Introduction
Pancreatic related cancer is the ninth most common
cancer in Western Europe and the fifth common cause of death from
cancer (1). The estimated overall
5-year survival in ductal pancreatic carcinoma is less than 5 per
cent (2). Hence, there is need for
therapy improvements and to understand mechanisms behind metastases
to improve postoperative survival of patients with periampullary
cancer, since circulating tumor cells (CTC) in patients with
gastrointestinal carcinomas are assumed to enter the portal
circulation as an initial phase of the metastatic process (3,4). Previous
research has indicated numbers of CTC in blood to predict disease
prognoses in breast, colon and prostate carcinomas (5–7). There are
also indications that CTC may have prognostic value in patients
with pancreatic cancer (8–10), suggesting the existence of CTC
subgroups with aggressively metastasizing phenotypes (11,12), where
increased CTC numbers in portal blood predict liver metastases and
reduced survival in pancreatic carcinoma (13,14), and
colorectal cancer (15). However, the
nature of CTC may be elusive, since cytological characteristics
imply similar CTC in both benign and malignant diagnosis (16). Identification and analyses of CTC may
despite such uncertainties contribute to facilitate diagnosis;
estimate prognosis and better understand the biology of metastases
of periampullary carcinoma. Therefore, the aim of the present study
was to evaluate methodological possibilities to confirm a
statistically significant fractional uptake of CTC across
liver-lung compartments during tumor resections aimed at cure in
limited number of patients, as a future model for isolation of CTC
clones retained across hepatico-lung compartments during
surgery.
Materials and methods
Patients and tumor tissues
Patients (n=17) were included when referred to the
Department of Surgery at Sahlgrenska University Hospital and
scheduled for pancreatic-duodenal surgery aimed at cure (Whipples
operation or total pancreatectomy) due to assumed ductal pancreatic
carcinoma or periampullary cancer according to preoperative
examinations (Table I).
Arterial-portal blood samples from 10 cancer patients were analyzed
by IsofluxR to detect and count CTC. Blood samples from
additional 7 cancer patients were analyzed by flowcytometry (FACS)
to evaluate the occurrence of various CTC markers in cancer
patients with periampullary tumors. Body weight [73.8±16 kg,
standard deviation (SD)] and length (173±7 cm, SD) were recorded
before operations and used in estimations of liver blood flow
compared to expected normal values (1.4–1.5 l/min/1.73
m2 body surface area) (17). All patients were stable during
operations; the mean blood loss was 750±290 ml (SD) and the mean
operation time was 359±56 min (SD). None of the patients received
blood transfusion during operation.
 | Table I.Diagnosis and tumor stage of patients
with cancer.a |
Table I.
Diagnosis and tumor stage of patients
with cancer.a
| Factor | Age at surgery | Sex | Adenocarcinoma | TNM |
|---|
| Isoflux |
|
|
|
|
| 1 | 75 | F | Pancreas | T3N0M0 |
| 2 | 59 | F | Pancreas | T3N1M1 |
| 3 | 72 | F | Pancreas | T3N1M0 |
| 4 | 72 | F | Pancreas | T3N0M0 |
| 5 | 80 | M | Pancreas | T3N1M0 |
| 6 | 57 | M | Pancreas | T2N1M0 |
| 7 | 71 | F | Pancreas | T3N1M0 |
| 8 | 75 | F | Papillary
intestinal | T3N1M0 |
| 9 | 68 | M | Bile ducts | T3N1M0 |
| 10 | 74 | M | Bile ducts | T3N1M0 |
| Flow cytometry
(FACS) |
|
|
|
|
| 11 | 74 | M | Duodenum | T4N1M0 |
| 12 | 71 | F | Pancreas | T3N1M0 |
| 13 | 77 | M | Pancreas | T3N1M0 |
| 14 | 75 | F | Pancreas | T3N1M0 |
| 15 | 72 | F | Pancreas | T3N1M0 |
| 16 | 54 | M | Pancreas | T3N1M0 |
| 17 | 37 | M | Pancreas | T1N1M0 |
Certified pathologists evaluated tissue biopsy
specimens. Postoperative histopathology confirmed 13
adenocarcinomas of the pancreas, 2 bile duct cancers and 2 duodenal
cancers. Bile duct cancers are sometimes hard to differentiate from
pancreatic carcinoma and may have similar tumor biology. Duodenal
cancers may be more diverse perhaps with different characteristics
compared to pancreatic adenocarcinoma. Histopathology and the
specific origin of periampullary cancer is usually not known until
after operations. This explains why our study group represents a
mix of different tumors; however, all with epithelial upper
gastro-intestinal origin.
Ki-67 analyses (in percent) were performed on
resected tumor tissue to achieve estimates of proliferation
activity among tumor cells in representative tumor tissue
specimens. Tumor tissue mass in the entire resected material was
estimated for measurements of tumor volume in three appropriate
directions assuming that one cm3 tissue corresponded to
one gram wet tissue weight; and that 1 mg tumor tissue wet weight
contained approximately 106 cells (18), perhaps variable among different kind
of tumors (19). The average
proportion of tumor cells in tumor tissue was confirmed to be at
least 50% including viability proportion around 80% based on
microscopy. These estimates are used in our attempts to consider
flux aspects of blood concentrations of CTC numbers
(Discussion).
