centrifuged at 10,000 × g for 10 min at 4°C, and the
supernatants were collected. The protein concentration was
determined using a BCA Protein Assay kit (Generay, Shanghai,
China). Then, approximately 10 µg of protein was separated on a 10%
SDS-PAGE gel and transferred to a polyvinylidenedifluoride (PVDF)
membrane.
PVDF membranes were incubated with mouse anti-CHSY1
(1:200 dilution; ab153813), anti-NFκB p105/p50 (1:300 dilution;
ab131546), anti-B-cell lymphoma 2 (Bcl-2; 1:500 dilution; ab32124),
anti-truncated caspase-3/7 (1:1,000 dilution; ab2302),
anti-Bcl-2-associated X protein (Bax; 1:400 dilution; ab182733;
Abcam), anti-Pi-IκB (1:200 dilution; sc8404), or anti-GAPDH
antibody (1:1,500 dilution; sc47724; Santa Cruz Biotechnology,
Santa Cruz, CA, USA) at 4°C overnight. Then, the PVDF membranes
were subsequently incubated with a horseradish peroxidase
(HRP)-conjugated goat anti-mouse IgG (1:1,500 dilution; sc2005) or
goat anti-rabbit IgG (1:1,200 dilution; sc2030; Santa Cruz
Biotechnology) at 37°C for 1 h and detected with the EasyBlot ECL
kit (Sangon Biotech).
Statistical evaluation
For in vitro experiments, statistical
analyses were performed with SPSS 16.0 (SPSS, Chicago, IL, USA).
Data are expressed as the mean ± SD. Raw data was subjected to
Independent Samples t-test to analyze the difference between group
shCtrl and shCHSY1. The difference between multiple groups was
