Introduction
Lung cancer is the leading cause of cancer-related
mortality in many countries, called ‘a global scourge’ with a
dismal prognosis. Diagnosis is frequently made at an advanced stage
when prognosis is poor and therapeutic options are limited
(1). The molecular mechanisms
underlying global variations in lung cancer biology remains poorly
understood (2). Therefore, it is
necessary to identify multiple biomarkers for early detection and
prognosis. Transcriptional silencing of genes by CpG islands (CGIs)
methylation is now recognized as a crucial component in lung cancer
initiation and progression (3). In
addition, gene-specific hyper-methylation has emerged as an
important factor in the earliest stages of preinvasive lung cancer
related to tobacco smoking, a major etiological factor (4).
Tumor suppressor p53 is a cellular gatekeeper that
guards against genetic instability and abnormality by sensing
multiple stress signals, including DNA damage, oncogene activation,
and hypoxia (5). Lung cancer has a
higher p53 mutation rate compared to other kinds of cancer
(6). Expression of p53 is
tightly controlled through multiple regulatory layers, but limited
information is available on how p53 is transcriptional and
epigenetically regulated (7).
Recently, WD40 repeat containing antisense to p53 (Wrap53) (also
known as WDR79/TCAB1) was found to be a natural antisense
transcript (NAT) of p53 that regulates endogenous p53 mRNA levels
by targeting the 5′untranslated region (UTR) (8). Transcripts initiated from exon 1α, 1β,
and 1γ are called Wrap53α, Wrap53β, and Wrap53γ,
respectively. Exon 1α directly overlaps the first exon of
p53 in an antisense fashion and forms an RNA-RNA hybrid with
p53 mRNA to protect it from degradation (8). Interestingly, Wrap53α transcript
is upregulated by cancer therapeutic drugs and miR-4732-5p has a
binding site in the 5′ UTR of the Wrap53α transcript
(9–11). However, overexpression of the WRAP53
protein, mainly produced from the Wrap53β transcript, is
linked to progression of several types of tumors, including lung
cancer (12–15). Importantly, neither β- or
γ-transcripts, nor WRAP53 protein, has any effects on p53 when
overexpressed or knocked down. Therefore, it is conceivable that
dysfunction of Wrap53α could contribute to tumorigenesis by
failing to sustain p53 expression and function in wild-type
(WT) p53-carrying tumors. In order to test this hypothesis
and understand the biological role of Wrap53α in lung
cancer, we investigated the methylation status of the
Wrap53α promoter in resected primary non-small cell lung
cancers (NSCLC) using methylation-specific polymerase chain
reaction (MSP) and assessed the correlation of these results with
clinicopathological characteristics.
Materials and methods
Patients and tissue samples
Tumor and corresponding non-malignant lung tissue
specimens (n=146) were provided by the National Biobank of Korea,
Kyungpook National University Hospital (KNUH; Daegu, Korea), which
is supported by the Ministry of Health, Welfare, and Family
Affairs. The present study was conducted with the approval of the
Ethics Committee of KNUH (no. 2014-04-210) and written informed
consent was obtained from all of the participants prior to
obtaining the samples. The clinicopathological characteristics of
the patients are summarized in Table
I.
 | Table I.Correlation between Wrap53α
promoter methylation status and characteristics of non-small cell
lung cancer patients. |
Table I.
Correlation between Wrap53α
promoter methylation status and characteristics of non-small cell
lung cancer patients.
| Variables | Methylation, n
(%) | P-value |
|---|
| All subjects
(n=146) | 12 (8.2) |
|
| Age (years) |
|
|
| ≤64
(n=76) | 7 (9.2) | 0.66 |
| >64
(n=70) | 5 (7.1) |
|
| Sex |
|
|
| Men
(n=98) | 8 (8.2) | 0.97 |
| Women
(n=48) | 4 (8.3) |
|
| Smoking status |
|
|
| Ever
(n=101) | 8 (7.9) | 0.84 |
| Never
(n=45) | 4 (8.9) |
|
| Histological
types |
|
|
| SQC
(n=43) | 2 (4.7) | 0.31 |
| ADC
(n=103) | 10 (9.7) |
|
| Pathologic
stage |
|
|
| Stage I
(n=91) | 4 (4.4) | 0.03 |
| Stage
II–IIIA (n=55) | 8 (14.6) |
|
| p53
mutations |
|
|
|
Negative (n=87) | 10 (11.5) | 0.08 |
|
Positive (n=59) | 2 (3.4) |
|
Genomic DNA isolation and methylation
analysis
Genomic DNA was extracted using a QIAamp DNA Mini
kit (QIAGEN, Valencia, CA). After treatment of the genomic DNA with
sodium bisulfite, the methylation status of Wrap53α promoter
encompassing the transcription start site was analyzed using MSP
with primers specific for either unmethylated or methylated
alleles. The primer sequences for Wrap53α were described in
Table II. All polymerase chain
reaction (PCR) amplifications were carried out using reagents
supplied in a GeneAmp DNA Amplification kit with AmpliTaq Gold as
the polymerase (PE Applied Biosystems, Foster City, CA, USA) on a
PTC-100 thermal cycler (MJ Research, Watertown, MA, USA). CpGenome™
Universal methylated and unmethylated DNA (Chemicon, Temecula, CA,
USA) was used as a positive control for the methylated and
unmethylated genes, respectively. Negative control samples without
DNA were included for each set of PCR. PCR products were analyzed
on 2% agarose gel, stained with ethidium bromide, and visualized
under UV light. Each MSP was repeated at least once to confirm the
results.