Blood sampling and CTC analyses
Blood samples were collected after surgical
dissections before the removal of the tumor. Blood sampling was
performed by direct puncture of the portal vein simultaneously with
arterial blood sampling through a catheter in the radial artery
inserted before the start of the operation. Complications were not
observed related to collection of portal blood in any patient.
Quantification of CTC was performed by commercially
available equipment (IsofluxR; Westburg-Fluxion
Bioscience Amsterdam, Holland), while qualitative CTC analyses were
performed by an in-house Flow cytometric method (FACS), which,
however, did not allow quantification of CTC in blood. Samples for
Isoflux measurements were from females (60%) and males (40%), while
samples for FACS were 43 and 57% from females and males,
respectively (Table I). Blood samples
were collected in 10 ml syringes (~8 ml) and transferred to special
tubes for cell separation (BD Vacutainer CPT sodium
heparin/Ficoll). Duplicate samples were collected to confirm that
freezing of blood samples before flow cytometric analyses was
appropriate. Peripheral venous blood samples were obtained from
healthy blood donors for FACS (n=10) analyses.
Blood samples were centrifuged for 20 min in room
temperature at 1,800 × g in a swing-out centrifuge. Cell layer and
plasma were transferred to a new tube and washed twice with RPMI
(RPMI-1640; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), at 300
× g for 10 min at room temperature. Cell pellets were immediately
used for downstream Isoflux analyses. Blood samples for FACS
analyses were frozen; cell pellets were resuspended in 400 µl fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA), transferred to a cryo tube and diluted with 400 µl of 50%
FBS, 30% RPMI, 20% DMSO (Gibco; Thermo Fisher Scientific,
Inc./Lonza Group, Ltd., Basel, Switzerland/VWR International GmbH,
Darmstadt, Germany). Samples were contained in −80°C until use.
Frozen cell samples were washed twice with RPMI, centrifuged at 300
× g for 5 min at room temperatures before downstream analysis by
FACS.
Immune-magnetic enrichment of CTCs
with isoflux
Immune-magnetic enrichment with Isoflux is a system,
where CTC of epithelial origin are captured with different
antibodies. The CTC isolation is accomplished with immune-magnetic
beads linked to antibodies added to the blood sample targeting
markers on the cell surface of the CTC. Through a magnetic field
the CTC are then separated from the other blood cells. We used
epithelial cellular adhesion molecule (EpCAM) and cytokeratin
(CK-7, −8, −18, −19) antibodies for CTC capture and detection by
Isoflux; and cluster of differentiation 45 (CD45) antibodies to
identify leukocytes. Isoflux has been validated for CTC
measurements in cancer patients (20,21). A
similar method for CTC enumeration and one of most utilized
worldwide is the CellSearchR system, which applies
EpCAM+, CKs+ (CK-8, −18, −19) and CD45- as standard for CTC
detection in breast, colorectal or prostate cancer (22). A difference between
IsofluxR and CellSearchR is the use of CK-7
for enrichment of CTC, which seems to be important in periampullary
cancer (23) also suggested by the
present study (Tables II and
IV).
 | Table II.Number of CTCs detected with
immune-magnetic enrichment with isoflux (EpCAM-CKs) in each
patient. |
Table II.
Number of CTCs detected with
immune-magnetic enrichment with isoflux (EpCAM-CKs) in each
patient.
| Patient | CTC, amount in
portal sample | CTC, portal
concentration per ml | CTC, amount in
arterial sample | CTC, arterial
concentration per ml |
|---|
| 1 | 0 | 0 | 0 | 0 |
| 2 | 2 | 0.25 | 0 | 0 |
| 3 | 6 | 1 | 2 | 0.33 |
| 4 | 0 | 0 | 0 | 0 |
| 5 | 4 | 0.5 | 2 | 0.25 |
| 6 | 10 | 1.25 | 3 | 0.38 |
| 7 | 3 | 0.38 | 1 | 0.14 |
| 8 | 5 | 0.63 | 1 | 0.2 |
| 9 | 8 | 1 | 5 | 0.71 |
| 10 | 6 | 0.75 | 3 | 0.38 |
 | Table IV.Results from flow cytometric analyses
with expression of markers detected in each blood sample. |
Table IV.
Results from flow cytometric analyses
with expression of markers detected in each blood sample.