analyzed by one-way ANOVA/post hoc Tukey Test. The difference of
CHSY1 expression between tumor tissues and adjacent normal tissues
was analyzed with paired Student's t-test. P<0.05 was considered
to indicate a statistically significant difference.
For the association analysis of CHSY1 expression
with the prognosis of patients, the Kaplan-Meier method was used,
and a log-rank test was employed to analyze the difference.
P<0.05 was considered to indicate a statistically significant
difference.
Results
CHSY1 is clinically associated with
colorectal cancer
To explore the relationship of CHSY1 with colorectal
cancer, we collected a total of 21 tumor samples and adjacent
normal samples. Then, the level of CHSY1 was detected using the
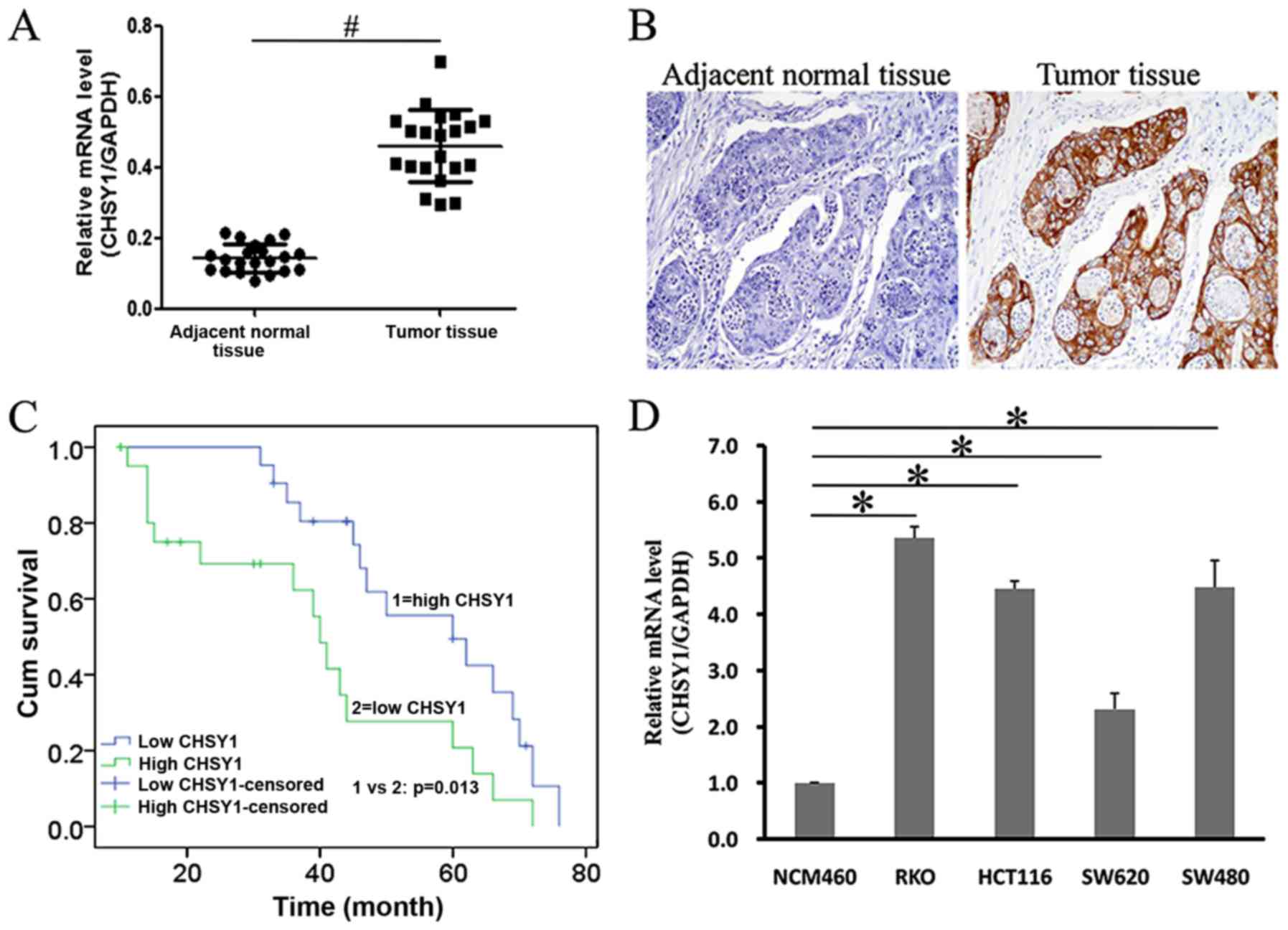
RT-qPCR method. As shown in Fig. 1A,
CHSY1 was more highly expressed in tumor samples than in adjacent
normal samples. IHC staining also demonstrated that CHSY1 was
expressed highly in tumor tissues and was weak in the adjacent
normal control (Fig. 1B and Table I). Furthermore, higher CHSY1
expression was associated with a poorer prognosis, such that the
5-year survival rate of patients with high CHSY1 (tumor: Normal ≥2)
was significantly lower than those with low CHSY1 expression
(tumor: Normal <2) (20% vs. 45%) (Fig.
1C). In addition, we found that CHSY1 was more highly expressed