 | Table II.Primer sequences used for MSP and
sqPCR. |
Table II.
Primer sequences used for MSP and
sqPCR.
| Primer | Forward primer (5′
to 3′) | Reverse primer (5′
to 3′) |
|---|
| MSP |
|
|
|
U-MSP |
AATATATGGAGTTGAGAGTTT |
AAAAACATACTTTCCACAACA |
|
M-MSP |
AATATACGGAGTCGAGAGTTC |
AAAAACGTACTTTCCACGACG |
| sqPCR |
|
|
|
Wrap53α |
CGGAGCCCAGCAGCTACC |
TTGTGCCAGGAGCCTCGCA |
|
Wrap53β |
GTCCCGGCTCCGCGGGTTC |
GGCTGAGGACATCAGAGAATACCAGC |
|
P53 |
GACGGTGACACGCTTCCCTGGAT |
CGTGCAAGTCACAGACTTGGCTGTC |
|
GAPDH |
CATGACAACTTTGGTATCGTG |
GTGTCGCTGTTGAAGTCAGA |
Cell culture, total RNA isolation, and
semi-quantitative (sq)-PCR
Ten human NSCLC cell lines (A549, HCC827, H23, H358,
H520, H522, H1299, H1703, H2009 and PC9) were obtained from the
American Type Culture Collection (ATCC, Manassas, VA). All cells
were propagated according to instructions from the ATCC. HCC827
cells were treated with 20 µM 5-AzadC for 3 days and the culture
media was changed daily. Total RNA was extracted from cultured
cells and primary tumor tissues using TRIzol (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). After removal of
residual DNA, first-strand cDNA was synthesized from total RNA
using SuperScript preamplification (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions. The
resulting cDNA was amplified with Wrap53α- and p53-specific
primers as previously described (8).
The following thermocycling conditions were applied: 95°C for 2
min, then 30 cycles of 95°C for 1 min, 58°C for 1 min and 72°C for
1 min, and a final extension at 72°C for 10 min. Amplification of
GAPDH was used as an internal loading control. All primer
sequences were described in Table
II.
Mutational analysis of p53 gene
P53 mutational analysis of the entire coding
regions (exons 2–11), including exon/intron boundaries, was
performed by PCR-based direct sequencing. The primers and
conditions for PCR reactions were described previously (16). Sequencing was done using an ABI Prism
3100 Genetic Analyzer (PE Applied Biosystems). All sequence
variants were confirmed by sequencing the products of independent
PCR amplifications in both directions.
Statistical analysis
The associations between methylation status and
clinicopathological characteristics were analyzed using a
chi-square test for categorical variables. Logistic regression
analysis was conducted to estimate the association between
methylation status and the covariates of age, sex, exposure to
tobacco smoke, and histology. The overall survival (OS) of NSCLC
patients according to methylation status of the Wrap53α
promoter was compared using the Kaplan-Meier method and the
log-rank test. Hazard ratios (HRs) and 95% confidence intervals
(CIs) were estimated using a multivariate Cox proportional hazard
model. Data were analyzed using SAS v9.4 software (SAS Institute,
Inc., Cary, NC, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Methylation status and expression of
Wrap53α gene in NSCLC samples
We have analyzed the methylation status of the human
Wrap53α gene in 146 primary NSCLCs and their corresponding
nonmalignant lung tissues using MSP. There were no classical CGIs
in the 5′-flanking region of the human Wrap53α gene,
including the first exon, but 13 CGIs were found from −150 to +30
bp upstream of the transcription start site. Thus, we designed the
MSP primer pairs to cover this region. Methylated alleles of
representative samples were shown as in Fig. 1A. Unmethylated bands were detected in
most of the nonmalignant and malignant tissues (data not shown),
thus confirming the integrity of the DNA in those samples.