| Pt | Blood | EpCAM | MIC-A | CD34 | CD133 | VAP | CK18 | CK19 |
|---|
| 11 | Portal | ++ | +++ | − | + | − | − | − |
| 11 | Arterial | ++ | +++ | +++ | ++ | − | − | − |
| 12 | Portal | ++ | +++ | − | ++ | − | − | +/− |
| 12 | Arterial | ++ | +++ | + | ++ | − | − | − |
| 13 | Portal | + | +++ | − | − | − | − | ++ |
| 13 | Arterial | ++ | +++ | − | − | − | − | ++ |
| 14 | Portal | ++ | +++ | + | ++ | − | − | − |
| 14 | Arterial | ++ | +++ | − | ++ | − | − | − |
| 15 | Portal | ++ | +++ | + | ++ | − | − | − |
| 15 | Arterial | ++ | +++ | ++ | ++ | − | − | − |
| 16 | Portal | ++ | +++ | +/− | ++ | − | − | + |
| 16 | Arterial | ++ | +++ | + | ++ | − | − | + |
| 17 | Portal | ++ | +++ | +/− | ++ | − | − | − |
| 17 | Arterial | ++ | +++ | +/− | ++ | − | − | − |
Blood samples from 10 cancer patients were run
according to the protocol for the Isoflux equipment provided by the
manufacturer (CTC Enrichment kit 630–0124, revision B; Fluxion
Biosciences, Alameda, CA, USA). Briefly, cell pellets were
immediately resuspended in 200 µl RPMI and Fc blocking was added.
CTC beads (pre-labeled with EpCAM antibody) were prepared and cell
solution transferred to the CTC bead solution. The tubes were
rinsed with 300 µl binding solution transferred to the bead+CTC
solution and incubated for at least 1 h at rotation in 4°C. The
Isoflux instrument was primed and samples were run according to the
protocol for CTC enrichment (version 2; Fluxion Biosciences).
Enriched cells were collected in a holder. Collected cells were
immediately diluted in binding buffer and enumeration was performed
according to Circulating Tumor Cell Enumeration kit (630–0126,
revision A; Fluxion Biosciences). Hoechst 33342 dye, anti-CD45+Cy3
and FITC-conjugated anti-Cytokeratin (CK-7, −8, −18 and −19) were
used to identify cells, which were counted in a fluorescence
microscope (Nikon Eclipse E400; Nikon Corporation, Tokyo, Japan).
Cells showing Hoechst+/CD45-/CKs+ staining were regarded as CTC
(not shown).
CTC detection by flow cytometry
Each blood sample was divided into 18 different
tubes with two different antibodies added to each tube. The total
blood volume could not be analyzed together with all antibodies
simultaneously, which may fail to identify CTC in tubes without
appropriate antibodies. Arterial blood samples from 7 cancer
patients and venous blood from 10 healthy individuals were analyzed
by flowcytometry (FACS) for the presence of CTC-markers, although
our protocol did not allow quantification of the number of
marker-positive cells. Cell pellets were resuspended in 1,800 µl
PBS, mixed by pipetting and aliquot into 18 tubes. Direct
conjugated antibodies [EpCAM FITC/PE (CD326,
130-080-301/130-091-253; Miltenyi Biotec GmbH, Bergisch Gladback,
Germany), MIC-A FITC/PE (MCA2403FT; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA/12-5788-42; eBioscience; Thermo Fisher
Scientific, Inc.), CD133 PE (AC133, 130-080-801; Miltenyi Biotec
GmbH), CD34 FITC (BD bioscience 555821), VAP-1 PE (TK8-14,
sc-33670; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)] were
added including negative controls (BD Biosciences, Franklin Lakes,
NJ, USA; Simul test FITC/PE, 382409), and samples were incubated
for 30 min in room temperature. Cells were washed with 1 ml of
PBS/BSA 5% (Sigma-Aldrich; Merck KGaA) and centrifuged at 300 × g
for 5 min at room temperature, resuspended in 200 µl PBS and added
to a 96-wells plate. 1 ml of 0.1% Saponin PBS/BSA 5% (Saponin;
Sigma-Aldrich; Merck KGaA) was added to 6 tubes for indirect
conjugated antibodies (Cytokeratin 18 and 19), and removed by
centrifugation at 300 × g for 5 min in room temperature.
Cytokeratin antibodies were added and samples were incubated for 30
min at 4°C. Blank control samples lacked primary antibody. Cells
were washed with 1 ml of 0.1% Saponin PBS/BSA 5%, centrifuged at
300 × g for 5 min at room temperature, secondary antibody (diluted
1:5 in PBS/BSA 5%) was added and samples were incubated 30 min at
4°C. Cells were washed with 1 ml of 0.1% Saponin PBS/BSA 5%,
centrifuged at 300 × g for 5 min at room temperature, resuspended
in 200 µl PBS and added to a 96-wells plate. Samples were run in
FACS guava easyCyte HT (EMD Millipore, Billerica, MA, USA) and
analyzed by Guava Software® (EMD Millipore).
Statistical analysis
Results are presented as the mean ± SD or SEM as
indicated. Statistical testing among groups was performed using
analysis of variance with Fisher's protected least significant
difference post hoc test (Statview 5.0.1; SAS Institute, Inc.,
Cary, NC, USA). P<0.05 was considered to indicate a
statistically significant difference and P<0.10 a trend to
significance in two-sided tests.