in colorectal cancer cell lines, including RKO, HCT116, and SW480,
than in NCM460 cells, a human colon epithelial cell (Fig. 1D). Therefore, CHSY1 was clinically
associated with colorectal cancer.
 | Table I.Mean score of CHSY1 expression in
tumor tissues or adjacent normal tissues by IHC analysis. |
Table I.
Mean score of CHSY1 expression in
tumor tissues or adjacent normal tissues by IHC analysis.
| Group | Adjacent normal
tissues | Tumor tissues | P-value |
|---|
| Score value | 0.52±0.51 | 4.24±2.43 | <0.05a |
CHSY1 was successfully knocked down by
lentivirus-mediated shRNA in colorectal cancer cells
The lentivirus vector was an efficient tool to carry
a particular gene into cells and was applied extensively. To reduce
the expression of CHSY1 in the RKO cell line, we synthesized a
shRNA fragment targeting CHSY1 (shCHSY1) and the negative control
(shCtrl) and prepared lentivirus particles carrying shCHSY1 or
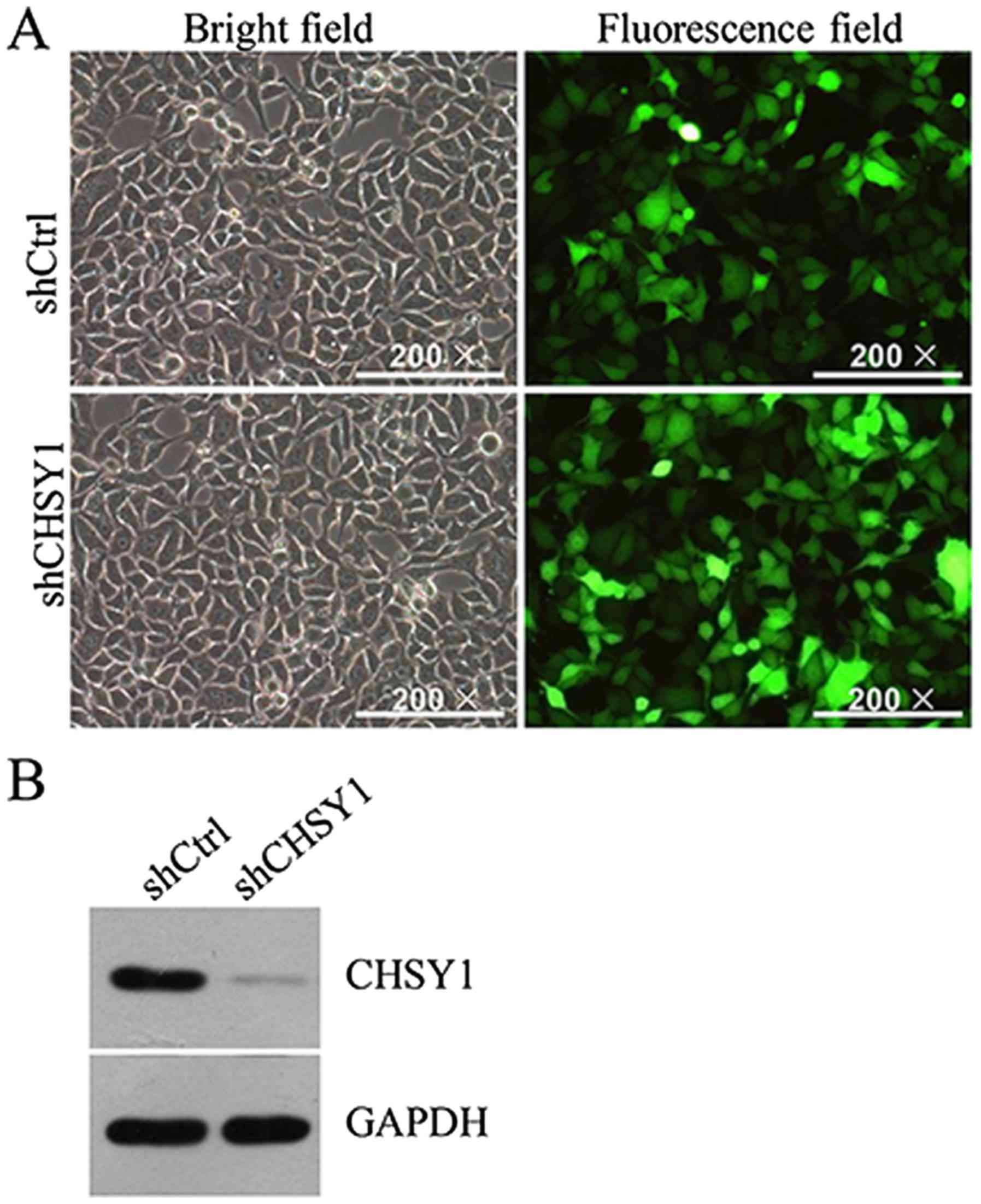
shCtrl. Because GFP was a tag in the lentivirus vector, the
infection efficiency of shCHSY1 in RKO cells could be monitored
directly under a microscope. As shown in Fig. 2A, the prepared lentivirus particles
efficiently infected RKO cells. And CHSY1 expression was
significantly reduced in RKO cells at protein level (Fig. 2B). Also, the mRNA level of CHSY1 was
decreased greatly by shCHSY1 in RKO cells. The knockdown efficiency