Bisulfite-sequencing of the representative PCR products confirmed
the assigned methylation status and showed that all cytosines at
non-CpG sites were converted to thymine (data not shown), ruling
out the possibility of incomplete bisulfite conversion.
Wrap53α methylation was exclusively detected in malignant
tissues at a frequency of 8.2% (12/146), suggesting that
Wrap53α promoter methylation may be a tumor-associated event
during NSCLC tumorigenesis.
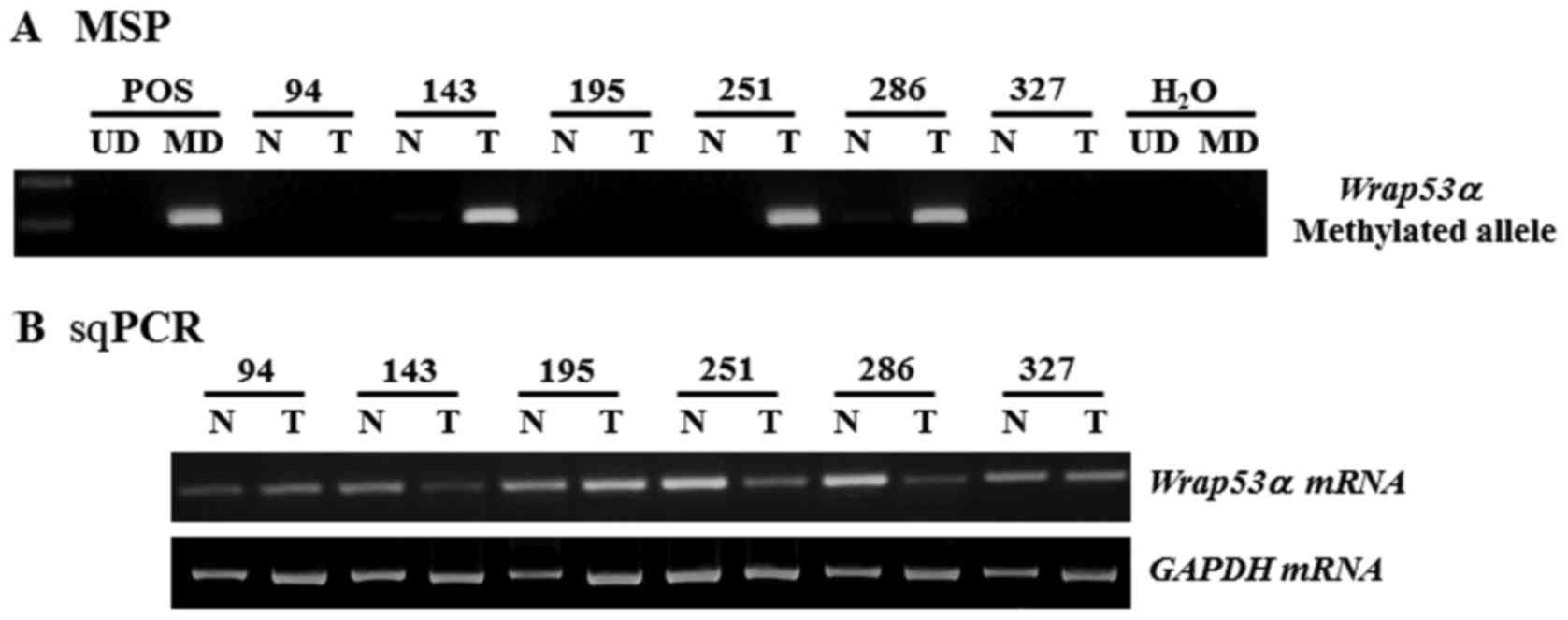 | Figure 1.Representative results of MSP and
sqPCR analysis of Wrap53α gene in NSCLC patients. (A) The
methylation status of the Wrap53α promoter in NSCLCs was
analyzed by MSP. CpGenome™ Universal MD or UD was used
as a POS for the methylated or unmethylated products, respectively.
Water was used as a negative control. (B) Expression of
Wrap53α mRNA was measured in primary tissues from NSCLC
patients by sqPCR. Amplified products were run on 2% agarose gel
and appeared at positions corresponding to the expected base pair
lengths. Amplification of GAPDH was used as an internal
loading control. sqPCR, semi-quantitative polymerase chain
reaction; N, non-malignant tissue; T, tumor tissues; M-MSP,
amplified product with primers that recognize the methylated
sequences; Wrap53α, WD repeat containing antisense to TP53α;
NSCLC, non-small cell lung cancer; MD, methylated DNA; UD,
unmethylated DNA; POS, positive control. |
To determine whether CpG methylation was involved in
the regulation of Wrap53α expression, we analyzed
Wrap53α mRNA levels in representative tissue specimens.
sqPCR analysis showed low or undetectable levels of Wrap53α
transcripts in tumor tissues with a methylated allele, whereas high
levels were detected in tumor and non-tumor lung tissues with an
unmethylated allele (Fig. 1B). We
have further confirmed these results in 10 human NSCLC cell lines.