Results
Immune-magnetic enrichment of CTC by
IsofluxR
Portal and arterial blood samples were analyzed in
10 patients with Immune-magnetic enrichment with Isoflux. Ten
patients showed between 0 and 5 CTC in arterial blood and between 0
and 10 CTC in portal blood. Seven patients showed CTC in both
portal and arterial blood, while two patients showed no CTC in
either portal or arterial blood. In one patient CTC was detected in
portal blood, but not in arterial blood. Corresponding tissue
analyses showed no signs of tumor dissemination to regional lymph
nodes in CTC negative patients (Tables
I and II). The amount of CTC was
significantly higher in portal blood [58±14 per 100 ml, (SE)]
compared to arterial blood [24±7 per 100 ml, (SE)] equivalent to a
fractional uptake of at least 40% across liver- and lung
compartments in patients with periampullary tumors (P<0.05;
Table III). Proliferation Ki-67
index was 17.8±5.6% (SE) in the tumors (Table III).
 | Table III.CTCs in portal and arterial blood
from cancer patients associated with the tumor mass and Ki-67 index
of tumor cells. |
Table III.
CTCs in portal and arterial blood
from cancer patients associated with the tumor mass and Ki-67 index
of tumor cells.
| Variable | Tumor volume,
cm3 | Ki-67, % | CTC, amount in
portal samplesc | CTC, amount in
arterial samplesc | CTC, portal
concentration per 100 ml | CTC, arterial
concentration per 100 ml |
|---|
| Patients
(n=10) | 26.6±10.1 | 17.8±5.6 |
4.4±1.0a | 1.7±0.5 | 58±14b | 24±7 |
Flow cytometry
Portal and arterial blood were evaluated in 7 cancer
patients with FACS. All these patients were positive to MIC-A and
EpCAM, in both portal and arterial blood. All patients except one
were also positive or weakly positive to CD133 (Table IV). Corresponding histopathology
showed tumor dissemination to regional lymph nodes (Table I).
Overall the combination of Isoflux and FACS
measurements indicated that present enrichments of CTC from blood
were based on highly specific epitopes (cell markers), while our
subsequent quantification markers may have been less than optimal.
None of our 10 healthy blood donors were positive to any of the
markers in FACS analyses (not shown).
Discussion
The importance of CTC detection has deserved great
interest as a possibility to collect liquid tissue samples from
patients where direct tissue sampling is difficult. CTC are,
however, extremely rare compared to the number of other blood
cells, which makes them difficult to identify, count and analyze
(24). The validity and relevance of
achieved information is thus dependent on the specificity of the
markers suggested to be representative for CTC from different
tissue origin in a certain condition; i.e., disease stage,
treatments etc. In the present investigation CTC were detected by
FACS analyses in all investigated blood samples from our cancer
patients, positive to EpCAM, MIC-A and CD133, with high
specificity, since all individuals in a normal group of blood
donors were negative to all applied markers. A combination of
markers in a panel may then be recommended in future studies on
pancreatic carcinoma. Our FACS analyses confirmed that our applied
and fixed markers in the Isoflux platform for immunomagnetic
enrichments of CTC were appropriate for quantitative estimates of
CTC in the present methodological evaluation.
EpCAM is a protein in the membrane surrounding
epithelial cells, contributing to cell adhesion. Epithelial cells
are normally not found in the blood circulation. Therefore, EpCAM
are considered specific for CTC, since blood cells do not express
EpCAM (25). A problem with
epithelial-based methods for CTC detection may be epithelial to
mesenchymal transition (EMT), since EpCAM may not be expressed at
cell surface of CTC when EMT occurs. There is increasing evidence,
that EMT is important for increased aggressiveness, metastatic
potential and drug resistance in cancers of epithelial origin,
including pancreatic cancer (26–28). Thus,
EMT may explain falsely low numbers of CTC detected by the Isoflux
system in patients. Another circumstance may be that CTC are
detected by CK-7, −8, −18, and −19 in the Isoflux system.
Cytokeratin antibodies −18, −19 were applied in our FACS analyses,
but found to be negative in most of the samples (Table IV), perhaps due to down regulation of
epithelial markers in EMT (29); or
to less than standardized methods and optimal biomarkers for CTC
detection in pancreatic cancer, although the presence of CK-7 in
Isoflux enrichment may be compensatory (8,23).
However, significant differences in numbers of cells between portal
and arterial blood from the same patients should be less hampered
by the use of less than optimal markers, although marker
combinations with high sensitivity and specificity should always be
strived for in future studies.
CTC in portal blood from patients with pancreatic
cancer has been reported earlier (30,31). With
the use of a CellSearchR system, a cutoff amount of 5
CTC or more is utilized to define results as significantly above
blank levels, in patients with breast and prostate cancer (32,33), while
there is no agreed cut-off level in patients with pancreatic
cancer. Again, simultaneous measurements of CTC in portal and
arterial blood should make the level of blank cut off levels less
critical, but may impact the sensitivity to determine statistical
differences in fractional uptake across blood compartments.
False positive results using epithelial markers of
CTC are well-recognized in the literature, since antibodies
designed for epithelial cells occasionally may stain both
hematopoietic and plasma cells (34).
