Introduction
The majority of gastrointestinal tract malignancies
are classified as adenocarcinomas (MAs), which remain a leading
cause of death worldwide (1).
Mucinous MA in the gastrointestinal tract is different from
classical MA in terms of morphological characteristics (2). The currently accepted definition of
mucinous MA, which was initially proposed by Jass et al
(3), is characterized as abundant
mucous secretions comprising a minimum of 50% extracellular mucin
produced by neoplastic cells (4). MA
is still considered an unfavourable and unfamiliar subtype of the
disease (5). Nevertheless, the
epidemiology of MA is not well illustrated, particularly in terms
of demographic characteristics, incidence and survival outcomes for
different sites of the gastrointestinal tract. Heterogeneous MA has
been reported to be associated with inconsistent clinicopathologic
and biologic characteristics (6–8). The
prognostic impact of different anatomic sites in the
gastrointestinal tract on MA is unclear.
The purpose of this study was to explore the
evolving epidemiology and treatment response of MA in the
gastrointestinal tract over a 15-years period using the database of
the Surveillance, Epidemiology, and End Results (SEER) program. We
queried and utilized data from 2000 to 2014 in a site-stratified
survival analysis of the oesophagus, stomach, small intestine,
appendix, colon and rectum, focusing on the role of the primary
tumour site as a prognostic factor related to the long-term
survival. To our knowledge, this is the first large
population-based study to clarify epidemiological and survival
changes in MA according to the anatomical distribution in the
gastrointestinal tract. Findings in this study may help to
elucidate the carcinogenesis of MA in the various tumour locations
of the alimentary tract.
Materials and methods
Data source
We applied for and obtained research files in
November 2016 from the National Cancer Institute's SEER database
(Program, released April 2017, based on the November 2016
submission), which is a comprehensive source of population-based
information covering 28% of the U.S. population. Strict quality
control is maintained by the SEER Quality Improvement program,
which establishes standards for cancer registries and maintains
them through continual monitoring, assessment, and education. We
obtained permission to access the SEER database with the ID no.
10947-Nov2016 via the Internet access method. Cases of invasive
gastrointestinal mucinous MA (ICD-O-3 8480/3, 8481/3) that were
reported to the SEER program between 2000 and 2014 were included in
the study.
Classification of gastrointestinal
mucinous MA
We used SEER histologic grade information to
classify cases as grade I, well differentiated; grade II,
moderately differentiated; grade III, poorly differentiated; and
grade IV, undifferentiated or anaplastic. Grade III and grade IV
were combined into 1 category for all analyses.
The SEER staging system was used for analysis.
Tumors were classified as localized, regional, or distant. A
localized GIMA was defined as an invasive neoplasm confined
entirely to the organ of origin. A regional GIMA was defined as a
neoplasm that i) extended beyond the limits of the organ of origin
directly into surrounding organs or tissue, ii) involved regional
lymph nodes, or iii) fulfilled both of the aforementioned criteria.
Finally, a distant GIMA was defined as a neoplasm that spread to
parts of the body remote from the primary tumor.
Statistical analysis
One way ANOVA test with Student-Newman-Keuls post
hoc test was used to compare the difference of continuous data.
Chi-square test was used to compare the difference of categorical
data. Incidence rates were age adjusted to the 1970 standard
million U.S. population and expressed as cases per 100,000 persons.
To maximize the representativeness of our study, we calculated the
2000–2014 incidences and survival using SEER 18 databases. The time
of follow-up for all analyses was from the date of diagnosis until
death, date of last contact, or the deadline of the study.
To evaluate the most recent trends in survival, we
conducted multivariable survival analyses of the SEER 18 data
(2000–2014). Two cohorts were identified for multivariable survival
analyses: the total SEER 18 gastrointestinal mucinous MA cohort,
which comprised all patients with gastrointestinal mucinous MA in
SEER 18, and the distant gastrointestinal mucinous carcinoma
cohort. Overall survival (OS) and the Cox proportional hazards
model were used in the multivariable analysis. Covariates for this
analysis included factors known to influence the prognosis of
gastrointestinal mucinous MA, including grade, age, race, sex,
stage, site, and time interval from diagnosis.
The incidence (including annual percentage change)
was calculated using SEER*Stat software, version 8.3.4
(Surveillance Research Program, National Cancer Institute). In this
software, the annual percentage change was calculated by fitting a
least-squares regression to the natural logarithm of the rates,
using the calendar year as a regress or variable, and age-adjusted
incidence rates were computed using weighted proportions of
corresponding age groups in the 2000 US standard population.
All other statistical analyses were performed using
IBM SPSS software for Windows, version 19.0 (IBM Corporation,
Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Demographic characteristics
A total of 51,632 cases of gastrointestinal MA were
reported in the SEER program during the period from 2000 to 2014.
In total, 26,058 (50.5%) were women, and 25,574 (49.5%) were men.
Additionally, 82.9% of the patients were white, 10.7% were African
American, 5.6% were Asian/Pacific Islander, and 0.5% were American
Indian/Alaskan native. The baseline characteristics in the present
study are shown in Table I. The mean
ages at diagnosis of patients with the disease in all sites or in
the appendix were 68.5 and 59.2 years, respectively, and the age at
diagnosis of patients with appendiceal MA was significantly younger
than that of patients with the disease in other sites (P<0.01
for all comparisons). In male individuals, a higher proportion of
the disease was located in the oesophagus, stomach and small
intestine than in the appendix, colon and rectum (P<0.01).
Tumour extension at the time of diagnosis differed by different
disease locations. Distant metastasis in patients with colorectal
MA was less common than in those with MA in other locations
(P<0.01).
 | Table I.Characteristics of patients with
gastrointestinal mucinous adenocarcinoma, SEER, 2000-2014. |
Table I.