Comparison of methylation status with sqPCR findings demonstrated
that Wrap53α mRNA was present in all examined cell lines
except a HCC827 cell line that had methylated alleles (Fig. 2A). After treatment with the
demethylating agent 5-AzadC for 3 days, HCC827 cells exhibited the
disappearance of methylated alleles and induced the re-expression
of Wrap53α mRNA, resulting in increased p53 mRNA
levels (Fig. 2B). These results
suggest that CpG island methylation may be a mechanism for
downregulating the expression of Wrap53α gene.
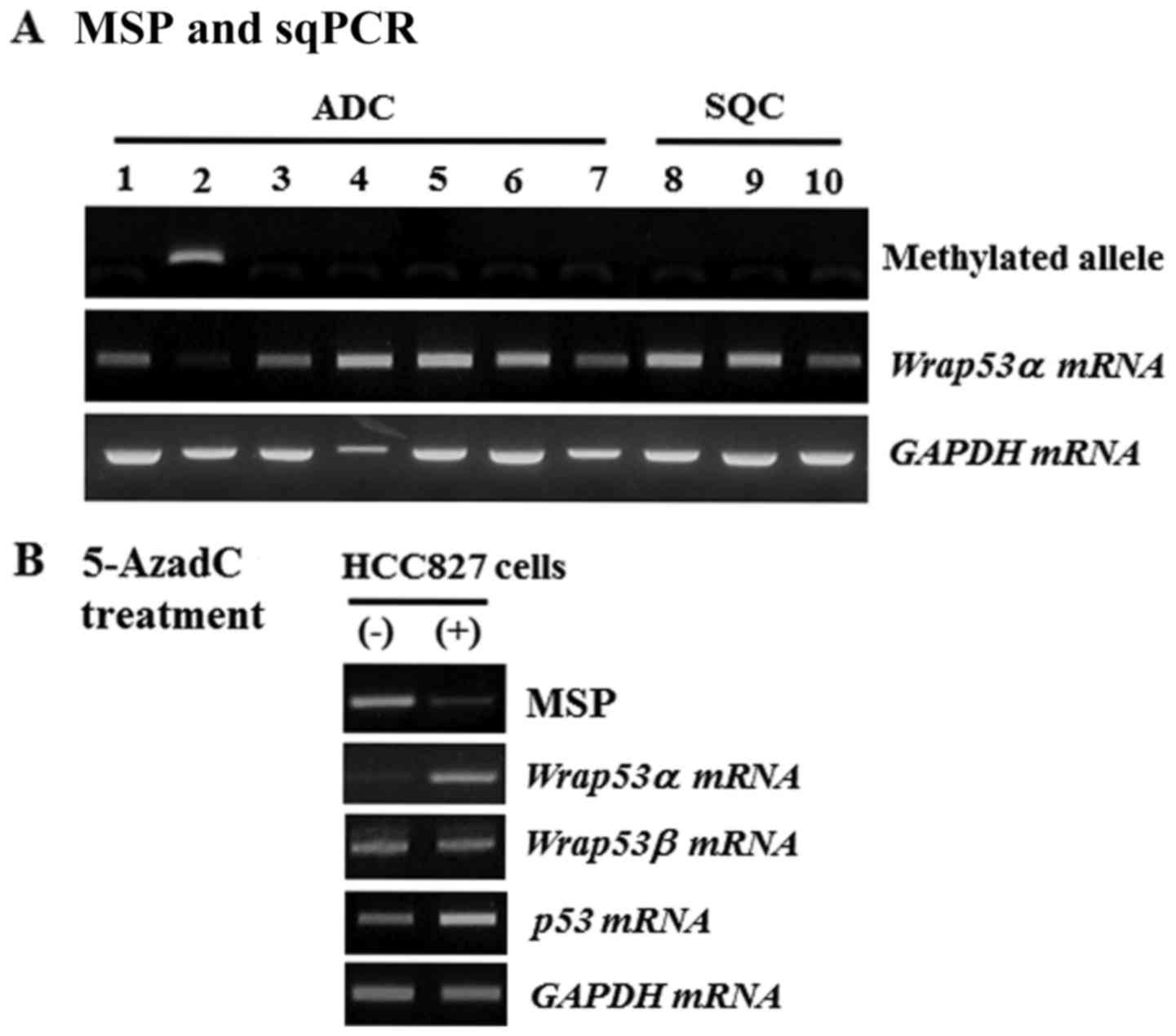 | Figure 2.MSP and sqPCR analysis of
Wrap53α gene in NSCLC cell lines. (A) The methylation status
of the Wrap53α promoter was analyzed in 10 cell lines by
MSP. Expression of Wrap53α mRNA was performed on the same
cell lines by sqPCR. Lane 1, A549; Lane 2, HCC827; Lane 3, H23;
Lane 4, H358; Lane 5, H522; Lane 6, H1299; Lane 7, PC9; Lane 8,
H520; Lane 9, H1703; Lane 10, H2009. Lanes 1–7, ADC; Lanes 8–10,
SQC. (B) Methylation status and expression of Wrap53α was
analyzed in HCC827 cells following 20 µM 5-AzadC treatment for 3
days. Simultaneously, Wrap53α and p53 mRNA levels
were measured. GAPDH was amplified as an internal loading
control. (−), vehicle alone; (+), 5-AzadC addition; MSP,
methylation-specific polymerase chain reaction; sqPCR,
semi-quantitative polymerase chain reaction; Wrap53α, WD
repeat containing antisense to TP53α; NSCLC, non-small cell lung
cancer; ADC, adenocarcinoma; SQC, squamous cell carcinoma. |
Association of Wrap53α promoter
methylation with clinicopathological parameters and clinical
outcomes
Wrap53α promoter methylation was
significantly more frequent in stages II–IIIA tumors than stages I