Also, non-malignant cells with EpCAM+, CK+ and CD 45-may appear in
the circulation of individuals with benign conditions such as
inflammation and intestinal polyps (35). Our FACS analyses provided various
antibodies with purpose to evaluate the presence of biomarkers in
pancreatic cancer, such as MHC class I polypeptide-related sequence
A (MIC-A); a stress-inducible glycoprotein expressed as a
trans-membrane protein or released as a soluble protein and binding
to a receptor expressed in natural killer cells and various T
cells. In a variety of epithelial malignancies MIC-A expression is
increased, including pancreatic cancer, and correlate with the
extent of tumor burden (36,37). CD133 is a trans-membrane glycoprotein
expressed on the cell surface and found on ductal cells of
pancreatic tumors. In earlier studies CD133 expression was
significantly associated with lymphatic metastasis and prognosis in
pancreatic cancer (38). CD133 is
regarded a cancer stem cell marker with evidence that CD133
expression contributes to EMT induction and metastasis in
pancreatic cancer (39,40). Thus, optimal combinations of markers
for detection and counting of CTC in portal and arterial blood from
periampullary cancers remain to be determined.
With all possible limitations in mind we confirmed a
statistically significant difference in the amount of CTC in
portal-vs. arterial blood with the Isoflux method in a limited
number of patients at surgery aimed at cure. This difference
indicates that more tumor cells appeared in the portal circulation
compared with the level of tumor cells in the arterial compartment
where cells are considered to immediate and complete mixing; i.e.,
the same number of cells in any simultaneously collected arterial
blood sample within the body. A portal-arterial difference
indicates that CTC appear either from the tumors, as expected, or
from any other tissue in the splanchnic bed; or that tumor cells
disappear from the circulation across liver-lung compartments; a
potential trap for subsequent metastases. This represents a
different concept compared to measurements of portal-venous
differences (13,15,30), which
include a variety of additional tissues as muscles, nerves,
bone-marrow among others, that may provide CTC with different
tissue origins.
CTC are extremely rare in blood compared to other
cells, but in a surgical perspective it may be that time course of
CTC appearance in portal blood should predict the intra-and
perioperative risk for establishments of hepatic metastases
particularly. Therefore, it was interesting to estimate how CTC
related to tumor burden. An estimated and assumed average portal
blood flow around 1,208 ml/min (17);
a mean tumor weight of 26.6 g and a fractional level of at least
50% tumor cells, with 80% tumor cell viability (estimated by
microscopy) in our tumor tissue specimens, would translate into a
viable tumor cell burden in pancreas corresponding to
11*109 cells on average in our patients
(26.6×109×0.8×0.5), since a tumor reaching the size of 1
cm3 (approximately 1 g wet weight) is usually estimated
to contain around 109 cells (18,19). This
would correspond to an implied appearance rate of
0.59*106 tumor cells per day [(0.58–0.24)×1208×60×24)]
into the portal circulation; or corresponding to a tumor mass
fraction of 0.005% per day at operation
(0.59×106/11×109). This implies a low release
fraction rate of tumor cells and probably a low efficient process
to support metastatic and systemic disease progression considering
experimental evidence with less than 2% of injected tumor cells in
experimental liver and lung metastases in mice following bolus and
intravenously injected malignant cells (41). Seen together our estimates suggest a
risk fraction for metastases at the level of 0.00010% per day of
all CTCs (0.00005×0.02). Thus, it seems that spread of
periampullary cancer appears to be a low-efficient process, perhaps
in agreement with others suggestion that pancreatic carcinoma has a
total disease progression across 20 years, with only late clinical
symptoms (42). Our figures equal to
a release of approximately 410 CTC per minute during surgical
resection and tumor removal, which is a quite new perspective of
the risk for per-operative spread during resections.
With above perspectives, it may also be interesting
to consider the meaning of Ki-67 proliferation index; i.e., the
number of proliferating viable cells in percent of all evaluated
tumor cells. Then, the question is the rate unit? Assuming that
26.6±10 g of tumor mass with Ki-67 index of 17.8±5.6% (Table III), corresponding to net tumor
growth during 3 years is equal to 0.97*107 tumor cells
per day (26.6×109×0.8×0.5/3×365); which may suggest that
the proliferation index has a time unit corresponding to at least
17 days, to be compared to a maximum release of tumor cells into
the portal circulation of 0.59×106 cells per day
[(0.97×107+0.59×106)/0.59×106].