Characteristics of patients with
gastrointestinal mucinous adenocarcinoma, SEER, 2000-2014.
|
|
| No. of patients
(%) |
|
|---|
|
|
|
|
|
|---|
| Characteristic | Total (N=51,632) | Esophagus
(N=769) | Stomach
(N=2,055) | Small intestine
(N=711) | Appendix
(N=2,894) | Colon (N=39,714) | Rectum (N=5,489) | P-value |
|---|
| Mean age at
diagnosisa | 68.54 | 66.47 | 69.27 | 65.83 | 59.24 | 69.81 | 64.63 | P<0.01 |
| Sexb |
|
|
|
|
|
|
| P<0.01 |
| Male | 25,574 (49.5) | 666 (86.6) | 1,380 (67.2) | 391 (55.0) | 1,301 (45.0) | 18,521 (46.6) | 3,315 (60.4) |
|
|
Female | 26,058 (50.5) | 103 (13.4) | 675 (32.8) | 320 (45.0) | 1,593 (55.0) | 21,193 (53.4) | 2,174 (39.6) |
|
| Raceb |
|
|
|
|
|
|
| P<0.01 |
|
Caucasian | 42,805 (82.9) | 735 (95.6) | 1,499 (72.9) | 552 (77.6) | 2,402 (83.0) | 33,105 (83.4) | 4,512 (82.2) |
|
|
African-American | 5,544 (10.7) | 21 (2.7) | 315 (15.3) | 122 (17.2) | 244 (8.4) | 4,311 (10.9) | 531 (9.7) |
|
|
Other | 3,283 (6.4) | 13 (1.7) | 241 (11.7) | 37 (5.2) | 248 (8.6) | 2,298 (5.7) | 446 (8.1) |
|
| Gradeb |
|
|
|
|
|
|
| P<0.01 |
| Grade
I | 5,477 (10.6) | 37 (4.8) | 104 (5.1) | 69 (9.7) | 963 (33.3) | 3,825 (9.6) | 479 (8.7) |
|
| Grade
II | 28,479 (55.2) | 212 (27.6) | 610 (29.7) | 346 (48.7) | 878 (30.3) | 23,490 (59.1) | 2,943 (53.6) |
|
| Grade
III+ IV | 10,994 (21.3) | 351 (45.6) | 919 (44.7) | 150 (21.1) | 299 (10.3) | 8,167 (20.6) | 1,108 (20.2) |
|
|
Unknown | 6,682 (12.9) | 169 (22.0) | 422 (20.5) | 146 (20.5) | 754 (26.1) | 4,232 (10.7) | 959 (17.5) |
|
| Tumor
extensionb |
|
|
|
|
|
|
| P<0.01 |
|
Localized | 14,306 (27.7) | 154 (20.0) | 355 (17.3) | 131 (18.4) | 527 (18.2) | 11,677 (29.4) | 1,462 (26.6) |
|
|
Regional | 24,002 (46.5) | 285 (37.1) | 786 (38.2) | 318 (44.7) | 649 (22.4) | 19,153 (48.2) | 2,811 (51.2) |
|
|
Distant | 12,295 (23.8) | 263 (34.2) | 757 (36.8) | 223 (31.4) | 1,624 (56.1) | 8,418 (21.2) | 1,010 (18.4) |
|
|
Unknown | 1,029 (2.0) | 67 (8.7) | 157 (7.6) | 39 (5.5) | 94 (3.2) | 466 (1.2) | 206 (3.8) |
|
Incidence
The incidence of gastrointestinal MA accounts for