tumors (P=0.03) (Table I). In
addition, its methylation was more frequently detected in p53
mutation-negative cases than in p53 mutation-positive cases with
borderline significance (P=0.08). However, no significant
correlation was observed between its methylation and any other
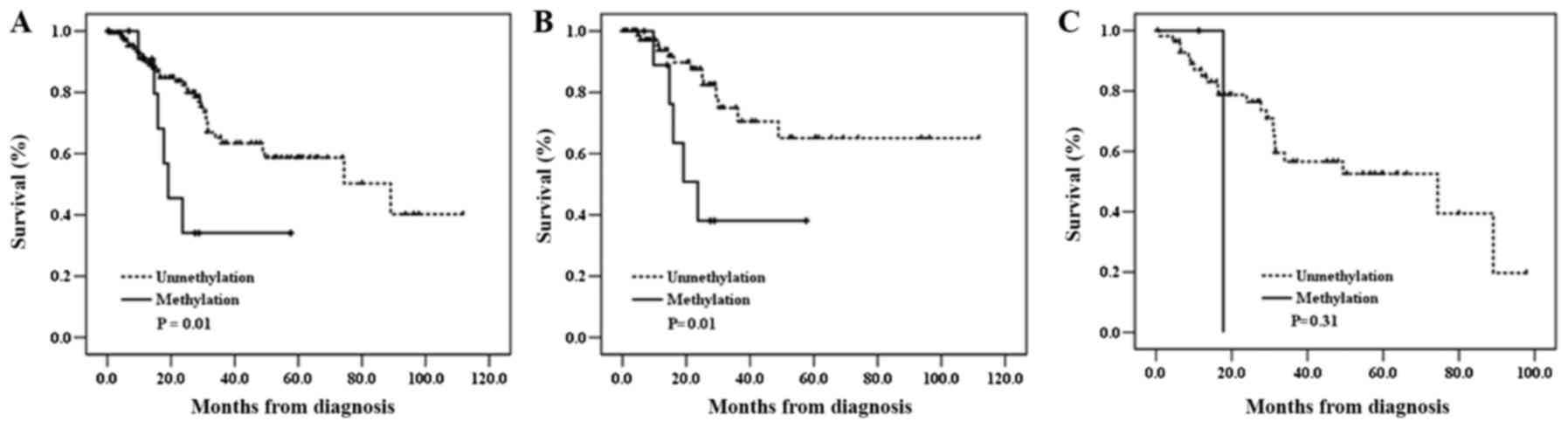
factors, such as age, sex, or smoking status (Table I). Next, Kaplan-Meier survival
analysis was carried out to determine the prognostic potential of
Wrap53α promoter methylation. Interestingly, the patients
with the methylation had worse OS compared to those without
Wrap53α methylation [log-rank P
(PL-R)=0.01] (Table
III and Fig. 3). When stratified
according to clinicopathological characteristics of patients,
Wrap53 promoter methylation was significantly associated
with an unfavorable survival in a subset of patients including
younger, female, never-smoker, squamous cell carcinoma, and
p53 mutation-negative (PL-R=0.0003, 0.03, 0.02,
0.01, and 0.01, respectively) (data not shown). To evaluate the
Wrap53α promoter methylation as an independent prognostic
factor in NSCLC, we further analyzed the data using the Cox
proportional hazards regression adjusting for possible confounders
of survival. Methylation of Wrap53α promoter was
significantly associated with worse OS of total patients [adjusted
HR (adjHR)=2.44, 95% CI=0.98–6.04, P=0.05]. Notably,
Wrap53α promoter methylation significantly associated with
poor OS in p53 mutation-negative patients
(adjHR=2.92, 95% CI=1.00–8.60, P=0.05; Table III) but not in patients with p53
mutations. Moreover, Wrap53α promoter methylation exhibited
a trend toward worse OS in patients with stages II–IIA
(adjHR=2.76, 95% CI=0.93–8.22, P=0.07) (data not shown).