This implies that cell cycle rates may be low in pancreatic
malignant cells, perhaps in agreement with the lack of correlation
between tumor volumes and the release of tumor cells into portal
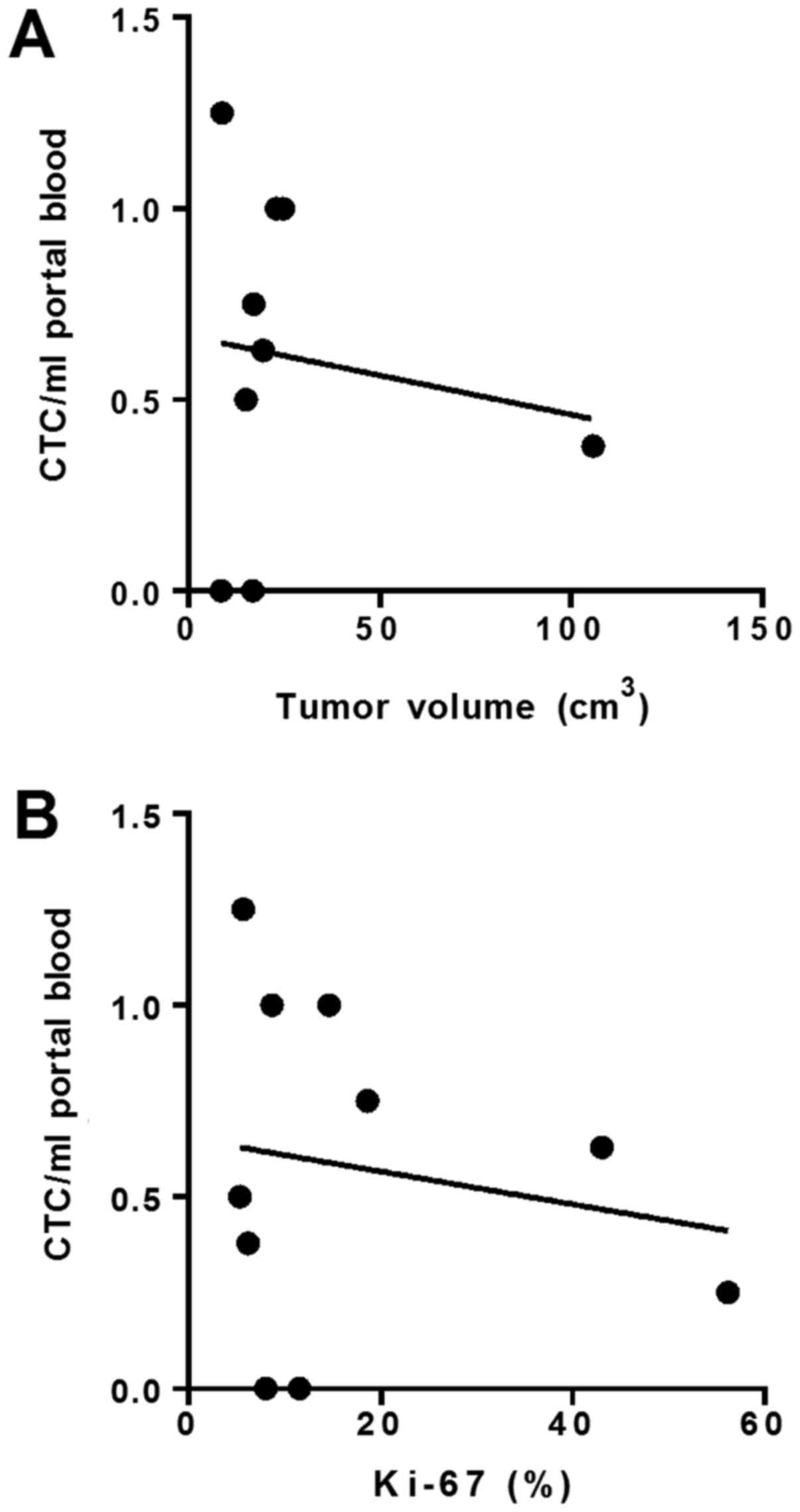
blood (Fig. 1).
In conclusion, the present study applied a ready to
use and commercially available instrument (IsofluxR) to
confirm a statistically significant fractional uptake of presumably
malignant cells from periampullary tumors during surgical
resections aimed at cure, assessed in a limited number of patients
with pancreatic malignancy. This finding may represent a future
surgical model to define and characterize tumor cells that
disappear across hepatico-lung compartments, which may in part
represent tumor clones with high metastatic potential during
pancreatic resection. A next step should be to confirm that CTC,
retained across the liver or lungs during a first circulation
passage, are truly malignant originating from the gastro-intestinal
solid tumor.
Acknowledgements
The authors would like to thank Westburg/Fluxion
Bioscience for providing technical support regarding the Isoflux
equipment and Director M. Wolving and Mr. S. De Lara, biomedical
scientists at the Department of Pathology, Sahlgrenska University
Hospital (Gothenburg, Sweden), for providing assistance with
complementary tissue analyses. They would also like to thank Dr
Christina Biörserud (Department of Surgery, and Gastrosurgical
Research and Education, Institute of Clinical Sciences, Sahlgrenska
Academy, University of Gothenburg, Gothenburg, Sweden) for
providing practical support in the selection of patients.
Funding
The present study was supported by grants from
Cancerfonden (grant nos. 2015/400 and 2017/401) and the Albert
Ekmans foundation.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
KL and PN are responsible for the conception and
design of the study, as well as obtaining financial support; AA and
BI were responsible for performing isoflux analyses; AA and AN
performed the FACS analyses; CV and JBF conducted the Ki-67
analyses; CE and CV collected the blood samples, and CE was in
charge of collecting the portal and arterial blood samples during
the operations. CV, AA and KL drafted and wrote the manuscript. All
authors participated in revising the manuscript.
Ethics approval and consent to
participate
The Regional Ethical Review Board of Gothenburg
approved the present study (462–11) and all participants provided
written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Steliarova-Foucher E,
Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D and
Bray F: Cancer incidence and mortality patterns in Europe:
Estimates for 40 countries in 2012. Eur J Cancer. 49:1374–1403.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Azevedo AS, Follain G, Patthabhiraman S,
Harlepp S and Goetz JG: Metastasis of circulating tumor cells:
Favorable soil or suitable biomechanics, or both? Cell Adh Migr.