was ~70%. Therefore, CHSY1 was successfully knocked down in the RKO
cell line.
CHSY1 serves critical roles in the
proliferation of colorectal cancer cells
To examine the effects of CHSY1 knockdown on cell
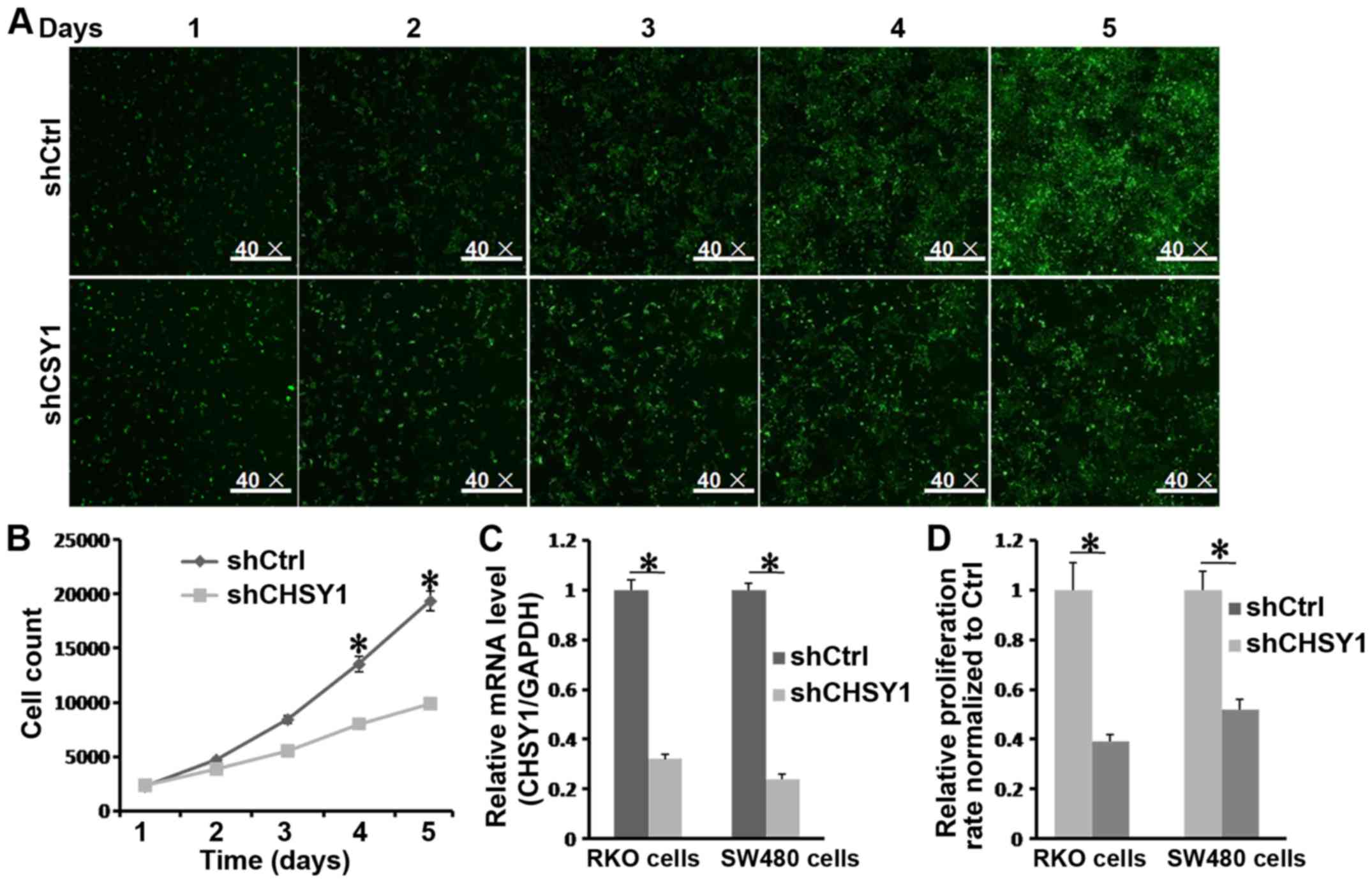
proliferation, we treated RKO cells seeded in a 96-well plate with
shCHSY1 or shCtrl and monitored the cells for 5 consecutive days
with Cellomics. As shown in Fig. 3A,
the GFP intensity in the shCtrl group was higher than that in the
shCHSY1 group, which indicated that RKO cells treated with shCtrl
underwent significant expansion after culture for 5 days. This was
further supported by Fig. 3B and D.
The cell number in the shCtrl group doubled compared to that in the
shCHSY1 group. In addition, CHSY1 was effectively reduced in SW480
cells (Fig. 3C), and the
proliferation of SW480 cells was inhibited after CHSY1 knockdown
(Fig. 3D). Therefore, we hypothesized
that CHSY1 was essential for cell proliferation in colorectal
cancer.
Knockdown of CHSY1 induces apoptosis
in RKO cells
In tumor cells, apoptosis was often suppressed by a
driver gene. Not surprisingly, we found that decreased expression
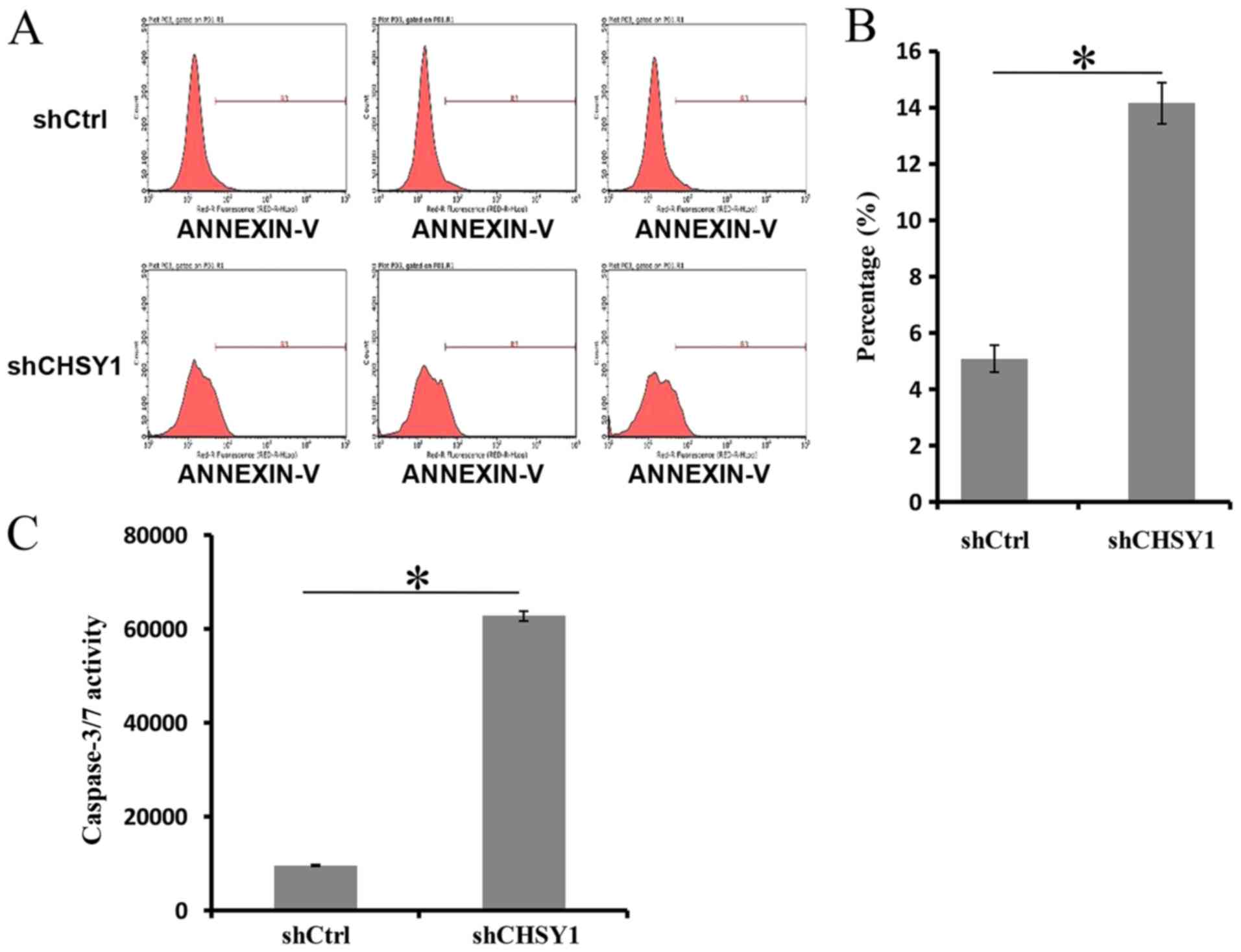
of CHSY1 increased cell apoptosis in RKO cells. As demonstrated in
Fig. 4A and B, the apoptosis rate of
cells treated with shCtrl was 5.09%, whereas it was 14.15% in the