approximately 5.3–8.7% of all gastrointestinal malignant neoplasms.
This proportion differed by the location of the disease (0.9–1.9%
in the oesophagus, 1.3–3% in the stomach, 2.7–4.5% in the small
intestine, 24.4–33.9% in the appendix, 8.1–11.8% in the colon and
3.1–6.5% in the rectum). The annual age-adjusted incidence of
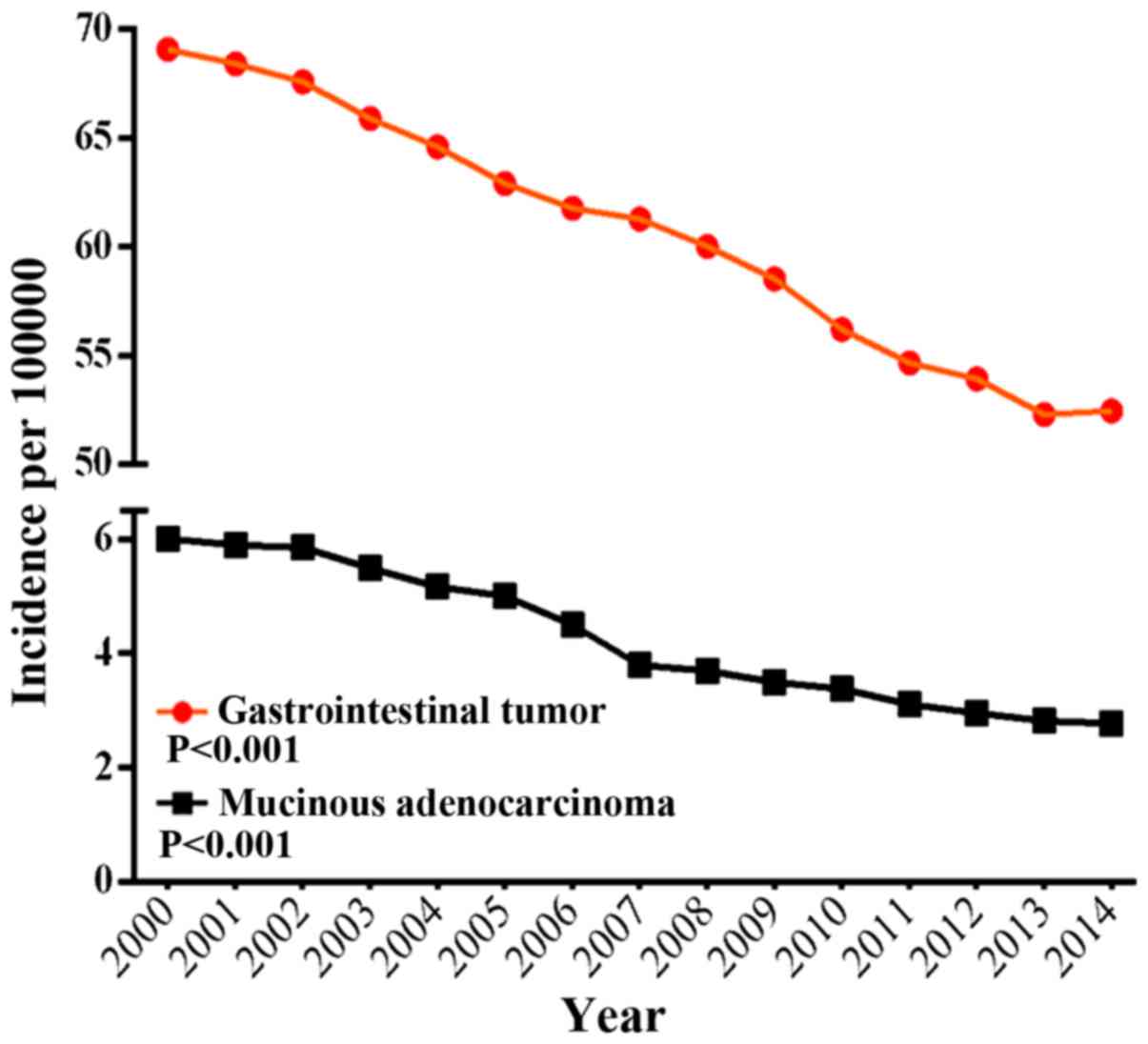
gastrointestinal mucinous MA was 6.007 per 100,000 persons in 2000
and decreased to 2.779 per 100,000 persons in 2014, as shown in
Fig. 1. Age-specific incidence rates
were calculated for 3 age groups: Younger than 50 years, 50 to 64
years, and 65 years or older. The most dramatic decrease in
incidence was noted in patients 65 years or older with a 58.8%
decrease to 13.602 per 100,000 persons, and, in those 50 to 64
years, to 4.539 per 100,000 persons; those younger than 50 years
had a more modest 26.3% decrease to 0.524 per 100,000 persons. The
annual percentage change for age-adjusted incidences from 2000 to
2014 in SEER 18 was −6.12 per 100,000 persons (P<0.001).
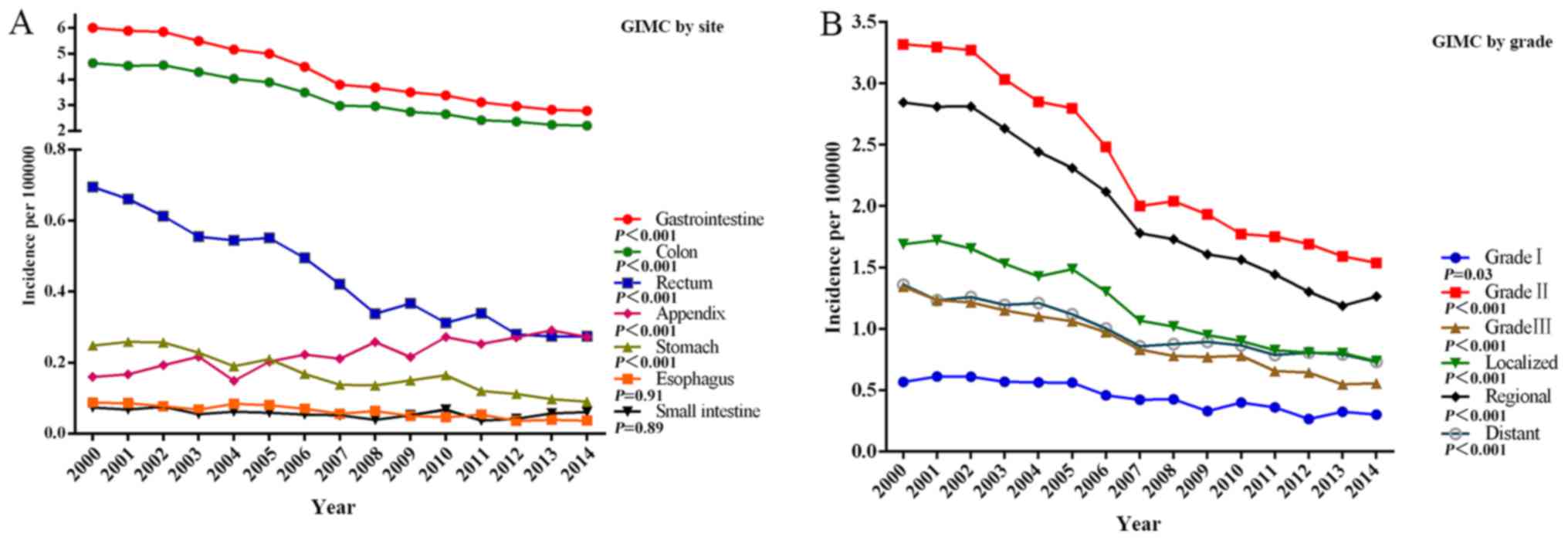
The decrease in the incidence of gastrointestinal
mucinous MA from 2000 to 2014 occurred across all stages, grades
and sites apart from the appendix (a 1.7-times increase) (Fig. 2A). The decreases in the incidence for
various sites ranged from 63.5% in the stomach to 17.6% in the
small intestine. Among stage groups, the incidence decreased the
most in localized gastrointestinal mucinous MA from 1.688 per
100,000 persons in 2000 to 0.736 per 100,000 persons in 2014
(P<0.001) (Fig. 2B). Among the
different grade groups, the incidence of Grade II gastrointestinal
mucinous MA decreased dramatically from 1.343 per 100,000 persons
in 2000 to 0.556 per 100,000 persons in 2014 (P<0.001) (Fig. 2B). In SEER 18 (2000–2014), the highest
incidences were 3.333 per 100,000 persons in the colon, 0.448 per
100,000 persons in the rectum, 0.224 per 100,000 persons in the
appendix, 0.171 per 100,000 persons in the stomach, 0.062 per
100,000 persons in the oesophagus, and 0.057 per 100,000 persons in
the small intestine.