These results suggest that Wrap53α may play an important
role in lung cancer pathogenesis and its methylation could be
considered as a prognostic marker for NSCLC patients.
 | Table III.Overall survival according to
methylation of the Wrap53α promoter in non-small cell lung
cancer patients. |
Table III.
Overall survival according to
methylation of the Wrap53α promoter in non-small cell lung
cancer patients.
|
|
|
|
|
| Crude | Adjusted |
|---|
|
|
|
|
|
|
|
|
|---|
| Methylation
negative/positive | Cases (n) | Mortality
(%)a | 5 SYRb | PLR | HR (95% CI) | P-value | PHT | HR (95%
CI)c | P-value | PHT |
|---|
| All subjects | 146 |
|
|
|
|
|
|
|
|
|
|
Negative | 134 | 35 (26.1) | 58 |
| 1 |
|
| 1 |
|
|
|
Positive | 12 | 6 (50.0) | 34 | 0.01 | 2.88
(1.19–6.96) | 0.02 |
| 2.44
(0.98–6.04) | 0.05 |
|
| p53-mutation
negative |
|
|
|
|
|
|
|
|
|
|
|
Negative | 77 | 13 (16.9) | 65 |
| 1 |
|
| 1 |
|
|
|
Positive | 10 | 5 (50.0) | 38 | 0.01 | 3.59
(1.26–10.20) | 0.02 |
| 2.92
(0.99–8.60) | 0.05 |
|
| p53-mutation
positive |
|
|
|
|
|
|
|
|
|
|
|
Negative | 57 | 22 (38.6) | 53 |
| 1 |
|
| 1 |
|
|
|
Positive | 2 | 1 (50.0) | 0 | 0.32 | 2.73
(0.35–21.19) | 0.34 | 0.81 | 1.90
(0.21–16.86) | 0.57 | 0.73 |
Discussion
Although the majority of investigations concerned
with P53 have focused on coding regions, recent studies have
highlighted the significant roles that regulatory elements located
in p53 mRNA play, particularly the 5′UTR that displays high
conservation and immutability (17,18).
Wrap53 antisense RNA targets p53 mRNA via the 5′UTR
and increases p53 protein levels, indicating that dysfunction of
Wrap53 itself may be a separate cause of cancer.
Wrap53 has three different start exons: Exon 1α, 1β, and 1γ.
Exon 1α and 1γ match the first exon and intron, respectively, of
p53 in a cis-antisense manner. Exon 1β does not produce
transcripts that are complementary to any section of p53
(8). Moreover, knockdown of
Wrap53α reduces p53 abundance (8). There are several studies focusing on the
function and expression of Wrap53β transcript in tumor
progression (12–15), however, the exact function of have no
information about is available. Thus, we focused on the methylation
status of the Wrap53α promoter. The discovery of
Wrap53α would elucidate the role of NAT-mediated gene
regulation in the P53 pathway. NATs, as a member of long non-coding
RNAs, occur ubiquitously in mammals and are crucial players in
carcinogenesis, invasion, and metastasis (19). These RNAs regulate gene expression
through direct interaction with sense transcripts or indirect
interactions with other targets, such as DNA methyltransferases
(DNMTs), histone acetylases and histone deacetylases. Many NATs
interact with cancer relevant genes such as p53, p15, p21,
RB1, and PTEN (20). Taken
together, our findings provide new insights that NATs could be a
potential rich sources of biomarkers for use in diagnosis and
prognosis of cancer.