9:345–356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rhim AD, Mirek ET, Aiello NM, Maitra A,
Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK,
Vonderheide RH, et al: EMT and dissemination precede pancreatic
tumor formation. Cell. 148:349–361. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Giuliano M, Giordano A, Jackson S, De
Giorgi U, Mego M, Cohen EN, Gao H, Anfossi S, Handy BC, Ueno NT, et
al: Circulating tumor cells as early predictors of metastatic
spread in breast cancer patients with limited metastatic
dissemination. Breast Cancer Res. 16:4402014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Iinuma H, Watanabe T, Mimori K, Adachi M,
Hayashi N, Tamura J, Matsuda K, Fukushima R, Okinaga K, Sasako M
and Mori M: Clinical significance of circulating tumor cells,
including cancer stem-like cells, in peripheral blood for
recurrence and prognosis in patients with Dukes' stage B and C
colorectal cancer. J Clin Oncol. 29:1547–1555. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thalgott M, Heck MM, Eiber M, Souvatzoglou
M, Hatzichristodoulou G, Kehl V, Krause BJ, Rack B, Retz M,
Gschwend JE, et al: Circulating tumor cells versus objective
response assessment predicting survival in metastatic
castration-resistant prostate cancer patients treated with
docetaxel chemotherapy. J Cancer Res Clin Oncol. 141:1457–1464.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tjensvoll K, Nordgård O and Smaaland R:
Circulating tumor cells in pancreatic cancer patients: Methods of
detection and clinical implications. Int J Cancer. 134:1–8. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Okubo K, Uenosono Y, Arigami T, Mataki Y,
Matsushita D, Yanagita S, Kurahara H, Sakoda M, Kijima Y, Maemura K
and Natsugoe S: Clinical impact of circulating tumor cells and
therapy response in pancreatic cancer. Eur J Surg Oncol.
43:1050–1055. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xie ZB, Yao L, Jin C and Fu DL:
Circulating tumor cells in pancreatic cancer patients: Efficacy in
diagnosis and value in prognosis. Discov Med. 22:121–128.
2016.PubMed/NCBI
|
|
11
|
Barriere G, Riouallon A, Renaudie J,
Tartary M and Rigaud M: Mesenchymal characterization: Alternative
to simple CTC detection in two clinical trials. Anticancer Res.
32:3363–3369. 2012.PubMed/NCBI
|
|
12
|
Yu M, Bardia A, Wittner BS, Stott SL, Smas
ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, et al:
Circulating breast tumor cells exhibit dynamic changes in
epithelial and mesenchymal composition. Science. 339:580–584. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Arnoletti JP, Zhu X, Almodovar AJ,
Veldhuis PP, Sause R, Griffith E, Corpus G, Chang JC, Fanaian N and
Litherland SA: Portal venous blood circulation supports
immunosuppressive environment and pancreatic cancer circulating
tumor cell activation. Pancreas. 46:116–123. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tien YW, Kuo HC, Ho BI, Chang MC, Chang
YT, Cheng MF, Chen HL, Liang TY, Wang CF, Huang CY, et al: A high
circulating tumor cell count in portal vein predicts liver
metastasis from periampullary or pancreatic cancer: A high portal
venous CTC count predicts liver metastases. Medicine (Baltimore).
95:e34072016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Connor AA, McNamara K, Al-Sukhni E, Diskin
J, Chan D, Ash C, Lowes LE, Allan AL, Zogopoulos G, Moulton CA and
Gallinger S: Central, but not peripheral, circulating tumor cells
are prognostic in patients undergoing resection of colorectal
cancer liver metastases. Ann Surg Oncol. 23:2168–2175. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rosenbaum MW, Cauley CE, Kulemann B, Liss
AS, Castillo CF, Warshaw AL, Lillemoe KD, Thayer SP and Pitman MB:
Cytologic characteristics of circulating epithelioid cells in
pancreatic disease. Cancer Cytopathol. 125:332–340. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bradley SE, Ingelfinger FJ, Bradley GP and
Curry JJ: The estimation of hepatic blood flow in man. J Clin
Invest. 24:890–897. 1945. View Article : Google Scholar
|
|
18
|
DeVita VT Jr, Young RC and Canellos GP:
Combination versus single agent chemotherapy: A review of the basis
for selection of drug treatment of cancer. Cancer. 35:98–110. 1975.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Del Monte U: Does the cell number 10(9)
still really fit one gram of tumor tissue? Cell Cycle. 8:505–506.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Harb W, Fan A, Tran T, Danila DC, Keys D,
Schwartz M and Ionescu-Zanetti C: Mutational analysis of
circulating tumor cells using a novel microfluidic collection
device and qPCR assay. Transl Oncol. 6:528–538. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu L, Mao X, Imrali A, Syed F, Mutsvangwa
K, Berney D, Cathcart P, Hines J, Shamash J and Lu YJ: Optimization
and evaluation of a novel size based circulating tumor cell
isolation system. PLoS One. 10:e01380322015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miller MC, Doyle GV and Terstappen LW:
Significance of circulating tumor cells detected by the CellSearch
system in patients with metastatic breast colorectal and prostate
cancer. J Oncol. 2010:6174212010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Goldstein NS and Bassi D: Cytokeratins 7,
17 and 20 reactivity in pancreatic and ampulla of vater
adenocarcinomas. Percentage of positivity and distribution is
affected by the cut-point threshold. Am J Clin Pathol. 115:695–702.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Joosse SA and Pantel K: Biologic
challenges in the detection of circulating tumor cells. Cancer Res.