Survival outcome
The median OS for all patients was 54.8 months.
Patients with localized gastrointestinal MA showed better survival
than individuals with regional or distant disease (P<0.001 for
120 months vs. 71.9 months vs. 13.9 months, respectively). The
median OS of patients with good, average and
poor/no-differentiation were 96.6, 69.9 and 31.4 months,
respectively, and the difference in the median OS between these
subgroups was statistically significant (P<0.001). The best and
worst median OS were observed in the appendix (86.6 months),
oesophagus (11.51 months), and stomach (10.63 months). A similar
trend was observed for OS (data not shown). The significant
differences between them were observed using a Kaplan-Meier Curve
(P<0.001 for log-rank test).
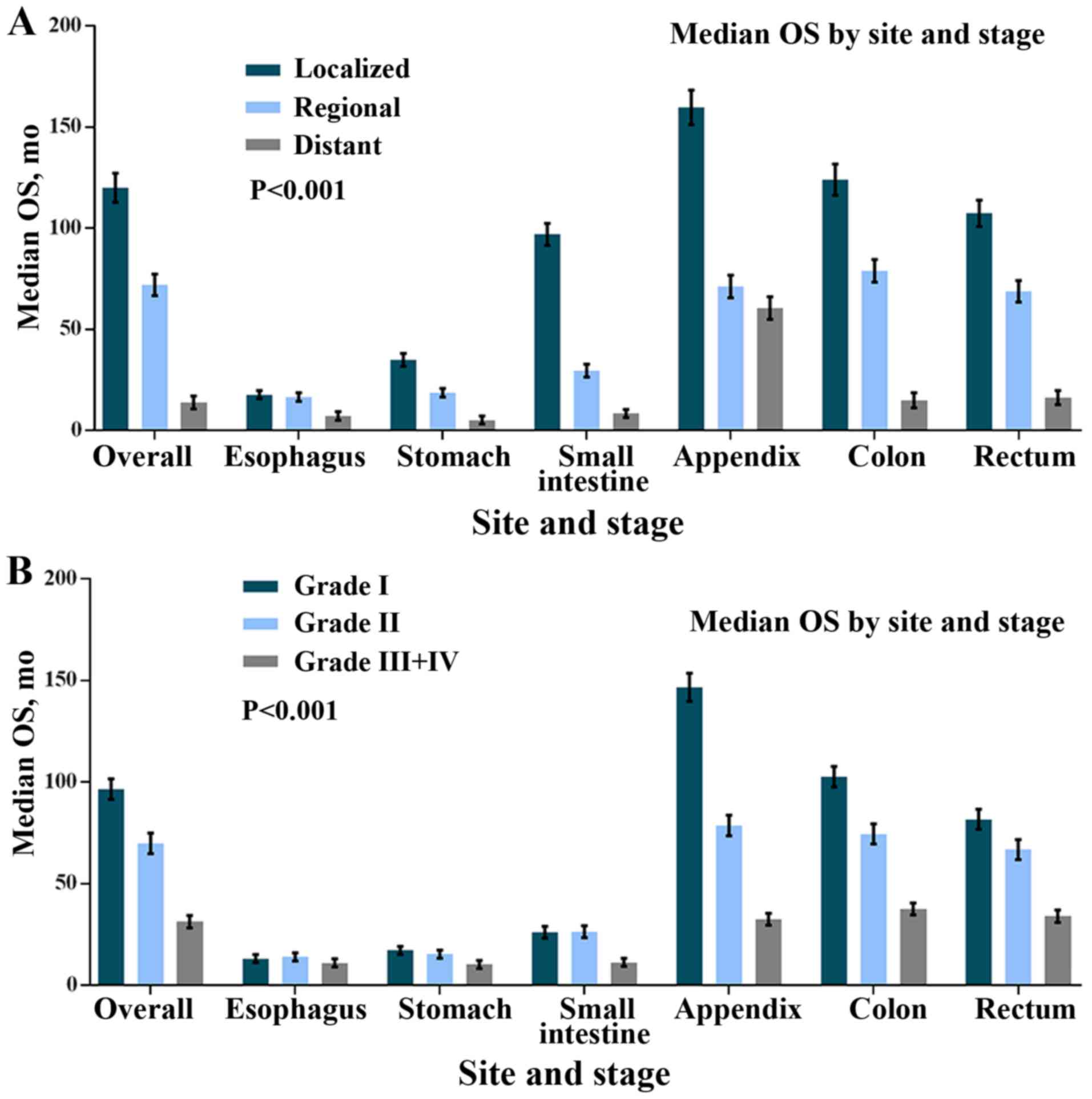
According to different sites and stages, we
evaluated survival patterns in localized, regional and distant
disease. In localized disease, the median OS ranged from 17.4
months for the oesophagus to 159.7 for the appendix. The median OS
ranged from 16.5 months for oesophageal MA to 78.9 months for
colonic MA in terms of regional disease. Appendiceal MA had the
best median OS (60.4 months), whereas stomach and oesophageal MA
conferred the worst survival (5.2 and 7.25 months, respectively) in
terms of distant MA. A similar trend was observed for OS (data not
shown). All of the median OS differences were statistically
significant (P<0.001 for the log-rank test) (Fig. 3A).
Next, we evaluated the median OS according to the
site and the grade. Patients with Grade I appendiceal MA had the
longest median OS (146.7 months). For Grade III/IV MA, appendiceal
MA had a worse median OS than colonic MA (32.5 vs. 37.6 months,
respectively). Oesophageal, gastric, and small intestinal MA had
the worst median OS (11, 10.3 and 11.3 months, respectively). A
similar trend was observed for OS (data not shown). All of the
differences between median OS were statistically significant
(P<0.001 for the log-rank test) (Fig.
3B).