Although the molecular mechanisms contributing to
promoter methylation of Wrap53α remain elusive, there is
evidence that malignant transformation associated with chronic
inflammation, persistent viral infection, cigarette smoking, and
oxidative stress can upregulate the expression and activity of
DNMTs through transcriptional and post-translational regulation
(21–24). Interestingly, Lin et al
(25) have shown that dysregulation
of p53 control leads to DNMT1 and DNMT3A
overexpression, resulting in promoter hypermethylation of multiple
tumor suppressor genes in NSCLC patients (26). Thus, it is likely that the
overexpressed DNMT can induce Wrap53α hypermethylation.
It is noteworthy that Wrap53α methylation was
significantly associated with unfavorable survival in a subset of
NSCLC patients, especially for p53 mutation-negative
patients. The downregulation of Wrap53α transcripts by
promoter methylation could destabilize p53 mRNA to reduce
tumor suppressor activity of the WT P53, contributing to poor
prognosis. Smoking causes a high percentage of missense mutations
in the DNA-binding domain of p53, producing a striking
gain-of function phenotype (6). In
addition, mutant P53 can drive cancer by subverting multiple tumor
suppression pathways independent of WT p53 (27). Mutations of the p53 gene
usually but not always lead to an increased half life of the
p53 protein, and result in a nuclear accumulation of protein
(27). Consequently, p53 alteration
that can be detected as either protein overexpression or mutation
makes the problem more complicated. Alternatively, alterations in
regulators of P53 provide an alternative way to deregulate P53
function in p53 WT tumors, but are likely redundant in tumors that
already have a dysfunctional P53 protein. Indeed, we found that
Wrap53α methylation showed a tendency for p53
mutation-negative patients, indicating the absence of concomitant
alterations in p53 and Wrap53α. Mutual exclusive
alterations is frequently observed in cancer, being believed to
occur between functionally related genes (28). Taken together, it could be speculated
that the effect of Wrap53α promoter methylation on the
clinical outcome could be more noticeable in patients with WT P53.
Therefore, our findings suggest that the close interplay between
the Wrap53α and p53 might be involved in the
pathogenesis of NSCLC. However, further studies with larger sample
sizes are required to establish that Wrap53α methylation is
a useful prognostic indicator for patients with NSCLC.
The present study has shown that the Wrap53α
promoter was methylated in a subset of NSCLCs, and its methylation
was significantly associated with unfavorable OS of patients,
particularly in patients with p53 mutation negative, suggesting
that Wrap53α methylation status could be informative for
prediction of NSCLC prognosis. Although the current study did not
have a full viewpoint because of the small sample size and no
information regarding P53 protein expression, it is the first
report to demonstrate an aberrant methylation of the Wrap53α
promoter in NSCLC and may provide new pieces in the P53 targeting
puzzle for cancer therapy.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
Korea Health Technology R&D Project through the Korea Health
Industry Development Institute (KHIDI), funded by the Ministry of
Health and Welfare (grant no. HI4C0402).
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author on reasonable request.
Authors' contributions
DSK contributed to the experimental design and
implementation, performed the experiments and data analysis, and
drafted the manuscript. WKL performed the statistical analyses. JYP
contributed to experimental implementation, interpreted the patient
data and modified the manuscript. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of KNUH (no. 2014-04-210). Written informed consent was
obtained from all participants or their families prior to obtaining
the samples.
Patient consent for publication
All participants provided written informed consent
for the publication of any associated data and accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McIntyre A and Ganti AK: Lung cancer-A
global perspective. J Surg Oncol. 115:550–554. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kerr KM, Galler JS, Hagen JA, Laird PW and
Laird-Offringa IA: The role of DNA methylation in the development
and progression of lung adenocarcinoma. Dis Markers. 23:5–30. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brzeziańska E, Dutkowska A and Antczak A:
The significance of epigenetic alterations in lung carcinogenesis.
Mol Biol Rep. 40:309–325. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Green DR and Kroemer G: Cytoplasmic
functions of the tumour suppressor p53. Nature. 458:1127–1130.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gibbons DL, Byers LA and Kurie JM:
Smoking, p53 mutation, and lung cancer. Mol Cancer Res. 12:3–13.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Saldaña-Meyer R and Recillas-Targa F:
Transcriptional and epigenetic regulation of the p53 tumor
suppressor gene. Epigenetics. 6:1068–1077. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mahmoudi S, Henriksson S, Corcoran M,
Méndez-Vidal C, Wiman KG and Farnebo M: Wrap53, a natural p53
antisense transcript required for p53 induction upon DNA damage.
Mol Cell. 33:462–471. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bug M and Dobbelstein M: Anthracyclines
induce the accumulation of mutant p53 through E2F1-dependent and
-independent mechanism. Oncogene. 30:3612–3624. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yuan JM, Li XD, Liu ZY, Hou GQ, Kang JH,
Huang DY and Du SX: Cisplatin induces apoptosis via upregulating
Wrap53 in U-2OS osteosarcoma cells. Asian Pac J Cancer Prev.