73:8–11. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Munz M, Baeuerle PA and Gires O: The
emerging role of EpCAM in cancer and stem cell signaling. Cancer
Res. 69:5627–5629. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Arumugam T, Ramachandran V, Fournier KF,
Wang H, Marquis L, Abbruzzese JL, Gallick GE, Logsdon CD, McConkey
DJ and Choi W: Epithelial to mesenchymal transition contributes to
drug resistance in pancreatic cancer. Cancer Res. 69:5820–5828.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shah AN, Summy JM, Zhang J, Park SI,
Parikh NU and Gallick GE: Development and characterization of
gemcitabine-resistant pancreatic tumor cells. Ann Surg Oncol.
14:3629–3637. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Boyer B, Vallés AM and Edme N: Induction
and regulation of epithelial-mesenchymal transitions. Biochem
Pharmacol. 60:1091–1099. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu S, Liu S, Liu Z, Huang J, Pu X, Li J,
Yang D, Deng H, Yang N and Xu J: Classification of circulating
tumor cells by epithelial-mesenchymal transition markers. PLoS One.
10:e01239762015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bissolati M, Sandri MT, Burtulo G, Zorzino
L, Balzano G and Braga M: Portal vein-circulating tumor cells
predict liver metastases in patients with resectable pancreatic
cancer. Tumour Biol. 36:991–996. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Catenacci DV, Chapman CG, Xu P, Koons A,
Konda VJ, Siddiqui UD and Waxman I: Acquisition of portal venous
circulating tumor cells from patients with pancreaticobiliary
cancers by endoscopic ultrasound. Gastroenterology.
149:1794–1803.e4. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jiang ZF, Cristofanilli M, Shao ZM, Tong
ZS, Song EW, Wang XJ, Liao N, Hu XC, Liu Y, Wang Y, et al:
Circulating tumor cells predict progression-free and overall
survival in Chinese patients with metastatic breast cancer,
HER2-positive or triple-negative (CBCSG004): A multicenter,
double-blind, prospective trial. Ann Oncol. 24:2766–2772. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shaffer DR, Leversha MA, Danila DC, Lin O,
Gonzalez-Espinoza R, Gu B, Anand A, Smith K, Maslak P, Doyle GV, et
al: Circulating tumor cell analysis in patients with progressive
castration-resistant prostate cancer. Clin Cancer Res.
13:2023–2029. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mavroudis D: Circulating cancer cells. Ann
Oncol. 21 Suppl 7:vii95–vii100. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pantel K, Deneve E, Nocca D, Coffy A,
Vendrell JP, Maudelonde T, Riethdorf S and Alix-Panabières C:
Circulating epithelial cells in patients with benign colon
diseases. Clin Chem. 58:936–940. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dambrauskas Z, Svensson H, Joshi M,
Hyltander A, Naredi P and Iresjö BM: Expression of major
histocompatibility complex class I-related chain A/B (MICA/B) in
pancreatic carcinoma. Int J Oncol. 44:99–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xu X, Rao GS, Groh V, Spies T, Gattuso P,
Kaufman HL, Plate J and Prinz RA: Major histocompatibility complex
class I-related chain A/B (MICA/B) expression in tumor tissue and
serum of pancreatic cancer: Role of uric acid accumulation in
gemcitabine-induced MICA/B expression. BMC Cancer. 11:1942011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Maeda S, Shinchi H, Kurahara H, Mataki Y,
Maemura K, Sato M, Natsugoe S, Aikou T and Takao S: CD133
expression is correlated with lymph node metastasis and vascular
endothelial growth factor-C expression in pancreatic cancer. Br J
Cancer. 98:1389–1397. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nomura A, Banerjee S, Chugh R, Dudeja V,
Yamamoto M, Vickers SM and Saluja AK: CD133 initiates tumors,
induces epithelial-mesenchymal transition and increases metastasis
in pancreatic cancer. Oncotarget. 6:8313–8322. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ding Q, Miyazaki Y, Tsukasa K, Matsubara
S, Yoshimitsu M and Takao S: CD133 facilitates
epithelial-mesenchymal transition through interaction with the ERK
pathway in pancreatic cancer metastasis. Mol Cancer. 13:152014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fidler IJ: Tumor heterogeneity and the
biology of cancer invasion and metastasis. Cancer Res.
38:2651–2660. 1978.PubMed/NCBI
|
|
42
|
Yachida S, Jones S, Bozic I, Antal T,
Leary R, Fu B, Kamiyama M, Hruban RH, Eshleman JR, Nowak MA, et al:
Distant metastasis occurs late during the genetic evolution of
pancreatic cancer. Nature. 467:1114–1117. 2010. View Article : Google Scholar : PubMed/NCBI
|