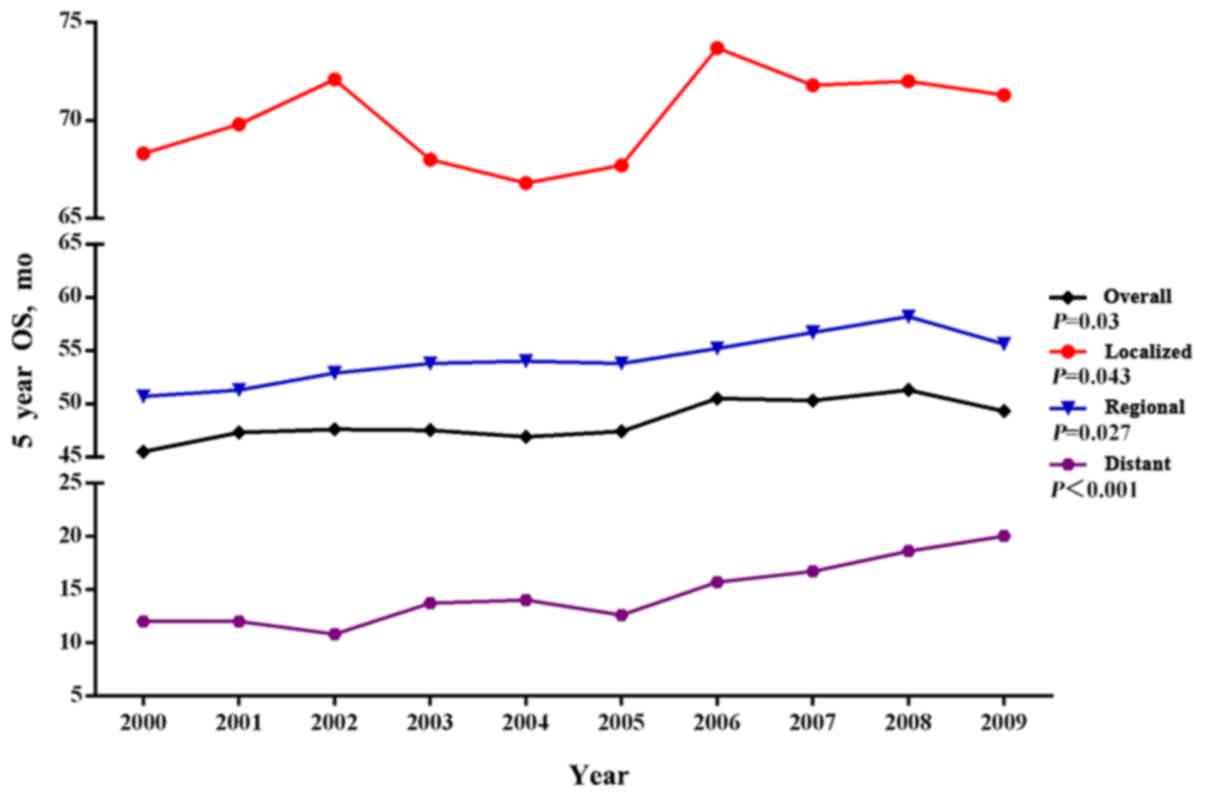
Finally, we focused on the SEER 18 cohort
(2000–2012) to evaluate the most recent trends in OS for localized,
regional and distant gastrointestinal MA. The OS of
gastrointestinal MA improved slowly between 2000 and 2009 (Fig. 4). The improvement in the median OS
over the same time interval was more pronounced in the subgroup of
patients with distant gastrointestinal MA (19.9 months for 2012 vs.
10.3 months for 2000).
Multivariable Cox analysis for OS
We next performed a multivariable Cox analysis with
measurements of hazard ratios (HRs) and 95% confidential intervals
(CIs). Age, stage and site were all found to have significant
correlations with survival. Compared with grade I gastrointestinal
MA, patients with grade II (HR, 1.11; 95% CI, 1.07–1.16) and grade
III/IV (HR, 1.40; 95% CI, 1.34–1.47) disease had poor OS. Worse OS
was observed in regional (HR, 1.42; 95% CI, 1.37–1.46) and distant
(HR, 4.93; 95% CI, 4.77–5.10) gastrointestinal MA than in localized
gastrointestinal MA. In subgroups stratified by site, the worst OS
was observed in oesophageal MA (HR, 1.92; 95% CI, 1.76–2.09), and
gastric MA also showed a worse OS (HR, 1.82; 95% CI, 1.72–1.93)
than rectal MA (Table II).
 | Table II.Multivariable survival analysis of
patients with gastrointestinal mucinous carcinoma diagnosed from
2000 to 2014. |
Table II.
Multivariable survival analysis of
patients with gastrointestinal mucinous carcinoma diagnosed from
2000 to 2014.
|
| HR (95% CI) |
|---|
|
|
|
|---|
| Covariate | Total SEER18
GIMC | Distant GIMC |
|---|
| Year |
|
2000–2004 | 1 (Reference) | 1 (Reference) |
|
2005–2009 | 0.93
(0.91–0.96) | 0.87
(0.83–0.91) |
|
2010–2014 | 0.92
(0.83–0.91) | 0.83
(0.78–0.87) |
| Sex |
|
Male | 1 (Reference) | 1 (Reference) |
|
Female | 0.93
(0.91–0.95) | 0.94
(0.90–0.97) |
| Race |
|
Caucasian | 1 (Reference) | 1 (Reference) |
|
African-American | 1.11
(1.07–1.15) | 1.13
(1.07–1.20) |
|
Other | 0.86
(0.82–0.91) | 0.95
(0.87–1.03) |
| Age at diagnosis,
years |
|
≤49 | 1 (Reference) | 1 (Reference) |
|
50–64 | 1.19
(1.13–1.24) | 1.15
(1.08–1.23) |
|
≥65 | 2.29
(2.20–2.39) | 1.70
(1.60–1.81) |
| Grade |
| Grade
I | 1 (Reference) | 1 (Reference) |
| Grade
II | 1.11
(1.07–1.16) | 1.21
(1.11–1.31) |
| Grade
III+IV | 1.40
(1.34–1.47) | 1.56
(1.43–1.70) |
| Site |
|
Esophagus | 1.92
(1.76–2.09) | 1.63
(1.41–1.88) |
|
Stomach | 1.82
(1.72–1.93) | 1.83
(1.65–2.02) |
| Small
intestine | 1.61
(1.47–1.77) | 1.65
(1.41–1.92) |
|
Appendix | 0.45
(0.42–0.48) | 0.43
(0.39–0.47) |
|
Colon | 0.93
(0.90–0.97) | 1.14
(1.06–1.22) |
|
Rectum | 1 (Reference) | 1 (Reference) |
| Stage |
|
Localized | 1 (Reference) | NA |
|
Regional | 1.42
(1.37–1.46) | NA |
|
Distant | 4.93
(4.77–5.10) | NA |
We evaluated the most recent trends in OS between
2000–2004, 2005–2009 and 2010–2014 using the SEER 18 cohort. The
patients who were diagnosed during 2010–2014 and during 2005–2009
had an 8% (HR, 0.92, 95% CI, 0.83–0.91) and 7% (HR, 0.89, 95% CI,
and 0.91–0.96) decreased risk of death, respectively, compared with
patients who were diagnosed between 2000 and 2004. The improvement
in survival over those time intervals was more pronounced in the
subgroup with distant gastrointestinal mucinous carcinoma (HR,
0.87; 95% CI, 0.83–0.91 for 2005–2009 and HR, 0.83; 95% CI,
0.78–0.87 for 2009–2014 compared with 2000–2004). All of the above
comparisons were considered to be statistically significant at
P<0.001.