shCHSY1 group. The difference between the two groups was
significant (P<0.05). Then, the activity of caspase-3/7 was
determined in RKO cells, and the activity of caspase-3/7 in the
shCHSY1 group was approximately 6-foldthat in the shCtrl group
(Fig. 4C). The above data suggested
that CHSY1 played important roles in the apoptosis of RKO
cells.
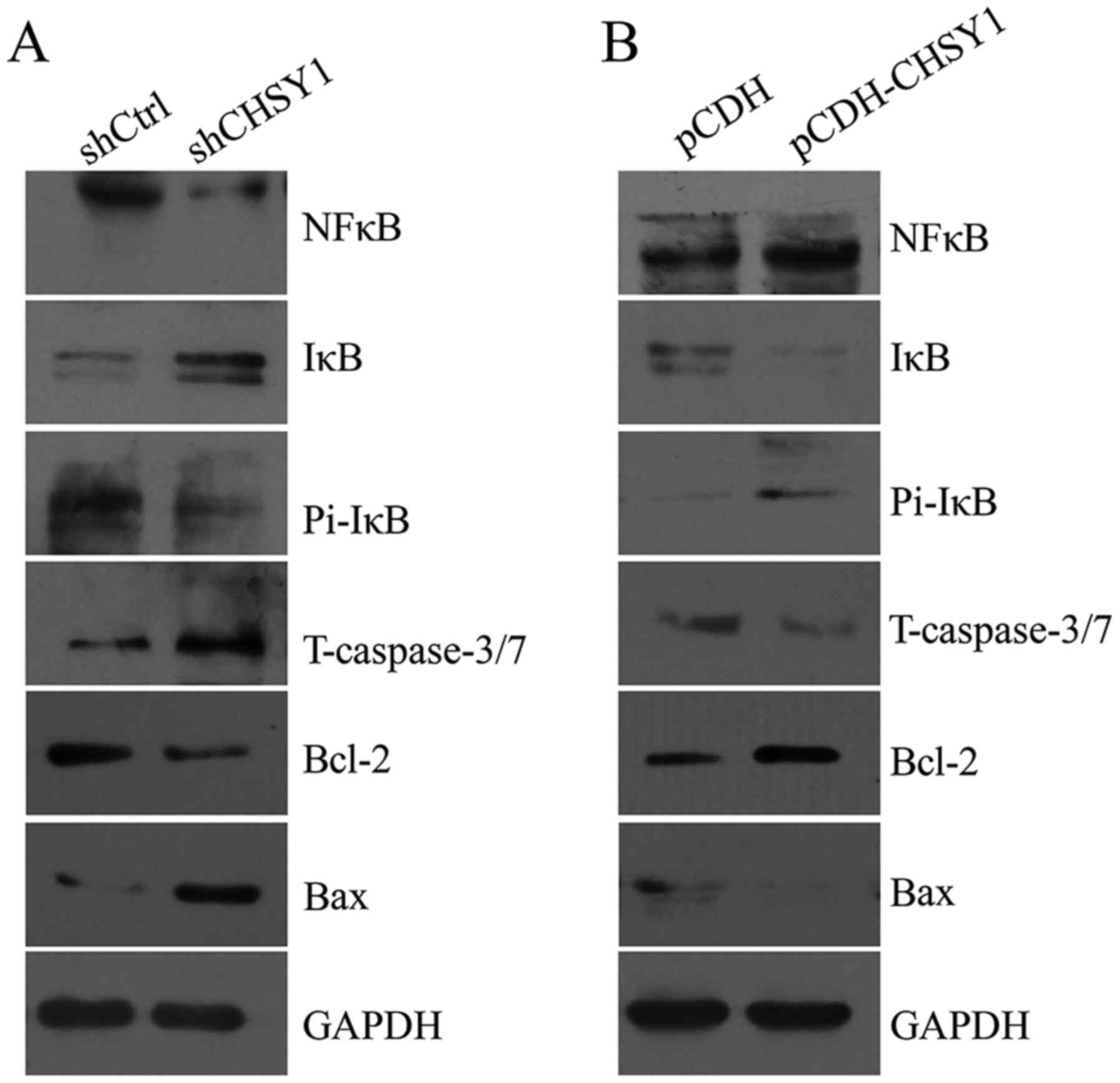
CHSY1 regulates NFκB and caspase-3/7
signaling in RKO cells
To elucidate the mechanism by which CHSY1 affects
cell proliferation and apoptosis in colorectal cancer, we
determined the critical signaling molecules in proliferation and
apoptosis. As shown in Fig. 5,
expression of the anti-apoptotic molecule Bcl-2 was decreased after
CHSY1 was knocked down in RKO cells. In contrast, the level of the
pro-apoptotic molecule Bax increased significantly, and the level
of truncated caspase-3/7 was also increased. Additionally, we found
that the phosphorylation level of IκB was decreased, whereas total
IκB was increased after CHSY1 knockdown. Moreover, the expression
of NFκB was also reduced. Conversely, overexpression of CHSY1
increased the level of NFκB and the phosphorylated level of IκB;
however, total IκB expression was decreased. Moreover, Bcl-2 was
upregulated, whereas Bax and truncated caspase-3/7 levels were
reduced. Therefore, we hypothesized that CHSY1 regulated cell
proliferation and apoptosis via regulation of the NFκB and/or
caspase-3/7 signaling pathway in RKO cells.
Discussion
Colorectal cancer is one of the most common
malignant diseases in the world and threatens the life of humans.
The most effective weapon against colorectal cancer is just a
scalpel, but we are currently losing the war. One of the major
causes of the poor prognosis in colorectal cancer is the
heterogeneity of the cancer (21).
Although a number of factors have been identified, none are able to
fully explain the formation or progression of cancer. In this
study, we demonstrated that CHSY1 was expressed more highly in
colorectal cancer tissues and colorectal cancer cell lines than in
control tissues and lines. Moreover, higher CHSY1 expression was
associated with a worse 5-year survival rate. These data indicate
that CHSY1 may play critical roles in the progression of colorectal
cancer. In a previous study, CHSY1 was significantly upregulated in
stage I colorectal cancer but not in stage II or III (14). In this study, we showed that CHSY1 was
more highly expressed in both stage II and III colorectal cancer
tissues than in the adjacent normal control. The difference may be
partially attributed to the origin of the tumor tissues. However, a
large cohort of patient tissues was necessary to clearly explain
this paradox and to confirm the discovery in this study.