12:3465–3469. 2011.PubMed/NCBI
|
|
11
|
Pouladi N, Kouhsari SM, Feizi MH, Gavgani
RR and Azarfam P: Overlapping region of p53/wrap53 transcripts:
Mutational analysis and sequence similarity with microRNA-4732-5p.
Asian Pac J Cancer Prev. 14:3503–3507. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mahmoudi S, Henriksson S, Farnebo L,
Roberg K and Farnebo M: WRAP53 promotes cancer cell survival and is
a potential target for cancer therapy. Cell Death Dis. 2:e1142011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang H, Wang DW, Adell G and Sun XF:
WRAP53 is an independent prognostic factor in rectal cancer-a study
of Swedish clinical trial of preoperative radiotherapy in rectal
cancer patients. BMC Cancer. 12:2942012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rao X and Huang D, Sui X, Liu G, Song X,
Xie J and Huang D: Overexpression of WRAP53 is associated with
development and progression of esophageal squamous cell carcinoma.
PLoS One. 9:e916702014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yuan XS, Cao LX, Hu YJ, Bao FC, Wang ZT,
Cao JL, Yuan P, Lv W and Hu J: Clinical, cellular, and
bioinformatic analyses reveal involvement of WRAP53 overexpression
in carcinogenesis of lung adenocarcinoma. Tumor Biol.
39:10104283176943092017. View Article : Google Scholar
|
|
16
|
Lee EB, Jin G, Lee SY, Park JY, Kim MJ,
Choi JE, Jeon HS, Cha SI, Cho S, Kim CH, et al: TP53 mutations in
Korean patients with non-small cell lung cancer. J Korean Med Sci.
25:698–705. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Swiatkowska A, Zydowicz P, Sroka J and
Ciesiołka J: The role of the 5′ terminal region of p53 mRNA in the
p53 gene expression. Acta Biochim Pol. 63:645–651. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alonso S, Izarirre N, López S,
Smith-Zubiaga I, Hervella M, Boyano MD, Arroyo-Berdugo Y,
Gardeazabal J, Díaz-Ramón JL, Sánchez Díez A, et al: The diversity
profile of TP53 is influenced by positive selection on the
immediately upstream locus WDR79. Hum Hered. 69:34–44. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nie L, Wu HJ, Hsu JM, Chang SS, Labaff AM,
Li CW, Wang Y, Hsu JL and Hung MC: Long non-coding RNAs: Versatile
master regulators of gene expression and crucial players in cancer.
Am J Transl Res. 4:127–150. 2012.PubMed/NCBI
|
|
20
|
Su WY, Xing H and Fang JY: Natural
antisense transcripts regulate gene expression in an epigenetic
manner. Biochem Biophys Res Commun. 396:177–181. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kanai Y: Alterations of DNA methylation
and clinicopathological diversity of human cancers. Pathol Int.
58:544–558. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu Q and Ni X: ROS-mediated DNA
methylation pattern alterations in carcinogenesis. Curr Drug
Targets. 16:13–19. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin RK and Wang YC: Dysregulated
transcriptional and post-translational control of DNA
methyltransferases in cancer. Cell Biosci. 4:462014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jin T, Hao J and Fan D: Nicotine induces
aberrant hypermethylation of tumor suppressor genes in pancreatic
epithelial ductal cells. Biochem Biophys Res Commun. 499:934–940.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin RK, Wu CY, Chang JW, Juan LJ, Hsu HS,
Chen CY, Lu YY, Tang YA, Yang YC, Yang PC and Wang YC:
Dysregulation of p53/Sp1 control leads to DNA methyltransferase-1
overexpression in lung cancer. Cancer Res. 70:5807–5817. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tang YA, Tsai YT, Lin RK, Hsu HS, Chen CY
and Wang YC: Dysregulation of p53 and RB transcriptional control
leads to overexperssion of DNA methyltransferase in lung cancer. J
Cancer Res Pract. 1:14–27. 2014. View Article : Google Scholar
|
|
27
|
Yue X, Zhao Y, Xu Y, Zheng M, Feng Z and
Hu W: Mutant p53 in cancer: Accumulation, gain-of-function, and
therapy. J Mol Biol. 429:1595–1606. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yeang CH, McComick F and Levine A:
Combinatorial patterns of somatic gene mutations in cancer. FASEB
J. 22:2605–2622. 2008. View Article : Google Scholar : PubMed/NCBI
|