Discussion
Several epidemiological studies have previously
reported the clinical features and prognosis of MA in different
primary tumour sites (6–8). However, there is a scarcity of
large-scale studies that examine site-specific MA differences
specifically in the gastrointestinal tract. In contrast to previous
studies, our study is the first population-based study focusing
solely on the demographic characteristics, incidence and clinical
outcomes of MA in different sites of the gastrointestinal tract.
The analysis of the different primary site distributions revealed
that the incidence of gastrointestinal MA has decreased in the
whole gastrointestinal tract, except for in the appendix. Moreover,
a statistically significant improvement in the prognosis of
gastrointestinal MA was observed.
In this large, nationally representative study of
more than 51,600 MA patients, a steadily decreasing trend in the
incidence of gastrointestinal malignancies was found. From 2000 to
2014, the annual incidence of MA had a similar trend to classical
MAs but decreased at a higher speed; this observation is in
agreement with other studies (8–10).
However, a notable observation on the age distribution was that the
decreased incidence of MA was associated mainly with people older
than 50, particularly for people 65 or older. US demographic data
demonstrated a rapidly aging population from 2000 to 2014 (11). The decrease in the incidence of
classical MA and MA suggests that the epidemic peak of
gastrointestinal malignancies has passed. A high body mass index
and unhealthy lifestyle factors, such as the excessive consumption
of red meat and alcohol, are known to be risk factors for
gastrointestinal neoplasms (12,13). The
promotion of a healthy lifestyle and the reduced exposure to these
risk factors may be potentially responsible for the decreasing
incidence of MA that we observed. Similarly, gastroenterological
endoscopic techniques allow for early detection and resection,
which are helpful for preventing tumour progression at an early
stage (14,15). People younger than 50 underwent less
frequent endoscopy screening than people who were 50 years and
older, which was another possible reason.
These findings, in agreement with other studies
(16), have shown that the majority
of gastrointestinal MAs occurred in the colorectal. Based on these
observations, MAs were less frequently found in the proximal
gastrointestinal tract, such as in the oesophagus, stomach and
small intestine. Additionally, the proportion of MAs in the
oesophagus, stomach, and small intestine was relatively higher in
males than in females, whereas MAs in the colon and rectum were
more frequently observed in females. The site-specific difference
between male and female patients in the proximal and distal
gastrointestinal tract should be highlighted.
Moreover, disparities were seen in survival rates
according to anatomic location. MAs in the upper gastrointestinal
tract were associated with poorer survival (17). Specifically, our data revealed that
oesophageal, gastric and small intestinal MA tend to have worse
prognoses with a higher incidence of metastases than colorectal MA
at the time of diagnosis. Compared with colorectal MA, MA located
in the upper gastrointestinal tract may have an underlying
aggressive molecular profile, suggesting that different types of
cancer progression and carcinogenesis may be involved between the
proximal and distal gastrointestinal tract. Knowledge of
site-specific differences may be useful in making better
therapeutic guidelines and treatment decisions. An increase in
screening endoscopy might help with earlier detection of these
lesions and thus lead to faster treatment. Microsatellite
instability and BRAF mutations are common in patients with
MA and are frequently associated with poor outcomes and metastatic
risk (2,18). In future work, the estimation of
tumour aggressiveness should be performed in light of
microsatellite instability and BRAF mutational status.
Underlying site-specific biological carcinogenesis or environmental
backgrounds should be targeted in more studies to improve outcomes
for MA in the upper gastrointestinal tract.
Gastrointestinal MA is a disease entity with
decreased incidence rates across all anatomic sites, except for the
appendix. The incidence of appendiceal MA showed an increasing
trend of more than 1.7 times over a period of 15 years. This
increase might partially be attributed to improved detection
through advances in endoscopic and radiologic imaging techniques.
Compared with MA located in any of the other sites, appendiceal MA
has been associated with a younger age at presentation and larger
proportions of appendiceal malignancies, which is a finding
consistent with other reports (19,20).
According to the different grade levels, appendiceal MA appeared to
have variable survival outcomes; better OS time than any other
sites of the gastrointestinal tract was commonly seen in most
stages and grades of appendiceal MA, except for Grade III/IV. Our
study showed, with convincing data, that poorly differentiated
grade III/IV appendiceal MA was directly correlated with a poorer
prognosis, even worse than the median OS for colonic MA. This
finding is in accordance with the 7th AJCC TNM classification of
appendiceal neoplasms.