Nearly all types of cancers are characterized by
rapid expansion and evasion from drug-induced apoptosis (22). Here, we showed that CHSY1 was
essential for the proliferation of colorectal cancer cell lines,
including RKO cells and SW480 cells. The NFκB signaling pathway was
often activated in tumors and promoted cell proliferation (23,24). In
RKO cells, the level of NFκB and phosphorylated IκB was decreased,
whereas total IκB was increased after CHSY1 knockdown. Conversely,
CHSY1 overexpression increased the level of NFκB and phosphorylated
IκB in RKO cells. In tumors, IκB was phosphorylated and separated
from the NFκB molecule. When the level of phosphorylated IκB
decreased, NFκB was bound by IκB, and the expression of downstream
genes was inhibited (25). Therefore,
CHSY1 suppressed cell proliferation via regulation of the NFκB
signaling pathway in colorectal cancer.
Additionally, in RKO cells, CHSY1 could suppress
cell apoptosis, which may partially account for the poor prognosis
of colorectal cancer patients. The major mechanism of a large
number of chemotherapeutic drugs in the clinic is apoptosis
(26,27). Generally, chemotherapeutic drugs
further clear remaining cancer cells by inducing cell apoptosis
after the surgical removal of solid tumor tissues, and patients do
benefit from this treatment. However, a small cohort of cancer
cells is able to evolve and evade the apoptosis induced by
drugs.
Apoptosis is a programmed cell death process, and
the caspase cascade response is active when apoptosis begins. In
general, the activity of caspase-3/7 is greatly enhanced in the
apoptosis process (28,29). In this study, CHSY1 was shown to
contribute to the anti-apoptotic ability of RKO cells, and
caspase-3/7 was highly activated after CHSY1 was knocked down in
RKO cells. Furthermore, we found that Bcl-2 was decreased, whereas
Bax increased after knockdown of CHSY1. Bcl-2 is an antagonistic
gene of apoptosis (28), but Bax
often promotes the progression of apoptosis (28,29). The
expression pattern suggested that caspase-3/7-mediated apoptosis
signaling was activated when CHSY1 was knocked down in RKO
cells.
However, these results were just based on RKO cells.
And it is necessary to confirm the role of CHSY1 in another cell
line such as SW480 cells. Also, the in vivo function of
CHSY1 gene in colorectal cancer will be explored and the dominant
molecular mechanism of CHSY1 in colorectal cancer will be an
emphasis in future.
In summary, we demonstrated that CHSY1 played a
tumor-promoting role in colorectal cancer by regulating the NFκB
and/or caspase-3/7 signaling pathway. Additionally, this study
suggests that CHSY1 is a potential target for colorectal cancer
therapy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LZ designed the whole study, carried out the
experiments and wrote the manuscript. JQ analyzed the data. XL
contributed to the acquisition of data, carried out the western
blot experiments, participated in the draft and revision of
manuscript and studied the references.. AZ and ZZ participated in
the experiments and interpreted the immunohistochemistry data. QF
reviewed the manuscript and contributed to the acquisition of
data.
Ethics approval and consent to
participate
All study procedures were approved by the
Institutional Review Board of Jiangxi Provincial People's Hospital,
and a written informed consent form was collected from each
patient.
Patient consent for pubication
The patients provided written informed consent for
the publication of any associated data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Martel-Pelletier J, Boileau C, Pelletier
JP and Roughley PJ: Cartilage in normal and osteoarthritis
conditions. Best Pract Res Clin Rheumatol. 22:351–384. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Holmborn K, Habicher J, Kasza Z, Eriksson
AS, Filipek-Gorniok B, Gopal S, Couchman JR, Ahlberg PE, Wiweger M,
Spillmann D, et al: On the roles and regulation of chondroitin
sulfate and heparin sulfate in zebrafish pharyngeal cartilage
morphogenesis. J Biol Chem. 287:33905–33916. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Winship A, Van Sinderen M, Heffernan-Marks
A and Dimitriadis E: Chondroitin sulfate proteoglycan protein is
stimulated by interleukin 11 and promotes endometrial epithelial
cancer cell proliferation and migration. Int J Oncol. 50:798–804.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
du Souich P, García AG, Vergés J and
Montell E: Immunomodulatory and anti-inflammatory effects of
chondroitin sulphate. J Cell Mol Med. 13:1451–1463. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kalathas D, Theocharis DA, Bounias D,
Kyriakopoulou D, Papageorgakopoulou N, Stavropoulos MS and Vynios
DH: Alterations of glycosaminoglycan disaccharide content and
composition in colorectal cancer: Structural and expressional
studies. Oncol Rep. 22:369–375. 2009.PubMed/NCBI
|
|
6
|
Ogawa H, Hatano S, Sugiura N, Nagai N,
Sato T, Shimizu K, Kimata K, Narimatsu H and Watanabe H:
Chondroitin sulfate synthase-2 is necessary for chain extension of
chondroitin sulfate but not critical for skeletal development. PLoS
One. 7:e438062012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hashimoto SI, Ogoshi K, Sasaki A, Abe J,
Qu W, Nakatani Y, Ahsan B, Oshima K, Shand FH, Ametani A, et al:
Coordinated changes in DNA methylation in antigen-specific memory
CD4 T cells. J Immunol. 190:4076–4091. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wilson DG, Phamluong K, Lin WY, Barck K,
Carano RA, Diehl L, Peterson AS, Martin F and Solloway MJ:
Chondroitin sulfate synthase 1 (Chsy1) is required for bone
development and digit patterning. Dev Biol. 363:413–425. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Y, Laue K, Temtamy S, Aglan M, Kotan
LD, Yigit G, Canan H, Pawlik B, Nürnberg G, Wakeling EL, et al:
Temtamy preaxial brachydactyly syndrome is caused by
loss-of-function mutations in chondroitin synthase 1, a potential
target of BMP signaling. Am J Hum Genet. 87:757–767. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yin L: Chondroitin synthase 1 is a key
molecule in myeloma cell-osteoclast interactions. J Biol Chem.