Therefore, grade III/IV appendiceal MA should be
considered an unfavourable, high-risk disease. This is another
reason why we should pay more attention to gaining a comprehensive
understanding of how grade and stage at the time of diagnosis
affect MA. According to a new proposal by the AJCC TNM
classification of appendiceal neoplasms, low-grade appendiceal
mucinous neoplasm (LAMN) should be classified as a distinct entity
disease that has a possible relationship to pseudomyxoma peritonei
(PMP) (21–23). To improve the survival of these
patients, it is therefore important to detect appendiceal MA and to
perform curative resection at an early stage (24). Better-tailored approaches to patient
management on the basis of clinicopathologic characteristics, which
enable the targeting of specific tumour phenotypes in patients with
appendiceal MA, best suit the needs of individual patients. This
observation also highlights the importance of MA screening with
routine endoscopy to identify and treat these tumours at an early
stage.
Consistent with the current knowledge that the grade
and stage of MA are directly correlated with the prognostic outcome
(25,26), our study reveals that advanced stage
MA and a poorly differentiated grade are independently associated
with poorer outcomes and more metastatic risk, (27,28); these
findings are in agreement with other reports. Interestingly, what
is promising is that survival outcomes for MA have been improving
over a period of 15 years, especially for patients with advanced
stage disease and distant metastasis. This is probably mainly
explained by the remarkable advances that have been made in the
treatment of patients with advanced stage MA. Although surgery is
the mainstay of treatment for gastrointestinal MA, the availability
of chemotherapy or chemoradiotherapy for the treatment of
gastrointestinal malignancies is already commonly used (29). In addition, the benefits of adjuvant
treatment in patients with gastrointestinal MA are applicable to
those with MA (30). While advanced
stage MAs behave more aggressively, patients have also been treated
with multidisciplinary strategies, which is a possible reason for
the obvious improvement in survival rates (31). Moreover, a multidisciplinary approach
has become the standard treatment for the management of patients
with advanced stage MA (29). Joint
efforts from surgeons, pathologists, oncologists and radiologists
have been made to better tailor approaches to patient management on
an individualized basis.
There are several limitations to this study due to a
lack of relevant clinical information regarding neoadjuvant
chemotherapy or chemoradiotherapy strategies. Additionally, several
known prognostic indicators were not captured by the SEER database,
such as microsatellite instability and BRAF, p53, and
p16 mutational status, which might provide a more detailed
analysis of incidence and survival in patients with MA. In future
studies, we will work to study the impact of these factors on the
prognosis of GIMA. Furthermore, the WHO classification of tumours
of the digestive system changed according to novel molecular
findings over the 15-year period of this study. MA at the same
primary site may exhibit different molecular characteristics, which
probably leads to a certain bias in this study. Such drawbacks are
inherent to any retrospective population-based study. We believe
that the size of the present study is the largest to date and that
the long duration of follow-up provides a comprehensive
epidemiologic picture to understand gastrointestinal MA. Further
research about site-specific molecular and clinicopathologic
characteristics of MA are necessary.
MA in different primary sites of the
gastrointestinal tract exhibits different clinical and biological
characteristics. All sites along the alimentary tract, with the
exception of the appendix, showed a decrease in the incidence of
MA. Increased OS was observed in patients with MA in most areas of
the gastrointestinal tract, especially in patients with advanced
stage disease. Given the noted changes in the incidence of MA and
the survival of patients with MA, clinicians should consider the
primary tumour site and focus on the prognostic implications of the
primary tumour site.
Acknowledgements
Not applicable.
Funding
The study was supported by The National Natural
Science Foundation of China (grant nos. 81860433 and 81860466).
Availability of data and materials
These data are publicly available for use in
accordance with a limited use agreement for SEER research data:
SEER Program (https://seer.cancer.gov) SEER*Stat
Database: Incidence-SEER Program (www.seer.cancer.gov) SEER*Stat Database:
Incidence-SEER 18 Regs Research Data + Hurricane Katrina Impacted
Louisiana Cases, November 2016 Sub (1973–2014 varying)-Linked To
County Attributes-Total U.S., 1969–2015 Counties, National Cancer
Institute, DCCPS, Surveillance Research Program, released April
2017, based on the November 2016 submission.
Authors' contributions
CY, ZZ and HL were involved in the study conception
and design. ZZ, YL, HY, AW, HL and CY collected and assembled data;
prepared the figures and tables; provided final approval of the
manuscript to be published; and are accountable for all aspects of
the study. CY, ZZ and YL were involved in data analysis and
interpretation. CY and ZZ wrote the manuscript.
Ethics approval and consent to
participate
The study was reviewed by the Institutional Review
Board of the Second Affiliated Hospital of Nanchang University. It
was determined to be a retrospective analysis of publicly
available, de-identified data and was determined to be exempt from
requiring written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AJCC
|
American Joint Committee on Cancer
|
|
SEER
|
surveillance, epidemiology, and end
results
|
|
MA
|
mucinous adenocarcinoma
|
|
OS
|
overall survival
|
|
CI
|
confidential interval
|
|
HR
|
hazard ratio
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nitsche U, Zimmermann A, Späth C, Muller
T, Maak M, Schuster T, Slotta-Huspenina J, Kaser SA, Michalski CW,
Janssen KP, et al: Mucinous and signet-ring cell colorectal cancers
differ from classical adenocarcinomas in tumor biology and
prognosis. Ann Surg. 258:775–783. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jass JR, Sobin LH and Watanabe H: The
World Health Organization's histologic classification of
gastrointestinal tumors. A commentary on the second edition.
Cancer. 66:2162–2167. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pihl E, Nairn RC, Hughes ES, Cuthbertson
AM and Rollo AJ: Mucinous colorectal carcinoma: Immunopathology and
prognosis. Pathology. 12:439–447. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hugen N, Brown G, Glynne-Jones R, de Wilt
JH and Nagtegaal ID: Advances in the care of patients with mucinous
colorectal cancer. Nat Rev Clin Oncol. 13:361–369. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yin C, Li D, Sun Z, Zhang T, Xu Y, Wang Z
and Xu H: Clinicopathologic features and prognosis analysis of
mucinous gastric carcinoma. Med Oncol. 29:864–870. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
van den Heuvel MG, Lemmens VE, Verhoeven
RH and de Hingh IH: The incidence of mucinous appendiceal
malignancies: A population-based study. Int J Colorectal Dis.
28:1307–1310. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hyngstrom JR, Hu CY, Xing Y, You YN, Feig
BW, Skibber JM, Rodriguez-Bigas MA, Cormier JN and Chang GJ:
Clinicopathology and outcomes for mucinous and signet ring
colorectal adenocarcinoma: Analysis from the national cancer data
base. Ann Surg Oncol. 19:2814–2821. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Du W, Mah JT, Lee J, Sankila R,
Sankaranarayanan R and Chia KS: Incidence and survival of mucinous
adenocarcinoma of the colorectum: A population-based study from an
Asian country. Dis Colon Rectum. 47:78–85. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xie L, Villeneuve PJ and Shaw A: Survival
of patients diagnosed with either colorectal mucinous or
non-mucinous adenocarcinoma: A population-based study in Canada.
Int J Oncol. 34:1109–1115. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Geronimus AT, Bound J and Ro A:
Residential mobility across local areas in the United States and
the geographic distribution of the healthy population. Demography.
51:777–809. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aleman JO, Eusebi LH, Ricciardiello L,
Patidar K, Sanyal AJ and Holt PR: Mechanisms of obesity-induced
gastrointestinal neoplasia. Gastroenterology. 146:357–373. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shivanna Mysuru L and Urooj A: A review on
dietary and non-dietary risk factors associated with
gastrointestinal cancer. J Gastrointest Cancer. 47:247–254. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Veitch AM, Uedo N, Yao K and East JE:
Optimizing early upper gastrointestinal cancer detection at
endoscopy. Nat Rev Gastroenterol Hepatol. 12:660–667. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Songur Y, Okai T, Watanabe H, Fujii T,
Motoo Y and Sawabu N: Preoperative diagnosis of mucinous gastric
adenocarcinoma by endoscopic ultrasonography. Am J Gastroenterol.
91:1586–1590. 1996.PubMed/NCBI
|
|
16
|
Numata M, Shiozawa M, Watanabe T, Tamagawa
H, Yamamoto N, Morinaga S, Watanabe K, Godai T, Oshima T, Fujii S,
et al: The clinicopathological features of colorectal mucinous
adenocarcinoma and a therapeutic strategy for the disease. World J
Surg Oncol. 10:1092012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tang X, Zhang J, Che X, Lan Z, Chen Y and
Wang C: The clinicopathological features and long-term survival
outcomes of mucinous gastric carcinoma: A consecutive series of 244
cases from a single institute. J Gastrointest Surg. 20:693–699.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nitsche U, Rosenberg R, Balmert A,
Schuster T, Slotta-Huspenina J, Herrmann P, Bader FG, Friess H,
Schlag PM, Stein U and Janssen KP: Integrative marker analysis
allows risk assessment for metastasis in stage II colon cancer. Ann
Surg. 256:763–771. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Widmann B, Warschkow R, Schmied BM, Marti
L and Steffen T: Impact of mucinous histology on the prognosis of
stage I–III Adenocarcinomas of the appendix: A population-based,
propensity score-matched analysis. J Gastrointest Surg.
20:1493–1502. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hsu JT, Chen HM, Liao CH, Yeh CN, Yeh TS,
Hwang TL, Jan YY and Chen MF: Clinicopathologic features and
predictors for survival of mucinous and non-mucinous appendiceal
adenocarcinoma. Dig Surg. 25:369–375. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ramaswamy V: Pathology of mucinous
appendiceal tumors and pseudomyxoma peritonei. Indian J Surg Oncol.
7:258–267. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Carr NJ, Bibeau F, Bradley RF, Dartigues
P, Feakins RM, Geisinger KR, Gui X, Isaac S, Milione M, Misdraji J,
et al: The histopathological classification, diagnosis and
differential diagnosis of mucinous appendiceal neoplasms,
appendiceal adenocarcinomas and pseudomyxoma peritonei.
Histopathology. 71:847–858. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Misdraji J: Mucinous epithelial neoplasms
of the appendix and pseudomyxoma peritonei. Mod Pathol. 28 Suppl
1:S67–S79. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sugarbaker PH: The natural history, gross
pathology, and histopathology of appendiceal epithelial neoplasms.
Eur J Surg Oncol. 32:644–647. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ooki A, Akagi K, Yatsuoka T, Asayama M,
Hara H, Yamamoto G, Nishimura Y and Yamaguchi K: Inverse effect of
mucinous component on survival in stage III colorectal cancer. J
Surg Oncol. 110:851–857. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hsu JT, Wang CW, Le PH, Wu RC, Chen TH,
Chiang KC, Lin CJ and Yeh TS: Clinicopathological characteristics
and outcomes in stage I–III mucinous gastric adenocarcinoma: A
retrospective study at a single medical center. World J Surg Oncol.
14:1232016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jimi S, Hotokezaka M, Ikeda T, Uchiyama S,
Hidaka H, Maehara N, Ishizaki H and Chijiiwa K: Clinicopathological
features, postoperative survival and prognostic variables for
cancer-related survival in patients with mucinous colorectal
carcinoma. Surg Today. 45:329–334. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kawamura H, Kondo Y, Osawa S, Nisida Y,
Okada K, Isizu H, Uebayasi T, Takahasi M and Hata T: A
clinicopathologic study of mucinous adenocarcinoma of the stomach.
Gastric Cancer. 4:83–86. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fiorica F, Stefanelli A, Pascale G and
Fisichella R: Elderly gastrointestinal cancer patients and
radiochemotherapy: A review. Clin Ter. 165:57–61. 2014.PubMed/NCBI
|
|
30
|
Sandler S: Esophagogastric junction and
gastric adenocarcinoma: Neoadjuvant and adjuvant therapy, and
future directions. Oncology (Williston Park). 28:505–512.
2014.PubMed/NCBI
|
|
31
|
Hogan J, Burke JP, Samaha G, Condon E,
Waldron D, Faul P and Coffey JC: Overall survival is improved in
mucinous adenocarcinoma of the colon. Int J Colorectal Dis.
29:563–569. 2014. View Article : Google Scholar : PubMed/NCBI
|