280:15666–15672. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Momose T, Yoshimura Y, Harumiya S, Isobe
K, Kito M, Fukushima M, Kato H and Nakayama J: Chondroitin sulfate
synthase 1 expression is associated with malignant potential of
soft tissue sarcomas with myxoid substance. Hum Pathol. 50:15–23.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tian J, Ling L, Shboul M, Lee H, O'Connor
B, Merriman B, Nelson SF, Cool S, Ababneh OH, Al-Hadidy A, et al:
Loss of CHSY1, a secreted FRINGE enzyme, causes syndromic
brachydactyly in humans via increased NOTCH signaling. Am J Hum
Genet. 87:768–778. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu CH, Lan CT, Chou JF, Tseng TJ and Liao
WC: CHSY1 promotes aggressive phenotypes of hepatocellular
carcinoma cells via activation of the hedgehog signaling pathway.
Cancer Lett. 403:280–288. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kalathas D, Theocharis DA, Bounias D,
Kyriakopoulou D, Papageorgakopoulou N, Stavropoulos MS and Vynios
DH: Chondroitin synthases I, II, III and chondroitin sulfate
glucuronyltransferase expression in colorectal cancer. Mol Med Rep.
4:363–368. 2011.PubMed/NCBI
|
|
15
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pabla B, Bissonnette M and Konda VJ: Colon
cancer and the epidermal growth factor receptor: Current treatment
paradigms, the importance of diet, and the role of chemoprevention.
World J Clin Oncol. 6:133–141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sauer R, Liersch T, Merkel S, Fietkau R,
Hohenberger W, Hess C, Becker H, Raab HR, Villanueva MT, Witzigmann
H, et al: Preoperative versus postoperative chemoradiotherapy for
locally advanced rectal cancer: Results of the German
CAO/ARO/AIO-94 randomized phase III trial after a median follow-up
of 11 years. J Clin Oncol. 30:1926–1933. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Luo Y, Tsuchiya KD II, Park D, Fausel R,
Kanngurn S, Welcsh P, Dzieciatkowski S, Wang J and Grady WM: RET is
a potential tumor suppressor gene in colorectal cancer. Oncogene.
32:2037–2047. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fisher R, Pusztai L and Swanton C: Cancer
heterogeneity: Implications for targeted therapeutics. Br J Cancer.
108:479–485. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xia JT, Chen LZ, Jian WH, Wang KB, Yang
YZ, He WL, He YL, Chen D and Li W: MicroRNA-362 induces cell
proliferation and apoptosis resistance in gastric cancer by
activation of NF-κB signaling. J Transl Med. 12:332014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cui H, Yuan J, Du X, Wang M, Yue L and Liu
J: Ethyl gallate suppresses proliferation and invasion in human
breast cancer cells via Akt-NF-κB signaling. Oncol Rep.
33:1284–1290. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li F, Zhang J, Arfuso F, Chinnathambi A,
Zayed ME, Alharbi SA, Kumar AP, Ahn KS and Sethi G: NF-κB in cancer
therapy. Arch Toxicol. 89:711–731. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Makin G and Hickman JA: Apoptosis and
cancer chemotherapy. Cell Tissue Res. 301:143–152. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dandekar DS, Lopez M, Carey RI and
Lokeshwar BL: Cyclooxygenase-2 inhibitor celecoxib augments
chemotherapeutic drug-induced apoptosis by enhancing activation of
caspase-3 and −9 in prostate cancer cells. Int J Cancer.
115:484–492. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wong RS: Apoptosis in cancer: From
pathogenesis to treatment. J Exp Clin Cancer Res. 30:872011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vermeulen K, Van Bockstaele DR and
Berneman ZN: Apoptosis: Mechanisms and relevance in cancer. Ann
Hematol. 84:627–639. 2005. View Article : Google Scholar : PubMed/NCBI
|